Weight loss, behavioral change, and structural neuroplasticity in children with obesity through a multidisciplinary treatment program
Funding information: Fonds Wetenschappelijk Onderzoek, Grant/Award Number: 3F000714; Nederlandse Organisatie voor Wetenschappelijk Onderzoek, Grant/Award Number: 639.072.411; Netherlands Organization for Scientific Research (NWO), Grant/Award Number: 639.072.411; Research Foundation Flanders (FWO), Grant/Award Number: 3F000714
Abstract
This study evaluated the effect of a multidisciplinary treatment program for children with obesity (OB) on motor competence, executive functioning (EF), and brain structure. Nineteen children with OB (7–11 years), who attended a multidisciplinary treatment program consisting of diet restriction, cognitive behavioral therapy, and physical activity, were compared with an age-matched control group of 24 children with a healthy weight (HW), who did not follow any treatment. For both groups, anthropometric measurements and tests of motor competence and EF were administered twice, with 5 months between pretest and posttest. Additionally, children’s brain structure was assessed by performing a magnetic resonance imaging (MRI) scan at the pretest and posttest, which included a T1 anatomical scan, diffusion MRI scan, and magnetization transfer imaging scan. Compared to HW controls, children with OB lost a considerable amount of their body mass (p ≤ .001) and significantly improved their balance skills (p ≤ .001), while no transfer effects of the program were observed for EF. Furthermore, the program resulted in a significant increase in total (p ≤ .001) and cerebellar (p ≤ .001) gray matter volume in children with OB, while no change was observed in the HW controls. Finally, only weak to moderate (nonsignificant) correlations could be observed between structural brain alterations, weight-related changes, and behavioral improvements. Altogether, this is the first longitudinal study showing behavioral and structural brain alterations in response to a multidisciplinary weight loss program for children with OB. Our findings support the need for multidimensional intervention (and prevention) measures for children with OB to deal with this multifactorial health problem.
1 INTRODUCTION
The high prevalence of obesity (OB) in children highlights the need for intervention programs that tackle this multifactorial health problem (Ng et al., 2014). Fortunately, clinical research has been able to identify a number of multidisciplinary treatment programs that have shown to be effective in terms of weight loss (e.g., D’Hondt et al., 2011; Nemet et al., 2005; Walsh, Palmer, Welsh, & Vos, 2014). Yet, while weight management is perhaps the major outcome variable, evidence indicates that related factors need to be considered too in order to maintain the weight effect and provoke long term behavioral changes. Successful engagement in physical activities, for example, requires a sufficient level of gross and fine motor competence (Robinson et al., 2015). In addition, high level of executive functioning (EF) is an important requisite to control behaviors, such as meal and activity planning, proportion control, and activity-related decision making (Liang, Matheson, Kaye, & Boutelle, 2014). Both motor competence and EF have been shown to be affected in a significant proportion of children with OB (D’Hondt, Deforche, De Bourdeaudhuij, & Lenoir, 2008, 2009; Gentier, Augustijn, et al., 2013; Gentier, D’Hondt et al., 2013; Liang et al., 2014; Reinert, Po, & Barkin, 2013). Therefore, it is important that multidisciplinary treatment programs for children with OB not only result in weight loss, but also improve children’s level of motor competence and EF.
In addition to this, neuroimaging studies have shown that childhood OB is associated with alterations in brain structure. Specifically, compared to healthy weight (HW) controls, children with OB have reduced gray matter (GM) volumes in the cerebellum, thalamus, precentral/postcentral gyrus, superior parietal lobule and middle temporal gyrus, whereas increased GM volumes are found in the pallidum and hippocampus (de Groot et al., 2016; Moreno-López, Soriano-Mas, Delgado-Rico, Rio-Valle, & Verdejo-García, 2012; Ou, Andres, Pivik, Cleves, & Badger, 2015). As these regions have shown to be important in motor competence and EF, authors suggested that the alterations in GM volume could be related to deficits in these behaviors. Furthermore, diffusion magnetic resonance imaging (MRI) studies have found increased fractional anisotropy (FA) values in the fronto-occipital fasciculus and superior corona radiate (Ou et al., 2015), and reduced FA values in the superior cerebellar peduncle in children with OB compared to HW controls (Augustijn et al., 2017). This latter study also demonstrated that reduced white matter (WM) organization in the cerebellar peduncles was accompanied with more pronounced motor deficits in children with OB. Given the neural basis of behavioral problems in children with OB, it is important to evaluate the effect of programs at the neural level too, as a better understanding in neural plasticity is likely to advance treatment design for OB.
To date, only a few longitudinal studies in children with OB have examined the effect of a multidisciplinary treatment program on children’s motor performance or EF. D’Hondt et al. (2011), for example, showed that a 4-month multidisciplinary treatment program consisting of diet restriction, regular physical activity, and cognitive behavioral therapy significantly improved gross motor performance in children with overweight or OB (7–13 years, N = 36). Additionally, Gentier et al. (2015) demonstrated that the same program for children with OB (aged 10.1 ± 1.4 years old, N = 26) resulted in a significant enhancement in perceptual-motor function. In the study of Kulendran et al. (2014), adolescents with OB (10–17 years, N = 53) significantly improved EF performance after a short-term (2–8 weeks) multidisciplinary treatment program, which focused on core skills of behavioral change (e.g., goal setting and planning) and physical activity. Clearly, these studies show that a multidisciplinary program can improve motor competence and EF, however, it is not known whether these changes are accompanied with structural brain alterations in brain regions related to motor competence and EF.
