Longitudinal white matter microstructural change in Parkinson's disease
Funding information: United States Department of Veterans Affairs Clinical Sciences Research and Development, Grant/Award Number: 101CX000555; University of Wisconsin Alzheimer's Disease Research Center Clinical Core, Grant/Award Number: P50 AG033514
Abstract
Postmortem studies of Parkinson's disease (PD) suggest that Lewy body pathology accumulates in a predictable topographical sequence, beginning in the olfactory bulb, followed by caudal brainstem, substantia nigra, limbic cortex, and neocortex. Diffusion-weighted imaging (DWI) is sensitive, if not specific, to early disease-related white matter (WM) change in a variety of traumatic and degenerative brain diseases. Although numerous cross-sectional studies have reported DWI differences in cerebral WM in PD, only a few longitudinal studies have investigated whether DWI change exceeds that of normal aging or coincides with regional Lewy body accumulation. This study mapped regional differences in the rate of DWI-based microstructural change between 29 PD patients and 43 age-matched controls over 18 months. Iterative within- and between-subject tensor-based registration was completed on motion- and eddy current-corrected DWI images, then baseline versus follow-up difference maps of fractional anisotropy, mean, radial, and axial diffusivity were analyzed in the Biological Parametric Mapping toolbox for MATLAB. This analysis showed that PD patients had a greater decline in WM integrity in the rostral brainstem, caudal subcortical WM, and cerebellar peduncles, compared with controls. In addition, patients with unilateral clinical signs at baseline experienced a greater rate of WM change over the 18-month study than patients with bilateral signs. These findings suggest that rate of WM microstructural change in PD exceeds that of normal aging and is maximal during early stage disease. In addition, the neuroanatomic locations (rostral brainstem and subcortical WM) of accelerated WM change fit with current theories of topographic disease progression.
1 INTRODUCTION
Parkinson's disease (PD) is the second most prevalent neurodegenerative disease and most common movement disorder next to essential tremor (Alves, Forsaa, Pedersen, Dreetz Gjerstad, & Larsen, 2008; Tanner & Aston, 2000). PD is characterized by motor symptoms of tremor, bradykinesia, rigidity, and postural instability, as well as nonmotor symptoms of cognitive decline, sleep disorders, and sensory symptoms, all of which negatively impact quality of life (Alves et al., 2008; Schrag, Jahanshahi, & Quinn, 2000). Symptom severity is typically quantified using the Unified Parkinson's Disease Rating Scale (UPDRS; Fahn & Elton, 1987). The UPDRS contains the Hoehn and Yahr (1967) [HY] scale, a widely used clinical staging system, in which stage 1 indicates unilateral motor signs, stage 2 bilateral signs, stage 3 bilateral signs with postural instability, and stage 4 and 5 degrees of physical dependency. Traditionally believed to be a result of dopaminergic cell loss in the substantia nigra (SN) pars compacta, recent evidence suggests that PD is a multisystem brain disorder affecting additional nondopaminergic neuronal systems (Alves et al., 2008; Helmich, Hallett, Deuschl, Toni, & Bloem, 2012; Perry et al., 1991). Postmortem studies in PD suggest that Lewy bodies accumulate first in the olfactory bulb, followed sequentially by caudal brainstem, SN, limbic cortex, and neocortex and that greater neocortical Lewy body accumulation is associated with bradykinetic onset and cognitive decline (Braak et al., 2003; Braak, Rüb, Jansen Steur, Del Tredici, & de Vos, 2005; Selikhova et al., 2009). While regional progressive brain atrophy has been reported in longitudinal studies in PD, it is debated whether the rate of atrophy is greater in PD than in normal aging or specifically related to cognitive decline (Agosta et al., 2013; Agosta et al., 2014; Baba et al., 2012; Burton, McKeith, Burn, & O'Brien, 2005; Camicioli et al., 2011; Compta et al., 2013; Hanganu et al., 2014; Hu et al., 2001; Ibarretxe-Bilbao et al., 2012; Melzer et al., 2015; Paviour, Price, Jahanshahi, Lees, & Fox, 2006; Ramírez-Ruiz et al., 2005; Tessa et al., 2014; Wen et al., 2015).
With this multisystem hypothesis in mind, we used magnetic resonance diffusion-weighted imaging (DWI), an in vivo technique for the quantitative characterization of cellular water diffusion, to map whole brain white matter (WM) microstructural indices of fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD; Alexander, Lee, Lazar, & Field, 2007; Basser & Pierpaoli, 1996; Beaulieu, 2002; Goveas et al., 2015; Johansen-Berg & Behrens, 2009; Jones & Leemans, 2011). While DWI is sensitive to early disease-related WM change, the exact inferences that can be drawn from these measures remain somewhat controversial (Jones, Knösche, & Turner, 2013). In general, aging and disease processes are expected to produce reductions in FA due to axonal degeneration and demyelination, increases in MD and RD due to loss of tissue boundaries, and increase in AD as a possible result of higher directional coherence among remaining axons (Burzynska et al., 2010; Goveas et al., 2015; Johansen-Berg & Behrens, 2009; Madden, Bennett, & Song, 2009; Phillips et al., 2014; Takahashi, Ono, Harada, Maeda, & Hackney, 2000).
