Individual differences in pain sensitivity are associated with cognitive network functional connectivity following one night of experimental sleep disruption
Funding information: National Institutes of Health, Grant/Award Numbers: NIH F32 HL143941, NIH K23 DA035915, NIH P30 NR014131, NIH R01 DA0329922, NIH T32 NS7020110; Blaustein Pain Research Award
Abstract
Previous work suggests that sleep disruption can contribute to poor pain modulation. Here, we used experimental sleep disruption to examine the relationship between sleep disruption-induced pain sensitivity and functional connectivity (FC) of cognitive networks contributing to pain modulation. Nineteen healthy individuals underwent two counterbalanced experimental sleep conditions for one night each: uninterrupted sleep versus sleep disruption. Following each condition, participants completed functional MRI including a simple motor task and a noxious thermal stimulation task. Pain ratings and stimulus temperatures from the latter task were combined to calculate a pain sensitivity change score following sleep disruption. This change score was used as a predictor of simple motor task FC changes using bilateral executive control networks (RECN, LECN) and the default mode network (DMN) masks as seed regions of interest (ROIs). Increased pain sensitivity after sleep disruption was positively associated with increased RECN FC to ROIs within the DMN and LECN (F(4,14) = 25.28, pFDR = 0.05). However, this pain sensitivity change score did not predict FC changes using LECN and DMN masks as seeds (pFDR > 0.05). Given that only RECN FC was associated with sleep loss-induced hyperalgesia, findings suggest that cognitive networks only partially contribute to the sleep-pain dyad.
1 INTRODUCTION
Evidence consistently shows that sleep disturbance leads to a variety of neurobiological and behavioral adaptations (Bosch et al., 2013; Short & Banks, 2014; Verweij et al., 2014). Even one night of total sleep deprivation is associated with brain changes, especially in neural networks underlying cognitive control, affect, and memory (Krause et al., 2017). However, these neurobiological differences are not always linked with detrimental behavioral responses. Some individuals actually benefit from acute sleep disturbance (Chuah, Venkatraman, Dinges, & Chee, 2006; Sletten, Segal, Flynn-Evans, Lockley, & Rajaratnam, 2015), begging the question: what mechanisms drive diverging responses to various types of sleep loss?
Among aberrant sequelae associated with sleep loss, maladaptive changes in pain perception represent one of the largest public health concerns. First, emerging evidence suggests that chronic sleep loss predicts new cases of chronic pain and exacerbations of existing pain symptoms (see Finan, Goodin, & Smith, 2013 for a review). Second, there is a high prevalence and rate of comorbidity between chronic pain and chronic sleep disturbance (Alsaadi et al., 2014; Karaman et al., 2014; Koffel et al., 2016; Tang, Wright, & Salkovskis, 2007). There are many protocols to experimentally alter sleep in order to explore mechanisms of the sleep-pain dyad, such as total sleep deprivation (i.e., preventing individuals from sleeping at all through >24-hr period), sleep restriction (i.e., limiting sleep under 8 hr), and forced awakenings (i.e., interrupting sleep at various intervals throughout the night). Because chronic pain patients tend to experience difficulty staying asleep—characterized by multiple night time awakenings (i.e., sleep maintenance insomnia)—the forced awakenings paradigm is a particularly relevant tool for understanding the relationship between sleep disruption and pain sensitivity (Rosseland, Pallesen, Nordhus, Matre, & Blågestad, 2018).
Pain itself is a complex experience that is influenced by sensory, affective, and cognitive processes (Apkarian, Bushnell, Treede, & Zubieta, 2005; Bushnell, Čeko, & Low, 2013; Price, 2002). While various networks contribute to this perception, brain regions associated with cognitive control are critical for our ability to modulate pain. Notably, cognitive control exerts top-down influence over sensory and affective pain components (Seminowicz & Ceko, 2015). For this reason, the present study focused on the relationship between sleep disruption-induced hyperalgesia and function across networks associated with cognitive control.
Two key networks involved in cognitive pain control are the executive control network (ECN) and default mode network (DMN). The ECN predominately includes the dorsolateral prefrontal cortex (dlPFC) and posterior parietal areas, and the DMN is composed of the medial prefrontal cortex (mPFC), posterior cingulate cortex/precuneus, and inferior parietal lobules. Functionally, the ECN is linked with attention to relevant information and suppression of irrelevant information (Awh, Vogel, & Oh, 2006; Gazzaley & Nobre, 2012; Smith et al., 2018), and the DMN is associated with self-referential thought and focus on internal milieu (Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010; Lorenzi et al., 2011; Raichle, 2015).
Previous work suggests that the functional dynamics of these networks can contribute to the detection and inhibition of nociceptive inputs. For example, greater ECN-mPFC functional connectivity (FC) was associated with better cognitive modulation of pain among healthy individuals (Kong et al., 2013). Commensurate with this finding, another study demonstrated greater ECN-DMN FC in individuals who learned and practiced cognitive modulation strategies over 21 days compared to participants who completed a treatment control (Kucyi, Salomons, & Davis, 2016). These results suggest that ECN-DMN FC is positively associated with pain modulation and is responsive to intervention.
