Cofluctuation analysis reveals aberrant default mode network patterns in adolescents and youths with autism spectrum disorder
Lei Li and Xiaoran Su contributed equally to this work.
Funding information: Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine, Grant/Award Number: ZYYCXTD-D-202003; Fundamental Research Funds for Central Universities, Grant/Award Number: ZYGX2019Z017; National Social Science Foundation of China, Grant/Award Number: 20&ZD296; National Natural Science Foundation of China, Grant/Award Numbers: 62036003, 81871432, 82121003
Abstract
Resting-state functional connectivity (rsFC) approaches provide informative estimates of the functional architecture of the brain, and recently-proposed cofluctuation analysis temporally unwraps FC at every moment in time, providing refined information for quantifying brain dynamics. As a brain network disorder, autism spectrum disorder (ASD) was characterized by substantial alteration in FC, but the contribution of moment-to-moment-activity cofluctuations to the overall dysfunctional connectivity pattern in ASD remains poorly understood. Here, we used the cofluctuation approach to explore the underlying dynamic properties of FC in ASD, using a large multisite resting-state functional magnetic resonance imaging (rs-fMRI) dataset (ASD = 354, typically developing controls [TD] = 446). Our results verified that the networks estimated using high-amplitude frames were highly correlated with the traditional rsFC. Moreover, these frames showed higher average amplitudes in participants with ASD than those in the TD group. Principal component analysis was performed on the activity patterns in these frames and aggregated over all subjects. The first principal component (PC1) corresponds to the default mode network (DMN), and the PC1 coefficients were greater in participants with ASD than those in the TD group. Additionally, increased ASD symptom severity was associated with the increased coefficients, which may result in excessive internally oriented cognition and social cognition deficits in individuals with ASD. Our finding highlights the utility of cofluctuation approaches in prevalent neurodevelopmental disorders and verifies that the aberrant contribution of DMN to rsFC may underline the symptomatology in adolescents and youths with ASD.
1 INTRODUCTION
Autism spectrum disorders (ASDs) refer to neurodevelopmental disorders characterized by impairments in social communication and interaction and stereotyped behavior and interests (American Psychiatric Association, 2013). Although these disorders have common central features, including poor social reciprocity, lack of emotional interaction, and inability to develop relationships, their broad range and individual variability contribute to the challenges in ASDs diagnosis and treatments (Elsabbagh & Johnson, 2016; Hobson & Meyer, 2005).
Previous neuroimaging studies identified functional connectivity (FC) for assessing the temporal correlation among brain areas (Rogers et al., 2007). Previous studies reported that ASD was associated with altered FC patterns in a pair of cortical midline brain regions, namely, the posterior cingulate cortex (PCC) and ventromedial prefrontal cortices, which constituted the hubs of the default mode network (DMN) (Assaf et al., 2010; Sutterer & Tranel, 2017). This network involved internally directed attention, self-referential thought, and social cognition (Uddin et al., 2007). Many studies showed that the self-reflective and social cognition deficits observed in ASD were associated with the alterations in FC within DMN nodes and from the DMN nodes to the entire brain (Washington et al., 2014; Yerys et al., 2015). Increased within network connectivity between core DMN nodes was reported in children with ASD (Lynch et al., 2013; Uddin et al., 2013). Moreover, some study found that participants with ASD exhibited over-connectivity between the medial and anterolateral temporal cortex and an aberrantly weak connectivity of the precuneus with visual cortex and basal ganglia (Lynch et al., 2013). This study also reported a significant correlation between aberrant connectivity patterns and the severity of social impairment.
