Intelligence, educational attainment, and brain structure in those at familial high-risk for schizophrenia or bipolar disorder
Funding information: Australian National Health and Medical Research Council Grants, Grant/Award Numbers: 1037196, 1063960, 1066177, 510135, 1176716; Canadian Institutes of Health Research, Grant/Award Numbers: 103703, 106469, 142255; Departament de Salut de la Generalitat de Catalunya, Grant/Award Number: SLT002/16/00331; Deutsche Forschungsgemeinschaft, Grant/Award Number: 1617; Development Service Merit Review Award, Grant/Award Number: I01CX000227; Dokuz Eylul University Department of Scientific Research Projects Funding, Grant/Award Number: 2012.KB.SAG.062; e:Med program, Grant/Award Numbers: O1ZX1314B, O1ZX1314G; Ege University School of Medicine Research Foundation, Grant/Award Number: 2009-D-00017; Fundacio Marato TV3, Grant/Award Number: 091630; Geestkracht program of the Netherlands Organisation for Health Research and Development, Grant/Award Number: 10-000-1002; Generalitat de Catalunya, Grant/Award Number: 2017SGR01271; German Federal Ministry for Education and Research; Medical Research Council, Grant/Award Number: G0901310; Ministerstvo Zdravotnictví Ceské Republiky, Grant/Award Numbers: NR8786, NT13891; National Alliance for Research on Schizophrenia and Depression, Grant/Award Numbers: 17319, 20244, 26731; Swiss National Centre of Competence in Research Robotics, Grant/Award Number: 51NF40-185897; National Institute of Mental Health, Grant/Award Numbers: 1S10OD017974-01, P30 NS076408, R01 MH052857, R01 MH080912, R01 MH113619, U01 MH108150, R01 MH085667; National Institute on Aging, Grant/Award Number: T32AG058507; National Institutes of Health, Grant/Award Numbers: P41 EB015922, R01 MH111671, R01 MH116147, R01 MH117601, R01MH121246, R03 MH105808, U54EB020403; Research Council of Norway, Grant/Award Number: 223273; Spanish Ministry of Economy and Competitiveness/Instituto de Salud Carlos III, Grant/Award Numbers: CPII19/00009, PI070066, PI1100683, PI1500467, PI18/00976; Stanley Medical Research Institute; Swedish Research Council, Grant/Award Numbers: K2007-62X-15077-04-1, K2008-62P-20597-01-3, K2010-62X-15078-07-2, K2012-61X-15078-09-3; Swiss National Science Foundation, Grant/Award Number: 32003B_156914; VIDI, Grant/Award Numbers: 452-11-014, 917-46-370; Wellcome Trust, Grant/Award Numbers: 085475/B/08/Z, 085475/Z/08/Z; Wellcome Trust Research Training Fellowship, Grant/Award Number: 064971; ZonMw, Grant/Award Number: 908-02-123
Abstract
First-degree relatives of patients diagnosed with schizophrenia (SZ-FDRs) show similar patterns of brain abnormalities and cognitive alterations to patients, albeit with smaller effect sizes. First-degree relatives of patients diagnosed with bipolar disorder (BD-FDRs) show divergent patterns; on average, intracranial volume is larger compared to controls, and findings on cognitive alterations in BD-FDRs are inconsistent. Here, we performed a meta-analysis of global and regional brain measures (cortical and subcortical), current IQ, and educational attainment in 5,795 individuals (1,103 SZ-FDRs, 867 BD-FDRs, 2,190 controls, 942 schizophrenia patients, 693 bipolar patients) from 36 schizophrenia and/or bipolar disorder family cohorts, with standardized methods. Compared to controls, SZ-FDRs showed a pattern of widespread thinner cortex, while BD-FDRs had widespread larger cortical surface area. IQ was lower in SZ-FDRs (d = −0.42, p = 3 × 10−5), with weak evidence of IQ reductions among BD-FDRs (d = −0.23, p = .045). Both relative groups had similar educational attainment compared to controls. When adjusting for IQ or educational attainment, the group-effects on brain measures changed, albeit modestly. Changes were in the expected direction, with less pronounced brain abnormalities in SZ-FDRs and more pronounced effects in BD-FDRs. To conclude, SZ-FDRs and BD-FDRs show a differential pattern of structural brain abnormalities. In contrast, both had lower IQ scores and similar school achievements compared to controls. Given that brain differences between SZ-FDRs and BD-FDRs remain after adjusting for IQ or educational attainment, we suggest that differential brain developmental processes underlying predisposition for schizophrenia or bipolar disorder are likely independent of general cognitive impairment.
1 INTRODUCTION
Schizophrenia and bipolar disorder are highly heritable disorders with a shared genetic architecture (Anttila et al., 2018; Lee et al., 2013; Lichtenstein et al., 2009). Both patient groups are characterized by overlapping patterns of structural brain abnormalities (Arnone et al., 2009; Ellison-Wright & Bullmore, 2010; Haijma et al., 2013; Hibar et al., 2016; Hibar et al., 2018; Ivleva et al., 2017; McDonald et al., 2004; Okada et al., 2016; van Erp et al., 2016, 2018). In contrast, our recent ENIGMA–Relatives meta-analysis showed that their family members—who share the risk for the disorder but generally are not confounded by medication use or other illness related factors—show divergent patterns of global brain measures (de Zwarte, Brouwer, Agartz, et al., 2019). That study found that first-degree relatives of patients diagnosed with bipolar disorder (BD-FDRs) had a larger intracranial volume (ICV) which was not present in first-degree relatives of patients diagnosed with schizophrenia (SZ-FDRs). When we adjusted for ICV, no differences were found between BD-FDRs and controls but SZ-FDRs still showed significantly smaller brain volumes, diminished cortical thickness and larger ventricle volume compared to controls. These findings suggest that individuals at familial risk for either bipolar disorder or schizophrenia may show disease-specific deviations during early brain development.
Differential neurodevelopmental trajectories in schizophrenia and bipolar disorder have also been linked to intelligence quotient (IQ) development and school performance (Parellada, Gomez-Vallejo, Burdeus, & Arango, 2017). Schizophrenia has been associated with poorer cognitive performance, as well as decreases in cognitive performance over time, years before onset (Agnew-Blais & Seidman, 2013; Dickson, Laurens, Cullen, & Hodgins, 2012; Hochberger et al., 2018; Kendler, Ohlsson, Sundquist, & Sundquist, 2015; Khandaker, Barnett, White, & Jones, 2011; Reichenberg et al., 2005; Woodberry, Giuliano, & Seidman, 2008), while premorbid IQ or educational attainment are often not affected or are even higher in individuals who later develop bipolar disorder (MacCabe et al., 2010; Smith et al., 2015; Tiihonen et al., 2005; Zammit et al., 2004).