Interactions between these networks are susceptible to experimental sleep manipulation. In this regard, greater ECN-DMN FC has been documented in healthy individuals after one night of experimental sleep deprivation (Bosch et al., 2013). Additional measures reflecting increased functional coupling among DMN and ECN nodes, such as a reduction in anticorrelation and loss of functional segregation, following sleep deprivation have also been reported (De Havas, Parimal, Soon, & Chee, 2012; Ben Simon, Lahav, Shamir, Hendler, & Maron-Katz, 2018). Per se, sleep deprivation is associated with the separate phenomena of hyperalgesia and greater ECN-DMN coupling; however, it is unclear how sleep disruption via forced awakenings might influence the relationship between ECN and DMN.
The present study used one night of forced awakenings to determine the effects on pain sensitivity and cognitive networks associated with pain modulation. The limited timeframe of the sleep manipulation used in this study may help to identify early brain responses to sleep disruption that contribute to sleep loss-related hyperalgesia. Based on the complex literature described above, we hypothesize that changes in ECN-DMN FC following sleep disruption will be associated with changes in pain sensitivity. However, given that previous studies suggest that the association between changes in ECN-DMN FC and pain sensitivity could be either positive or negative, the potential direction of this association remains unclear.
2 METHODS
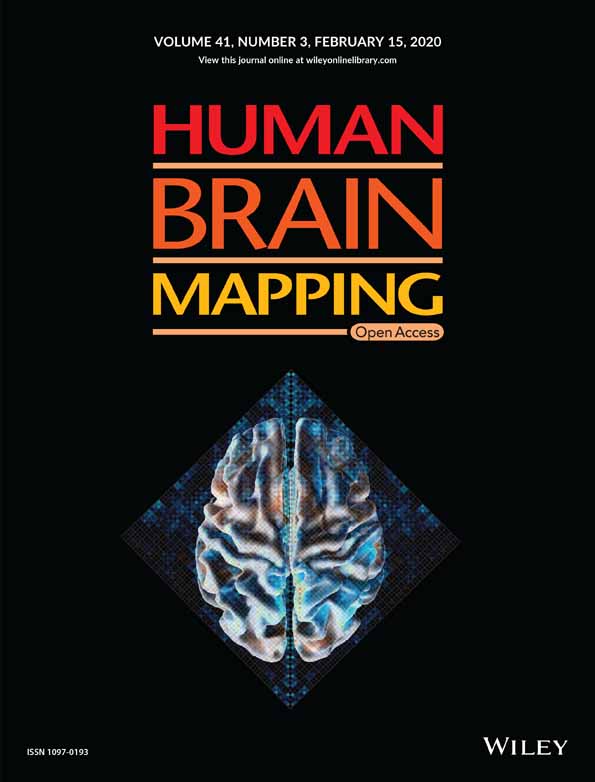
The present analyses were completed on data from an fMRI task that was part of a larger neuroimaging protocol used to examine the effects of one night of experimental sleep disruption via forced awakenings on the neural correlates of pain sensitivity and affective pain modulation in healthy individuals (Seminowicz et al., 2019). The primary data examined in the present study include (a) pain ratings and stimuli temperatures from a noxious thermal stimulation task (i.e., we did not use fMRI data from this task) and (b) a simple motor task (SMT) designed to elicit intrinsic network connectivity without the risk of participants falling asleep. Pain ratings and stimuli temperatures were computed in a change score (described below) and used as a predictor of individual differences in pain sensitivity following sleep loss. More information about the task used to derive the pain ratings and stimuli temperatures is provided in Supporting Information.
The neuroimaging protocol was further nested within a larger study (R01DA032922) that included additional nights for each sleep conditions and separate daytime procedures conducted on the day following the MRI scan; these data are not examined for the present study. All procedures were approved by Institutional Review Boards (IRB) at Johns Hopkins University and University of Maryland, Baltimore in conformation to the ethical guidelines of the 1975 Declaration of Helsinki. All participants provided written informed consent to complete study procedures and were notified of their right to withdraw from study procedures at any time without penalty. Figure 1 demonstrates the methods used in the present study.
2.1 Participants
SMT fMRI data were collected from 22 healthy individuals after uninterrupted and experimentally disrupted sleep. Participants were eligible if they met the following criteria: (a) met Research Diagnostic Criteria for Normal Sleepers, (b) scored <5 on the Pittsburgh Sleep Quality Index and <10 on the Epworth Sleepiness scale, (c) had average total sleep time between 6.5 and 8.5 hr/night and sleep efficiency >85% as reported on 2 weeks of sleep diaries and captured via actigraphy monitoring, (d) showed a stable sleep phase within 21:00 and 10:00, (e) polysomnography-confirmed apnea-hypopnea index <10, (f) denied a lifetime history of pain persisting >6 months or acute pain as measured on the McGill Pain Questionnaire and baseline sleep diaries, (g) reported no significant medical or psychiatric morbidity within 6 months of study participation and obtained T-scores <64 on the Brief Symptom Inventory global scales, (h) no lifetime history of bipolar disorder, psychotic disorder, recurrent major depression, posttraumatic stress disorder, substance abuse, reported history of traumatic brain injury, or seizures, (i) reported no tobacco/nicotine use and low caffeine use (<2 cups per day), (j) had a BMI <35, and (k) no MRI contraindications.
