Longitudinal stability of the brain functional connectome is associated with episodic memory performance in aging
Funding information: H2020 European Research Council, Grant/Award Number: 802998; Norges Forskningsråd, Grant/Award Numbers: 249795, 276082; Western Norway Health Authority, Grant/Award Numbers: 911397, 911593, 911687
Abstract
The brain functional connectome forms a relatively stable and idiosyncratic backbone that can be used for identification or “fingerprinting” of individuals with a high level of accuracy. While previous cross-sectional evidence has demonstrated increased stability and distinctiveness of the brain connectome during the course of childhood and adolescence, less is known regarding the longitudinal stability in middle and older age. Here, we collected structural and resting-state functional MRI data at two time points separated by 2–3 years in 75 middle-aged and older adults (age 49–80, SD = 6.91 years) which allowed us to assess the long-term stability of the functional connectome. We show that the connectome backbone generally remains stable over a 2–3 years period in middle and older age. Independent of age, cortical volume was associated with the connectome stability of several canonical resting-state networks, suggesting that the connectome backbone relates to structural properties of the cortex. Moreover, the individual longitudinal stability of subcortical and default mode networks was associated with individual differences in cross-sectional and longitudinal measures of episodic memory performance, providing new evidence for the importance of these networks in maintaining mnemonic processing in middle and old age. Together, the findings encourage the use of within-subject connectome stability analyses for understanding individual differences in brain function and cognition in aging.
1 INTRODUCTION
Recent advances in brain imaging have documented that the functional organization of the human brain largely complies with a spatiotemporal hierarchical network structure or “connectome” that remains relatively stable across task and context (Finn et al., 2015; Kaufmann, Alnaes, Brandt, et al., 2017). The connectome backbone comprises idiosyncratic features, allowing the identification of individuals much like a brain-based fingerprint (Finn et al., 2015; Kaufmann, Alnaes, Doan, et al., 2017; Miranda-Dominguez et al., 2014). Moreover, the stability or degree of distinctiveness of the connectome has been associated with individual differences in various traits and conditions. The short-term stability of the connectome was found to increase with increasing age in youth and was overall found to be lower in children and adolescents with higher levels of common symptoms of mental disorders (Kaufmann, Alnaes, Doan, et al., 2017), and in adult patients with severe mental disorders compared to healthy peers (Kaufmann et al., 2018). Further, the neural networks contributing the most to an individual's connectome fingerprint—the frontoparietal and the default mode networks (DMN)—were also associated with individual differences in cognitive abilities (Finn et al., 2015). Jointly, these findings hold great promise for using brain network approaches to advance our understanding of individual variations in cognition, behavior, and neuropsychiatric disorders, including an extension to the study of cognitive aging and neurodegenerative disease.
Alterations in the brain gray and white matter structural and functional connectivity are among the hallmarks of cognitive aging (Ferreira & Busatto, 2013; Fjell, Westlye, et al., 2009; Westlye et al., 2010). These age-related decrements in brain connectivity are paralleled by decline in numerous cognitive functions, likely related to impaired communication between brain regions necessary for maintaining cognitive function (Ferreira & Busatto, 2013; Fjell et al., 2016; Fjell, Sneve, Grydeland, Storsve, & Walhovd, 2017). Notably, both cortical and subcortical networks are vulnerable to aging (Ferreira & Busatto, 2013; Sala-Llonch, Bartres-Faz, & Junque, 2015) with some networks showing increased and others decreased resting-state connectivity with increasing age (Buckner, 2004; Mowinckel, Espeseth, & Westlye, 2012). Moreover, the extent and rate of change show strong heterogeneity across networks, with frontoparietal and DMNs, repeatedly identified as the most discriminative of individuals, being particularly sensitive to the aging process (Sala-Llonch et al., 2015). Together these findings suggest that alterations in the structural and functional connectivity of the brain may be related to how well the individual functional connectome backbone is preserved. Furthermore, it raises the question whether longitudinal stability of the individual connectome is sensitive to concurrent cognitive changes in aging.