Over the years evidence has accumulated showing that structural neuroplasticity may occur in response to task-specific training, that is, isolated motor or cognitive practice, both in healthy participants (Draganski et al., 2004; Moreno, Lee, Janus, & Bialystok, 2015; Thomas & Baker, 2013) and clinical populations (for a review, see Caeyenberghs et al., 2018). To our knowledge such training studies are nonexisting in populations with (pediatric) OB, however, two studies did examine the effect of a nonspecific 8-month cardiovascular exercise program on brain structure and behavior in children who are overweight (Krafft et al., 2014; Schaeffer et al., 2014). In the first study, Schaeffer et al. (2014) examined training-induced changes in WM structure of the uncinate fasciculus, a tract involved in verbal memory proficiency. Their findings revealed a greater increase in FA together with a larger decrease in radial diffusivity (RD) in the exercise (8–11 years, N = 10) compared to the control group (Schaeffer et al. 2014). In line with these results, Krafft et al. (2014) showed that the same training program did affect EF and WM structure in the superior longitudinal fasciculus, with greater increase in EF and WM structure being associated with a larger participation rate in the exercise compared to the control group. Although these results are promising, they are limited to the effect of isolated cardio-vascular exercise, which are known to result in only modest reductions in terms of weight loss (Thorogood et al., 2011). Therefore, it would be very useful to investigate the effect of a multidisciplinary treatment program, with greater effects on weight reduction, on behavior and brain structure in children with OB. These programs combine diet restriction, behavior change guidance, and regular physical activity. As the amount of weight loss and behavioral improvement as a result of (obesity) treatment may vary among individuals, it is worthwhile to examine whether brain characteristics can predict which patients benefit the most from the multidisciplinary treatment program (de Lange et al., 2016; Drijkoningen, Caeyenberghs, et al., 2015; King, Hopkins, Caudwell, Stubbs, & Blundell, 2008). Earlier research in the elderly (de Lange et al., 2016) and brain injured patients (Drijkoningen, Caeyenberghs, et al., 2015) demonstrated that the individual response to treatment (i.e., cognitive or balance training) could indeed be accounted for by differences in WM structure prior to the onset of the treatment.
In the present study, we evaluated the effects of an existing multidisciplinary treatment program for children with OB, as offered in a specialized rehabilitation center (Zeepreventorium, De Haan, Belgium), on motor competence, EF, and brain structure. Previous studies have demonstrated the efficacy of this multidisciplinary treatment program on weight loss (Δ17.9–21.7%; D’Hondt et al., 2011; Gentier et al., 2015). Our hypotheses were as follows: First, we expected that deficits in motor competence and EF at baseline would improve in children with OB after the treatment program. Second, we predicted training-induced alterations in GM volume and WM organization of brain regions related to motor competence and EF, expressed as changes in GM volume, FA, mean diffusivity (MD), axial diffusivity (AD), RD, and magnetization transfer ratio (MTR). Third, we hypothesized that inter-individual differences in GM volume and WM organization at baseline would predict behavioral improvements. Finally, weight loss and behavioral improvements in children with OB were expected to correlate with neuroplasticity changes.
2 METHODS
2.1 Participants
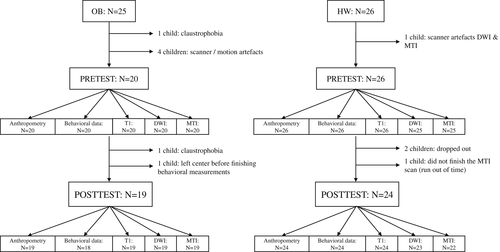
Fifty-one children (39.2% girls, 9.5 ± 0.9 years, range 7.8–11.6 years) participated in this study, including 25 children with OB (48% girls; 9.6 ± 0.9 years) who attended a multidisciplinary rehabilitation program (as discussed in detail in the next section) at the Zeepreventorium (De Haan, Belgium). All of these children with obesity were referred to the program by their general practitioner, but had no underlying endocrine diseases or obesity-related genetic disorders, such as Prader-Willi syndrome. In addition, children with mental retardation (IQ < 70) were excluded. The other 26 children (30.8% girls; 9.5 ± 1.1 years) within the study sample were recruited and contacted via a database with 959 children from local primary schools, taking into account their HW status (based on cut off points of Cole & Lobstein, 2012) and their age that should match that of the children in the OB group. All HW children were not involved in any specialized motor or cognitive interventions other than their usual activities inside and outside the school-context between the premeasurement and postmeasurement.
The protocol of the study was approved by the Ethical Committee of the Ghent University Hospital. For each participant informed consent was signed by both the parents and the school or treatment center staff.
2.2 Treatment program
The 25 recruited children with OB all followed a multidisciplinary treatment program in the rehabilitation center “Zeepreventorium” (De Haan, Belgium), with a total duration of 11 months. As previous work has revealed that the first 4–5 months are the most successful in terms of weight loss (i.e., 17.9 ± 3.1%; D’Hondt et al., 2011), we administered the postmeasurements 5 months after the start of the program. During that period, the children were full-time residents at the rehabilitation center, whereby they also received primary school education. Only at the weekends (i.e., three times a month), they went home to spent time with their families. The multidisciplinary treatment program (i.e., calorie intake and energy expenditure) focused on three central pillars, including diet, regular physical activity, and cognitive behavioral therapy of which the two former were tailored to the needs of each individual child. Tailoring was based on children’s body weight, energy intake, and usual habitual physical activity levels at the start of the treatment. Diet consisted of three main meals and three healthy snacks daily (total intake: 1593 ± 595 kcal/day) and weekly information sessions (30 minutes/week) on how to make healthy food choices. Regular physical activity (total energy expenditure: 2032 ± 26 kcal/day) was provided in the form of physiotherapy sessions as well as in guided exercise sessions (minimum 3 hr/week) with a focus on aerobic activities such as walking, swimming, soccer, and dancing. Additionally, every child participated in supervised team sports (e.g., soccer, basketball, and volleyball) and group games (e.g., obstacle swim, relay race, and dance shows) for approximately 1 hr a day (5 hr/week). Finally, during cognitive behavioral therapy sessions (30 minutes/week), children were taught how to use solution-based thinking to improve behavioral modification.
2.3 Procedure and materials
All children underwent anthropometric measurements, motor assessments, neuropsychological testing, and an MRI scan. All measurements were taken at the start of the treatment program (within the range of 1 month) and after 5 months among the children with OB (OB: 147 ± 21 days). For the age-matched control children, all measurements were administered at equal pretest/posttest intervals (HW: 154 ± 12 days). Due to practical reasons (e.g., habituation time for children before scanning, children get quickly tired) (Byars et al., 2002), assessment of the MRI scans and motor/neuropsychological testing took place on separate days, with an average interval of 11.4 ± 14.7 days between both sessions. Anthropometric measurements were assessed at the day of the MRI scan.