Existing FC methods typically assume that time series maintain their characteristics over time. Therefore, they are usually estimated over the course of an entire scan session (Rogers et al., 2007). Recent evidence emphasized the importance of dynamic functional interactions, in which sliding window techniques were used to track fluctuations in FC across time. An arbitrary fixed window length was determined prior to analyses, and FC matrices were subsequently calculated for observations within that window. The window slides along the timeseries, and states are clustered on the basis of the dynamic FC matrices of each window (Allen et al., 2014). One study found hypervariability in ASD across numerous brain regions, thereby suggesting the presence of atypical network connectivity in multiple transient states,while falling short of statistical significance in static analysis (Mash et al., 2019). Other studies demonstrated dynamic characteristic as a function of ASD symptoms. Global alterations in dynamic FC density (FCD) variabilities and atypical dynamics of intra- and interhemispheric FCD variabilities were found in ASD (Guo et al., 2020). The within-network variance of DMN was significantly associated with the symptom severity of ASD. Besides, the atypical dynamic FC variance between DMN and sensorimotor cortex was associated with social deficits in ASD (He et al., 2018). In addition, the increased variance of widespread long-range dynamic functional connections was found in ASD, thereby suggesting that greater dynamic variance was a potential biomarker of ASD (Chen et al., 2017). Additionally, abnormal quantification of metrics of sliding window analysis, including dwell time (Yao et al., 2016) and transitions between brain states (De Lacy et al., 2017; Watanabe & Rees, 2017), was found in participants with ASD, hence supporting the evidence that ASD was characterized by transient states. Although early studies on dynamic FC and the symptom severity in ASD produced some results, the windowing procedure induced a blurring effect, thus making the localization of the time-varying connectivity in time and the assessment of the contributions made by each single frame impossible (Hindriks et al., 2016). Some emerging methods, such as coactivation patterns (CAPs), allow brain dynamics to be characterized at each single time point (Liu & Duyn, 2013). However, these approaches generally require the specification of a seed region or a threshold to determine high-activation frames. Thus, explaining precisely how these coactivity patterns are combined to give rise to the entire FC is elusive (Preti et al., 2017).
Recently, a comprehensive and mathematical method was proposed for the exact decomposition of FC into its frame-wise contributions, explicitly linking instantaneous patterns of cofluctuations to FC over long timescales (Esfahlani et al., 2020). They also found that FC and its system-level organization were driven by cofluctuations during high-amplitude frames, which were underpinned by the activation of the default mode and control networks. Meanwhile, 10 adults' data from the Midnight Scan Club (MSC, 10 resting state scans per subjects[Gordon et al., 2016]) were used to measure differential identifiability, which indicated how much more similar FC patterns were to intra-subject than to inter-subject. The results indicated that the cofluctuations of high-amplitude frames carried reliably individualized and distinguishable information. Despite this methodological innovation, latest studies produced interesting results. A recent study used two independent sampling datasets (i.e., MSC and MyConnectome [Laumann et al., 2015], a project in which a single individual was scanned >100 times) to demonstrate that the FC of a few high-amplitude frames recapitulated time-averaged FC accurately. They also extended the prior study by classifying a small subset of high-amplitude frames as “event” (Betzel et al., 2022b). The use of cofluctuation analysis showed that functional dynamic in high-amplitude frames were partly shaped by the modular organization of structural connectivity (Pope et al., 2021).
Taken together, previous studies provided evidence of the abnormal FC of the DMN in ASD, but focused mostly on the entire scan session. The contribution of moment-to-moment-activity cofluctuations to the overall dysfunctional connectivity pattern in ASD remains poorly understood. Notably, adolescence or youth (ages 8–24) is a particularly critical window for social development, thus it is an important time to investigate the neurobiological mechanism implicated in social behaviors. The current study applied the cofluctuation analysis to rs-fMRI in a large multisite sample of adolescents and youths from the ABIDE repository (ASD = 354, typically developing controls [TD] = 446). We speculate that adolescents and youths with ASD would show abnormal cofluctuations in a few frames, and such abnormalities may underline their social deficits.