The present analyses were completed on data from 19 individuals (14 women; mean age = 23.95 years (SD = 4.17)) after two participants were excluded for incomplete fMRI data, and one individual was found to be an outlier with influential, extreme values for pain ratings. Seven participants identified as White Non-Hispanic, two individuals identified as White Hispanic, five participants identified as Black Non-Hispanic, one individual identified as Black Hispanic, two participants identified as mixed race, and one individual identified as Other Hispanic. Participant demographics and psychosocial function are listed in Table 1.
| Sex | Male | 6 |
| Female | 13 | |
| Age | 24 (4.3) | |
| Race | Black | 5 |
| White | 10 | |
| Asian | 2 | |
| Other/mixed | 2 | |
| Ethnicity | Hispanic | 5 |
| Non-Hispanic | 14 | |
| Education | Current student | 5 |
| Some college | 5 | |
| College graduate | 1 | |
| Master's degree | 3 | |
| Doctoral degree | 5 |
- Note: Sex, race, ethnicity, and education are represented as frequencies. Age is represented as mean (SD).
2.2 Experimental design and statistical analyses
2.2.1 Sleep monitoring and manipulation
Participants completed two nights of sleep monitoring at the Johns Hopkins Clinical Research Unit (CRU). Neuroimaging data analyzed for this study were collected following a night of 8-hr sleep opportunity (uninterrupted sleep, US) and following a night of sleep fragmentation via forced awakenings (FA). The order of these study visits was counterbalanced among participants to control for temporal confounds.
Uninterrupted sleep
For the US condition, participants were given the opportunity for 8 hours of sleep at the CRU without experimental disruption.
Sleep disruption procedure
The present study used a previously described sleep disruption protocol (see Finan et al., 2017, for a complete description). We chose sleep disruption (i.e., FA) based on previous work suggesting that this type of paradigm results in greater impairment of endogenous pain modulation compared to sleep restriction (Smith, Edwards, Mccann, & Haythornthwaite, 2007).
Briefly, the night was divided into eight 1-hr intervals, with one randomly chosen interval in which no sleep was allowed. The remaining seven 1-hr intervals were further divided into 20-min tertiles, so that one tertile per hour interval was randomly selected for a forced awakening. CRU staffs were responsible for waking up participants at these randomly chosen times and ensuring that participants remained awake throughout the interval. The maximum sleep allowed for the FA night was 280 min.
2.2.2 MRI session
All structural and functional MRI scanning was completed at the University of Maryland, Baltimore Magnetic Resonance Research Center between 1 p.m. and 5 p.m. Data were acquired using a Siemens 3T Tim Trio scanner with a 32-channel head coil. Structural images were collected using a high-resolution T1-weighted MPRAGE anatomical scan with the following parameters: 240 slices, TR 2,300 ms, TE 2.98 ms, flip angle 9°, FOV 256 mm, matrix 256 × 256 mm, resolution 1.0 × 1.0 mm, slice thickness 1 mm, no gap. Participants also completed 3 fMRI runs of an experimental pain task, during which a noxious thermal stimulus was applied to the left medial forearm (fully described in Seminowicz et al. (2019) and Supporting Information). Neuroimaging data from this task were not used in the present study, but pain ratings aggregated over 15 stimulation periods were used to derive the predictor variable for FC analyses. Please see Supporting Information for complete methods related to the noxious thermal stimulation task.
The present study aimed to examine changes in FC as they related to individual differences in pain sensitivity before and after sleep disruption. Due to concerns that participants might fall asleep in the scanner using a traditional resting-state task, we instead collected data using a 10-min SMT, which required participants to respond to the direction of a projected arrow (left or right). Arrows were sequentially displayed for 500 ms seconds at a time, with a total of 150 trials. Left and right arrows were shown in equal proportion. Interstimulus intervals were jittered and ranged from 1s to 4 s each. Task stimuli were programmed and run using Presentation (Neurobehavioral Systems, Berkeley, CA) on a desktop computer in the scanner suite. Participants used an angled mirror mounted on the MRI head coil to view task images via a translucent screen with back-project images. The following instructions were provided: During this time, we will help you stay awake by having you engage in a simple task that involves using your response box. You will look up and see a screen, and on the screen an arrow pointing either left or right will flash periodically. When the right facing arrow flashes, you should press 3 (the button farthest to the right). When the left facing arrow flashes you should press 1 (the button farthest to the left).
Previous work has shown minimal FC differences between traditional resting-state tasks (i.e., acquired during a steady-state period of non-goal-directed activity) and simple motor tasks, such as the one we employed (Baliki, Geha, Apkarian, & Chialvo, 2008; Jurkiewicz, Crawley, & Mikulis, 2018). At each visit, the SMT scanning sequence included an interleaved T2*-weighted, echo planar imaging (EPI) sequence with the following parameters: spin-echo, 658 volumes/run, 66 slices, TR: 907 ms, TE: 30 ms, flip angle 52°, FOV 224 mm, matrix size 110 × 102 mm, resolution 2.0 × 2.0 mm, slice thickness 2.0 mm, multiband factor 6.
2.2.3 Pain sensitivity change score
The present study aimed to examine changes in FC among cognitive networks as it related to sleep loss-induced hyperalgesia. In order to examine this aim, we computed a pain sensitivity change score as a predictor variable. For the parent study, participants underwent a quantitative sensory testing session before scanning at both study visits to derive participant-tailored temperatures for the fMRI noxious stimulation task. Temperatures rated between 5 and 8 out of 10 were selected. During fMRI scanning, these tailored temperatures were presented concomitant with music of neutral valence. Pain ratings were made following the delivery of each noxious thermal stimulus, which ended at the same time the music clip ended. The same procedure was employed at each study visit (see Supporting Information for additional details). Due to the aims of the parent study, music stimuli were paired with each noxious thermal stimulus. The parent study also overlaid positive-valence song clips on noxious thermal stimuli in separate blocks, but pain ratings associated with those blocks were not used in the present study because they were associated with pain inhibition (Seminowicz et al., 2019).