Although aging brings about decline in numerous cognitive faculties, episodic memory is one of the most studied. Age-related declines in episodic memory have been reliably identified in both cross-sectional (Hedden & Gabrieli, 2004; Nyberg, Lovden, Riklund, Lindenberger, & Backman, 2012; Ronnlund, Nyberg, Backman, & Nilsson, 2005) and longitudinal (Lundervold, Wollschlager, & Wehling, 2014; Nyberg, 2017) studies of healthy elderly individuals. Moreover, impaired episodic memory is a core cognitive symptom of several neurodegenerative disorders, of which Alzheimer disease is the most studied (Gallagher & Koh, 2011). Such age-related changes in memory have been related to altered structural and functional connectivity in prefrontal networks and the DMN (Fjell et al., 2015; Nyberg, 2017; Salami, Pudas, & Nyberg, 2014; Staffaroni et al., 2018; Westlye, Lundervold, Rootwelt, Lundervold, & Westlye, 2011) but more recently also to specific subcortical systems (i.e., the thalamus, amygdala and basal ganglia) (Fjell et al., 2016; Rieckmann, Johnson, Sperling, Buckner, & Hedden, 2018; Ystad, Eichele, Lundervold, & Lundervold, 2010). This is not surprising given the centrality of subcortical nuclei, which are connected to virtually all parts of the cortex (Sah, Faber, Lopez De Armentia, & Power, 2003; Shepherd, 2013), and that neurotransmitters affecting episodic memory target both cortical and subcortical brain structures (Backman, Nyberg, Lindenberger, Li, & Farde, 2006). Moreover, the hippocampal subsection of the DMN and the basal ganglia are conventionally viewed as parallel learning and memory systems (DeCoteau et al., 2007), which may act competitively or cooperatively depending on the context. Accordingly, age-related changes in resting-state functional connectivity of both systems have been linked to deficits during mnemonic processing (Rieckmann et al., 2018; Staffaroni et al., 2018), and disorders predominantly targeting the striatal system have also been associated with memory impairments already in the earliest stages of the disease (Solomon et al., 2007).
Episodic memory performance in aging has additionally been linked to the morphology of the aging brain, including global cortical volume (Harrison, Weintraub, Mesulam, & Rogalski, 2012), cortical thickness (Harrison, Maass, Baker, & Jagust, 2018), and hippocampal volume (Ezzati, Katz, Lipton, Zimmerman, & Lipton, 2016; Ystad et al., 2009). Both a constitutionally larger gray matter volume and a slower rate of gray matter loss throughout life, may endow individuals with larger gray matter reserves which may translate into increased capacity to resist age related-memory decline (Cabeza et al., 2018). Although speculative, it is possible that this gray matter reserve exert its effect on episodic memory performance through an impact on the brain functional connectome (Smith et al., 2019). As such, indices of brain gray matter volume could be associated with both connectome stability and episodic memory performance.
In the present study, we investigated the longitudinal stability of the connectome backbone in middle and older age, and how this relates to changes in episodic memory, hippocampal, and global cortical volume. We obtained T1-weighted structural and resting-state functional MRI (rsfMRI) data from 75 middle-aged and older adults (age 49–80, SD = 6.91 years) at two time-points separated by 2–3 years, and assessed the longitudinal stability of the whole-brain functional connectome and a set of subnetworks. We hypothesized that connectome stability would decrease as a function of increasing age as well as smaller cortical and hippocampal volume, as a proxy of brain structural aging. Second, supported by studies linking age-related cognitive decline to structural and functional connectivity, we hypothesized that weaker connectome stability within subnetworks important for episodic memory would be associated with a steeper memory decline.
2 MATERIALS AND METHODS
2.1 Participants
Healthy volunteers were invited through advertisement to take part in a longitudinal study on cognitive aging involving extensive neuropsychological testing, MRI, and genotyping. Participants were assessed up to three times over a period of 6.5 years, of which rsfMRI data were acquired at Session 2 (MRI1) and Session 3 (MRI2). MRI1 and MRI2 were separated by 2–3 years (mean = 2.54, SD = ±0.28 years). General exclusion criteria included history of substance abuse, present neurological or psychiatric disorder or other significant medical conditions. The protocol was approved by the Regional Committee for Medical and Health Research Ethics of Southern and Western Norway, and all subjects gave written informed consent before participation.
The present study included 75 participants who underwent rsfMRI at MRI1 and MRI2. T1-weighted three-dimensional (3D) images were evaluated by an experienced neuroradiologist at inclusion, and the presence of brain tumors, cysts, recent infarctions or gross regional or global signal abnormalities lead to exclusion. No participants were excluded based on the neuroradiological evaluation. Moreover, none of the included participants was diagnosed with dementia or mild cognitive impairment (Mini Mental State Exam < 24) (Mungas, 1991). For further participant characteristics, please see Table 1.