2 MATERIALS AND METHODS
2.1 Participants
Original rs-fMRI data and phenotypes were downloaded from the ABIDE repository (ABIDEs I and II, http://fcon_1000.projects.nitrc.org/indi/abide/) (Di Martino et al., 2014; Martino et al., 2017). The inclusion criteria were detailed as follows: 1) subjects between 8 and 22 years of age; 2) participants with complete cortical coverage and available full IQ (FIQ), handedness, and eye state during scanning scores; 3) subjects with low levels of head motion during scanning (i.e., maximum motion <2 mm translation and 2° rotation and less than 30% frames with high frame-wise displacement [FD], as illustrated in preprocessing); 4) full anatomical and high-quality brain images determined by manual checking; 5) well-matched dataset between the ASD and TD groups for each site generated using a data-driven algorithm that maximizes the p values of group difference in terms of age, handedness, FIQ, eye status, and mean FD; and 6) sites with more than ten subjects per group left after the aforementioned selection procedure. Finally, a well-matched dataset of 800 subjects (ASD = 354, TD = 446) from 16 sites was constructed. The demographic data is shown in Table 1.
| ASD (n = 354) | TD (n = 446) | t/x2 | p value | |
|---|---|---|---|---|
| Age (years) | 13.47 ± 3.62 | 13.40 ± 3.26 | t(798) = 0.29 | .76a |
| Sex (male/female) | 301/53 | 382/64 | χ2 = 0.053 | .82b |
| Handedness (right/left/mixed) | 291/34/29 | 387/29/30 | χ2 = 1.25 | .54b |
| FIQ | 105.55 ± 17.07 | 112.97 ± 17.07 | t(798) = −7.00 | <.05a |
| Mean FD (mm) | 0.15 ± 0.05 | 0.63 ± 0.05 | t(798) = −1.26 | .21a |
| ADI_R (N = 257) | ||||
| Social | 19.68 ± 5.29 | |||
| Verbal | 15.41 ± 4.62 | |||
| RRB | 5.80 ± 3.30 | |||
| Onset | 3.03 ± 2.51 | |||
| ADOS gotham | ||||
| RRB (N = 204) | 2.03 ± 1.61 | |||
| Social (N = 227) | 8.03 ± 2.83 | |||
| Communication (N = 227) | 3.50 ± 1.64 | |||
| Total (N = 238) | 11.57 ± 4.03 | |||
- Abbreviations: ADI_R Autism Diagnostic Interview-Revised; ADOS, the Autism Diagnostic Observation Schedule; ASD, autism spectrum disorder; FD, frame-wise displacement; FIQ, the full-scale intelligence quotient; RRB, restricted and repetitive behaviors; TD, typically developing controls.
- N number of subjects.
- a The p value was obtained by two sample t-test, two tailed;
- b The p value was obtained by χ2 test.
2.2 Data preprocessing
The rs-fMRI data were preprocessed using the advanced edition of the data-processing assistant for rs-fMRI (DPARSFA v4.1, http://rfmri.org/DPARSF) toolbox in MATLAB (Yan & Zang, 2010). The first ten images of each subject were removed to ensure a steady-state longitudinal magnetization. Slice-timing correction and realignment were applied on the remaining functional images to correct the temporal differences and head motion. The corrected data were spatially normalized to the Montreal Neurological Institute stereotaxic space by using 12-parameter affine linear transformation and nonlinear deformation and resampled to 3 × 3 × 3 mm3. This process enabled us to directly compare responses among adolescents, youths, and young adults. Previous studies suggested that anatomical differences among children as young as seven were small relative to the resolution of fMRI data, which supported the usage of a common space in a group with a broad age range (Bedny et al., 2015; Burgund et al., 2002). Subsequently, all the normalized functional images were smoothened using a 6-mm full-width at half-maximum Gaussian kernel and then detrended. Next, the linear trends were removed. Signals from white matter and cerebrospinal fluid and 24 rigid body motion parameters were regressed out of the data. Subsequently, a bandpass filter (0.01–0.1 Hz) was applied on the regressed time series. Finally, given that FC was sensitive to the confounding factor of head motion, scrubbing was performed for motion correction to reduce the negative influence. When the FD exceeded 0.5 mm, the value of the signal at that point was removed. Growing evidence showed that the global signal, especially in studies of ASD (Gotts et al., 2013), may also contain valuable information (Fox et al., 2009; Schölvinck et al., 2010). Therefore, the global signal regression (GSR) was not applied.