A pain sensitivity change score was calculated using pain ratings and tailored temperatures. Temperatures ranged from 40°C to 50°C, so we subtracted 40 from each temperature denominator to make the scales of pain ratings and temperatures equal (0–10). Our approach of subtracting 40 from temperature values was intended for scaling purposes only and does not assume an association between the temperature value and pain rating value.

2.2.4 Behavioral data analyses
Using SPSS 25 (IBM), we ran paired-samples t tests to examine condition-based differences in the following variables: total sleep time (TST), stage N1, stage N2, slow wave sleep (SWS), rapid eye movement sleep (REM), Stanford Sleepiness Scale (SSS), SMT percent accuracy, pain ratings collected during the noxious thermal stimulation task, and thermal stimuli temperatures used during the noxious thermal stimulation task. No transformations were applied to these data.
2.2.5 Neuroimaging data processing
Functional MRI data preprocessing
SMT data were preprocessed in SPM12 v6906. The preprocessing pipeline included realignment (motion correction), coregistration of the T1 anatomical to the mean functional image, segmentation of the anatomical to six standard tissue probability maps in SPM12, normalization of functional images using forward deformation fields from anatomical segmentation, and spatial smoothing using a 6 mm full-width at half maximum (FWHM) kernel. Data were visually inspected for quality control to determine motion artifact. Further, the Artifact Detection Tool (ART; www.nitrc.org/projects/artifact_detect/) was also used to mark potentially confounding outliers in the SMT time series for exclusion (i.e., rotation >.02 rad from prior volume, or translation >.4 mm from the prior volume (Chai, Ofen, Gabrieli, & Whitfield-Gabrieli, 2014). No condition-based differences in outlier detection (p = .4) or motion correction (p = .38) were identified.
We used the CONN toolbox (Whitfield-Gabrieli & Nieto-Castanon, 2012) to complete denoising procedures and FC analyses. Gray matter, white matter, and CSF masks were created via anatomical segmentation and were eroded by one voxel to minimize partial volume effects (Whitfield-Gabrieli & Nieto-Castanon, 2012). Physiological confounds, including respiratory and cardiac activity, were accounted for by using the white matter and CSF masks as nuisance regressors during denoising (Behzadi, Restom, Liau, & Liu, 2007). Denoising including scrubbing of excessive motion and outliers detected via ART and regression of white matter and CSF masks (% volumes removed: mean = 1.9%, range = 0.53–5.34%). A stepwise, rather than simultaneous, regression approach was applied to maintain adequate degrees of freedom of the resting-state data (Patel & Bullmore, 2016). Data were bandpass filtered between .008 and .09 (Aurich, Alves Filho, Marques da Silva, & Franco, 2015).
Hybrid independent component analysis-seed-based functional connectivity
We conducted hybrid independent component analysis (ICA)-seed-based FC analyses on denoised fMRI data in the CONN toolbox. Previous work suggests that hybrid ICA-seed-based FC analyses result in better reproducibility of fMRI results than either ICA or seed-based analyses alone (Kelly et al., 2010). First, group ICA is a data-driven technique that identifies orthogonal sources of variance within the data (Calhoun, Adali, Pearlson, & Pekar, 2001), yielding independent components (ICs). Each IC contains brain regions with activity co-occurring across a unique time course, thought to represent a neural network (Calhoun, Adalı, & Pekar, 2004).
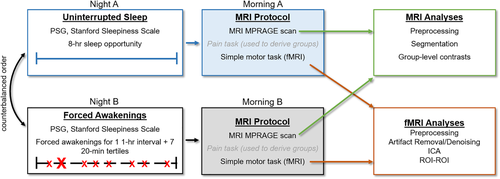
Consistent with steps described by Calhoun et al. (Calhoun et al., 2001), denoised data first underwent participant- and condition-level BOLD signal concatenation. We estimated the optimum number of ICs within the GIFT toolbox using minimum description length criterion (Li, Adali, & Calhoun, 2007), yielding 20 potential ICs. Dimensionality reduction was then conducted at the group-level to identify these 20 dimensions/ICs. To estimate IC maps at the group-level, the fastICA algorithm was used. Group-level IC maps are then back reconstructed on individual-level data via GICA1 backprojection to create individual-level IC spatial maps that can be pooled for second-level GLM statistics; however, only the group-level ICs were used to create seeds for subsequent analyses. Resultant group-level ICs were sorted to freely available spatial templates of RECN, LECN, and the DMN (Shirer, Ryali, Rykhlevskaia, Menon, & Greicius, 2012). The IC with the strongest match to each template (based on the Dice coefficient) was converted into a binarized map used in subsequent seed-based analyses (Table 2; Figure 2).