Both IQ and educational attainment are highly heritable (Devlin, Daniels, & Roeder, 1997; Heath et al., 1985; Tambs, Sundet, Magnus, & Berg, 1989). Consequently, similar patterns of cognitive performance and educational attainment are often found among relatives. Indeed, cognitive alterations have been reported in SZ-FDRs compared to controls (Hughes et al., 2005; Kremen, Faraone, Seidman, Pepple, & Tsuang, 1998; McIntosh, Harrison, Forrester, Lawrie, & Johnstone, 2005; Niendam et al., 2003; Sitskoorn, Aleman, Ebisch, Appels, & Kahn, 2004; Van Haren, Van Dam, & Stellato, 2019; Vreeker et al., 2016) and in BD-FDRs compared to controls (Vonk et al., 2012; Vreeker et al., 2016). Vreeker et al. (2016) showed, in a direct comparison, a discrepancy between IQ and educational attainment in SZ-FDRs and BD-FDRs: both groups showed lower IQ but similar educational attainment compared to controls. These findings suggest that, despite the high genetic and phenotypic overlap between intelligence and educational attainment in the general population (Sniekers et al., 2017; Strenze, 2007), it is important to differentiate between these two measures when investigating individuals at familial risk for mental illness.
Intelligence has consistently been associated with brain structure in the general population (McDaniel, 2005). Our recent schizophrenia family studies reported that IQ is intertwined with most of the brain abnormalities in SZ-FDRs (de Zwarte, Brouwer, Tsouli, et al., 2019; van Haren et al., 2020). Altered brain structure and cognitive deficits observed in schizophrenia patients could be a direct consequence of the association observed in the general population or alternatively, both could be caused by the disease through independent mechanisms. IQ and brain structure are both considered indirect measures for (early) neurodevelopment. Indeed, both brain structure and cognitive deficits in schizophrenia relatives are apparent in children and adolescents at high familial risk (van Haren et al., 2020), suggesting that individuals at familial risk for schizophrenia show altered neurodevelopment already early in life. This would suggest that genetic risk for the disease influences both cognition and the brain and we cannot study one without the other. However, in BD-FDRs, it remains unknown how IQ and risk for bipolar disorder interact with the brain. In particular, the relationship between IQ and the familial predisposition for a larger ICV in BD-FDRs is unclear. Hence, it is of interest to see how brain anomalies and intelligence differences are related to each other in those at familial risk for schizophrenia and bipolar disorder.
Here, through the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA)-Relatives Working Group, we performed meta-analyses of magnetic resonance imaging data sets consisting of SZ-FDRs and/or BD-FDRs, probands, and matched control participants. There were three main aims. First, we extended our findings of group differences in global brain measures between relatives and controls (and patients) for both disorders (de Zwarte, Brouwer, Agartz, et al., 2019) by adding local cortical measures. This allowed us to investigate whether the findings were limited to specific (functional) brain regions, or can be attributed to a more global mechanism. Previous ENIGMA meta-analyses have shown that patients with schizophrenia have widespread attenuation of cortical thickness and surface area (with largest effects in frontal and temporal lobe regions), with evidence for regional specificity only in the thickness findings (van Erp et al., 2018). In contrast, patients with bipolar disorder have shown thinner cortex in frontal, temporal and parietal regions, but no differences in surface area, compared to controls (Hibar et al., 2018). Based on the patient findings and our previous ENIGMA-Relatives findings for global brain measures—showing globally thinner cortex in SZ-FDRs and larger surface area in BD-FDRs—we expected to find subtle regional differences in these measures in the relatives. In particular, we predicted locally thinner cortex in SZ-FDRs with a similar pattern to previous observations in patients but with smaller effect sizes (van Erp et al., 2018). Based on the larger ICV and global surface area reported in our previous study, locally larger surface area in BD-FDRs was expected in contrast to previous bipolar patient findings (Hibar et al., 2018). Second, in cohorts that had information on current IQ and/or educational attainment (the latter is defined as years of education completed), we meta-analyzed the group effects of IQ and educational attainment between relatives and controls (and patients) for both disorders. We hypothesized that both SZ-FDRs and BD-FDRs would have, on average, lower current IQ than controls. Educational attainment findings in relatives have been inconsistent, with findings of both lower educational attainment and no detectable differences between relatives and controls; therefore, we expected subtle but significant differences between both SZ-FDRs and BD-FDRs and controls. Third, we investigated the influence of IQ and educational attainment on global and local brain differences between relatives and controls. We hypothesized that IQ will account for most of the brain abnormalities found in SZ-FDRs, while a lower IQ most likely would not explain our previously reported larger ICV in BD-FDRs because of the well- established positive relationship between overall head size and IQ (McDaniel, 2005). The moderating effect of educational attainment on brain abnormalities is expected to be less pronounced than that of IQ, as we are only expecting modest group differences in educational attainment between relatives and controls.
2 MATERIALS AND METHODS
2.1 Study samples
This study included 5,795 participants from 36 family cohorts (age range 6–72 years). In total, 1,103 SZ-FDRs (42 monozygotic co-twins, 50 dizygotic co-twins, 171 offspring, 728 siblings, 112 parents), 867 BD-FDRs (32 monozygotic co-twins, 33 dizygotic co-twins, 453 offspring, 331 siblings, 18 parents), 942 patients with schizophrenia, 693 patients with bipolar disorder and 2,190 controls were included (Tables 1 and 2). All family cohorts included their own control participants. Controls did not have a family history of schizophrenia or bipolar disorder. SZ-FDRs or BD-FDRs were defined by having a first-degree family member with schizophrenia or bipolar disorder, respectively, and not having experienced (hypo)mania and/or psychosis themselves. Demographic characteristics for each cohort and their inclusion criteria are summarized in Tables 1 and 2 and Table S1. The cohorts in the current meta-analysis overlap largely, but not completely with those in our previous meta-analysis (de Zwarte, Brouwer, Agartz, et al., 2019). All study centers obtained approval from their respective ethics committee for research, following the Declaration of Helsinki. Informed consent was obtained from all participants and/or parents, in the case of minors.