| MRI1 | MRI2 | t-Value | p-Value | |
|---|---|---|---|---|
| Age | 64.3 (6.9) | 66.8 (6.8) | 41.24 | <.001 |
| Years of education | 14.0 (2.9) | |||
| Women (%) | 65.3 | |||
| MMSE | 28.9 (1.0) | 29.1 (1.4) | 1.00 | .32 |
| IQ | 116 (11) | |||
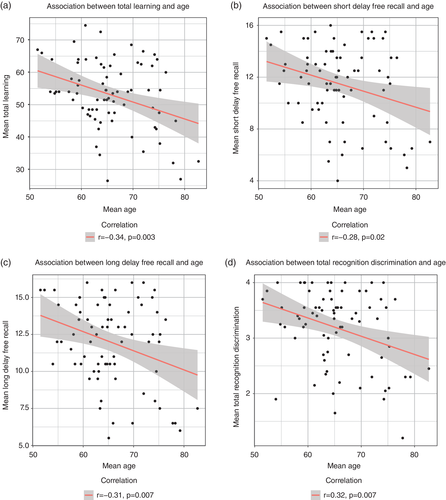
| CVLT, total learning | 56.7 (10.9) | 49.6 (11.3) | −8.01 | <.001 |
| CVLT, short delay recall | 12.3 (2.9) | 10.6 (3.6) | −5.61 | <.001 |
| CVLT, long delay recall | 12.9 (2.7) | 11.0 (3.4) | −6.86 | <.001 |
| CVLT, recognition discrimination | 3.4 (0.7) | 3.0 (0.8) | −4.87 | <.001 |
- Abbreviations: CVLT, California Verbal Learning Test; MMSE, Mini Mental State Exam.
2.2 Neuropsychological assessments
All participants completed an extensive set of neuropsychological tests at each assessment, and the test scores were evaluated by an experienced neuropsychologist. The battery included tests of executive functions, episodic memory, language, IQ, and mental processing speed. Episodic memory function was assessed using the Norwegian translation of the California Verbal Learning Test-Second Version (CVLT-II; Delis, Kramer, Kaplan, & Ober, 2000). A list of 16 words (List A) was presented five times. After each presentation, the participant had to repeat as many words as possible, and a total learning score was defined from the sum of correct responses across these five presentations. Upon completing the fifth trial, a new list was presented (List B), and subjects had to recall the words from List A immediately after List B (short delay free recall). Approximately 20 min later, subjects were asked to identify the words from List A again (long delay free recall). Finally, subjects were presented with a larger list that contained items from List A, List B as well as other various distracter items, and asked to identify the 16 items from List A (total recognition discrimination).
The CVLT-II assesses three essential features of episodic memory: learning, recall and recognition, represented by the variables total learning, free recall (short and long delay) and total recognition discrimination, respectively. To reduce data dimensionality, we used principal component analysis (PCA) to get a robust compound measure of episodic memory for the combined analyses with the neuroimaging data. The Kaiser–Meyer–Olkin value exceeded 0.8 and Bartlett's test of sphericity was highly significant, confirming that the data was suitable for PCA. In the sample, PCA captured 86.2% of the variance in one single component (PC1). Both the mean PC1 across MRI1 and MRI2 and the individual changes in PC1 from MRI1 and MRI2 were used for the imaging analyses.
2.3 MRI acquisition
Whole-brain, T2*-weighted, echo-planar images (TR = 2,000 ms, TE = 50 ms, flip angle 90°, voxel size 3.75 × 3.75 × 5.0 mm3) were acquired using a GE Signa Echospeed 1.5 T Scanner (General Electric Company; Milwaukee, WI) supplied with a standard eight-channel head coil. A total of 256 volumes (25 axial slices) were acquired, yielding a scan time of approximately 8 min. Participants were instructed to relax with their eyes closed, to think of nothing in particular, and not to fall asleep. Cushions and headphones were used to reduce subject motion and scanner noise. For anatomical comparison purposes, two T1-weighted 3D inversion recovery-prepared fast spoiled gradient-recalled series (TR = 9.11 ms, TE = 1.77 ms, flip angle 7°, voxel size 0.94 × 0.94 × 1.40 mm3) were acquired prior to the functional imaging. The imaging parameters were identical for both the T2* and the T1 series at the two time-points. rsfMRI data from MRI1 and MRI2 have been previously published (Hodneland, Ystad, Haasz, Munthe-Kaas, & Lundervold, 2012; Westlye et al., 2011; Ystad et al., 2010; M. Ystad et al., 2011); however, none of the studies have included longitudinal analyses.