Both cross-sectional and longitudinal studies of neurodegenerative disorders have reported encouraging results regarding the use of DWI as a biomarker for disease progression (Bozzali et al., 2002; Canu et al., 2010; Goveas et al., 2015; Gregory et al., 2015; Harrington et al., 2016; Mayo et al., 2017; Phillips et al., 2014; Phillips et al., 2016; Poudel et al., 2015; Stricker et al., 2009; Weaver et al., 2009; Weiler et al., 2015; Yu et al., 2009). While studies in PD have reported cross-sectional differences in DWI measures between PD and control subjects (Agosta et al., 2014; Bertrand et al., 2012; Duncan et al., 2016; Goveas et al., 2015; Hattori et al., 2012; Melzer et al., 2013; Rae et al., 2012; Zhang et al., 2011), information regarding differential progression of WM change between PD patients and normal aging adults is relatively limited (Melzer et al., 2015; Rossi, Ruottinen, Saunamäki, Elovaara, & Dastidar, 2014; Zhang et al., 2016).
The purpose of this 18-month neuroimaging study was to map regional differences in the rate of change in magnetic resonance imaging (MRI)-based measures of brain WM microstructural integrity between PD patients and age-matched controls using a voxel-wise approach. Since PD is a progressive neurodegenerative disorder, we also investigated associations between disease duration, clinical disease stage measured using the HY scale, and clinical severity measured using the UPDRS. We hypothesized that the rate of microstructural decline in PD would exceed that of normal aging. We also hypothesized that PD patients with early stage, unilateral disease (HY < 2) might show a different rate of change in WM microstructural indices than those with bilateral disease (HY ≥ 2).
2 METHODS
2.1 Participants
A total of 85 participants were recruited through the William S. Middleton V.A. Hospital, University of Wisconsin-Madison clinics, and Wisconsin Alzheimer's Disease Research Center as part of the Longitudinal MRI in Parkinson's Disease (LMPD) study. Exclusion criteria included cardiovascular, cerebrovascular, other major neurological or psychiatric disease (determined from participant interviews and review of medical charts and prior brain imaging as necessary), significant cognitive impairment [Mini Mental State Examination (MMSE) score of less than 27 (Folstein, Folstein, & McHugh, 1975)], and for PD participants, motor symptom onset earlier than 45 years of age, as well as more than one first-degree relative with PD. At sequential visits, 18 months apart, all participants underwent brain MRI, neuropsychological assessment, and Unified Parkinson's Disease Rating Scale (UPDRS; Fahn & Elton, 1987) scoring by a movement disorders neurologist (C. G.), off anti-Parkinson medications for 12–18 hr. Seventy-two participants (29 PD and 43 controls) met study and imaging criteria for analysis and participated in the 18-month follow-up. All participants provided written informed consent before participation, and all study procedures were approved by the University of Wisconsin Institutional Review Board as well as the Research and Development committee of the W.S. Middleton V.A.
2.2 Brain image acquisition
A GE 750 Discovery 3T MRI system (General Electric Healthcare, Waukesha, WI) with an eight-channel phased array head coil was used to acquire brain MRIs at baseline and follow-up visits. Two structural MRI sequences, a high resolution 3D brain volumetric (BRAVO) T1-weighted inversion prepared sequence of inversion time (TI) = 450 ms, repetition time (TR) = 8.2 ms, echo time (TE) = 3.2 ms, flip angle = 12°, acquisition matrix = 256 × 256, field of view (FOV) = 256 mm, slice thickness = 1.0 mm and a 3D T2-weighted fluid-attenuated inversion recovery (FLAIR) sequence of TR = 6,000 ms, TE = 124 ms, TI = 1867 ms, flip angle = 90°, acquisition matrix = 256 × 256, FOV = 256 mm, and slice thickness = 2.0 mm with no gap were acquired for WM lesion segmentation and regional brain volume comparisons. DWI data were acquired with a diffusion-weighted echo planar imaging sequence with ASSET parallel imaging (under sampling R = 2 in the phase encoding direction) and high order shimming prior to the acquisition of each sequence. Data were acquired in 40 encoding directions at b = 1,300 s/mm2 in addition to eight nondiffusion weighted volumes (b = 0). The cerebrum was covered in 52 contiguous, 2.9 mm thick axial slices of TR = 8,000 ms, TE = 86.3 ms, FOV = 240 mm, matrix = 96 × 96 to yield 2.5 × 2.5 × 2.9 mm3 resolution which was interpolated in-plane on the scanner to 0.9375 × 0.9375 × 2.9 mm3 voxels.