| Independent component | Cluster hemisphere | Cluster coordinates | Cluster size | p-value (<.05 FWE) | Region |
|---|---|---|---|---|---|
| RECN | Right | 34 18 54 | 5,746 | <.001 | dlPFC/MFG |
| Right | 44 −44 54 | 3,287 | <.001 | LOC | |
| Left | −34 −56 52 | 1,726 | <.001 | SPL | |
| Right | 64 −32 −14 | 810 | <.001 | MTG | |
| Left | −30 −62 −32 | 681 | <.001 | Cerebellum | |
| Right | 6 −26 32 | 544 | <.001 | PCC | |
| Left | −42 50 −10 | 333 | <.001 | Frontal pole | |
| Left | −10 −78 −26 | 315 | <.001 | Cerebellum | |
| Left | −64 −44 −22 | 309 | <.001 | ITG | |
| Right | 32 33 −6 | 243 | <.001 | Insula cortex | |
| Right | 10 −58 46 | 233 | <.001 | Precuneus | |
| Left | −52 16 40 | 53 | <.001 | MFG | |
| Left | −34 4 30 | 45 | <.001 | Ventral premotor | |
| Left | −38 −28 8 | 19 | .005 | Planum temporale | |
| LECN | Left | −46 12 36 | 7,986 | <.001 | MFG |
| Left | −34 −60 52 | 3,536 | <.001 | LOC | |
| Left | −56 −50 −10 | 1,461 | <.001 | ITG | |
| Right | 30 −62 46 | 284 | <.001 | LOC | |
| Right | 44 34 8 | 230 | <.001 | IFG | |
| Right | 12 −76 −24 | 157 | <.001 | Cerebellum | |
| Right | 28 −70 −46 | 143 | <.001 | Cerebellum | |
| Right | 28 −62 −30 | 136 | <.001 | Cerebellum | |
| Right | 4 8 28 | 75 | <.001 | MCC | |
| Left | −6 −34 42 | 46 | <.001 | PCC | |
| Right | 54 −48 −18 | 41 | <.001 | ITG | |
| Right | 50 −12 60 | 31 | <.001 | Precentral gyrus | |
| Left | −10 −20 72 | 25 | <.001 | Precentral gyrus | |
| DMN | Midline | 6 −46 20 | 8,750 | <.001 | Precuneus/PCC |
| Left | −44 −62 32 | 2,529 | <.001 | LOC | |
| Right | 40 −64 44 | 1,941 | <.001 | LOC | |
| Midline | 0 52 −12 | 1,872 | <.001 | vMPFC/ACC | |
| Left | −22 30 42 | 436 | <.001 | SFG | |
| Right | 22 32 38 | 357 | <.001 | SFG | |
| Right | 34 −80 −6 | 204 | <.001 | LOC | |
| Left | −58 −22 −18 | 69 | <.001 | MTG | |
| Left | −8 50 −42 | 54 | <.001 | Cerebellum | |
| Right | 54 −10 −18 | 34 | <.001 | MTG | |
| Left | −24 −88 −8 | 23 | .003 | Fusiform gyrus | |
| Left | −18 −96 4 | 20 | .005 | Occipital pole | |
| Right | 10 −46 −46 | 19 | .01 | Cerebellum |
- Abbreviations: ACC, anterior cingulate cortex; IFG, inferior frontal gyrus; ITG, inferior temporal gyrus; LOC, lateral occipital cortex; MFG, middle frontal gyrus; MTG, middle temporal gyrus; OFC, orbitofrontal cortex; PCC, posterior cingulate cortex; SFG, superior frontal gyrus; SPL, superior parietal lobule.
Binarized IC masks were added to the CONN toolbox as a priori regions of interest (ROIs). Further, the Power 264-region atlas was used to define 10 mm ROI spheres across the whole brain (Power et al., 2011). This whole-brain atlas describes subgraphs of functionally integrated nodes as defined by graph theory analyses on resting-state fMRI data, rather than structurally defined. First-level analyses were completed in CONN to extract time series data within each ROI and compute Fisher's r-to-Z transformed bivariate correlations among these ROIs.
GLM analyses
Contrast images were entered into a second-level seed-to-ROI analysis to determine FC differences between US and FA across the sample. The pain sensitivity change score described above was inserted as a regressor of interest, and RECN, LECN, and DMN masks were defined as seeds. In total, there were six primary sets of GLM tests, including three seed-to-ROI tests (i.e., RECN, LECN, and DMN as seeds) and three tests examining associations among RECN, LECN, and DMN masks (i.e., RECN-LECN, LECN-DMN, RECN-DMN). ROIs in the seed-to-ROI analyses included 264 spheres from the Power 264 atlas. A conservative analysis-level threshold across seeds was applied as a correction for multiple comparisons (pFDR < .05). We used a two-tailed test given the nature of our competing directional hypotheses.
2.2.6 Data availability statement
We have shared the resultant ROI maps from the present study in neurovault.org (Gorgolewski et al., 2015), which is a public repository for neuroimaging statistical maps. Data are located at the following link: https://neurovault.org/collections/ALMIXGCQ/
2.2.7 Validation analyses
Given the small sample size, we attempted to replicate findings using a distinct dataset. These analyses were exploratory in nature and not part of the primary aim. However, the complex and unique experimental approach used in our study limited our ability to test replication in an experimentally matched dataset. Instead, we examined whether clinical pain ratings moderated the relationship between sleep quality (i.e., Pittsburgh Sleep Quality Inventory scores) and FC values that resulted from our primary analyses. This validation sample included 30 individuals with migraine who reported poor sleep quality (i.e., PSQI >5). Methods from the study associated with our validation sample are reported at the following link: https://clinicaltrials.gov/ct2/show/NCT02133209.