2.4 MRI processing and analysis
T1-weighted 3D MR was processed using the longitudinal pipeline in FreeSurfer v 5.3 (http://surfer.nmr.mgh.harvard.edu), which enables fully automated volumetric segmentation of neuroanatomical structures and longitudinal comparisons. The processing steps included motion correction and averaging, removal of nonbrain tissue and automated Talairach transformation. Tessellation of the gray/white matter boundary together with surface deformation following intensity gradients to optimally place the gray/white/CSF borders allowed segmentation of cortex as well as subcortical white matter and deep gray matter structures. All segmented scans were visually inspected.
rsfMRI data were processed using FMRI Expert Analysis Tool (FEAT), as implemented in FMRIB Software Library (FSL (Smith et al., 2004; Woolrich et al., 2009), (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/)), and included motion correction, spatial smoothing using a six mm full-width at half-maximum Gaussian kernel as well as high-pass temporal filtering (90 s). To minimize the influence of noise (e.g., related to participant motion and vascular artifacts), we applied FMRIB's independent component analysis-based Xnoisifier (FIX (Salimi-Khorshidi et al., 2014)), which uses single-session multivariate exploratory linear optimized decomposition into independent components (MELODIC (Beckmann, DeLuca, Devlin, & Smith, 2005)) to decompose the individual fMRI data sets. Using default options, components were classified as noise and non-noise variability, respectively, using a standard training set supplied with FIX. Components identified as noise and the estimated participant motion parameters were regressed out of the data, and we manually inspected the resulting cleaned fMRI data sets.
The fMRI volumes were registered to the participants' skull-stripped T1-weighted scans using the FMRIB linear image registration tool (FLIRT, (Jenkinson & Smith, 2001)) implementing boundary-based registration. The T1-weighted volume was nonlinearly warped to the Montreal Neurological Institute MNI-152 template using FMRIB's nonlinear image registration tool (FNIRT (Anderson, Jenkinson, & Smith, 2007)), and the resulting nonlinear transform was applied to the rsfMRI data. To control subsequent analyses for data quality and motion confounds, we utilized quality assurance scripts released by Roalf et al. (2016) and calculated estimates of temporal signal-to-noise ratio (tSNR). One estimate of tSNR per subject and run was calculated by computing voxel-wise mean and SD of the time series (after correcting for linear trends) and averaging the ratio of mean and SD across voxels in the individual brain mask from FSL FEAT. In addition, we estimated an individual mean motion parameter by taking the mean of the relative frame-to-frame displacement (including both rotation and translation) of the raw data.
2.5 Individual level fingerprinting using fMRI data
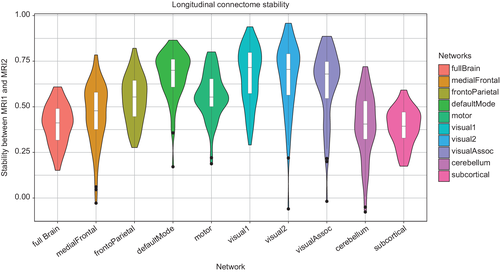
We used a functional whole-brain atlas consisting of 268 regions of interest (ROIs) (Shen, Tokoglu, Papademetris, & Constable, 2013) and estimated the pairwise Pearson correlations between all ROIs independently for each of the two time points (MRI1 and MRI2). ROIs were excluded if they were not covered by a minimum of 10% of voxels in all subjects, leading to the exclusion of 20 ROIs in total (Figure S1, Supporting Information). The whole-brain connectivity matrix from each individual at each time point was then transformed into a vector of size 1 × 30,628 (248 ROIs and 30,628 network links between them). Next, we computed the connectome stability in line with the approach by Kaufmann et al. (2018) which involved computing the within-subject Spearman correlation coefficient between MRI1 and MRI2 networks.
In addition to parcellating the brain into 248 nodes, we also clustered these nodes based on Yeo et al.'s network scheme (Buckner, Krienen, Castellanos, Diaz, & Yeo, 2011), yielding nine large-scale networks (i.e., medial frontal, frontoparietal, default mode, motor, visual 1, visual 2, visual association, cerebellum, subcortical) (Finn et al., 2015; Kaufmann, Alnaes, Doan, et al., 2017). In line with the whole-brain analysis, we calculated between time points connectome stability scores for each of these nine networks.
2.6 Statistical analysis
All statistical analyses were performed in R (version 3.5.0; R Development Core Team, 2018). Longitudinal analyses modeling the relationship between episodic memory performance and age-, sex, session, and time between sessions were performed using linear mixed effects models (lme4 package in R (Bates, Maechler, Bolker, & Walker, 2015)). Separate models were run for each CVLT-II variable (i.e., learning, short delay free recall, long delay free recall, and total recognition discrimination). As fixed effects, we entered age (mean across MR1 and MR2), sex, session, and time between sessions (without interaction terms) into the model. In addition to these fixed-effects, the model included subject ID as random factor, modeling the individual level intercept. p-Values were obtained by likelihood ratio χ2 tests of the full model with the effect in question compared to a model without the effect in question.