2.3.1 Anthropometric measurements
Body height (0.1 cm; Harpenden, Holtain Ltd., Crymych, UK), body mass (0.1 kg) and fat percentage (0.1%; Tanita, BC420SMA, Weda B.V., Naarden, Holland) were measured barefoot and in minimal clothing. Based on body height and body mass, participants’ BMI (kg/m2) was calculated and used to classify children as being HW or OB (Cole & Lobstein, 2012). Finally, waist circumference was measured using a flexible tape measure to the nearest 0.1 cm.
2.3.2 Behavioral measurements
Motor competence
Motor competence was assessed with the Movement Assessment Battery for Children, 2nd Edition (MABC-2; (Henderson, Sugden, & Barnett, 2007; Smits-Engelsman, Henderson, Sugden, & Barnett, 2010). It is a reliable and valid test battery to identify and describe motor difficulties in children, aged 3–16 years old (Brown & Lalor, 2009; Smits-Engelsman et al., 2010). Age-based scaled scores were calculated for manual dexterity (three test items), ball skills (two test items) and static and dynamic balance (three test items), and an overall test score was computed. A total score at or below the fifth percentile indicates significant motor problems, whereas a score between the sixth and 16th percentile is indicative of potential motor problems (i.e., “at risk”). A more detailed description of the eight test items can be found in the Supporting Information.
Executive functioning
The Cambridge Neuropsychological Test Automated Battery (CANTAB® [Cognitive assessment software], Cambridge Cognition (2017), all rights reserved, www.cantab.com) is a valid and reliable instrument to assess EF in subjects, aged 4–90 years old, with and without neurocognitive disorders (Lowe & Rabbitt, 1998). Three tasks were administered for this study: (a) the intra-extra dimensional shifting task to assess attention shifting by calculating the total number of errors, adjusted by adding 50 errors for each stage that was not completed; (b) the rapid visual processing task to measure updating abilities (i.e., total hits) and inhibition control (i.e., total false alarms); and (c) the stockings of Cambridge to measure planning and decision making by calculating the number of problems that were solved in the minimum number of moves. All tests were administered randomly across subjects and were displayed on a tablet PC (Gigabyte tablet S1080) with a 10.1 inch touch-sensitive screen, which was placed 30 cm in front of the participant. A more detailed description of the three tasks can be found in the Supporting Information.
2.4 MRI acquisition
MRI acquisition was performed on a 3T Siemens Magnetom Trio MRI scanner system (Siemens, Erlangen, Germany) with a 32-channel phased-array head coil. Because an MRI scanner can be intimidating for young children (e.g., large machine, narrow bore, loud noises), the following steps were undertaken to prepare the child for the MRI scan: (a) a comic strip about the MRI procedure was provided to read at home, (b) the researcher guided the child in the MRI room and explained the different steps, (c) the child selected a DVD to watch during the scanning, and (d) the researcher made sure communication was possible before, during, and after the MRI scan (Byars et al., 2002; de Bie et al., 2010). In total, three MRI sequences were administered. First, an axial three-dimensional T1-weighted image with magnetization-prepared rapid gradient echo was acquired for anatomic detail (i.e., 144 slices of 1 mm thick covering the cerebrum, brainstem, and cerebellum; repetition time (TR) = 1,590 ms; echo time (TE) = 4.18 ms; field of view (FOV) = 256 × 256 mm2; matrix size = 256 × 256; voxel size = 1.0 × 1.0 × 1.0 mm3). Second, a diffusion-weighted imaging (DWI) sequence was acquired using single-shot echo planar imaging with a twice-refocused spin echo sequence to reduce eddy-current induced distortions. Sixty-four directions (b-value = 1,200 s/mm2) and one baseline image without diffusion-weighting (b-value = 0 s/mm2) were acquired (i.e., 60 contiguous slices of 2.5 mm thick covering the cerebrum, brainstem, and cerebellum; TR = 10,800 ms; TE = 83 ms; FOV = 240 × 240 mm2; matrix size = 96 × 96; voxel size = 2.5 × 2.5 × 2.5 mm3). Finally, two series of three-dimensional magnetization transfer imaging (MTI) scans, one with and one without an off-resonance saturation pulse, were acquired (i.e., 60 slices of 2.4 mm thick covering cerebrum, brainstem, and cerebellum; TR = 50 ms; TE = 4.59 ms; FOV = 230 × 230 mm2; matrix size = 128 × 128; voxel size = 2.2 × 1.8 × 2.4 mm3).
2.5 Image processing
2.5.1 Gray matter
An illustration of the preprocessing pipeline is shown in Figure 1. The T1-weighted images were preprocessed using the longitudinal pipeline of FreeSurfer version 5.3. (http://surfer.nmr.mgh.harvard.edu; Reuter, Schmansky, Rosas, & Fischl, 2012), which was run on the high-performance computing infrastructure of Multi-modal Australian ScienceS Imaging and Visualization Environment (MASSIVE; Goscinski et al., 2014). The following multistep procedure was performed: (a) the raw data were visually inspected to check for any artifacts using MRIcron (Rorden & Brett, 2000), (b) the T1-weighted images were run through the cross-sectional pipeline to perform surface reconstruction and segmentation for all time points of all subjects independently, (c) for each subject, a template was created from all time points to estimate the average subject anatomy (base), (d) information from the first (cross-sectional) and second (base) step were used to initialize the longitudinal processing, which includes spatial and intensity normalization, talairach registration, brainmask creation, subcortical segmentation, surface reconstruction, cortical atlas registration, and parcellations, and (e) GM volume was calculated using an automated parcellation approach based on the subject-specific Desikan-Killiany atlas (Desikan et al., 2006; Fischl et al., 2004). To check the quality of (sub)cortical segmentation, surface reconstruction and atlas parcellation, all preprocessed images were visually inspected using the QA tool (version 1.1; Koh, Lee, Pacheco, Pappu, & Vinke, 2017). In a next step, a more detailed inspection of the quality of the preprocessing was applied by inspecting and looping through the different image maps in different orthogonal views (i.e., axial, sagittal, and coronal).