3 RESULTS
3.1 Sleep manipulation check
To examine the efficacy of our sleep disruption manipulation we examined condition-based differences in several sleep parameters. As expected, there was a significant decrease in minutes of TST, stage N2, SWS, and REM sleep phases, and a significant increase in stage N1 sleep. Participants reported significantly greater sleepiness during the FA compared to US condition. Table 3 provides means, SD values, and paired-samples t test results for these variables. Combined, these results indicate the efficacy of the experimental manipulation.
| Variable | Mean (SD) | Paired-samples t test | |
|---|---|---|---|
| US | FA | ||
| TST (min) | 415.9 (81.9) | 269.3 (53.9) | t17 = 5.2, p < .001 |
| N1 (min) | 17.5 (9.3) | 25.1 (11.3) | t17 = −2.9, p = .009 |
| N2 (min) | 191.6 (77.8) | 121.3 (30.3) | t17 = 3.6, p = .002 |
| SWS (min) | 113.6 (24.6) | 81.0 (24.6) | t17 = 2.2, p = .045 |
| REM (min) | 88.6 (28.4) | 42.8 (29.3) | t17 = 4.6, p < .001 |
| SSS | 1.9 (.6) | 2.98 (.9) | t13 = −5.1, p < .001 |
| SMT % accuracy | 74.8% (25.3) | 63.3% (22.7) | t18 = 2.5, p = .021 |
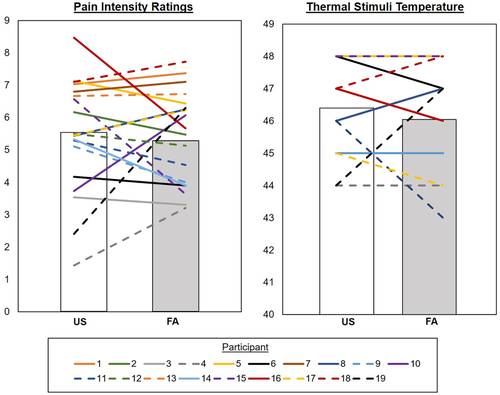
| Pain ratings | 5.4 (1.8) | 5.4 (1.4) | t18 = 0.05, p = .96 |
| Thermal stimulus temperatures | 46.4 (1.4) | 46.1 (1.6) | t18 = 1.1, p = .29 |
| Pain sensitivity change score | .04 (.31); range = −.81 – .55 | – | |
- Note: Thermal stimuli temperatures were derived during a quantitative sensory testing session that took place outside of the scanner. Pain ratings were collected during a noxious thermal stimulation fMRI task that used the derived stimulus temperatures. The pain sensitivity change score accounts for changes in both thermal stimulus temperatures and pain ratings between US and FA.
3.2 Pain ratings, thermal stimuli temperatures, and SMT performance
Table 3 lists statistical information about condition-based differences in pain ratings, thermal stimuli temperatures, and SMT performance. Neither pain ratings nor thermal stimuli temperatures significantly differed between the US and FA conditions. On the SMT, participants’ accuracy in identifying arrow directions was better during US compared to FA (74.8% and 63.3% accuracy, respectively). The low accuracy scores were due to missed trials or incorrect responses.
3.3 Left and right ECN and DMN functional connectivity
3.3.1 RECN as a seed
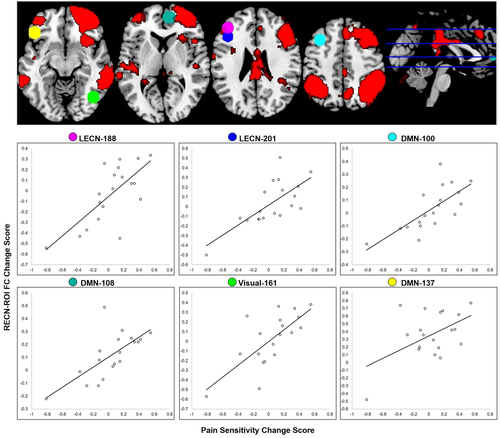
Several ROIs from the Power atlas demonstrated significant FC changes with the RECN seed associated with pain sensitivity change scores (omnibus model: F(4,14) = 25.28, pFDR = .05; individual ROI statistics reported in Table 4; Figure 4). Specifically, there was a positive association between increased pain sensitivity and increased RECN FC to left dlPFC (part of the LECN), left medial and inferior frontal gyri (MFG, IFG, respectively; part of the DMN), and the right paracingulate gyrus (part of the DMN). There was also a positive association between increased pain sensitivity and decreased RECN FC to the right lateral occipital cortex (LOC; part of the DMN). Table 4 provides additional information about these ROIs, and Table 5 provides descriptive statistics for FC values based on condition.
| Seed | Power 264 atlas ROI | Associated network | Region | Coordinates | t-stat | pFDR | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| RECN | 188 | LECN | l-dlPFC | −42 | 38 | 21 | 4.21 | .03 |
| 201 | LECN | l-dlPFC | −42 | 25 | 30 | 4.24 | .03 | |
| 100 | DMN | l-MFG | −35 | 20 | 51 | 4.35 | .03 | |
| 137 | DMN | l-IFG | −46 | 31 | −13 | 4.23 | .03 | |
| 108 | DMN | r-paracingulate gyrus | 9 | 54 | 3 | 3.97 | .04 | |
| 161 | Visual | r-LOC | 42 | −66 | −8 | 4.28 | .03 | |
- Abbreviations: dlPFC, dorsolateral prefrontal cortex; DMN, default mode network; FDR, false discovery rate; l-, left; LECN, left executive control network; IFG, inferior frontal gyrus; MFG, medial frontal gyrus; LOC, lateral occipital cortex; r-, right; RECN, right executive control network; ROI, region of interest.