| Total | IQ scores | Educational attainment | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls | Patients | Relatives | Controls | Patients | Relatives | Controls | Patients | Relatives | |||||||||||||
| Sample | N | M/F | Age | N | M/F | Age | N | M/F | Age | N | IQ | N | IQ | N | IQ | N | EA | N | EA | N | EA |
| BPO-FLB | 7 | 3/4 | 12.9 (1.3) | 9 | 5/4 | 13.3 (2.6) | 22 | 10/12 | 10.0 (3.5) | 7 | 91.0 (10.2) | 5 | 91.2 (16.1) | 7 | 95.4 (17.3) | — | — | — | |||
| Cardiff | 79 | 28/51 | 39.8 (8.7) | 120 | 42/78 | 41.9 (8.1) | 33 | 13/20 | 45.9 (6.9) | — | — | — | — | — | — | ||||||
| CliNG-BDa | 19 | 6/13 | 30.9 (9.6) | — | 19 | 6/13 | 31.9 (5.0) | — | — | — | 12 | 14.9 (3.3) | — | 10 | 15.2 (3.2) | ||||||
| DEU | 27 | 11/16 | 32.9 (8.8) | 27 | 10/17 | 36.3 (9.5) | 23 | 11/12 | 31.3 (8.9) | — | — | — | 21 | 13.1 (4.1) | 24 | 12.9 (2.9) | 14 | 11.6 (3.1) | |||
| EGEU | 33 | 13/20 | 33.6 (7.8) | 27 | 16/11 | 36.7 (7.8) | 27 | 10/17 | 34.5 (9.5) | — | — | — | 28 | 11.6 (3.8) | 26 | 10.8 (4.2) | 23 | 10.8 (4.4) | |||
| ENBD-UT | 36 | 13/23 | 34.8 (11.7) | 72 | 23/49 | 36.9 (12.4) | 52 | 10/42 | 44.3 (13.6) | 27 | 101.0 (14.5) | 40 | 97.0 (12.0) | 19 | 99.2 (14.4) | 26 | 15.2 (3.0) | 55 | 14.7 (2.3) | 46 | 15.1 (2.3) |
| FIDMAG-Clinic | 61 | 12/49 | 41.1 (10.1) | 18 | 3/15 | 42.6 (8.8) | 18 | 5/13 | 45.1 (10.0) | 61 | 112.9 (13.6) | 14 | 101.9 (13.1) | 16 | 105.8 (16.7) | — | — | — | |||
| Geneva | 19 | 10/9 | 20.1 (2.7) | — | 18 | 9/9 | 19.4 (3.1) | — | — | — | — | — | — | ||||||||
| IDIBAPSa | 53 | 21/32 | 12.3 (3.6) | — | 61 | 31/30 | 12.4 (3.4) | 53 | 106.1 (12.4) | — | 61 | 107.0 (13.0) | — | — | — | ||||||
| IoP-BD | 39 | 9/30 | 35.4 (11.2) | 34 | 15/19 | 40.6 (13.1) | 17 | 4/13 | 43.1 (14.6) | — | — | — | 31 | 15.2 (2.6) | 26 | 15.4 (3.2) | 14 | 16.4 (2.6) | |||
| MFS-BDa | 54 | 25/29 | 40.2 (15.3) | 38 | 15/23 | 41.0 (11.7) | 41 | 17/24 | 49.3 (9.6) | 39 | 110.8 (16.1) | 31 | 97.4 (11.7) | 34 | 100.0 (10.3) | 35 | 14.1 (3.9) | 35 | 13.9 (3.3) | 31 | 14.6 (4.0) |
| MooDS-BDa | 63 | 25/38 | 30.3 (9.5) | — | 63 | 25/38 | 30.4 (9.4) | 62 | 99.4 (5.5) | — | 62 | 101.5 (5.8) | 33 | 15.4 (2.4) | — | 34 | 17.2 (2.8) | ||||
| MSSM | 52 | 25/27 | 35.2 (13.0) | 41 | 21/20 | 44.3 (11.9) | 50 | 26/24 | 33.8 (8.3) | — | — | — | — | — | — | ||||||
| Olin | 68 | 25/43 | 32.2 (11.7) | 108 | 34/74 | 34.5 (12.3) | 78 | 30/48 | 32.0 (13.0) | 54 | 107.0 (15.0) | 95 | 102.9 (15.6) | 68 | 105.6 (15.1) | 40 | 15.2 (2.4) | 74 | 14.6 (2.2) | 40 | 14.6 (2.2) |
| ORBIS-I | 32 | 12/20 | 20.7 (3.3) | 6 | 0/6 | 22.9 (4.0) | 39 | 13/26 | 19.8 (3.2) | — | — | — | — | — | — | ||||||
| ORBIS-II | 18 | 7/11 | 23.0 (3.5) | 8 | 3/5 | 24.0 (5.0) | 26 | 10/16 | 19.9 (4.0) | — | — | — | — | — | — | ||||||
| PENS-BDa | 16 | 6/10 | 45.9 (10.1) | 20 | 14/6 | 46.9 (10.4) | 9 | 5/4 | 40.4 (6.3) | 16 | 115.7 (13.8) | 20 | 103.7 (15.8) | 9 | 101.3 (18.0) | 16 | 16.0 (1.3) | 19 | 14.8 (2.7) | 9 | 15.0 (1.6) |
| PHCP-BDa | 38 | 21/17 | 38.4 (13.7) | 29 | 7/22 | 32.2 (11.6) | 7 | 2/5 | 51.0 (6.1) | 38 | 106.3 (11.8) | 29 | 101.8 (8.8) | 7 | 100.6 (9.1) | 29 | 16.0 (2.5) | 18 | 14.8 (1.8) | 7 | 15.4 (1.5) |
| STAR-BDa | 83 | 39/44 | 49.0 (10.4) | 25 | 7/18 | 45.8 (10.1) | 21 | 6/15 | 47.9 (11.3) | — | — | — | 81 | 11.9 (2.9) | 25 | 12.9 (3.6) | 21 | 11.5 (2.5) | |||
| SydneyBipolarGroup | 117 | 54/63 | 22.2 (3.9) | 59 | 17/42 | 25.1 (3.6) | 150 | 65/85 | 19.9 (5.4) | 116 | 117.6 (10.3) | 57 | 116.2 (12.3) | 147 | 114.5 (10.6) | 24 | 17.1 (3.2) | 32 | 16.4 (2.3) | 30 | 15.9 (2.2) |
| UMCU-BD twinsa | 110 | 40/70 | 39.3 (9.2) | 52 | 13/39 | 39.6 (9.7) | 27 | 9/18 | 41.7 (9.3) | 48 | 98.0 (13.5) | 22 | 92.4 (13.2) | 14 | 95.4 (14.1) | 108 | 13.4 (2.7) | 47 | 12.7 (2.6) | 26 | 12.1 (2.6) |
| UMCU-DBSOSa | 40 | 21/19 | 12.7 (2.1) | — | 66 | 37/29 | 14.7 (2.7) | 40 | 117.1 (13.0) | — | 63 | 106.7 (18.3) | — | — | — | ||||||
- a Overlapping controls with schizophrenia sample from the same site, that is, with CliNG-SZ (n = 10), IDIBAPS (n = 53), MFS-SZ (n = 54), MooDS-SZ (n = 36), PENS-SZ (n = 16), PHCP-SZ (n = 38), STAR-SZ (n = 73), UMCU-UTWINS (n = 19), UMCU-DBSOS (n = 40).