2.3 Image preprocessing
Diffusion-weighted imaging data were preprocessed using a robust pipeline also used in a number of previous studies (Adluru et al., 2014; Ly et al., 2016; Merluzzi et al., 2016; Racine et al., 2017). DWI data from baseline and follow-up visits were first converted from Digital Imaging and Communication in Medicine (DICOM) format to Neuroimaging Informatics Technology Initiative (NIFTI) format and visually inspected for artifacts. DTIprep was then used to detect and remove motion-affected volumes from the DWI series (https://www.nitric.org/projects/dtiprep/; Oguz et al., 2014). Then, the eddy-correct tool of the Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL; Version 5.0; Oxford Centre for Functional MRI of the Brain; http://fsl.fmrib.ox.ac.uk/fsl/) was used to correct for image distortions caused by eddy currents and the output transformations were used to reorient the diffusion gradients (Andersson & Sotiropoulos, 2016; Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012). Brain tissue extractions were then obtained from the eddy-corrected diffusion weighted images using FSL's Brain Extraction Tool (Smith, 2002). Finally, diffusion tensors were fitted using a nonlinear least squares method in Camino (http://cmic.cs.ucl.ac.uk/camino/; Alexander & Barker, 2005; Cook et al., 2006; Jones & Basser, 2004).
Iterative within- and between-subject tensor-based registration was performed according to methods described previously (Keihaninejad et al., 2013). First, using Diffusion Tensor Imaging Toolkit (DTI-TK; http://www.nitric.org/projects/dtitk/), an optimized DWI spatial normalization and atlas construction tool, subject-specific templates were created via iterative rigid, affine, and deformable alignment following bootstrapping of each participant's tensor maps (Wang et al., 2011; Zhang et al., 2007; Zhang, Yushkevich, Alexander, & Gee, 2006). Subject-specific templates were then used to create a population level template with DTI-TK. Following population space template production, subject-specific FA, MD, RD, and AD maps were normalized to population space and smoothed with a 6 mm-Gaussian kernel to reduce any residual misalignment. Then, subject-specific change maps were produced for each DWI measure by taking the difference between follow-up and baseline normalized maps (Follow-up – Baseline). To restrict our analyses to investigating only WM, an explicit WM mask was produced by binarizing the population space mean FA at ≥0.2. All diffusion weighted images were visually inspected for missing data and artifacts after preprocessing and prior to analysis.
2.4 White matter hyperintensity segmentation
Segmentation of WM hyperintensities (WMHs) was performed from T1- and T2-weighted FLAIR volumes using the lesion growth algorithm (LGA; Schmidt et al., 2012) as implemented in the lesion segmentation tool (LST) toolbox version 2.0.15 (http://www.statistical-modelling.de/lst.html) for Statistical Parametric Mapping (SPM), using techniques reported previously by our group (Birdsill et al., 2014). T1-weighted images were first segmented into the three main tissue classes. This information was then combined with the coregistered FLAIR images to calculate lesion belief maps. Initial binary lesion maps were obtained by thresholding with an initial threshold of к = 0.3, chosen based on visual inspection of binary lesion maps at three different initial thresholds (к = 0.3, 0.5, and 0.7). Binary lesion maps were subsequently used as seeds for the LGA to grow lesion probability maps (LPMs) along hyperintense voxels within the corresponding FLAIR images. To ensure the validity and congruence of the LPMs at each time point, the LST longitudinal pipeline was used to correct LPMs by comparing each participant's FLAIR images and original LPMS produced by the LGA at consecutive time points to determine if lesion changes were significant or due to natural FLAIR signal variation (Schmidt et al., 2012).
To use the LPMs as imaging covariates for longitudinal DWI change voxel-wise statistics, the LPMs, T1-, and T2-weighted FLAIR volumes for the follow-up study visit were coregistered and spatially normalized to the study-specific DWI population template in SPM. The transformed LPMs were then binarized and smoothed with a 6 mm-Gaussian kernel to reduce residual misalignment between lesion and DWI maps. All output images were visually inspected.
For the purpose of comparing the volume of cerebrovascular change between groups, we also extracted the total lesion volume (mL), for each participant at both time points, from the corrected LPMs using the lesion extraction functionality in LST at a threshold of 0.5. TLVs were then divided by the respective intracranial volumes (ICV) and multiplied by 100 to convert them to units of % ICV (Birdsill et al., 2014). To calculate within-subject TLV change, baseline %ICV was subtracted from follow-up: (WMHΔ = WMH Follow-up – WMH Baseline).
2.5 Neuropsychological assessments
Neuropsychological testing was conducted at the baseline and follow-up visits, immediately prior to the brain MRI, with PD participants in the off-medication state. The cognitive battery was designed to evaluate performance within the domains of baseline literacy, language, verbal memory, and executive function. These tests included the Wide Range Achievement Test – Fourth Edition (WRAT-IV) reading test (Wilkinson & Robertson, 2006), Boston Naming Test (BNT; Kaplan, Goodglass, & Brand, 1983), Controlled Oral Word Association Test (COWAT; Benton & Hamsher, 1976), Hopkins Verbal Learning Test (HVLT; Benedict, Schretlen, Groninger, & Brandt, 1998), Wechsler Adult Intelligence Scale – Third Edition (WAIS-III) digit span (Wechsler, 1997), Stroop Task (Golden, 1975), Trail Making Tests A and B (TMT B; Reitan & Wolfson, 1993), and the Wisconsin Card Sort Test – 64 Card Version (WCST-64; Heaton, 1993; Heaton, Chelune, Talley, Kay, & Curtiss, 1993). Summary raw scores from each test were converted to Z-scores based on population norms. Then, cognitive change scores were generated for each test by subtracting the baseline z-score from the follow-up (Cognitive measure z-scoreΔ = zFollow-up – zBaseline).