| Seed | Power 264 atlas ROI | Associated network | Region | FC mean (SD) | Correlation with pain sensitivity changes (r, p) | |
|---|---|---|---|---|---|---|
| US | FA | |||||
| RECN | 188 | LECN | l-dlPFC | 0.54 (0.27) | 0.58 (0.26) | .72, .001 |
| 201 | LECN | l-dlPFC | 0.60 (0.23) | 0.64 (0.28) | .72, .001 | |
| 100 | DMN | l-MFG | 0.42 (0.18) | 0.45 (0.22) | .73, .000 | |
| 137 | DMN | l-IFG | 0.14 (0.16) | 0.26 (0.23) | .69, .001 | |
| 108 | DMN | r-paracingulate gyrus | 0.03 (0.21) | 0.05 (0.24) | .72, .001 | |
| 161 | Visual | r-LOC | 0.28 (0.2) | 0.27 (0.28) | .51, .03 | |
- Abbreviations: dlPFC, dorsolateral prefrontal cortex; DMN, default mode network; FDR, false discovery rate; l-, left; LECN, left executive control network; IFG, inferior frontal gyrus; MFG, medial frontal gyrus; LOC, lateral occipital cortex; r-, right; RECN, right executive control network; ROI, region of interest.
3.3.2 Null results
Although the RECN seed demonstrated significant associations between six RECN-ROI pairs and pain sensitivity change scores, the additional five primary analyses did not pass our significance threshold corrected for multiple comparisons. Specifically, neither the LECN nor DMN seed demonstrated significant seed-to-ROI FC associations with pain sensitivity changes (pFDR > .05). Further, FC between ECN and DMN seeds did not show significant pairwise associations between FC and pain sensitivity change scores (pFDR: RECN-LECN = .06, RECN-DMN = .16, LECN-DMN = .63).
3.4 Validation analyses
We tested whether clinical pain ratings moderated the relationship between PSQI scores and FC values from the six significant RECN-ROI pairs on a separate sample of migraine patients who reported poor sleep quality. Among these six ROIs, there was only one significant moderation effect of pain on the relationship between RECN-right paracingulate gyrus (F(1,29) = 6.4, p = .02). The other RECN-ROI moderation analyses were not significant (ps range = .37–.91). Because this was not an ideal validation sample, however, we do not further interpret results.
4 DISCUSSION
The present study examined the association between cognitive network FC and individual differences in sleep loss-induced hyperalgesia following one night of sleep disruption. In a healthy sample of normal sleepers, we used an experimental manipulation to produce multiple, prolonged FA during one night of sleep. We broadly hypothesized that neural systems associated with cognitive pain control would be affected by sleep loss and that these changes would be related to changes in pain sensitivity. Overall, we saw variability in the magnitude and direction of changes in participants’ pain sensitivity following one night of acute sleep loss, with an overall positive association between pain sensitivity changes and changes in RECN FC to several brain regions (i.e., dlPFC, MFG, IFG, paracingulate gyrus, and LOC). These results, however, are qualified by null findings across five additional a priori tests. Taken together, results only partially support our hypothesis of greater ECN-DMN FC in individuals with higher pain sensitivity following sleep disruption, suggesting that cognitive network alterations only partially contribute to sleep loss-induced hyperalgesia.
4.1 Pain sensitivity following forced awakenings
When combining pain ratings and thermal stimuli temperatures, some individuals displayed hyperalgesia while others showed decreased pain sensitivity. Even across pain ratings, which were obtained following tonic noxious thermal stimuli that were paired with neutral-valenced music, there were no overall mean changes in pain sensitivity across the sample after a single night of FA sleep. Although these findings are somewhat inconsistent with previous research demonstrating hyperalgesia following sleep loss, it should be noted that most of those studies were conducted over multiple nights of accumulated sleep loss. In fact, this study's parent project recently found that two nights of sleep disruption via FA-induced hyperalgesia in a larger sample (n = 79), reducing both heat pain threshold and cold pain tolerance. The present study's design using one night of FA did not induce a ubiquitous hyperalgesic response; however, results cautiously show the early changes in RECN FC contributes to sleep disruption-induced hyperalgesia.
Further, differences in quantitative sensory testing across studies make comparisons across studies challenging. In the present study, we analyzed pain ratings obtained following the application of tonic noxious thermal stimuli that were paired with neutral-valenced music, as the parent study design did not include noxious thermal stimuli presented alone (see Seminowicz et al., 2019). As with many quantitative sensory testing studies, the uniqueness of our study design may limit the generalizability of the present results. We encourage future replication attempts. Cautiously, these results suggest varied responses to acute sleep loss in healthy adults, with some individuals evidencing greater pain vulnerability to sleep loss, which may have more significant clinical implications (Lautenbacher, Kundermann, & Krieg, 2006).
4.2 RECN functional connectivity changes following forced awakenings
Our study specifically examined FC of bilateral ECNs and DMN to functionally defined ROIs throughout the brain (Power et al., 2011), and FC among bilateral ECN and DMN masks. The ECN is a group of brain regions associated with top-down modulation of sensory stimuli via goal-directed attentional amplification of relevant information, as well as goal-directed suppression of irrelevant information (Awh et al., 2006; Gazzaley & Nobre, 2012; Smith et al., 2018). Importantly, the ECN is engaged during cognitive pain control in individuals with and without chronic pain (Ceko et al., 2015; Ceko et al., 2015; Kong et al., 2013; Rainville, 2002; Sevel, Letzen, Staud, & Robinson, 2016; Villemure & Bushnell, 2002). The DMN, on the other hand, is a group of brain regions associated with self-referential thought and focus on internal milieu (Andrews-Hanna et al., 2010; Lorenzi et al., 2011; Raichle, 2015). Its activity is typically anticorrelated with ECN activity during cognitive tasks so that better suppression of the DMN is associated with better cognitive performance (Fox et al., 2005; Seminowicz & Davis, 2007; Smith, Sip, & Delgado, 2015). Because evidence links ECN and DMN interactions with cognitive control of sensory inputs that are susceptible to perturbations from sleep disruption, the combined previous findings were suggestive of ECN-DMN FC as a putative mechanism for sleep loss-induced hyperalgesia.