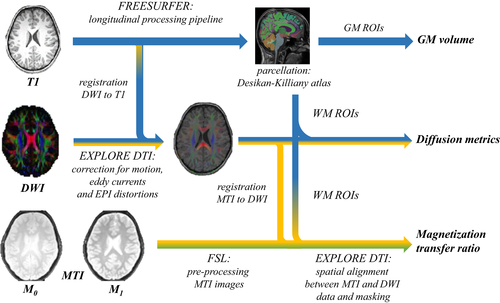
2.5.2 White matter
Diffusion-weighted imaging
The DWIs were analyzed and processed using ExploreDTI version 4.8.6. (Leemans, Jeurissen, Sijbers, & Jones, 2009). The images were processed using the following multistep procedure: (a) evaluation of any obvious artifacts in the data by inspecting and looping through the different DWIs in different orthogonal views (i.e., axial, sagittal, and coronal), looking at the average residuals per DWI volume and across the DWIs for each voxel, and inspecting the outlier profiles (Tournier, Mori, & Leemans, 2011), (b) the DWI data sets were corrected for subject (head) motion, eddy current induced distortions, and geometric deformations due to susceptibility artifacts (Irfanoglu, Walker, Sammet, Pierpaoli, & Machiraju, 2011; Leemans & Jones, 2009), (c) the same quality assessment procedure as described in step (a) was performed to check the quality of the corrected DWIs, (d) after robust tensor estimation with REKINDLE and the iteratively reweighted linear least squares approach (Tax, Otte, Viergever, Dijkhuizen, & Leemans, 2015; Veraart, Sijbers, Sunaert, Leemans, & Jeurissen, 2013), the FA MD, RD, and AD were extracted using an automated atlas-based approach based on the subject-specific Desikan-Killiany atlas obtained from FreeSurfer.
Magnetization transfer imaging
The raw MTI data were first inspected for artifacts using MRIcron (Rorden & Brett, 2000). Then, the following steps were performed using FMRIB Software Library 5.0.7 (FSL, Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012): (a) the MTI images without saturation pulse (M0) were registered to the images with saturation pulse (M1, reference) using the FMRIBs Linear Image Registration Tool (FLIRT, Andersson, Jenkinson, Smith, & Andersson, 2007) in order to remove possible tilts in the brain due to head movements between scans, (b) the Brain Extraction Toolbox (Smith, 2002) was used to remove non-brain tissue, (c) the MTR was calculated on a voxel-by-voxel basis using the equation [(M0 − M1)/M0], whereby M1 and M0 represent signal intensities with and without saturation pulse. After preprocessing of the MTI data, the images were registered to the FA map of the DWI data using rigid transformations in ExploreDTI 4.8.6. After visually inspection of the quality of the registration, the MTR values were calculated for each region of interest.
2.5.3 Selection of regions of interest
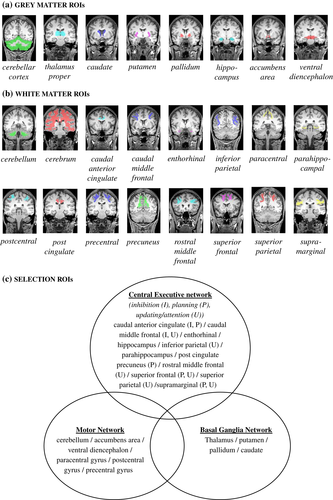
The Desikan-Killiany atlas (Desikan et al., 2006; Fischl et al., 2004) was used for parcellation of the regions of interest (ROIs). This subject-specific atlas was created during the preprocessing of the T1-weighted images in FreeSurfer. The GM and WM ROIs were selected based on (a) previous MRI studies in participants with OB demonstrating significant group differences in GM volume and WM organization (Alosco et al., 2014; Augustijn et al., 2017; de Groot et al., 2016; Kullmann, Schweizer, Veit, Fritsche, & Preissl, 2015; Kullmann et al., 2016; Moreno-López et al., 2012; Ou et al., 2015); (b) previous neuroplasticity studies in overweight (Krafft et al., 2014; Schaeffer et al., 2014) and other clinical populations (for a review, see Caeyenberghs et al., 2018); (c) anatomical hypothesis (Metzler-Baddeley, Caeyenberghs, Foley, & Jones, 2016; Olesen, Westerberg, & Klingberg, 2004; Thomas & Baker, 2013). Specifically, GM and WM regions were selected that are mainly involved in motor competence and/or EF. This resulted in eight GM and 16 WM ROIs (see Figure 2a,c). In addition, four global metrics of the GM were extracted, including (a) total intracranial volume to control for potential differences in total brain volume, (b) total GM volume, (c) cortical GM volume, and (d) subcortical GM volume. To reduce multiple comparisons, morphological and microstructural measures of the ROIs were averaged across hemispheres, because we did not expect any laterality effects.
2.6 Statistical analysis
Statistical analyses were performed using SPSS Statistics (22.0). Changes in anthropometric measurements, motor competence, EF, and brain structure were analyzed in 2 (time: pre vs. post) × 2 (group: OB vs. HW) repeated measures analysis of variance (ANOVA). In addition, Pearson correlations were performed for two purposes: (a) to evaluate the predictive value of brain structure at the pretest on changes in weight-related measures (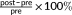 ) and behavioral measurements (post − pre); and (b) to examine whether the changes in anthropometric and behavioral measurements were associated with neuroplastic changes (post − pre) in the group of children with OB. To reduce the number of multiple comparisons for the correlational analyses, only anthropometric/behavioral measurements and measures of brain regions which showed significant main or interaction effects were included. False discovery rate (FDR) corrections were made to correct for multiple comparisons. The significance threshold was set at p < .05.