2.6 Statistics
Voxel-wise analyses were performed using Wake Forest University's Biological Parametric Mapping toolbox (BPM; Casanova et al., 2007) for SPM5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/). Four separate analysis of covariance (ANCOVA), models were constructed to compare the rate of change in each DWI measure (FA, MD, RD, and AD) between PD and control groups. In these models, participants' DWI difference maps were the voxel-wise dependent variable, while lesion probability maps at follow-up were included as an image covariate. Age, sex, years of formal education, and time between visits (months) were included as nonimage covariates. The lesion probability maps were included to control for DWI changes accounted for by regional microvascular disease rather than the effects of PD. In addition, regression models were constructed within the PD group to investigate the association between DWI metrics and disease features, such as disease duration and change in UPDRS scores between visits. An explicit WM mask was applied for all voxel-wise analyses. To control for false positives, Monte Carlo simulations with 10,000 iterations (3dClustSim; AFNI; http://afni.nimh.nih.gov; Cox, 1996; Saad & Reynolds, 2012) were used to compute the voxel-probability and minimum cluster-extent threshold needed to obtain a 0.05 family wise alpha with the original uncorrected per-voxel threshold of p < .001. A minimum cluster extent of 43 contiguous voxels was determined to be significant (p < .05, cluster-corrected).
To investigate the effects of disease stage on the rate of change in DWI-based measures, we employed a three-group BPM ANCOVA model that included controls, PD patients with unilateral signs (HY < 2), and PD patients with bilateral signs (HY ≥ 2; Hoehn & Yahr, 1967). These ANCOVA analyses comparing PD subgroups split by HY stage included the same image and nonimage covariates.
Clinical variables were analyzed using International Business Machines' Statistical Program for the Social Sciences (IBM SPSS; Version 22.0; https://www.ibm.com/analytics/us/en/technology/spss/) Except for categorical variables such as sex or side of disease onset, which were evaluated using Fisher's exact test, comparisons between PD and control groups were carried out using two-tailed independent samples t-tests. One-way ANOVAs were utilized to compare differences in means of more than two groups (i.e., HY < 2, HY ≥ 2, and controls). Post hoc analyses of significant ANOVAs were done using Tukey's test. A significance level of p < .05 was used for all analyses.
2.7 Gray matter segmentation
Regional gray matter segmentation was performed after the voxel-wise DWI analyses for the sole purpose of investigating changes in regional brain volume over the study interval that could potentially explain different rates of WM change. After visual inspection for quality, T1-weighted volumes were automatically segmented using the FreeSurfer image analysis suite version 5.1.0 according to FreeSurfer's RB_all_2008–03-26.gca atlas (http://surfer.nmr.mgh.harvard.edu; Fischl et al., 2002). As mentioned previously, subject- and visit-specific intracranial volumes were also derived via FreeSurfer segmentation. Based on the distribution of voxel-wise results from the DWI analyses, which were located in brainstem and deep subcortical WM, the brainstem was selected as the representative region for the evaluation of volume change. Volume change was calculated for each participant by taking the difference in regional volumes (mm3) between visits while correcting for the respective ICVs. Mean regional volume change was compared between controls and PD subgroups via one-way ANOVA with a significance of p < .05.
3 RESULTS
3.1 Demographics and group characteristics
Table 1 summarizes the demographic and disease characteristics of the PD and control groups. Age, years of formal education, sex, and time between visits did not differ significantly between groups. For PD participants (29 total: 16 HY < 2, and 13 HY ≥ 2), mean age at diagnosis was 62.1 (±9.1) years and mean disease duration was 3.7 (±3.2) years. Of PD participants, 17 experienced right, 10 left, and 2 symmetrical motor symptom onset. All but two PD participants were taking anti-Parkinson medications (dopamine agonists, levodopa, amantadine, and/or monoamine oxidase inhibitors) at the time of study enrollment. PD patients, as expected, had higher scores on both the total and motor sub-section of the UPDRS than controls at baseline. Over the 18-month study period, the PD group's UPDRS total score increased more than controls (p < .05), with a trend toward greater increase in the motor sub score (p = .06). White matter hyperintensity volume (WMH), at baseline and follow-up visits, did not differ significantly between PD and control groups (p = .130 and .117, respectively). No significant difference in WHM volume change was observed between groups (p = .225).