| Total | IQ scores | Educational attainment | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls | Patients | Relatives | Controls | Patients | Relatives | Controls | Patients | Relatives | |||||||||||||
| Sample | N | M/F | Age | N | M/F | Age | N | M/F | Age | N | IQ | N | IQ | N | IQ | N | EA | N | EA | N | EA |
| C-SFS | 23 | 11/12 | 40.2 (11.1) | 25 | 13/12 | 40.8 (10.8) | 23 | 8/15 | 42.1 (11.9) | — | — | — | 20 | 15.2 (2.4) | 23 | 14.2 (3.1) | 19 | 16.1 (2.7) | |||
| CliNG-SZa | 20 | 11/9 | 35.7 (12.2) | — | 20 | 11/9 | 36.1 (6.4) | — | — | — | 14 | 15.1 (2.1) | — | 14 | 14.0 (2.6) | ||||||
| EHRS | 89 | 44/45 | 21.0 (2.5) | 31 | 19/12 | 21.8 (3.7) | 90 | 44/46 | 21.2 (3.1) | 82 | 101.9 (12.9) | 22 | 87.9 (14.5) | 90 | 97.6 (13.5) | — | — | — | |||
| HUBIN | 102 | 69/33 | 41.9 (8.9) | 104 | 78/26 | 41.3 (7.7) | 33 | 23/10 | 39.4 (7.8) | 69 | 102.0 (16.5) | 73 | 89.1 (20.4) | 19 | 106.6 (12.4) | 90 | 14.3 (3.0) | 93 | 12.5 (2.7) | 30 | 12.9 (2.3) |
| IDIBAPSa | 53 | 21/32 | 12.3 (3.6) | — | 37 | 21/16 | 11.0 (3.3) | 53 | 106.1 (12.4) | — | 37 | 97.6 (14.2) | — | — | — | ||||||
| IoP-SZ | 67 | 35/32 | 40.8 (12.2) | 54 | 39/15 | 34.8 (10.8) | 18 | 8/10 | 33.0 (12.4) | 41 | 119.6 (13.9) | 37 | 91.5 (15.8) | 12 | 102.2 (12.6) | 57 | 14.1 (2.4) | 41 | 13.3 (3.1) | 14 | 13.5 (2.9) |
| LIBD | 361 | 162/199 | 32.5 (9.9) | 211 | 161/50 | 35.2 (10.2) | 240 | 99/141 | 36.2 (9.6) | 361 | 109.6 (9.2) | 211 | 95.4 (11.6) | 240 | 107.3 (10.8) | 259 | 17.5 (2.7) | 165 | 14.8 (2.4) | 201 | 16.3 (2.4) |
| Maastricht-GROUP | 87 | 33/54 | 30.8 (10.8) | 88 | 59/29 | 28.2 (7.0) | 96 | 50/46 | 29.5 (8.7) | 87 | 111.3 (15.0) | 87 | 96.7 (14.3) | 96 | 108.9 (16.2) | — | — | — | |||
| MFS-SZa | 54 | 25/29 | 40.2 (15.3) | 42 | 31/11 | 36.4 (9.8) | 56 | 21/35 | 49.4 (8.4) | 35 | 107.8 (14.1) | 39 | 106.4 (16.1) | 35 | 107.9 (16.8) | 35 | 14.1 (3.9) | 39 | 13.9 (3.2) | 41 | 14.1 (3.0) |
| MooDS-SZa | 65 | 26/39 | 30.6 (10.1) | — | 63 | 24/39 | 30.6 (8.2) | 63 | 100.0 (5.0) | — | 61 | 97.5 (12.3) | 37 | 15.1 (2.3) | — | 35 | 16.1 (2.5) | ||||
| PENS-SZa | 16 | 6/10 | 45.9 (10.1) | 20 | 13/7 | 47.4 (9.5) | 11 | 4/7 | 48.3 (8.9) | 16 | 115.7 (13.8) | 20 | 102.9 (15.3) | 11 | 105.0 (14.8) | 16 | 16.0 (1.3) | 19 | 12.7 (1.6) | 11 | 14.5 (1.8) |
| PHCP-SZa | 38 | 21/17 | 38.4 (13.7) | 41 | 30/11 | 42.2 (11.6) | 13 | 4/9 | 45.4 (11.4) | 38 | 106.3 (11.8) | 41 | 93.3 (11.7) | 13 | 99.5 (10.5) | 29 | 16.0 (2.5) | 38 | 13.8 (2.3) | 12 | 15.8 (3.0) |
| STAR-SZa | 73 | 33/40 | 49.0 (10.4) | 31 | 18/13 | 49.7 (8.9) | 29 | 17/12 | 49.8 (9.6) | — | — | — | 73 | 12.0 (3.0) | 31 | 12.9 (3.4) | 28 | 12.0 (3.8) | |||
| UMCU-DBSOSa | 40 | 21/19 | 12.7 (2.1) | — | 40 | 12/28 | 13.7 (3.0) | 40 | 117.1 (13.0) | — | 40 | 100.6 (19.2) | — | — | — | ||||||
| UMCU-GROUP | 167 | 83/84 | 27.7 (8.2) | 162 | 130/32 | 27.0 (5.8) | 201 | 95/106 | 27.7 (7.1) | 164 | 111.9 (14.8) | 153 | 93.5 (15.5) | 199 | 101.4 (14.3) | 83 | 14.0 (2.1) | 83 | 11.2 (3.0) | 119 | 13.5 (2.7) |
| UMCU-Parents | 41 | 14/27 | 52.8 (4.6) | — | 44 | 13/31 | 52.9 (4.3) | 41 | 119.0 (13.1) | — | 44 | 116.9 (14.7) | 41 | 12.5 (3.1) | — | 43 | 12.1 (3.8) | ||||
| UMCU-UTWINSa | 184 | 84/100 | 31.8 (13.0) | 56 | 33/23 | 35.6 (10.6) | 45 | 29/16 | 37.0 (11.9) | 168 | 106.0 (13.3) | 45 | 96.7 (15.0) | 38 | 107.2 (15.1) | 94 | 13.9 (2.4) | 39 | 11.4 (3.4) | 34 | 13.1 (2.9) |
| UNIBA | 78 | 52/26 | 31.4 (8.6) | 77 | 58/19 | 33.9 (8.2) | 44 | 23/21 | 33.8 (8.9) | 64 | 108.1 (12.7) | 60 | 74.5 (17.0) | 33 | 94.6 (17.2) | 22 | 15.7 (3.3) | 45 | 11.4 (3.3) | 13 | 13.0 (4.4) |
- a Overlapping controls with bipolar sample from the same site, i.e. with CliNG-BD (n = 10), IDIBAPS (n = 53), MFS-BD (n = 54), MooDS-BD (n = 36), PENS-BD (n = 16), PHCP-BD (n = 38), STAR-BD (n = 73), UMCU-BD twins (n = 19), UMCU-DBSOS (n = 40).
2.2 Intelligence quotient
Twenty-five family cohorts had either full scale IQ scores or estimated IQ scores available for most of their participants. In total, 4,095 participants with a measure of IQ were included; 968 SZ-FDRs, 507 BD-FDRs, 788 patients with schizophrenia, 313 patients with bipolar disorder and 1,549 controls (Tables 1 and 2; Table S2 for IQ test battery description).
2.3 Educational attainment
Educational attainment was measured as years of completed education. These data were available in 27 family cohorts. Subjects were included if they were at least 25 years old to avoid the bias of including participants still in school. In total, 3,056 participants were included; 614 SZ-FDRs, 306 BD-FDRs, 616 patients with schizophrenia, 381 patients with bipolar disorder and 1,139 controls (Tables 1 and 2; Table S3 for educational attainment description).