2.3 Regions-of-interest parcellation
In the current study, Schaefer et al.'s parcellation (Schaefer et al., 2018) scheme (resampled to MNI152NLin2009cAsym standard space) was used with 200 parcels, each of which was associated with one of brain network from Yeo et al.'s seven-network parcellation (Yeo et al., 2011), namely, the visual, somatomotor, dorsal attention, ventral attention, limbic, frontoparietal task control, and DMNs.
2.4 Cofluctuation time series
The strength of the FC between two brain regions was quantified as the Pearson correlation of their fMRI blood oxygen level-dependent (BOLD) time series, which was calculated as the mean value of their element-wise z-scores. The averaging step was omitted in the cofluctuation analysis. Let and be the time series recorded from voxels or parcels i and j, respectively. Similar to the Pearson correlation, we first obtained the z-score of each time series, , where and were the time averaged mean and SD, respectively. Subsequently, the cofluctuation of i with j was calculated as . This procedure was repeated for all pairs of parcels, thereby resulting in a set of cofluctuation (edge) time series. With N parcels, this set had pairs, each of length T. These elements represented the cofluctuation magnitude among the regions resolved at each moment in time.
A cofluctuation time series contains moments in time when many edges cofluctuate collectively. We identified these moments by calculating the amplitude (quantified by computing the root sum square [RSS]) across all cofluctuation time series and plotting this value as a function of time (Figure 1). We extracted the top 5% high-amplitude frames of all the time points (ordered by cofluctuation amplitude) and estimated the FC from those points alone. Then, we calculated the average RSS and activity patterns in the high-amplitude frames for each participant. In addition, the variances in the activity pattern of the high-amplitude frames in each group were obtained to characterize the fluctuation of these frames.
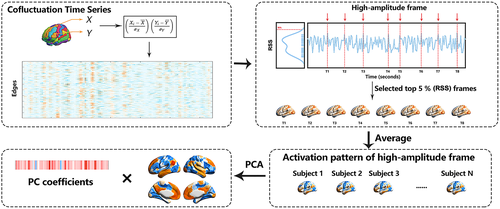
To understand what drove the high-amplitude frames better, we performed principal component analysis (PCA) on the activity patterns in the high-amplitude frames, which aggregated over all the subjects and scans. Then, the statistical significance of the regional PC scores was assessed nonparametrically by using permutation tests (Linting et al., 2011). The original data was permuted to obtain the permuted data set. The total number of possible permuted data set was , where was the number of participants, and was the number of the regional PC scores.
2.5 Statistical analysis
For the demographic data, the two-sample t test was used to evaluate the differences in age, FIQ, and mean FD. The χ2 test was performed on the handedness and the eye state.
In the current study, ComBat (Johnson et al., 2007) was used to reduce potential biases and non-biological variability induced by site and scanner effects. Notably, in the ComBat model, age, sex, handedness, mean FD, FIQ, and group as covariates were included to preserve important biological trends in the data and avoid overcorrection. ComBat harmonization analysis was performed using a publicly available MATLAB package hosted at https://github. com/Jfortin1/ComBatHarmonization (Yu et al., 2018). Next, the two-sample t test was conducted to assess the between-group differences (ASD vs. TD) in the RSS of the high-amplitude frames and the PCA coefficients. Additionally, two-way ANOVA was performed for PC1 coefficients using diagnosis (two levels: ASD and TD) and age (three levels: <12 years, 12–18 years and > 18 years) as between-subject factors. The gender, FIQ, handedness, and mean FD were taken as covariates in the model.
Given that the normality of data was vague, Spearman's correlation analysis was performed between the coefficient and social behavior scores for the ASD group. The significant threshold for multiple comparisons was set as FDR-corrected p < .05.