| PD (n = 29) | Control (n = 43) | p value | |
|---|---|---|---|
| Age at baseline (years) | 65.8 (9.4) | 66.7 (7.8) | .661 |
| Age at diagnosis (years) | 62.1 (9.1) | N/A | N/A |
| Mean disease duration (years) | 3.7 (3.2) | N/A | N/A |
| Sex (male/female) | 23/6 | 31/12 | .585 |
| Education (years) | 16.1 (2.8) | 17.1 (2.7) | .118 |
| Time between visits (months) | 18.2 (1.0) | 17.9 (0.8) | .199 |
| Side of onset (R/L/S) | 17/10/2 | N/A | N/A |
| UPDRSa motor sub scoreBaseline | 20.7 (11.0) | 1.5 (1.7) | <.001* |
| UPDRSa total scoreBaseline | 35.3 (14.3) | 3.1 (3.2) | <.001* |
| UPDRSa motor sub scoreΔ | 2.8 (6.8) | 0.3 (1.7) | .064 |
| UPDRSa total scoreΔ | 5.6 (11.0) | 0.9 (2.8) | .029* |
| Hoehn and Yahr stageBaseline | 16 (<2), 13 (≥2) | N/A | N/A |
| WMH (% ICV)Baseline | 0.37 (0.67) | 0.17 (0.23) | .130 |
| WMH (% ICV)Follow-up | 0.42 (0.83) | 0.17 (0.22) | .117 |
| WMH (% ICV)Δ | 0.06 (0.24) | 0.00 (0.05) | .225 |
- Note. Mean values with standard deviations denoted in parentheses. Between-group comparisons were performed by two-tailed independent samples t-test or Fisher's Exact Probability test.
- * p values < .05 are considered significant.
- ICV = intracranial volume; L = left; N/A = not applicable; PD = Parkinson's Disease; R = right; S = symmetrical; UPDRSa = unified Parkinson's disease rating scalea, scored with PD patients off of anti-Parkinson's disease medications for 12–18 hr; WMH = white matter hyperintensity volume.
3.2 Subgroup characteristics
The PD sample was divided into groups of subjects with unilateral (HY < 2) versus bilateral (HY ≥ 2) clinical signs at the baseline study visit for evaluating the effect of disease stage on rate of WM change. Subgroup characteristics are summarized in Table 2. There were no differences between the PD subgroups nor between each PD subgroup and the control group by age, sex, years of formal education, or time between study visits. Side of motor symptom onset did not differ between the PD subgroups. Neither WMH lesion volume at baseline and follow-up, nor rate of change in WMH volume, differed between subgroups (p > .272).
| HY < 2 (n = 16) | HY ≥ 2 (n = 13) | Control (n = 43) | p value | |
|---|---|---|---|---|
| Age at baseline (years) | 66.4 (10.1) | 65.1 (8.9) | 66.7 (7.8) | .829 |
| Age at diagnosis (years) | 64.0 (10.6) | 59.8 (6.4) | N/A | .206 |
| Disease duration (years) | 2.4 (1.4) | 5.2 (4.1) | N/A | .036* |
| Sex (male/female) | 12/4 | 11/2 | 31/12 | .695 |
| Education (years) | 15.9 (2.9) | 16.2 (2.7) | 17.1 (2.7) | .285 |
| Time between visits (months) | 18.3 (1.0) | 18.1 (1.1) | 17.9 (0.8) | .367 |
| Side of symptom onset (R/L/S) | 11/5/0 | 6/5/2 | N/A | .257 |
| UPDRSa motor sub scoreBaseline | 16.0 (7.7) | 26.6 (11.9) | 1.5 (1.7) | <.001* |
| UPDRSa total scoreBaseline | 29.6 (10.6) | 42.2 (15.6) | 3.1 (3.2) | <.001* |
| UPDRSa motor sub scoreΔ | 3.1 (5.9) | 2.4 (8.1) | 0.3 (1.7) | .074 |
| UPDRSa total scoreΔ | 4.3 (10.5) | 7.2 (11.8) | 0.9 (2.8) | .017* |
| WMH (% ICV)Baseline | 0.17 (0.21) | 0.61 (0.93) | 0.17 (0.23) | .272 |
| WMH (% ICV)Follow-up | 0.19 (0.24) | 0.70 (1.18) | 0.17 (0.22) | .283 |
| WMH (% ICV)Δ | 0.02 (0.06) | 0.10 (0.35) | 0.00 (0.05) | .305 |
- Note. Mean values with standard deviations denoted in parentheses. Between-group comparisons performed by one-way ANOVA or Fisher's exact test.
- * p values < .05 are considered significant.
- HY = Hoehn and Yahr stage; ICV = intracranial volume; L = left; N/A = not applicable; R = right; S = symmetrical; UPDRSa = unified Parkinson's disease rating scalea, scored with PD patients off of anti-Parkinson's disease medications for 12–18 hr; WMH = white matter hyperintensity volume.
As would be expected based on the typical progression of PD from unilateral to bilateral disease, disease duration and baseline UPDRS scores were lower for the unilateral (HY < 2) than for the bilateral (HY ≥ 2) PD subgroup (p < .05). There was no significant difference between PD subgroups in the mean change in UPDRS scores over the study interval. Four PD patients with unilateral clinical signs at baseline progressed to bilateral signs during the study.