) and behavioral measurements (post − pre); and (b) to examine whether the changes in anthropometric and behavioral measurements were associated with neuroplastic changes (post − pre) in the group of children with OB. To reduce the number of multiple comparisons for the correlational analyses, only anthropometric/behavioral measurements and measures of brain regions which showed significant main or interaction effects were included. False discovery rate (FDR) corrections were made to correct for multiple comparisons. The significance threshold was set at p < .05.
3 RESULTS
3.1 Final sample
As described previously, the initial sample included 25 children with OB and 26 children with a HW. In the group of children with OB, one child (boy, 8.4 years old) left the center before finishing the behavioral measurements. Additionally, MRI-data of two children (50% girl, 11.0 ± 0.2) with OB was missing due to claustrophobia and MRI-data of four children (50% girl, 9.6 ± 0.2) with OB had to be excluded from the study due to scanner/motion artifacts or low quality segmentation. This left us with a final OB sample of 19 children (47.4% girls, 9.4 ± 1.0 years old) with good quality pre- and post-MRI data and 18 children (50% girls, 9.5 ± 1.0 years old) with prebehavioral and postbehavioral data. In the group of children with a HW, two children (50% girl, 8.5 ± 0.3 years old) dropped out during the course of the study, because they had dental braces or were ill at the time of the posttest. In addition, DWI- and/or MTI-data of two control children (boys, 9.4 ± 0.1 years old) were missing (i.e., time constraints) or had to be excluded from the study due to motion/scanner artifacts. This resulted in a final sample of 24 HW controls (29.2% girls, 9.6 ± 1.2 years old; 23 DWI, 22 MTI) with good quality predata and postdata. An overview of the final sample is provided in Figure 3.
3.2 Anthropometry
Descriptive statistics of demographics and anthropometric measurements are shown in Table 1 (A and B). Significant time by group interaction effects were found for body weight (F[1,41] = 229.255, pFDR ≤ .001, η2 = 0.848), percentage body fat (F[1,41] = 86.501, pFDR ≤ .001, η2 = 0.678), waist circumference (F[1,41] = 20.571, pFDR ≤ .001, η2 = 0.340), and BMI (F[1,41] = 258.273, pFDR ≤ .001, η2 = 0.863). Specifically, a significant decrease in each of the weight-related measures was observed in children with OB (p ≤ .001) after the treatment program. Children with OB lost on average 15.6% (±4.9%) of their body weight and 5 out of 19 children could now be identified as overweight (average change in BMI: −18.4 ± 4.7%). The body weight (p = .004) of children with a HW slightly increased, whereas their percentage body fat (p = .887), waist circumference (p = .468) and BMI (p = .808) remained constant over the 5-month time interval between pretest and posttest.
| Pre | Post | |||
|---|---|---|---|---|
| OB (N = 19) | HW (N = 24) | OB (N = 19) | HW (N = 24) | |
| A. Demographics | ||||
| Gender (boys/girls) | 10/9 | 17/7 | 10/9 | 17/7 |
| Age (years) | 9.4 ± 1.0 | 9.6 ± 1.2 | 9.8 ± 1.0 | 10.0 ± 1.2 |
| B. Anthropometric measurements | ||||
| Body height (m) | 142.0 ± 6.8 | 139.4 ± 9.0 | 144.4 ± 7.2 | 142.3 ± 9.2 |
| Body weight (kg) | 63.8 ± 11.9 | 33.1 ± 5.7 | 53.7 ± 9.8 | 34.6 ± 6.0 |
| Body fat (%) | 45.2 ± 6.0 | 17.9 ± 4.5 | 33.7 ± 6.5 | 17.7 ± 4.1 |
| Waist circumference (cm) | 93.9 ± 7.7 | 61.4 ± 4.2 | 82.1 ± 6.5 | 60.3 ± 8.4 |
| Body mass index (kg/m2) | 31.50 ± 4.43 | 16.90 ± 1.17 | 25.71 ± 3.73 | 16.96 ± 1.21 |
| Pre | Post | |||
| OB (N = 20) | HW (N = 26) | OB (N = 18) | HW (N = 24) | |
| C. Motor competence | ||||
| General motor competence | 57.4 ± 20.3 | 77.1 ± 9.6 | 68.7 ± 20.5 | 82.8 ± 9.1 |
| Manual dexterity | 22.5 ± 9.3 | 28.0 ± 5.8 | 26.4 ± 8.6 | 32.0 ± 4.7 |
| Ball skills | 15.7 ± 7.0 | 19.1 ± 5.1 | 17.2 ± 5.9 | 19.5 ± 5.1 |
| Balance skills | 19.2 ± 9.0 | 30.0 ± 3.8 | 25.1 ± 8.3 | 30.9 ± 3.4 |
| D. Executive functioning | ||||
| IED (total errors) | 34.5 ± 19.2 | 31.5 ± 20.7 | 28.2 ± 22.5 | 24.0 ± 17.3 |
| RVP (total hits) | 6.3 ± 1.9 | 7.5 ± 1.1 | 6.7 ± 1.3 | 8.4 ± 1.8 |
| RVP (total false alarms) | 4.8 ± 8.7 | 1.0 ± 1.1 | 2.5 ± 2.2 | 1.3 ± 1.2 |
| SOC (problems solved in min moves) | 16.6 ± 4.5 | 19.3 ± 3.3 | 19.6 ± 3.4 | 21.5 ± 2.2 |
- IED = intra-extra dimensional shift; RVP = rapid visual processing; SOC = stockings of Cambridge.
3.3 Behavioral measurements
Mean and standard deviations of the behavioral measurements are provided in Table 1 (C and D).
3.3.1 Motor competence
Longitudinal analyses revealed a significant time by group interaction effect for balance skills (F[1,40] = 11.314, pFDR = .008, η2 = 0.220), but not for general motor competence, manual dexterity, and ball skills (pFDR > .05). Post hoc analysis revealed a significant improvement for balance skills in the group of children with OB (p ≤ .001), while no significant change was observed in children with a HW (p = .405). It should be noted that post hoc analysis also revealed that children with OB demonstrated reduced balance skills compared to HW controls at the pretest (p ≤ .001) and posttest (p = .003). Despite the absence of a significant interaction effect for the other (sub)scores, a main effect of group was observed for general motor competence and manual dexterity, but not for ball skills. Children with a HW had better general motor competence and manual dexterity compared to peers with OB both at the pretest and posttest. The absence of a significant main effect of time, then, indicated that there was no improvement in motor competence over time, regardless of weight status.