Next, we used a general linear model to test for associations between individual whole-brain connectome stability and age while controlling for sex, tSNR, mean motion, and time between sessions. The analysis was repeated for each of the nine subnetworks separately, based on studies reporting anatomical differences in the rate and degree of aging (Buckner, 2004). Beyond chronological age, we also tested for associations between cortical (defined as CortexVol in FreeSurfer) or hippocampus volume and connectome stability. As such, the general linear models were expanded to also include a predictor for mean (across MRI1 and MRI2) or longitudinal changes in total cortical or hippocampus volume while additionally controlling for total intracranial volume (ICV).
To explore the cognitive significance of the whole-brain as well as the subnetworks temporal stability, we used the PC1 obtained from the episodic memory compounds. In separate general linear models we tested for associations between the mean PC1 or changes (across time) in the PC1 and connectome stability, while covarying for sex, age, tSNR, mean motion, and time between sessions for each of the nine subnetworks.
To rule out confounding effects of potential extreme values on our results, we excluded subjects with CVLT-II performance, network stabilities, cortical volume or hippocampal volume values >|4| SD from the group mean from the statistical analyses. Throughout the manuscript, we report uncorrected p-values, with a significance threshold for all tests determined by the Benjamini–Hochberg false-discovery rate procedure at q = 0.05. In the figures, the regression lines represent the association between dependent and independent variables estimated without covariates.
3 RESULTS
3.1 Verbal episodic memory function
Table 1 summarizes the changes in mean scores for all episodic memory measures, supporting significantly lower performance scores in MRI2. The contribution of age, sex, and session intervals in predicting individual longitudinal episodic memory performance were assessed in linear mixed effect models, with separate models for each of the four CVLT-II variables. Standard likelihood-ratio χ2 tests revealed that sex and session were significant predictors of all four memory components (Table 2). As such, performance dropped from MRI1 to MRI2, and more so for males than females. In addition, higher age was associated with greater decrements of learning, long delay free recall, and total recognition discrimination (Figure 1).
| Estimatea | SE | Χ2 test | p-Value | pfdr-Value | |
|---|---|---|---|---|---|
| Learning | |||||
| Age | −0.37 | 0.14 | 6.21 | .01 | .03 |
| Sexb | −9.32 | 2.04 | 18.39 | <.001 | <.001 |
| Session | −7.09 | 0.88 | 46.83 | <.001 | <.001 |
| Time between sessions | 0.02 | 0.01 | 6.04 | .01 | .05 |
| Short delay recall | |||||
| Age | −0.08 | 0.04 | 3.57 | .06 | .06 |
| Sex | −3.17 | 0.59 | 24.12 | <.001 | <.001 |
| Session | −1.73 | 0.31 | 26.54 | <.001 | <.001 |
| Time between sessions | 0.004 | 0.003 | 2.71 | .1 | .13 |
| Long delay recall | |||||
| Age | −0.09 | 0.04 | 4.79 | .03 | .04 |
| Sex | −2.82 | 0.55 | 22.44 | <.001 | <.001 |
| Session | −1.85 | 0.27 | 36.90 | <.001 | <.001 |
| Time between sessions | 0.006 | 0.003 | 4.41 | .04 | .08 |
| Recognition discrimination | |||||
| Age | −0.02 | 0.01 | 5.83 | .02 | .03 |
| Sex | −0.64 | 0.14 | 19.21 | <.001 | <.001 |
| Session | −0.35 | 0.07 | 20.86 | <.001 | <.001 |
| Time between sessions | 0.001 | 0.0006 | 2.24 | .13 | .14 |
- a The estimate refers to the beta values obtained from the linear mixed effects models.
- b Using male as a reference.
3.2 Longitudinal stability of the connectome and its association to age
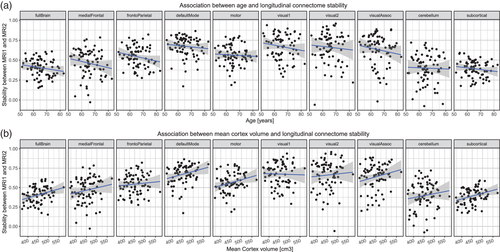
Figure 2 shows the results from the connectome stability analyses. In line with recent work in a longitudinal sample of youths (Miranda-Dominguez et al., 2018), the connectome fingerprint retained relatively stable across 2–3 years (mean Spearman correlations between scans for all subjects: ρ = 0.4, range: 0.15–0.60 for the full brain connectome). However, the stabilities differed for the various subnetworks, with the subcortical and the cerebellar networks having lower temporal stability than most cortical networks (Figures 2, S2, and S3). Figure 3a illustrates the association between connectome stability and age (mean across MRI1 and MRI2). The association between age and whole-brain connectome stability (slope [±SE] = −0.003 ± 0.002, t69 = −1.57, p = .12) as well as between age and subnetwork connectome stabilities (all p > .05) were subtle and none remained significant when accounting for multiple comparison.