3.3 Neuropsychological test scores
Summaries of the PD and control groups' neuropsychological test scores at baseline as well as mean change in performance during the 18-month study are shown in Supplemental Table 1 (PD vs. control) and Supplemental Table 2 (PD subgroups). At baseline, PD patients, as well as the unilateral PD subgroup, scored lower than the control group on the MMSE. However, the PD group was somewhat less educated than the control group (Table 1, p = .118), which may have influenced their performance on this global measure. In addition, the PD group scored lower than controls at baseline on the Trails B test, and the bilateral (HY ≥ 2) subgroup scored lower than the control group on the COWAT (p = .034). There were no significant differences between PD and control groups nor between PD subgroups and controls in z-score difference between baseline and follow-up for any of the cognitive assessments. However, some participants in both the control and PD groups did show cognitive decline. Although current criteria for mild cognitive impairment in PD (PD-MCI) are most specific for cross-sectional data (Litvan et al., 2012), we chose to define MCI as a decline of 1 or more SD on at least two tests over the study interval. Based on these criteria, eight PD (three: HY < 2, five: HY ≥ 2) and six Control participants progressed to MCI over the study interval. The ratio of participants who progressed to MCI did not differ between control and PD groups (p = .225), nor between control and PD subgroups (p = .176).
3.4 Parkinson's versus control comparison of FA, MD, RD, and AD change
Analyses of FA, MD, RD, and AD change maps in BPM ANCOVAs revealed different rates of DWI change between PD and control groups for all four DWI measurements over the course of the 18-month study. Representative statistical images of regional differences (p < .05, cluster-corrected) are shown in Figure 1, while result clusters are summarized in Table 3. The PD group showed a greater decrease in FA within the rostral midbrain and periaqueductal gray matter than the control group. The PD group also demonstrated a steeper increase in MD in bilateral midbrain tegmentum and left thalamus, as well as slightly more extensive increases in both RD and AD in the same regions in addition to the pontine crossing tract and posterior limb of the left internal capsule, respectively. Removal of the eight PD subjects and six controls who showed cognitive progression over the study interval from these analyses did not produce an appreciable change in the results.
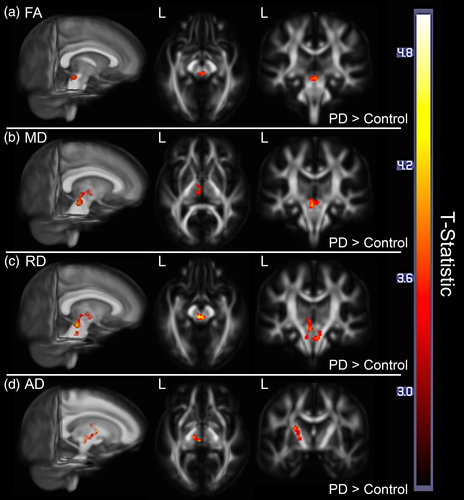
| Measure | Contrast direction | T-statistic | Location(s) | Cluster extent (mm3) |
|---|---|---|---|---|
| FA | PD decrease > control | 3.94 | B. midbrain tegmentum, periaqueductal gray matter | 344 |
| MD | PD increase > control | 4.14 | B. midbrain tegmentum, L. thalamus | 896 |
| RD | PD increase > control | 4.39 | B. midbrain tegmentum, B. pontine crossing tract, L. thalamus | 1,624 |
| AD | PD increase > control | 4.29 | L. post. limb of internal capsule, L. thalamus, L. midbrain tegmentum | 1,240 |
- Note. Contiguous clusters of at least 43 voxels were significant (p < .05, corrected for multiple comparisons at the cluster extent). Result cluster extent (mm3) was calculated by multiplying the number of voxels in a result cluster by DWI map voxel size (8 mm3 = 2 mm × 2 mm × 2 mm). Result locations were determined based on comparison to a WM atlas (Oishi, Faria, Van Zijl, & Mori, 2011).
- AD = axial diffusivity; B = bilateral; DWI = diffusion-weighted imaging; FA = fractional anisotropy; L = left; MD = mean diffusivity; Mid = middle; PD =Parkinson's disease; Post = posterior; R = right; RD = radial diffusivity; WM = white matter.
3.5 Association between disease duration and UPDRS change and DWI metric change
Voxel-based BPM regression analyses within the PD group revealed no association between change in either UPDRS total or motor sub-scores and progression of any of the four DWI metrics in cerebral WM. However, MD change within the right fornix/stria terminalis and lenticular fasciculus was negatively associated with disease duration (T = 6.47, Cluster extent = 440 mm3). In addition, AD change was negatively associated with disease duration within the left cerebral peduncle, midbrain tegmentum, and thalamus (T = 5.67, Cluster extent = 384 mm3). No association was observed between either FA or RD change and disease duration.