Using clinical cut-off scores of the MABC-2, 6 out of 15 children with OB improved their motor skills from mild (6th to 16th percentile) or severe (<5th percentile) motor problems at the pretest to a normal level of motor competence (>16th percentile) at the posttest. Additionally, one child improved from severe to mild motor impairments, whereas a decline from mild to severe motor problems was observed in one child with OB. In summary, after the multidisciplinary treatment program, 11 children with OB had normal motor competence (with only five at pretest), one child had mild motor problems (six at pretest) and seven children had severe motor competence (nine at pretest) after training.
3.3.2 Executive functioning
The 2 (time) by 2 (group) repeated measures ANOVAs (with age as covariate) revealed no significant time by group interaction effects for EF (pFDR < .05). However, significant main effects of group were observed for updating (F[1,40] = 13.462, pFDR = .004, η2 = 0.252) and planning (F[1,40] = 5.745, pFDR = .042, η2 = 0.126). Regardless of test moment, children with OB performed worse on all these measures of EF compared to HW controls, except for attention shifting and inhibition (pFDR > .05). Additionally, significant main effects of time were observed, indicating that—regardless of group—children had better updating abilities (F[1,40] = 5.726, pFDR = .044, η2 = 0.125) and skills (F[1,40] = 18.179, pFDR ≤ .001, η2 = 0.312) at the posttest compared to the pretest. No significant main effects of time or time by group interaction effects were observed for EF (pFDR < .05).
3.4 Image processing
Mean and standard deviations of the structural brain measurements are presented in Supporting Information Table S2 and S3.
3.4.1 Gray matter
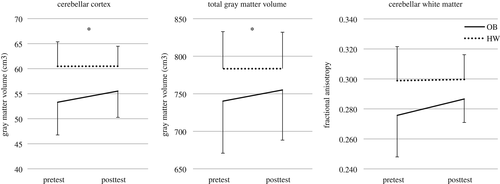
Total intracranial volume did not significantly differ between groups (p > .05). The time by group repeated measures ANOVA (with total intracranial volume and age as covariates) revealed a significant interaction effect for the cerebellar cortex (F[1,40] = 10.873, pFDR = .022, η2 = 0.214) and total GM volume (F[1,40] = 9.523, pFDR = .022, η2 = 0.192; see Figure 4). Specifically, GM volume in the cerebellar cortex and total GM volume increased significantly in children with OB (p ≤ .001), while no change was observed in children with a HW (p > .05). Moreover, post hoc analyses revealed that children with OB had reduced total (p = .034) and cerebellar (p ≤ .001) GM volume at the pretest compared to HW controls. In contrast to total GM volume (p = .120), the difference in cerebellar (p = .001) GM volume remained significant at the posttest. No significant interaction or main effects were observed for the other ROIs (pFDR’s < .05).
3.4.2 White matter
Diffusion-weighted imaging
The longitudinal analysis revealed no significant time by group interaction effects were observed for diffusion metrics (pFDR’s > .05). However, a significant main effect of group was found for FA in the cerebellum (F[1,39] = 10.036, pFDR = .048, η2 = 0.205), indicating reduced FA in the cerebellar WM in children with OB compared to HW peers both at the pretest and posttest (see Figure 4). No significant main effects of group were observed for the other diffusion metrics (pFDR’s > .05). Finally, significant main effects of time were observed for FA, MD, AD, and RD in different ROIs (i.e., pFDR < .05; see Supporting Information Table S3).
Magnetization transfer imaging
The time by group repeated measures ANOVA (with age as covariate) revealed no significant main or interaction effects for time and group as well as no significant interaction effect (pFDR’s > .05), which indicated that values of MTR did not differ between groups and did not change after training.
3.5 Pearson correlations
Results of the Pearson correlations are shown in Table 2. To reduce the number of multiple comparisons, only weight-related measures, behavioral measurements, and measures of brain regions which showed significant main or interaction effects were included. First, brain structure at the pretest could not significantly predict changes in weight-related measures and behavior in children with OB over time (pFDR’s > .05). In addition, no significant correlations between training-induced changes in brain structure and anthropometric/behavioral changes were observed in the group of children with OB (pFDR’s > .05).
| [A] | GM volume of the cerebellum | FA of the cerebellum | ||
|---|---|---|---|---|
| % change body weight | OB | r | −.302 | .273 |
| p | .209 | .259 | ||
| % change percentage body fat | OB | r | −.423 | .129 |
| p | .071 | .599 | ||
| % change waist circumference | OB | r | −.098 | .424 |
| p | .699 | .080 | ||
| % change BMI | OB | r | −.341 | .295 |
| p | .153 | .219 | ||
| ≠ balance skills | OB | r | −.043 | −.206 |
| p | .860 | .397 | ||
| [B] | ≠ total GM volume | ≠ GM volume of the cerebellum | ||
| % change body weight | OB | r | −.101 | −.230 |
| p | .681 | .344 | ||
| % change percentage body fat | OB | r | .104 | −.117 |
| p | .672 | .633 | ||
| % change waist circumference | OB | r | −.342 | −.317 |
| p | .152 | .186 | ||
| % change BMI | OB | r | −.057 | −.224 |
| p | .815 | .356 | ||
| ≠ balance skills | OB | r | .017 | .126 |
| p | .946 | .607 |
- % change =
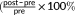 ); ≠ change = (post − pre); OB = obesity; HW = healthy weight; RVP = rapid visual processing; SOC = stockings of Cambridge; GM = gray matter; FA = fractional anisotropy; MTR = magnetization transfer imaging.
); ≠ change = (post − pre); OB = obesity; HW = healthy weight; RVP = rapid visual processing; SOC = stockings of Cambridge; GM = gray matter; FA = fractional anisotropy; MTR = magnetization transfer imaging.