2.4 Image acquisition and processing
Structural T1-weighted brain magnetic resonance imaging scans were acquired at each research center (Table S4). Cortical and subcortical reconstruction and volumetric segmentations were performed with the FreeSurfer pipeline (Table S4) (http://surfer.nmr.mgh.harvard.edu/fswiki/recon-all/; Fischl, 2012). The segmentations were quality checked according to the ENIGMA quality control protocol for subcortical volumes, cortical thickness and surface area (http://enigma.ini.usc.edu/protocols/imaging-protocols/). Global brain measures, regional cortical thickness, and surface area measures and subcortical volumes were extracted from individual images (Fischl & Dale, 2000; Fischl, Sereno, & Dale, 1999).
2.5 Statistical meta-analyses
All statistical analyses were performed using R (http://www.rproject.org). Linear mixed model analyses were performed within each cohort for bipolar disorder and schizophrenia separately, comparing relatives (per relative type) with controls and, if present, patients with controls, while taking family relatedness into account (http://CRAN.R-project.org/package=nlme; Pinheiro & Bates, 2000). Patients were analyzed as a sanity check as effects in patients are not the main focus of the study; for differences between patients and controls we refer to Supporting Information. Mean centered age, age squared, and sex were included as covariates. Brain measures were corrected for lithium use at time of scan in patients with bipolar disorder by adding a covariate (yes = 1/no = 0). All global brain measures and subcortical volume analyses were performed both with and without adjusting for ICV by including ICV as covariate. No interaction terms were modeled. All regional cortical thickness analyses were performed with and without correction for mean cortical thickness and all regional cortical surface areas with and without correction for total surface area to assess regional specificity. Analyses of multiscanner studies included binary dummy covariates for n − 1 scanners. Cohen's d effect sizes and 95% confidence intervals were calculated within each cohort separately and pooled per disorder for all relatives combined, and for patients as a group, using an inverse variance-weighted random-effects meta-analysis. All random-effects models were fitted using the restricted maximum likelihood method. False discovery rate (q < 0.05) thresholding across all global and subcortical phenotypes, and separately per regional phenotype, was used to control for multiple comparisons for the analyses between relatives and controls, and between patients and controls (Benjamini & Hochberg, 1995). Correlations between brain measures and IQ, brain measures and educational attainment, and between IQ and educational attainment were estimated by performing linear mixed model analyses in the overall sample and in the relative groups only, based on the gathered statistics of the local analyses. The resulting t-statistics were converted to correlation r with R package “esc” (http://CRAN.R-project.org/package=esc). Analyses were generally performed locally by the research center that contributed the cohort, using code created within the ENIGMA-Relatives Working Group (scripts available upon request). For some cohorts, data were sent to the main site for analysis.
3 RESULTS
3.1 Cortical thickness
SZ-FDRs had a thinner cortex in most cortical regions, compared to controls, with a thinner bilateral pars orbitalis surviving correction for multiple testing (left d = −0.17, right d = −0.16, q < 0.05 corrected; Figure 1a). There were no significant differences in regional cortical thickness in BD-FDRs compared to controls.
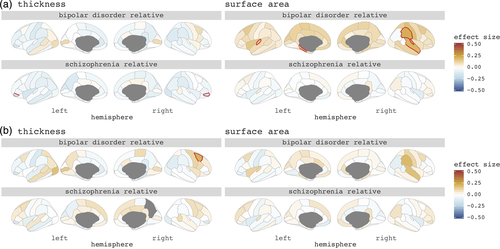
To investigate whether findings were driven by a global effect we corrected for mean cortical thickness. None of the findings survived correction for multiple testing in SZ-FDRs. BD-FDRs had a significantly thicker right caudal middle frontal cortex (d = +0.21, q < 0.05 corrected) (Figure 1b). For all regional cortical thickness effect sizes, and for the patient findings, see Figure 1, and Figures S1 and S2.
3.2 Cortical surface area
Differences between SZ-FDRs and controls were subtle and none were statistically significant. BD-FDRs had larger cortical surface areas in many cortical areas compared to controls, with a significantly larger cortical surface area in the left transverse temporal, left parahippocampal, right superior temporal, right supramarginal and right transverse temporal regions surviving correction for multiple testing (d's > +0.15, q < 0.05 corrected) (Figure 1a).
When controlling for total surface area to investigate regional specificity, none of the findings survived (Figure 1b). For all regional cortical surface area effect sizes, and for the patient findings see Figure 1, and Figures S3 and S4.
3.3 Intelligence quotient
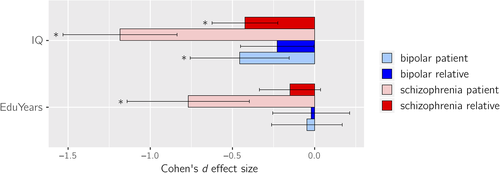
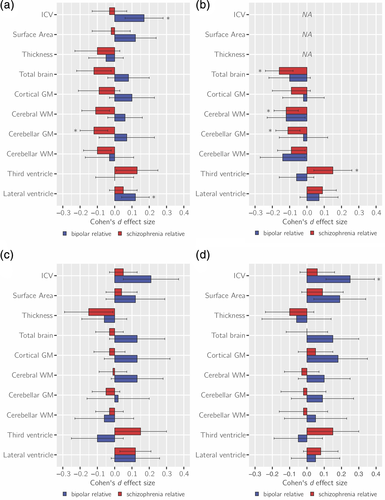
SZ-FDRs had significantly lower IQ compared to controls with a medium effect size d = −0.42 (p = 3 × 10−5). BD-FDRs showed mild IQ reductions compared to controls and of borderline significance with effect size d = −0.23 (p = .045; Figure 2). These findings translate to an average of 6.3 IQ points lower in SZ-FDRs and 3.5 IQ points lower in BD-FDRs compared to controls. In SZ-FDRs, most effect sizes of the global brain measures were slightly smaller after controlling for IQ; none of them survived correction for multiple testing (Figure 3c, Table S5, Figures S5 and S6). After controlling for IQ, most effect sizes of the global brain measures were slightly larger in BD-FDRs; however, after correction for multiple testing only larger caudate volume survived (d = +0.23; q < 0.05 corrected) (Figure 3c, Table S5, Figure S5 and S6). For all effect sizes and the effects in patients, see Table S5–S8, and Figure S5 and S6.
3.4 Educational attainment
Both SZ-FDRs and BD-FDRs did not differ from controls on years of education completed (Figure 2). After adjusting for educational attainment, the effect sizes in most global brain measures were slightly smaller in SZ-FDRs (none of which survived correction for multiple testing), while the effect sizes of the global brain measures were slightly larger in BD-FDRs, with a significantly larger ICV (d = +0.25, q < 0.05 corrected; Figure 3d, Table S5, Figure S5 and S6). For all effect sizes and the effects in patients, see Tables S5–S8, and Figure S5 and S6.
3.5 Correlations between IQ, educational attainment, and brain measures
The correlation between IQ and educational attainment in the total sample was r = .40 (p = 4 × 10−22). All correlations between IQ and global and subcortical brain measures were positive (ranging from r = .06 and r = .22 [q < 0.05 corrected], except for the third [r = −.04] and lateral ventricles [r = −.01]; Table S9; for the results in the SZ-FDRs or BD-FDRs subgroups see Table S10). A significant positive correlation was found between educational attainment and total brain, cortical gray matter, cerebellar gray and white matter, and hippocampal volume (r = .06–.08, q < 0.05 corrected; Table S9).