3.6 PD subgroup comparison of FA, MD, RD, and AD change
The BPM ANCOVA models used to map regional differences in the rate of change of DWI measures between the 16 unilateral PD (HY < 2 at baseline) subjects, 13 bilateral PD (HY ≥ 2 at baseline) subjects, and 43 age-matched controls showed a greater rate of DWI change in the unilateral PD subgroup relative to the control group: greater decrease in FA within the left thalamus, bilateral midbrain tegmentum, left middle cerebellar peduncle, right SN, right cerebral peduncle, and right posterior limb of internal capsule; greater increase in MD in the left thalamus and bilaterally in the midbrain, SN, pontine tegmentum, cerebral peduncles, fornix/stria terminalis, and posterior limbs of internal capsules; greater increase in RD in the left middle cerebellar peduncle and bilaterally in the thalamus, cerebral peduncles, rostral midbrain tegmentum, SN, fornix/stria terminalis, and posterior limbs of the internal capsule. Similarly, more extensive increase in AD was not only observed in the unilateral PD subgroup compared to controls within the brainstem, thalamus, and internal capsule, but also involved the hemispheric WM, including left cingulate gyrus WM, lateral occipital gyrus WM, superior longitudinal fasciculus, sagittal striatum, and fornix/stria terminalis. There were no regions of significantly different rates of FA, MD, RD, or AD change between bilateral PD and control subjects. Relative to the bilateral PD subgroup, the unilateral PD subgroup showed a greater rate of DWI change in the following regions: greater increase in MD in the caudal medulla, right middle cerebellar peduncle, and bilateral midbrain tegmentum, SN, and cerebral peduncles; greater increase in RD within the caudal medulla and unilaterally within the right midbrain tegmentum, SN, cerebral peduncle, and middle cerebellar peduncle; greater increase in AD in the caudal medulla, left midbrain tegmentum, left SN, and left cerebral peduncle. Unexpectedly, controls were observed to have a greater increase in both MD and RD within the right superior longitudinal fasciculus, as well as a greater increase in RD in the WM of the right superior parietal lobule, relative to the unilateral PD subgroup. Representative statistical images of regional differences (p < .05, cluster-corrected) are illustrated in Figure 2, while result clusters are summarized in Table 4.
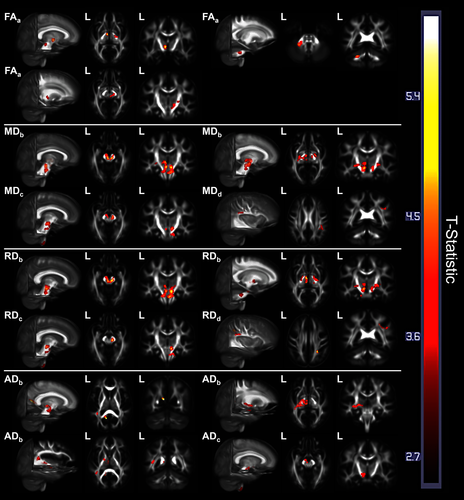
| Measure | Contrast direction | T-statistic | Location(s) | Cluster extent (mm3) |
|---|---|---|---|---|
| FA | U-PD decrease > control | 5.20 | L. thalamus, L. midbrain | 352 |
| U-PD decrease > control | 4.65 | L. mid. cerebellar peduncle | 624 | |
| U-PD decrease > control | 4.30 | B. midbrain tegmentum, R. SN, R. cerebral peduncle, R. post. limb of internal capsule | 1,568 | |
| MD | Control increase > U-PD | 3.79 | R. sup. long. fasciculus | 408 |
| U-PD increase > control | 5.27 | R. midbrain and pontine tegmentum, R. SN, R. cerebral peduncle, R. post. limb of internal capsule, R. fornix/stria terminalis | 2,768 | |
| U-PD increase > control | 5.12 | L. midbrain and pontine tegmentum, L. SN, L. thalamus, L. cerebral peduncle, L. post. limb of internal capsule, L. fornix/stria terminalis | 4,208 | |
| U-PD increase > B-PD | 5.02 | caudal medulla | 624 | |
| U-PD increase > B-PD | 4.67 | R. mid. cerebellar peduncle, R. cerebral peduncle, R. midbrain tegmentum, R. SN | 1,168 | |
| U-PD increase > B-PD | 4.23 | L. cerebral peduncle, L. midbrain tegmentum, L. SN | 504 | |
| RD | Control increase > U-PD | 5.07 | R. sup. long. fasciculus, R. sup. parietal lobule WM | 776 |
| U-PD increase > control | 5.71 | B. midbrain tegmentum, B. SN, B. thalamus, B. cerebral peduncle, R. post. limb of internal capsule, R. fornix/stria terminalis | 6,176 | |
| U-PD increase > control | 3.88 | L. post. limb of internal capsule, L. fornix/stria terminalis | 464 | |
| U-PD increase > control | 4.10 | L. mid. cerebellar peduncle | 448 | |
| U-PD increase > B-PD | 5.00 | R. midbrain tegmentum, R. SN | 480 | |
| U-PD increase > B-PD | 4.65 | caudal medulla | 600 | |
| U-PD increase > B-PD | 4.34 | R. mid. cerebellar peduncle, R. cerebral peduncle | 632 | |
| AD | U-PD increase > control | 6.09 | L. cingulate gyrus WM, L. splenium of corpus callosum, L. lateral occipital gyrus WM | 432 |
| U-PD increase > control | 4.62 | L. cerebral peduncle, L. midbrain tegmentum, L. SN, L. thalamus, L. sagittal striatum, L. fornix/stria terminalis, L. posterior limb and retrolenticular internal capsule | 3,912 | |
| U-PD increase > control | 4.05 | L. superior long. fasciculus | 368 | |
| U-PD increase > B-PD | 4.46 | caudal medulla | 544 | |
| U-PD increase > B-PD | 4.11 | L. cerebral peduncle, L. midbrain tegmentum, L. SN | 592 |
- Note. Contiguous clusters of at least 43 voxels were significant (p < .05, corrected for multiple comparisons at the cluster extent). Result cluster extent (mm3) was calculated by multiplying the number of voxels in a result cluster by DWI map voxel size (8 mm3 = 2 mm × 2 mm × 2 mm). Result locations were determined based on comparison to a WM atlas (Oishi et al., 2011).