2.6 Reproducibility analysis
Given that several critical strategies and parameter selections might influence the findings, the reproducibility of our findings, including global signal regression and the percentage of selected top high-amplitude frames, was further validated. In addition, we performed within-Group PCA in each group.
3 RESULTS
3.1 Group differences in high-amplitude cofluctuation events
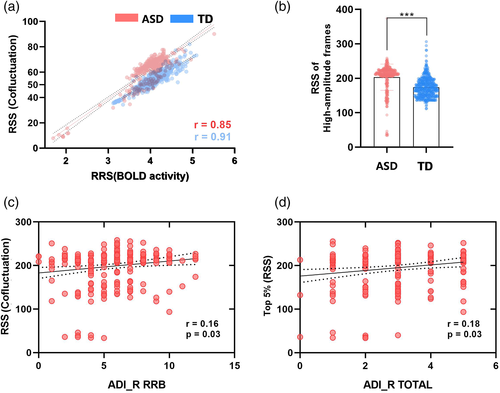
As a first point of results, we wanted to verify whether this mathematical approach enabled us to compare the cofluctuations of network organization with the fluctuations in the BOLD time series directly. Therefore, we calculated the RSS of the cofluctuation and z-scored fMRI BOLD time series. We found that across subjects, these time series were highly correlated in the ASD (r = 0.85) and TD groups (r = 0.91), indicating that high-amplitude frames had a nearly one-to-one correspondence with the high-amplitude BOLD fluctuations (Figure 2a). We found that the RSS of these high-amplitude frames was significantly higher (two-sample t-test, p < .0001; Figure 2b) in participants with ASD than those in the TD group. Additionally, we found a significant correlation (Figure 2c,d) between the RSS of high-amplitude frames and the restricted repetitive behaviors (RRB) (r = 0.16, p = .03) and the total score (r = 0.18, p = .03) in ADI-R (FDR-corrected).
3.2 Abnormal DMN patterns underlie the symptom severity in participants with ASD
We estimated the rsFC by only using the fMRI BOLD data for high-amplitude time points. Next, we calculated the similarity of the rsFC estimated during high-amplitude episodes with respect to the time-averaged rsFC estimated using the full time series. Findings showed that the high-amplitude networks were highly correlated with the rsFC, and this correlation was significantly lower in participants with ASD (r = 0.31 ± 0.15) than those in TD group (r = 0.46 ± 0.14, two-sample t-test, p < .0001; Figure 3a). Additionally, the between-group difference of variance in the activity pattern of the high-amplitude frames was determined by using two-sample t-test. In comparison with TD, the variance of the temporal cortex that belonged to the DMN was decreased in ASD (FDR, p < .05, Figure 3b).
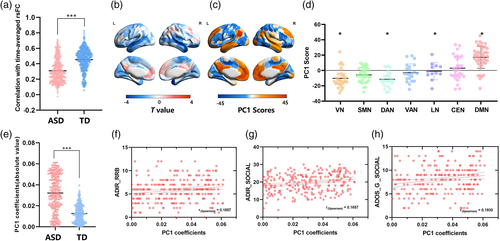
To better understand whether high-amplitude frames were underpinned by a specific brain activity pattern, we performed PCA on the activity patterns in high-amplitude frames in the ASD and TD groups. We focused on the first principal component (PC1), which explained 32% of the total variance. Then, we mapped the component scores for PC1 onto the cortical surface and found that PC1 corresponded to a mode of activity that emphasized correlated fluctuation of the DMN and anticorrelated fluctuations of the dorsal attention and visual network (Figures 3c,d). The significances of regional PC1 scores were obtained by permutation test (FDR-corrected, p < .05). The PC1 coefficients were much higher in participants with ASD than those in TD group (two-sample t-test, p < .0001; Figure 3e), indicating that the DMN was descriptive of primary activity patterns in participants with ASD but less in the TD group. Additionally, the abnormal coefficients were significantly associated with the social (Figure 3f) (r = 0.1857, p = .0241) and RRB scores (Figure 3g) (r = 0.1857, p = .0158) in ADI-R and social scores (r = 0.1930, p = .0158) in ADOS-G (Figure 3h). FDR-correction was used for multiple comparisons. The ANOVA results of PC1 coefficients exhibited significant diagnosis-related effects. However, no significant age-specific and diagnosis-by-age interaction effect was found in the coefficients (Table 2).