Among tested networks in the present study, however, changes in pain sensitivity were only associated with changes in RECN FC, and we did not observe significant changes in the association between pain sensitivity and ECN-DMN FC. Instead, there was a positive association between increased pain sensitivity following sleep deprivation and increased RECN FC to specific regions within the DMN and LECN as well as decreased RECN FC to a primary visual network region as a function of changes in pain sensitivity. These regions included the left dlPFC (LECN), left MFG/IFG and right paracingulate gyrus (DMN), and right LOC (primary visual network).
The dlPFC is among the brain regions commonly associated with pain vigilance, awareness, and attention (Lorenz, Minoshima, & Casey, 2003; Seminowicz et al., 2013; Seminowicz & Davis, 2007; Seminowicz & Moayedi, 2017; Valet et al., 2004). Findings from experimental sleep research suggest that individual differences in emotional responses following sleep loss are associated with changes in attention (Alfarra, Fins, Chayo, & Tartar, 2015). Attentional processes are well documented in the context of experimental pain so that pain vigilance contributes to exacerbated pain intensity and distraction is associated with lower pain report (for a review, see Wiech, Ploner, & Tracey, 2008). Individuals sleeping less than 6.5 hr per night have also been found to have impaired ability to derive analgesic benefits from distraction techniques (playing video games) during an experimental pain task. Further, because stronger anticorrelation between DMN-ECN activity is associated with better segregation of internally and externally directed awareness (Buckner, 2008; Horovitz et al., 2009; Larson-Prior et al., 2009), increased RECN-DMN FC—as identified in this study—potentially suggests poorer ability to modulate attention. The combined findings of increased intrinsic, interhemispheric dlPFC FC and RECN FC to regions within the DMN might represent heightened attention to sensory stimuli, contributing to greater pain sensitivity.
It is not fully clear why we observed lateralization effects of significantly altered RECN, but not LECN, FC. Although debated, there is some evidence suggesting a greater propensity of right hemisphere activity in relation to pain processing (Coghill, Gilron, & Iadarola, 2001; Ji & Neugebauer, 2009; Symonds, Gordon, Bixby, & Mande, 2006). Additionally, a previous study examining resting-state attentional networks in individuals with insomnia found altered right, but not left, hemisphere FC (Li et al., 2018). Although it is possible that RECN FC is one mechanism contributing to sleep loss-induced hyperalgesia, the fact that this was the only seed to demonstrate significant findings across all six a priori tests tempers the strength of inference. Future research is encouraged to measure whether other neural networks contribute to sleep loss-induced hyperalgesia.
4.3 Limitations
The present results should be interpreted in the context of several important limitations. First, the small sample size used in the present study, coupled with the high number of a priori tests, weakens the strength of inference. Second, previous studies using the FA protocol over two nights showed it maximally induced hyperalgesia (Iacovides, George, Kamerman, & Baker, 2017) and diminished pain inhibition (Finan et al., 2017; Smith et al., 2007). In the present study, we used a smaller time window (one night) than previously reported, which might account for our lack of robust hyperalgesia findings. Our previous data suggest that more than one night of FA sleep is beneficial for examining sleep-pain responses. Third, although the FA paradigm is thought to have face validity for the type of sleep disturbance profile commonly observed in patients with chronic pain (i.e., multiple prolonged awakenings with significant concomitant reduction in total sleep time) (Rosseland et al., 2018), it may not generalize fully to sleep disruption linked with chronic insomnia, which may be due to a variety of clinical factors that influence pain in addition to the multiple awakenings themselves. Our results should be extended to include patient populations that suffer from sleep loss. Fourth, as mentioned earlier, pain ratings were collected from a paradigm using neutral music overlay, so we could not rule out the possible influence of distraction on imaging findings. The primary findings should be replicated in a design that includes thermal stimuli presented alone. Fifth, we did not assess individuals’ cognitive strategies used to control pain. Given that there are individual differences in cognitive pain control strategies (Seminowicz, Mikulis, & Davis, 2004), future studies should measure how ECN-DMN FC patterns relate to strategy use following sleep disturbance.
5 CONCLUSION
The present findings suggest that healthy individuals have idiographic pain responses to one night of sleep disruption and that these responses are partially associated with changes in RECN FC to regions within the LECN and DMN. Future prospective studies are needed to examine whether additional neural networks further contribute to the sleep-pain dyad and whether RECN FC changes might act as one mechanism potentially involved in the chronification of pain via sustained sleep disruption.
ACKNOWLEDGMENTS
This research was supported with funds provided by NIH F32 HL143941 (JEL); NIH K23 DA035915 (PHF); Blaustein Pain Research Award (PHF); NIH R01 DA0329922 (MTS, MRI); NIH P30 NR014131 (MTS and PHF); and NIH T32 NS7020110 (BR, MTS). We would also like to thank Samuel Krimmel and Shana Burrowes for their time and guidance in preparing the present analyses.
CONFLICT OF INTEREST
The authors declare no competing financial interests.