4 DISCUSSION
In previous work from the ENIGMA-Relatives Working Group we showed that BD-FDRs had a larger ICV which was not found in SZ-FDRs; when we adjusted for ICV, no differences in global brain measures were found between BD-FDRs and controls, while SZ-FDRs had significantly smaller brain volumes, diminished cortical thickness, and larger ventricle volume compared to controls (de Zwarte, Brouwer, Agartz, et al., 2019). In this study, we extended the investigation to compare local cortical measures, IQ and educational attainment in SZ-FDRs and BD-FDRs with controls and investigated the effect of IQ and educational attainment on global and local brain measures in the relatives.
The main findings in the current study were that: (a) SZ-FDRs had a thinner cortex in most cortical regions, compared to controls, with a thinner bilateral pars orbitalis surviving correction for multiple testing. However, these findings may reflect a global effect rather than regionally specific effect. In contrast, BD-FDRs had a significantly thicker caudal middle frontal cortex when compared to controls that was only present when statistically controlling for global thickness and may thus reflect regionally specific sparing; (b) only BD-FDRs (and not SZ-FDRs) had larger cortical surface area in the temporal lobe, which was no longer present after statistically controlling for total surface area; (c) IQ was lower in both BD-FDRs and SZ-FDRs, while educational attainment did not differ between the relatives and controls; (d) there was a modest yet significant correlation between IQ and most brain measures in the full sample; however, statistically controlling for individual differences in IQ and educational attainment only minimally changed the group effects on the brain measures the expected direction, that is, effect sizes of brain measure differences between groups decreased for SZ-FDRs and increased for BD-FDRs after adjusting for IQ or educational attainment.
4.1 Cortical thickness and surface area in the relatives
SZ-FDRs had a thinner cortex in most brain areas. This pattern of findings is comparable to that in the included patient sample (Figure S1) as well as in the much larger sample of patients diagnosed with schizophrenia in an earlier ENIGMA study (van Erp et al., 2018). However, effect sizes are lower in SZ-FDRs. The most pronounced effect was observed in the bilateral pars orbitalis. This region has previously been associated with language function in those at familial risk for schizophrenia (Francis et al., 2012) and in individuals with nonclinical auditory verbal hallucinations (Van Lutterveld et al., 2014). When statistically controlling for mean cortical thickness this finding was no longer significant, suggesting that thinner cortex in SZ-FDRs is a global effect. In contrast, the pattern in BD-FDRs was diffuse with both thicker and thinner cortical regions, whereas bipolar patients showed globally thinner cortex (Figure S1), consistent with previous findings (Hibar et al., 2018). After correction for mean cortical thickness the right caudal middle frontal cortex was significantly thicker when compared to controls, suggesting regionally specific cortical thickness abnormalities in BD-FDRs.
Regional cortical surface area findings showed, on the one hand, that where the patients with schizophrenia had overall smaller surface area (Figure S3; van Erp et al., 2018), the effects in SZ-FDRs were even more subtle, and in both directions, compared to controls. On the other hand, BD-FDRs had widespread larger regional cortical surface area when compared to controls, in accord with our previous findings of larger total surface area in BD-FDRs (de Zwarte, Brouwer, Agartz, et al., 2019). While total surface area in patients with bipolar disorder was not significantly larger, we did see a pattern of mostly larger regional cortical surface area in the bipolar patients as well (Figure S3), with most prominent findings in the temporal lobe, in auditory regions associated with language (Crinion, Lambon-Ralph, Warburton, Howard, & Wise, 2003; Saur et al., 2008). This finding was not reported in the large ENIGMA bipolar disorder meta-analysis (Hibar et al., 2018); however, findings in that study were all corrected for ICV which most likely reduced the global surface area differences. In line with this, when we corrected for overall surface area, regional surface differences in BD-FDRs was no longer significant, suggesting that the larger surface area in BD-FRDs was a global effect.
Cortical thickness and surface area are highly heritable and largely influenced by independent genetic factors (Grasby et al., 2020; Strike et al., 2019). The latest cortical thickness and surface area genome-wide association study (GWAS) showed that the effects of genetic variants associated with surface area are more likely to be prenatal, while cortical thickness effects are more likely postnatal (Grasby et al., 2020), supporting the radial unit hypothesis that cortical thickness and surface area originate from two distinct processes in early brain development (Rakic, 1988). That BD-FDRs and SZ-FDRs show different patterns of abnormal cortical thickness and surface area, strengthens the notion that genetic predisposition may underlie distinct neurodevelopmental trajectories for these disorders early in life.
4.2 Discrepancy between IQ and educational attainment
Given the high genetic (rg = .7 [Sniekers et al., 2017]) and phenotypic (r = ~.5 [Strenze, 2007]) correlation between intelligence and educational attainment, educational attainment is often considered a proxy for IQ. In the current study, we found a phenotypic correlation of r = .4 between IQ and educational attainment. This implies that educational attainment is at most a weak proxy for IQ; it only explains 16% of the variance. We showed that IQ and educational attainment act differently in relatives, that is, lower IQ in SZ-FDRs and BD-FDRs than in controls, with larger alterations in SZ-FDRs, but no differences in educational attainment between SZ-FDRs and BD-FDRs as compared to controls. These findings are in line with an earlier study—of which a subset of the participants is included in the present study—investigating IQ and educational attainment in SZ-FDRs and BD-FDRs (Vreeker et al., 2016), suggesting that even though relatives have a lower IQ on average, gross school performance and engagement is not necessarily affected. It has previously been shown that differentiating intelligence from educational performance is important, as other factors besides intelligence are predictive of educational performance (Chamorro-Premuzic & Furnham, 2003; Deary, Strand, Smith, & Fernandes, 2007). In addition, completing a level of education gives little insight into the level of academic performance (e.g., grades). In fact, those measures are only partly correlated (Strenze, 2007). Perhaps, the modest cognitive alterations in the relatives cannot be picked up by a categorical measure such as educational attainment or the cognitive alterations must reach a certain threshold to lead to a lower level of school performance, which may be the case in patients with schizophrenia (who have the largest negative effect size for IQ and are significantly different from controls in educational attainment).
4.3 IQ, educational attainment and the brain
IQ and educational attainment both share genetic variance with ICV (rg = .29 and rg = .34, respectively (Okbay et al., 2016; Sniekers et al., 2017). Therefore, we speculated previously that the larger ICV reported in BD-FDRs could potentially be confounded by higher cognitive functioning (de Zwarte, Brouwer, Agartz, et al., 2019; de Zwarte, Brouwer, Tsouli, et al., 2019). Here, we showed that in the total sample ICV and all global brain measures were significantly correlated with IQ, except the ventricles, while correlations between the brain measures and educational attainment were much smaller. Adding to that, IQ was significantly lower in the relatives while this was not the case for educational attainment. Based on these findings, we propose that IQ is a more informative measure than educational attainment to explain variation in brain measures or group differences in brain measures.