| Sum of squares | Mean squares (MS) | F | p value | |
|---|---|---|---|---|
| Main effect of age | 0.002 | 0.001 | 3.068 | .061 |
| Main effect of diagnosis | 0.027 | 0.027 | 164.818 | .000 |
| Diagnosis-by-age interaction effect | 0.000 | 0.000 | 2.930 | .087 |
In addition, as shown in Figure S1, except for PC1, other components of the PCA were not related to the DMN. Notably, we found that the second principal component (PC2) corresponded to the limbic network (Figure S1a,b), whereas the third principal component (PC3) corresponded to a mode of activity that delineated regions in ventral attention and somatomotor network (Figure S1c,d).
3.3 Reproducibility results
To evaluate the stability and reproducibility of results, we repeated the main analysis by adopting different strategies and parameter selections. As a first point of validation, we calculated the results in the data with GSR. We verified that the PC1 with GSR was similar to the results without GSR (Figure S2). We re-analyzed the selection of the different percentages of top high-amplitude frames by using a broad range of threshold (0%–60%) to estimate whether it affected the results. We found that the correlation between rsFC estimated during high-amplitude frames and the time-averaged rsFC was greater in ASD than in the TD group over a range of thresholds (Figure S3a). We also found that the RSS of high-amplitude frames was greater in ASD (Figure S3b). In addition, the PC1 score patterns were robust (Figure S3c–f).
We noted that another strategy for comparing the ASD and TD was to analyze them by adopting within-GroupPCA in each group. We found PC1group explained 66.6% of the total variance in the ASD group and 20.9% of that in the TD group. Although the PC1group scores of the two groups exhibited similar activities in DMN (Figure S4), we found statistically significant differences in the dorsal attention, limbic, and default mode networks (Figure S4g–h) (ASD > TD, FDR-corrected, p < 0.05). To explore whether the lower explained variance of PC1group of TD was due to an over-decomposition of the components, we also retained the other principal components in the within-Group PCA. The first 10 components explained 84.1% of the total variance in the ASD group (Figure S4c), but only explained 63.53% of the total variance in TD (Figure S4f). Moreover, although the PC3group of the TD also emphasized the activity in the DMN, the remaining principal component in the ASD and TD groups did not exhibit this pattern (Figure S5). Viewed collectively, DMN made an overwhelming contribution to the FC in participants with ASD (PC1group) compared with the TD group (PC1group + PC3group).
4 DISCUSSION
Here, we used a cofluctuation approach to temporally unwrap the Pearson correlations to examine the relationships between the functional brain dynamics and dysfunctional symptomatology in ASD. This simple procedure enables us to decompose FC into individual co-fluctuation frames. The entire brain's FC and its system-level organization could be represented by a relatively small number of frames, which exhibit the strongest cofluctuation amplitude. We found that these frames showed fewer average amplitudes in participants with ASD than those in the TD group. Then, we performed PCA on the activity patterns in these high-amplitude frames, which aggregated over all subjects. We focused on the PC1 and found that it corresponded to DMN. Additionally, the abnormalities in the coefficients for PC1 were associated with the deficits of ASD. Our finding highlights the utility of cofluctuation approaches in prevalent neurodevelopmental disorders and verifies that altered DMNs may underline the social deficits in adolescents and youths with ASD.