3.3 The association between connectome stability and mean cortical and hippocampal volume
We next investigated if connectome stability was associated with cortical or hippocampal volume by expanding the general linear models to also include a predictor for mean (across MRI1 and MRI2) or time-dependent changes in total cortical volume while additionally controlling for ICV. Mean cortical volume was positively associated with DMN (slope = 1.66 × 10−6 ± 6.19 × 10−7, t66 = 2.68, p = .009, Figure 3b), subcortical (slope = 1.62 × 10−6 ± 5.22 × 10−7, t66 = 3.11, p = .003, Figure 3b), medial prefrontal (slope = 2.31 × 10−6 ± 8.17 × 10−7, t66 = 2.83, p = .006, Figure 3b), visual association (slope = 2.18 × 10−6 ± 8.91 × 10−7, t66 = 2.45, p = .02, Figure 3b) and whole-brain (slope = 1.60 × 10−6 ± 5.32 × 10−7, t66 = 3.01, p = .004, Figure 3b) connectome stability, indicating higher stability with larger brain cortical volumes. No significant associations emerged between the network stabilities and longitudinal changes in cortical volume (all p > .05). Finally, including hippocampal volume as a predictor in the models revealed no association between mean or changes in hippocampal volume and connectome stability (all p > .05).
3.4 The association between connectome stability and cross-sectional and longitudinal measures of episodic memory performance
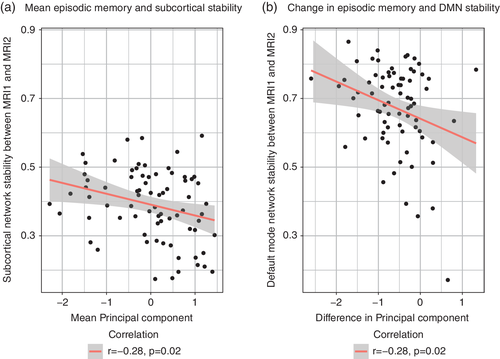
Based on studies linking changes in functional connectivity to episodic memory decline, we next investigated if episodic memory performance was related to longitudinal connectome stability. The analyses revealed a significant negative association between connectome stability and mean episodic memory performance (i.e., the mean of PC1 across MRI1 and MRI2) for the subcortical network (slope = −0.05 ± 0.02, t68 = −2.92, p = .005, Figure 4a), indicating higher subcortical network stability with lower mean episodic memory performance. Similar analyses for the other networks revealed no significant associations after correcting for multiple comparisons. Furthermore, the analyses revealed a significant negative association between DMN stability and change in episodic memory performance, indicating larger episodic memory decline between MRI1 and MRI2 in individuals with higher DMN stability (slope = −0.07 ± 0.02, t67 = −3.13, p = .003, Figure 4b). In addition, there was a nominal significant association between changes in episodic memory performance and subcortical network stability (slope = −0.04 ± 0.02, t68 = −2.21, p = .03). Finally, while mean cortical volume was associated with connectome stability, general linear models revealed no significant associations (all p > .05) between mean cortical volume and mean or changes in PC1 while adjusting for age, sex, ICV and time between sessions.
4 DISCUSSION
In this study, we tested the long-term stability of the functional connectome in middle- and older age, and how brain network stability relates to structural indices of aging and memory performance. In line with a recent study, we demonstrated relatively high stability of the connectome over a 2–3 years period (Horien, Shen, Scheinost, & Constable, 2019). While our analyses did not reveal a significant association with age, network stability was related to mean cortical volume across the two time points, suggesting that cortical morphology is associated with connectome stability. Supporting its relevance to cognitive aging, both cross-sectional and longitudinal measures of episodic memory were related to longitudinal stability of the DMN and the subcortical networks. The findings encourage the use of connectome stability for understanding individual differences related to brain aging and risk of neurodegenerative disease. Furthermore, the observation that individual variations in episodic memory decline relates to the stability of subcortical and DMNs provides new evidence for the importance of these networks in maintaining mnemonic functions in middle and older age.