As mentioned, only small-to-modest effects of IQ in relation to brain abnormalities in those at familial risk were reported, but these were in the expected direction. In SZ-FDRs, adjusting for IQ explained part of the effect of familial risk for schizophrenia in total brain, gray and white matter volumes (i.e., effect sizes decreased). This was previously shown in two twin studies (both included in this study; Bohlken, Brouwer, Mandl, Kahn, & Hulshoff Pol, 2016; Toulopoulou et al., 2015) and a study that included a subset of the present participants using a mega-analysis (de Zwarte, Brouwer, Tsouli, et al., 2019). Interestingly, adjusting for IQ resulted in an even larger ICV difference in BD-FDRs as compared to controls. Given that a larger ICV is associated with a higher IQ in healthy individuals, these findings suggest that the larger ICV in BD-FDRs is unrelated to differences in IQ (which was nonsignificantly lower in BD-FDRs compared to controls). Future work could compare the finding of ICV in risk for bipolar disorder to autism, another neurodevelopmental disorder. Patients with autism have larger ICV compared to controls (Van Rooij et al., 2018), while not having a functional benefit in form of higher intelligence. One could argue the same (to a much lesser extent) is true for the BD-FDRs. This may add another piece of information to disentangle risk for psychiatric disease, brain deficits and cognition.
Taken together, the study findings provide suggestive evidence for different genetic influences on neurodevelopmental processes in SZ-FDRs and BD-FDRs, leading to larger ICV and lower IQ in those at familial risk for bipolar disorder and lower IQ and but similar ICV in those at familial risk for schizophrenia compared to controls.
4.4 Limitations
A few limitations to this study should be taken into account. This work is a meta-analysis of multiple cohorts from research centers around the world. We were therefore not able to perform analyses such as predictive modeling which requires the raw data. One of the main reasons to choose this approach is that the actual imaging data does not have to be shared, which substantially increased our sample size. The study included heterogeneous samples (e.g., in acquisition protocols, scanner field strength, FreeSurfer version, IQ test battery, schooling systems, inclusion and exclusion criteria). Meta-analysis approaches find consistent effects despite this variance but cannot account for all sources of heterogeneity. One source of heterogeneity might also be the substantial age differences between the different cohorts. Both adult and children/adolescent cohorts were included in the analyses, and considering that the brains of the children and adolescents have not reached its adults size and that they have not yet reached the average age-at-onset, might have influenced the findings of the overall effects. In addition, the FDR groups consist of multiple first-degree relative types (parents, siblings, offspring, co-twins). We decided not analyze each relative type separately, as our prior study showed insufficient power to detect group differences between the different relatives subtypes (de Zwarte, Brouwer, Agartz, et al., 2019). Importantly, the composition of the SZ-FDRs and BD-FDRs groups differed. More SZ-FDRs were included, of whom a larger proportion were siblings, whereas there were more offspring in the BD-FDRs group. This indicates an overall systematic difference in the way bipolar and schizophrenia families were recruited and highlights that these are not epidemiologically acquired samples representing the entire population of relatives. This could confound the differences reported in the SZ-FDRs and BD-FDRs. Finally, we only analyzed current IQ and educational attainment as cognitive measures in relation to brain structure. Little to no information was available in the participating cohorts on some demographic features, such as parental socioeconomic status (SES), longitudinal cognitive performance (to address cognitive development over time) and other environmental factors that are potentially related to brain structure and to risk for schizophrenia and/or bipolar disorder.
5 CONCLUSIONS
In summary, investigating family members of patients with schizophrenia and bipolar disorder can provide insight into the effect of familial risk of these disorders on the brain and cognition. This study showed differential global cortical thickness and surface area abnormalities in SZ-FDRs and BD-FDRs. While present in both relative groups, cognitive alterations were more pronounced in SZ-FDRs, adding to the evidence that cognition is more affected in (risk for) schizophrenia than in (risk for) bipolar disorder. Brain differences in the relatives were related to cognitive alterations, as expected based on the well-established positive relationship between intelligence and brain. However, we found no evidence that the larger ICV in BD-FDRs was related to IQ, nor were differences in other brain measures between relatives and controls explained by IQ. This suggests that differential brain developmental trajectories underlying predisposition to schizophrenia or bipolar disorder are only minimally related to IQ. This study of schizophrenia and bipolar disorder relatives further disentangles the biological underpinnings of both disorders. The resulting findings may also inform the ongoing debate on whether schizophrenia and bipolar disorder should be conceptualized as different categories or whether they are part of a continuum of symptoms.
ACKNOWLEDGMENTS
The researchers and studies included in this article were supported by the Research Council of Norway (Grant No. 223273), National Institutes of Health (NIH) (Grant No. R01 MH117601 [to Neda Jahanshad], Grant Nos. R01 MH116147, R01 MH111671, and P41 EB015922 [to Paul M. Thompson], Grant No. U54EB020403 [to Paul M. Thompson, Christopher R. K. Ching, Theo G. M. van Erp and Sophia I. Thomopoulos], Grant No. R03 MH105808 [to Carrie E. Bearden and Scott C. Fears], Grant Nos. R01MH116147, and R01MH121246 [to Theo G. M. van Erp]) and National Institute on Aging (NIA) (Grant No. T32AG058507 [to Christopher R. K. Ching]). C-SFS: This work was supported by Canadian Institutes of Health Research. Vina M. Goghari was supported by a Canadian Institutes of Health Research New Investigator Award. Cardiff: This work was supported by the National Centre for Mental Health, Bipolar Disorder Research Network, 2010 National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Award (Grant No. 17319). CliNG: We thank Anna Fanelli, Kathrin Jakob, and Maria Keil for help with data acquisition. Clinic: This work was supported by the Spanish Ministry of Economy and Competitiveness/Instituto de Salud Carlos III (CPII19/00009), co-financed by ERDF Funds from the European Commission (“A Way of Making Europe”), and the Departament de Salut de la Generalitat de Catalunya (SLT002/16/00331). Eduard Vieta thanks the support of the Spanish Ministry of Science, Innovation and Universities (PI15/00283) integrated into the Plan Nacional de I+D+I y cofinanciado por el ISCIII-Subdirección General de Evaluación y el Fondo Europeo de Desarrollo Regional (FEDER); CIBERSAM; and the Comissionat per a Universitats i Recerca del DIUE de la Generalitat de Catalunya to the Bipolar Disorders Group (2017 SGR 1365) and the project SLT006/17/00357, from PERIS 2016-2020 (Departament de Salut). CERCA Programme/Generalitat de Catalunya. DEU: This work was supported by Dokuz Eylül Üniversitesi Department of Scientific Research Projects Funding (Grant No. 2012.KB.SAG.062). This report represents independent research funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley National Health Service Foundation Trust and King's College. London. The views expressed are those of the authors and not necessarily those of the National Health Service, NIHR, or Department of Health. EGEU: This work was supported by the Ege Üniversitesi School of Medicine Research Foundation (Grant No. 2009-D-00017). EHRS: The Edinburgh High Risk Study was supported by the Medical Research Council. ENBD_UT/BPO_FLB: This work was supported by the National Institute of Mental Health (Grant No. R01 MH085667). FIDMAG: This work was supported by the Generalitat de Catalunya (2017SGR01271) and several grants funded by Instituto de Salud Carlos III co-funded by the European Regional Development Fund/European Social Fund “Investing in your future”: Miguel Servet Research Contract (CPII16/00018 [to Edith Pomarol-Clotet]), Sara Borrell Research Contract (CD16/00264 [to Mar Fatjó-Vilas] and CD18/00029 [to Erick J. Canales-Rodríguez]), and Research Projects (PI15/00277 [to Erick J. Canales-Rodríguez], PI18/00810 [to Edith Pomarol-Clotet] and PI18/00877 [to Raymond Salvador]). The funding organizations played no role in the study design, data collection and analysis, or manuscript approval. Geneva: The study is supported by the Swiss National Center of Competence in Research; “Synapsy: the Synaptic Basis of Mental Diseases” (No.: 51NF40-185897), as well as a grant of the Swiss National Science Foundation (No.: 32003B_156914). GROUP: The infrastructure for the GROUP study was supported by the Geestkracht program of the ZonMw (Grant No. 10-000-1002). HUBIN: This work was supported by the Swedish Research Council (Grant Nos. K2007-62X-15077-04-1, K2008-62P-20597-01-3, K2010-62X-15078-07-2, K2012-61X-15078-09-3), regional agreement on medical training and clinical research between Stockholms Läns Landsting and the Karolinska Institutet, Knut och Alice Wallenbergs Stiftelse, and HUBIN project. IDIBAPS: This work was supported by the Spanish Ministry of Economy and Competitiveness/Instituto de Salud Carlos III (Grant Nos. PI070066, PI1100683, and PI1500467, PI18/00976) and Fundacio Marato TV3 (Grant No. 091630), co-financed by ERDF Funds from the European Commission (“A Way of Making Europe”), Brain and Behavior Research Foundation (NARSAD) 2017 Young Investigator Award (Grant No. 26731 [to Gisela Sugranyes]), Fundación Alicia Koplowitz and Ajut a la Recerca Pons Bartran. IoP-BD: The Maudsley Bipolar Twin Study was supported by the Stanley Medical Research Institute and NARSAD. IoP-SZ: This work was supported by a Wellcome Trust Research Training Fellowship (Grant No. 064971 to Timothea Toulopoulou), NARSAD Young Investigator Award [to Timothea Toulopoulou], and European Community's Sixth Framework Programme through a Marie Curie Training Network called the European Twin Study Network on Schizophrenia. LIBD: This work was supported by the National Institute of Mental Health Intramural Research Program (to Daniel R. Weinberger's laboratory). Lieber Institute for Brain Development (LIBD) is a nonprofit research institute located in Baltimore, MD. The work performed at LIBD was performed in accordance with an NIMH material transfer agreement with LIBD. MFS: The Maudsley Family Study cohort was supported by the NIHR Biomedical Research Centre for Mental Health at the South London and Maudsley NHS Foundation Trust at King's College London and by the NIHR Biomedical Research Centre at University College London Hospitals NHS Foundation Trust and UCL. Support to Elvira Bramon: The Wellcome Trust (Grant Nos. 085475/B/08/Z and 085475/Z/08/Z), Medical Research Council (Grant No. G0901310), British Medical Association Margaret Temple Fellowship 2016, Mental Health Research UK John Grace QC award and NIHR RfPB grant (Grant No. NIHR200756).MooDS: This work was supported by the German Federal Ministry for Education and Research grants NGFNplus MooDS (Systematic Investigation of the Molecular Causes of Major Mood Disorders and Schizophrenia) and Integrated Network IntegraMent (Integrated Understanding of Causes and Mechanisms in Mental Disorders) under the auspices of the e:Med program (Grant Nos. O1ZX1314B and O1ZX1314G) and Deutsche Forschungsgemeinschaft (Grant No. 1617 [to Andreas Heinz]). MSSM: This work was supported by National Institute of Mental Health (Grant Nos. R01 MH116147 and R01 MH113619). OLIN: This work was supported by NIH (Grant No. R01 MH080912). ORBIS Halifax and Prague samples: This work was supported by the Canadian Institutes of Health Research (Grant Nos. 103703, 106469, and 142255), Nova Scotia Health Research Foundation, Dalhousie Clinical Research Scholarship [to Tomas Hajek], 2007 Brain and Behavior Research Foundation Young Investigator Award [to Tomas Hajek], and Ministry of Health of the Czech Republic (Grant Nos. NR8786 and NT13891). PENS: This work was supported by a Department of Veterans Affairs Clinical Science Research and Development Service Merit Review Award (I01CX000227 [to Scott R. Sponheim]). PHCP: This work was supported by an NIMH Award (U01 MH108150 [to Scott R. Sponheim]) and by the NIH (P30 NS076408, 1S10OD017974-01). STAR: This work was supported by NIH (Grant No. R01 MH052857). SydneyBipolarGroup: The Australian cohort collection was supported by the Australian National Health and Medical Research Council Grants (Grant No. 510135 [to Philip B. Mitchell] and Grant No. 1037196 [to Philip B. Mitchell and Peter R. Schofield] and Grant No. 1176716 [to Peter R. Schofield]) and Project Grants (Grant No. 1063960 [to Janice M. Fullerton and Peter R. Schofield] and Grant No. 1066177 [to Janice M. Fullerton and Rhoshel K. Lenroot]), the Lansdowne Foundation and the Janette Mary O'Neil Research Fellowship [to Janice M. Fullerton]. UMCU: This work was supported by National Alliance for Research on Schizophrenia and Depression (Grant No. 20244 [to Manon H. J. Hillegers]), ZonMw (Grant No. 908-02-123 [to Hilleke E. Hulshoff Pol]), VIDI (Grant No. 452-11-014 [to Neeltje E. M. van Haren] and Grant No. 917-46-370 [to Hilleke E. Hulshoff Pol]), and Stanley Medical Research Institute.
CONFLICT OF INTEREST
Dr Yalin has been an investigator in clinical studies conducted together with Janssen-Cilag, Corcept Therapeutics, and COMPASS Pathways in the last 3 years. Dr Cannon reports that he is a consultant to Boerhinger Ingelheim Pharmaceuticals and Lundbeck A/S. Dr Meyer-Lindenberg has received consultant fees from Boehringer Ingelheim, BrainsWay, Elsevier, Lundbeck International Neuroscience Foundation, and Science Advances. Drs Ching, Jahanshad, and Thompson received partial research support from Biogen, Inc. (Boston, MA) for work unrelated to the topic of this manuscript. Dr Vieta has received grants and served as consultant, advisor or CME speaker for the following entities (work unrelated to the topic of this manuscript): AB-Biotics, Abbott, Allergan, Angelini, Dainippon Sumitomo Pharma, Galenica, Janssen, Lundbeck, Novartis, Otsuka, Sage, Sanofi-Aventis, and Takeda. The remaining authors report no biomedical financial interests or potential conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