ASD were widely considered associated with atypical patterns of functional brain connectivity in large-scale brain networks (Uddin et al., 2013; Vissers et al., 2012). Most of these studies focused on characterizing dynamic brain patterns and utilized sliding window dynamic FC approaches that are known for potential pitfalls, such as arbitrary window sizes (Lurie et al., 2020; Preti et al., 2017). Many novel studies verified the reliability of a recently-proposed method in several independently acquired datasets, which enabled us to unwrap Pearson correlations to generate the time series of interregional cofluctuations along network edges (Betzel et al., 2022; Betzel et al., 2022b; Liu et al., 2021; Pope et al., 2021). In the current study, we leveraged this cofluctuation method to decompose FC into its frame-wise contributions in participants with ASD. In the ASD and TD groups, the RSS of the cofluctuation was highly correlated with the z-scored fMRI BOLD in the high-amplitude frames, indicating that high-amplitude frames had a nearly one-to-one correspondence with high-amplitude BOLD fluctuations. Critically, the FC of these frames was exactly equal to the whole-brain static FC, which was consistent with previous findings (Esfahlani et al., 2020). Additionally, our findings hint at a crucial link between the instantaneous fluctuations in BOLD activity and the organization of the resting state FC. We demonstrated that the DMN was primarily responsible for driving the high-amplitude frames in the ASD and TD groups. Notably, other state-based analyses of brain dynamics reported similar patterns of activity (Cornblath et al., 2020; Karapanagiotidis et al., 2020). Although this mode made the greatest contribution, other modes were also likely to make nontrivial contributions, such as the control, ventral attention, and somatomotor network modes. All these patterns may recombine in different proportions according to task complexity and domain (Yarkoni et al., 2011) across individuals (Gratton et al., 2018).
The DMN is an important network that shows a substantial overlap with the “social brain” network (Blakemore, 2008), which has been hypothesized to be a candidate locus of pathology in ASD. This network includes the medial prefrontal cortex, the posterior cingulate cortex and the adjoining precuneus, the lateral parietal regions, and the temporal regions (Fox et al., 2005; Raichle et al., 2001). Some studies proposed the involvement of the DMN in processing one's own emotional state (Buckner et al., 2008), self-referential thinking (Gusnard et al., 2001; Gusnard & Raichle, 2001), thoughts about self-versus others and theory of mind (Li et al., 2014), and autobiographical memory (Andrews-Hanna et al., 2010).
We found that DMN showed aberrant contribution to rsFC in participants with ASD, which was associated with RRB and social deficits. Previous rsFC study demonstrated that DMN was among the most disrupted functional networks in ASD (Glerean et al., 2016; Moseley et al., 2015), which was associated with social deficits in children and adults with ASD (Padmanabhan et al., 2017). Our results are consistent with the wealth of static FC research, which implicates over-connectivity of the DMN as underlying social deficits in ASD (Anderson, 2008; Elton et al., 2016; Hogeveen et al., 2018; Redcay et al., 2013). Moreover, our results served as a compliment to static analyses by demonstrating that DMN dysfunction was not only limited to increase connectivity between nodes but also increase the contributions of momentary cofluctuations to the overall FC pattern. As mentioned earlier, in the within-Group-PCA, a particular activity pattern that involved the default regions was primarily responsible for the rsFC in the TD group. Although this mode made the greatest contribution, ventral attention and the somatomotor network also made contributions. However, the DMN made an overwhelming contribution to the FC in participants with ASD. Additionally, compared with TD, the variance of the DMN was decreased in ASD. These phenomena probably occurred because ASD decreased functional flexibility or the overly stable dynamic properties of the brain (Uddin, 2021). In the current study, the altered DMN patterns were highly correlated with the RRB in participants with ASD. The ability to flexibly switch among different patterns of thought and reference frames was a critical feature of the adaptive social function (Padmanabhan et al., 2017). Aberrancies in DMN patterns contributed to the atypical integration of information about the self in relation to “other” and impairments in the ability to attend to socially relevant stimuli flexibly (Padmanabhan et al., 2017). This finding is consistent with those of recent studies that reported on reducing transitions between the brain state configurations in ASD (Watanabe & Rees, 2017).