4 DISCUSSION
The present study evaluated the effect of an existing multidisciplinary treatment program for children with OB on weight-related measures, motor competence, EF, and brain structure. Consistent with previous studies examining the efficacy of this existing program on weight loss, our results clearly demonstrated that the children with OB lost a considerable amount of their weight. Although weight loss and changes in eating and exercise behavior are the main targets of the program, other factors, such as motor competence and EF, have to be considered to maintain these behavioral changes on the long term. In line with previous research (D’Hondt et al., 2011; Gentier et al., 2015), children with OB significantly improved their motor competence, their balance skills in particular, after following the program, while no such changes were observed in the HW controls over the duration of the study. Contrary, no transfer effects of the program were observed for EF. Furthermore, structural brain alterations were found in children with OB. More specifically, an increase in total GM volume and GM volume of the cerebellar cortex was observed, yet, no correlations between these changes in brain structure and those in anthropometrical and behavioral measurements were found.
The important finding of this study is that a multidisciplinary treatment program, combining diet, cognitive behavioral therapy, and physical activity, led to an increase in cerebellar and total GM volume in children with OB. No changes were observed in the HW control group, which indicates that the neuroplasticity found in the OB group was mainly driven by the residential program. The observed changes in GM volume might underlie different cellular and molecular biological mechanisms, such as axon sprouting, synaptogenesis, neurogenesis, gliogenesis, and/or vascular changes (Zatorre, Fields, & Johansen-Berg, 2012). Evidence from animal studies, for example, showed that motor skill learning in rats is associated with synaptogenesis (e.g., dendritic branching and increased astrocytes volume) in the motor cortex and the cerebellum (Kleim et al., 2002, 2007). In addition, increased physical activity in middle-aged monkeys has shown to alter vascular volume fraction in the motor cortex (Rhyu et al., 2010). Although these animal studies are useful to speculate about potential cellular and molecular changes underlying structural brain alterations, more advanced imaging techniques with sophisticated hardware are needed to provide better insights on GM microstructural changes in the human brain.
Changes in GM volume were only observed in the cerebellum, which is important as children and young adults with OB demonstrated reduced GM volume in the cerebellum compared to HW controls in cross-sectional studies (Ou et al., 2015; Taya, Sun, Babiloni, Thakor, & Bezerianos, 2015). Based on evidence from other neuroplasticity studies, several brain regions, including the cerebellum, have shown to be more susceptible to training than other brain regions (Caeyenberghs et al., 2018). Krafnick et al. (2011), for example, showed a significant increase in GM volume of the right anterior cerebellar cortex after reading training in children with dyslexia. Prosperini et al. (2014) and Drijkoningen et al. (2015) found significant changes in diffusion metrics in respectively the superior and inferior cerebellar peduncles in patients with multiple scleroses and traumatic brain injury in response to balance training. Altogether, our findings support literature showing that different types of training can alter the whole cerebellar cortex as well as specific subregions and/or tracts of the cerebellum.
To our knowledge, this is the first study examining the effect of a multidisciplinary treatment program on brain structure in a population with (pediatric) OB. Also in other clinical populations, studies examining structural brain changes as a result of a multidisciplinary treatment program are scarce. To date, only one exploratory study has examined the effect of a 9-month multidisciplinary treatment program, combining an aerobe exercise program, resistance training and occupational therapy, on brain structure and behavior in adults with Huntington’s disease (Cruickshank et al., 2015). In line with our results, researchers showed that a multidisciplinary rehabilitation program can alter regional GM volume. Although such training studies are nonexisting in populations with OB, previous work in children who are overweight revealed a positive effect of an isolated 8 month cardio-vascular exercise program on brain structure (Krafft et al., 2014; Schaeffer et al., 2014). Although this isolated cardio-vascular exercise program can alter brain structure, this type of training has shown to induce modest reductions in weight loss (Cohen’s d: −0.74 (Schaeffer et al., 2014) vs. −2.82 (present study); Thorogood et al., 2011). Therefore, specialized weight loss programs in rehabilitation centers, as used in the present study, are recommended as they target multiple factors of OB, induce structural brain alterations, and have shown to be more efficacious in terms of weight loss and BMI reduction (Moens, Braet, & Van Winckel, 2010).
Next to structural brain alterations, the multidisciplinary treatment program resulted in improvements in levels of motor competence, more specific balance skills, of children with OB. Consistent with previous research into the effectiveness of this multidisciplinary OB treatment, 40% of the children with OB demonstrated clinically significant improvements in general motor competence (D’Hondt et al., 2011). It is clear that the reduction in weight poses less challenges for the musculoskeletal system, especially in weight-bearing tasks such as dynamic balance skills, which might explain the gain in motor competence. As it is well known that the cerebellum plays a key role in motor control and motor learning (Glickstein & Doron, 2008; Koziol et al., 2014; Stoodley, Valera, & Schmahmann, 2012), one might speculate that these changes in balance performance could be related to the alterations in (cerebellar) GM volume. Based on the current findings, however, there does not seem to be a correlation between the changes in brain structure and motor competence. A possible explanation for the absence of a significant association is the fact that the total (cerebellar) GM volume was studied, whereas previous studies have shown that specific regions of the cerebellum are involved in postural control (Drijkoningen, Leunissen, et al., 2015; Morton & Bastian, 2004; Stoodley et al., 2012). Specifically, the medial zone, or vermis, is suggested to play a role in the regulation of extensor muscle tone, sustaining an upright position and dynamic balance control (Morton & Bastian, 2004). Moreover, the anterior lobe (i.e., lobule I–V) and lobule VI play a key role in timing, amplitude and trajectory of limb elevation and descent (sensorimotor processing) (Morton & Bastian, 2004). In this respect, it is possible that changes in balance skills are associated with changes in specific regions of the cerebellum involved in postural control rather than to total cerebellar GM volume. Therefore, it is recommended that future studies examine the role of specific lobules of the cerebellum in motor problems in children with obesity. Furthermore, correlation analysis revealed that baseline brain characteristics cannot predict which patients benefit the most from the multidisciplinary treatment program. As the program was tailored to the individual needs of the child, it is possible that the inter-individual variability in weight loss and postural improvement within the group of children with obesity was too small to observe significant correlations. Important to note, most structural neuroplasticity studies hardly observe significant correlations between structural brain differences/alterations and behavioral changes (Drijkoningen, Caeyenberghs, et al., 2015; Filippi et al., 2012; Schlaug, Marchina, & Norton, 2009; Sterling, Taub, Davis, Rickards, & Gauthier, 2013). This might be due to the small sample size of those studies (including the current one). Another explanation might be the difference in timing and rate of the neural and behavioral response to treatment (Caeyenberghs et al., 2018; Han, Davis, Chapman, & Krawczyk, 2017). Further research with multiple test moments and advanced statistical analysis is required to map the neural response to treatment and better understand the potential link between behavioral and structural changes to the brain.