Although previous studies have documented that the connectome individualizes during adolescence to form unique functional connectivity profiles (Kaufmann, Alnaes, Doan, et al., 2017), our knowledge regarding connectome stability in aging is limited. The high stability obtained in the present study suggests that the connectome backbone may represent a robust trait-like marker also in middle and old age, in spite of the vast changes in brain structural and functional connectivity associated with increasing age. Despite large efforts in linking cognitive decline to brain changes in aging, the majority of previous studies have not been able to separate state- or task-based variability from static, subject-unique features. The notion that the connectome backbone generally remains stable across contexts and cognitive tasks (Finn et al., 2017; Kaufmann, Alnaes, Brandt, et al., 2017), and that this stability may also be present in senescence, holds great promise for using connectome-based approaches to map clinically useful changes of brain functional connections also in middle and older age.
Although the stability of the connectome backbone was not significantly associated with age, the stability was related to individual differences in cortical volume. Aging is associated with structural changes of the cerebral cortex as indicated by widespread reductions in cortical thickness (Fjell, Westlye, et al., 2009) and gray matter volume (Good et al., 2001; Raz et al., 2005). However, individuals differ markedly in rate and degree of structural brain changes, and thus brain structure is relatively well preserved in some individuals into old age. Such individual differences are likely to be related to numerous factors, including individual variations in microvasculature (Dey, Stamenova, Turner, Black, & Levine, 2016) and white matter pathologies (Langen et al., 2017), processes which affect both local and distant brain connections and thus the connectome backbone. Of note, previous studies have reported an association between episodic memory maintenance and global cortical brain volume (Cook et al., 2017; Harrison et al., 2012). Moreover, we here report that global cortical volume relates to the connectome stability of medial prefrontal, DMN, visual association, and subcortical networks suggesting a mechanism by which cortical morphology could impact cognition. Thus, future studies may investigate if the association between cortical structure and age-related changes in episodic memory is mediated by the stability of the connectome, with consequences for our understanding of numerous neurodegenerative disorders.
The subcortical network had distinct lower stability than the cortical networks. Subcortical brain areas including the striatum, thalamus, and the hippocampus undergo substantial structural changes in aging (Bonifazi et al., 2018; Fama & Sullivan, 2015; Fraser, Shaw, & Cherbuin, 2015), which is likely to impact connectome stability. Indeed, less functional specialization including the separation of the striatal from the medial temporal lobe systems has been reported in aging (Rieckmann et al., 2018), and these age-related changes may involve both increased and decreased functional connectivity. In contrast, sensory cortical areas are more likely to remain differentiated, and even increase their modularity, as age progress (Geerligs, Renken, Saliasi, Maurits, & Lorist, 2015). In line with this notion, the visual sensory areas obtained the highest stability scores across the 2–3 years period. Finally, although reduced within-network amplitude and connectivity in the DMN is one of the most replicated findings in aging (Damoiseaux, 2017; Mowinckel et al., 2012), this network retained higher stability than other networks, including the cerebellar and the subcortical networks. Although this finding warrants replication, it indicates that the age-related changes in the DMN do not surpass the idiosyncratic patterns in functional connectivity that promotes successful identification (Horien et al., 2019).
The finding that the temporal stability of the subcortical network was inversely related to episodic memory performance may at first seem counterintuitive. However, increased subcortical network stability may not necessarily represent better brain maintenance. Increased network stability may partly reflect or come at the cost of decreased flexibility, including the task dependent engagement of cortico-subcortical networks in episodic memory tasks. In line with this notion, middle aged and older subjects experiencing increased striatal or hippocampal synchronization during rest, also had diminished cortical–subcortical connections and poorer memory performance in cross-sectional studies (Rieckmann et al., 2018; Salami et al., 2014). Moreover, the ability of neuronal networks to flexibly adapt in response to neurodegenerative changes may be a prerequisite for maintaining cognitive function in older age. As such, the reduced functional specialization and increased internetwork communication of subcortical structures (Rieckmann et al., 2018) could reflect diminished ability to flexibly adapt in response to neurodegenerative changes locally and elsewhere in the brain, which may translate into impaired episodic memory function. Finally, although individual differences in connectome stability may to some extent reflect cross-subject variability in connectivity strength, the stability is also likely to be influenced by the spatial configuration of the network (Bijsterbosch et al., 2018; Bijsterbosch, Beckmann, Woolrich, Smith, & Harrison, 2019). As such, various neurodegenerative events at play during aging which impact the precise shape, volume, and location of the subcortical brain regions, may influence episodic memory performance through an effect on the subnetwork's functional connectome.