Although a proliferation of resting-state connectivity studies on participants with ASD has been witnessed in the past few years, the results are inconsistent. These “inconsistencies” likely reflect developmental changes, as well as individual heterogeneity in ASD (Uddin et al., 2013). Existing studies demonstrated that spontaneous brain activity is aging globally (Xing, 2021). Over typical development, intrinsic FC within DMN nodes increased between childhood and adulthood (Supekar et al., 2010). In ASD, no consistent evidence of such increases in DMN connectivity with age was found (Vissers et al., 2012; Washington et al., 2014). Some studies found mixed patterns of under- and over-connectivity in adolescents with ASD relative to the TD group (Doyle-Thomas et al., 2015; Jann et al., 2015). Therefore, in the current study, ANOVA was further performed to probe the effect of age. The results exhibited significant diagnosis-related effects. However, no significant age-specific and diagnosis-by-age interaction effect was found in the coefficients, suggesting that the atypical spontaneous brain activity of DMN in ASD was not affected by age. Even so, the mechanism underlying the shift in DMN connectivity patterns in adolescence is not fully understood in ASD yet. As such, further longitudinal exploration is necessary to understand the developmental changes and their impact on symptomatology.
4.1 Limitations
A few limitations of the current work should be noted. First, our sample includes data collected at 16 different sites. Although the sample size and statistical power increase, the use of data across multiple sites presents its own limitations in that inter-site variability may affect the analyses. Although we used advanced multi-center correction methods (i.e., COMBAT) to reduce potential biases and non-biological variability induced by site and scanner effects, we could not be sure that inherent between-site effects were completely accounted for. Furthermore, similar to most ASD studies, our sample consisted mostly of males. Although sex was regressed in our statistical analysis, this imbalance of males to females may fail to account for differences in the brain activity of the two gender. To the best of our knowledge, ASD is a neurodevelopmental disorder. Unfortunately, ABIDE is a cross-sectional repository. Although the effects of age and interaction effect between age and group was analyzed in the current study, further studies using longitudinal data are needed to explore the developmental change in ASD. Another potential strategy, normative model, is analogous to growth charts used in pediatric medicine, mapping the height or weight as a function of age in a reference population (Cole, 2012; Marquand et al., 2019). This approach has been increasingly used to map variations between demographic, cognitive, clinical, or behavioral variables and quantitative brain readouts derived from neuroimaging (such as brain volume (Marquand et al., 2019, Wolfers et al., 2020, Ziegler et al., 2014), cortical thickness (Bethlehem et al., 2018; Zabihi et al., 2019), brain activity derived from task fMRI (Marquand et al., 2016) and rsFC (Kessler et al., 2016)), providing statistical inferences at the individual level based on the extent to which each individual deviate from the normative range. Importantly, previous multisite studies demonstrated stability and robustness of normal models across the life span (Bethlehem et al., 2022; Shan et al., 2022). However, measurement using in the current study might not be able to effectively capture the life-span developmental changes in ASD, and a potential reason might be the state-dependent nature of the moment-to moment activity cofluctuations in high-amplitude frames. Future studies are needed to examine age-dependent functional metrics for delineating the extent to which brain dynamics in ASD deviates from the normative range, from a developmental framework.
5 CONCLUSION
In summary, the findings of this study were built on the growing body of literature on the use of cofluctuation approach in the investigation of neurodevelopmental disorders, particularly ASD. We used this approach to decompose the functional connections into their exact frame-wise contributions and observed the aberrant DMN pattern and overly stable dynamic properties in participants with ASD. Consistent with previous findings, our results suggested that the dysfunction in the DMN is a potential endophenotype for the behavioral deficits in ASD.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81871432 [to XD], 82121003 [to HC], 62036003 [to HC], Fundamental Research Funds for Central Universities (Grant No.ZYGX2019Z017 [to XD]), Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (Grant No.ZYYCXTD-D-202003 [to HC]) and National Social Science Foundation of China(Grant No. 20&ZD296 [to XD]).
Open Research
DATA AVAILABILITY STATEMENT
Original rs-fMRI data and phenotypes were downloaded from the ABIDE repository (ABIDEs I and II, http://fcon_1000.projects.nitrc.org/indi/abide/).