In contrast to motor competence and brain structure, no transfer effects of the program were observed for measures of EF. As stringent external control exists in the rehabilitation center and children did not have to act independently, these cognitive control functions are responsible for weight management (i.e., EF requisite to control behaviors; Liang et al., 2014). The observed group differences in measures of EF, therefore, indicates the need for additional cognitive training in current multidisciplinary OB interventions in order to induce improvements in EF, and related regions, that are required for weight loss and maintenance. Verbeken et al. (2013), for example, demonstrated that EF training during the last stage of a similar multidisciplinary treatment program leads to increased updating abilities and better weight maintenance in children with OB (8–14 years) 8 weeks after the intervention. Other suggestions, such as computer or exergames, are promising to improve EF (Flynn, Richert, Staiano, Wartella, & Calvert, 2014; Verhelst, Vander Linden, Vingerhoets, & Caeyenberghs, 2017). In addition, Lamboglia et al. (2013) concluded in their review that an isolated training using exergames, that is, computer games combining computer games and physical exercise, may be useful in OB interventions as they can increase levels of physical activity, energy expenditure, heart rate, and reduce waist circumference in 6–15 year old children.
Interesting to note, no structural brain changes were observed in WM organization of children with OB over time. The main effect of group, however, showed that children with OB had reduced WM organization in the cerebellum (i.e., reduced FA) compared to HW controls. Despite these significant group differences, our results indicate that a multidisciplinary treatment program cannot alter WM microstructure in children with OB, which is in contrast with previous neuroimaging studies examining the effect of an 8-month isolated cardiovascular exercise program in children who are overweight (Krafft et al., 2014; Schaeffer et al., 2014). However, the absence of significant alterations in WM organization may be due to several factors, including technical limitations (e.g., crossing fibers; Jbabdi, Behrens, & Smith, 2010), selection of ROIs (e.g., regions vs. fiber tracts; Drijkoningen et al., 2015; Krafft et al., 2014; Schaeffer et al., 2014), duration and type of the program (e.g., 5 months vs. 8 months, isolated vs. multidisciplinary program; Krafft et al., 2014; Schaeffer et al., 2014), and the lack of power due to the relatively small sample size. Therefore, further research on structural neuroplasticity in children with OB is recommended.
The present study provides unique evidence for the efficacy of an existing multidisciplinary treatment program, with focus on diet, physical activity, and cognitive behavioral therapy, for children with OB on brain structure and behavior. However, there are some limitations that need to be addressed. In the present study, children with OB who followed a 5-month multidisciplinary treatment program were compared to a group of children with a HW. Previous longitudinal studies in children with developmental disorders, such as attention deficit hyperactivity disorder, have shown a pattern of delay in brain development, especially in cortical GM volume (Di Martino et al., 2014; Shaw et al., 2007). As childhood OB has been associated with different developmental disorders (Must et al., 2014), the inclusion of a control group of children with OB, who are not involved in a specific treatment program, would be of added value to examine whether the observed difference in changes of GM volume was the result of the program, an index of immaturity, or a combination of both. Additionally, specific details on training content, dose, and intensity of the subcomponents of the multidisciplinary treatment program are not available, which makes it difficult to explain which factors of the training program could have contributed to the observed findings. Furthermore, data of other developmental, psychiatric, and/or medical factors, such as number of years being obese, habitual level of physical activity, socioeconomic status, IQ, and/or comorbidities, were not available and, therefore, it was not possible to control for these potential confounders. Finally, in the present study, measurements were taken at two time points: at the start of the treatment program and after 5 months. Future follow-up studies with multiple test moments (as discussed earlier) are needed to evaluate the role of motor competence, EF, and brain structure as well as their mutual relationships in weight loss and maintenance.
Despite these limitations, this is the first longitudinal study examining the effect of a multidisciplinary treatment program on motor competence, EF, and brain structure in children with OB. Although isolated programs, with focus on one dimension of OB, have shown to alter brain structure and behavior, this type of treatment has shown to induce modest reductions in weight loss (Thorogood et al., 2011). Therefore, specialized multidisciplinary weight loss programs in rehabilitation centers, as used in the present study, are recommended as they target multiple factors of OB and have shown to be more efficacious in terms of weight loss and weight management (Moens et al., 2010). Moreover, the findings of the present study support the need for these more ecologically valid weight loss programs as they do not only result in a considerable amount of weight loss but also an altered brain structure and behavior. Finally, future studies are recommended to evaluate the effect of additional motor and EF training, in the form of exergames, to current multidisciplinary treatment programs for children with OB.
ACKNOWLEDGMENTS
The study was funded by the Ph.D. fellowship of the Research Foundation Flanders (FWO) awarded to Mireille Augustijn [3F000714]. The research of A. L. is supported by VIDI Grant 639.072.411 from the Netherlands Organization for Scientific Research (NWO). We are very grateful to all participants and their parents, the staff from the rehabilitation center “Zeepreventorium” (De Haan, Belgium), and the board of the participating schools. Finally, we would like to thank Kris Bakeland, Nele Bassier, Marieke Paredis, Hanne Lyskawa, and Teresa Gobert for their assistance in collecting the data.
CONFLICT OF INTEREST
None of the authors has a conflict of interest or financial ties to disclose.