In addition to the subcortical stability, subjects experiencing greater memory decline also had a more stable DMN during the 2–3 years period. This fits well with evidence that the DMN entails interacting subsystems that are implicated in episodic memory (Staffaroni et al., 2018), and that the longitudinal trajectory of DMN connectivity is associated with changes in episodic memory function in aging (Staffaroni et al., 2018). Moreover, increased DMN connectivity has been observed in mild cognitive impairment (Celone et al., 2006; Gardini et al., 2015; Jin, Pelak, & Cordes, 2012) preceding the profound reductions in whole-brain connectivity characteristic of Alzheimer disease. Although speculative, increased DMN stability may be a required permissive for the spread of pathological proteins, which eventually leads to aberrant network connectivity (de Haan, Mott, van Straaten, Scheltens, & Stam, 2012). In line with this heuristic, increased DMN synchrony over a lifetime is associated with total amyloid depositions in posterior DMN subsystems (Buckner et al., 2005). Moreover, the brains of healthy adults experiencing greater cognitive decline are more likely to harbor pathological proteins, including amyloid (Farrell et al., 2018). Irrespective of this our results support the view that memory impairment in old age depends on simultaneous changes in multiple memory systems as connectome stability of different subnetworks (DMN, subcortical) was associated with episodic memory performance (Fjell et al., 2016). Accordingly, a whole-brain approach may provide a more holistic approach to memory in aging than the consideration of single networks or brain areas.
The vast majority of studies investigating resting-state functional connectivity in association to age-related memory changes have been cross sectional. However, these studies do not allow determining whether the memory decline precedes the connectivity changes or the reverse. Among the few longitudinal exceptions, one study reported that the stability of the DMN was positively related to episodic memory maintenance in aging (Persson, Pudas, Nilsson, & Nyberg, 2014). Another study reported that better preservation of striatal- cortical connectivity over time yielded a more favorable memory outcome at follow-up testing (Fjell et al., 2016) possibly related to inhibition of subcortical intranetwork connectivity at rest (Salami et al., 2014). While these studies investigated how individual changes in a common template of brain functional organization relates to episodic memory, they did not explore how age-related changes in the connectome backbone may affect memory function in old age. Accordingly, our finding that the stability of the subcortical and the DMN connectome relates to episodic memory in aging suggests that individual differences in the organization of subcortical and DMNs influence how well mnemonic processes are maintained into old age.
The present study had some limitations. First, we note that the follow-up time of this study was relatively short, which may not be sufficient to detect reliable changes in functional connectivity in middle and older age. Thus, a longer follow-up period may have revealed a significant association between connectome stability and age. However, previous studies investigating longitudinal changes in functional brain connectivity in aging also used a follow-up time of approximately 3 years (Fjell et al., 2016). Moreover, gray matter atrophy (Storsve et al., 2014) and changes in diffusion MRI (Sexton et al., 2014) could be reliably tracked over a 3-year period, and possibly even shorter (Fjell, Walhovd, et al., 2009). Together, these studies suggest that a 3-year period is sufficient to detect age-related changes in functional connectivity. Moreover, we cannot rule out a practice effect regarding the episodic memory data in our subjects, which is a limitation in all longitudinal studies of neurocognitive aging. In the present study, an initial whole-brain approach was utilized, followed by predefined subnetwork analyses. Although the subnetworks represent relatively coarse subdivisions of the brain, they do not exclude parts of the connectome, which is the case in all seed-based approaches. However, it is still conceivable that a different parcellation scheme may have yielded different results. Thus, future work needs to investigate how the subnetwork stabilities change under different parcellation schemes in order to reveal how the various parts of the human functional connectome may show distinct stability in aging.
In summary, our results suggest that the connectome backbone remains relatively stable across 2–3 years in middle and older age. Individual differences in the stability of selected networks were associated with cortical volume and memory performance, supporting the neurocognitive relevance. Future large-scale longitudinal studies comprising genetics and rich cognitive and biological phenotyping with connectome-wide stability analyses could bring us closer to a mechanistic understanding of how age-related changes in neural events give rise to age-related cognitive decline, ranging from physiological changes to neurodegenerative disease.
ACKNOWLEDGMENTS
This work was supported by the Western Norway Health Authority (grant 911397 and 911687 to A.J.L., and grant 911593 to A.L.), the Research Council of Norway (249795 to L.T.W. and grant 276082 to T.K.), and the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (ERC Starting Grant, Grant agreement No. 802998). The authors would like to thank Dr Jonn-Terje Geitung and Haraldsplass Deaconess Hospital for access to MRI facility.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
Open Research
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.