- AD = axial diffusivity; B = bilateral; B-PD = bilateral PD (HY ≥ 2); DWI = diffusion-weighted imaging; FA = fractional anisotropy; L = left; Long = longitudinal; MD = mean diffusivity; Mid = middle; PD = Parkinson's disease; Post = posterior; R = right; RD = radial diffusivity; SN = substantia nigra; Sup =superior; U-PD = unilateral PD (HY < 2); WM = white matter.
3.7 Regional brain volume change
To ensure that the observed differences in rate of WM change between PD and control groups did not result from regional gray matter volume loss, we compared the rate of change in normalized, FreeSurfer-derived brainstem volumes between groups. Brainstem volumes were chosen for this comparison because the DWI voxel-wise results were located predominantly within the brainstem and deep subcortical WM. This comparison, via one-way ANOVA, yielded no difference between PD and control groups in the rate of brainstem volume change (p = .883). There was also no difference in mean brainstem volume between PD and control groups at baseline (p = .885).
4 DISCUSSION
This longitudinal MRI study used a voxel-wise approach to show differences in the rate of change in WM microstructural indices between PD patients and age-matched controls over 18 months. We also investigated the effect of disease stage, duration, and severity on the rate of DWI change in PD. These analyses revealed two major findings: first, the PD group showed greater decreases in fractional anisotropy, as well as greater increases in mean, axial, and radial diffusivity within the rostral brainstem, SN, and deep subcortical WM than the control group. The brainstem microstructural changes were not accounted for by volume loss. In addition, the rate of DWI change was greatest during early stage disease: within PD, disease duration was negatively associated with the magnitudes of both MD and AD change; furthermore, PD patients with early stage disease (HY < 2) experienced significantly greater rates of microstructural change compared with controls and the PD subgroup with bilateral disease (HY ≥ 2). Of the microstructural measures, MD and RD appeared to be the most sensitive for differential rates of change in the cerebral hemispheric WM.
Cross-sectional differences in brain DWI measures between PD patients and normal aged adults, as well as associations between clinical measures of motor, cognitive, and sensory function and measures of WM diffusion, have been reported in numerous studies (Bertrand et al., 2012; Duncan, Firbank, O'Brien, & Burn, 2013; Gallagher et al., 2013; Gattellaro et al., 2009; Goveas et al., 2015; Hattori et al., 2012; Karagulle Kendi, Lehericy, Luciana, Ugurbil, & Tuite, 2008; Kim et al., 2013; Melzer et al., 2013; Rae et al., 2012; Zhan et al., 2012; Zhang et al., 2011; Zheng et al., 2014); however, only a few studies of PD have evaluated DWI change over time (Melzer et al., 2015; Rossi et al., 2014; Zhang et al., 2016). Two of these studies (Melzer et al., 2015; Rossi et al., 2014) failed to observe significant differences in the rate of DWI change between PD patients and controls using a sensitive tract based spatial statistics (TBSS)-based voxel-wise approach. Compared with the present study, both of these studies included slightly fewer subjects and used fewer DWI encoding directions, which may have influenced their power to detect statistically significant differences in the subtle rate of microstructural change. Furthermore, Rossi et al. (2014) evaluated only FA change, which in our experience is less sensitive to Parkinson's-related differences than MD, RD, or AD measures.
A larger multicenter study (Zhang et al., 2016) of 122 PD (Average HY: 1.6 ± 0.5) and 50 control subjects from the Parkinson's Disease Progression Marker Initiative (PPMI) reported results that support those of the present study. Using an ROI-based approach, the investigators found greater reductions in FA as well as steeper increases in RD and AD in the SN, midbrain, and thalamus in PD subjects over 12 months relative to controls. Less significant differences in the rate of microstructural change were also seen within the corpus callosum, retrolenticular internal capsule, fronto-occipital fasciculus, and selected portions of frontal, temporal, and occipital WM (Zhang et al., 2016). Similar to the present study, no association between DWI change and UPDRS scores was observed, although an association between Montreal Clinic Assessment (MoCA; Nasreddine et al., 2005) scores and thalamic RD/AD was seen (Zhang et al., 2016). Considered together, the findings reported by Zhang et al. and those of the present study support an accelerated rate of microstructural change involving the rostral brainstem and subcortical WM in patients with early stage symptomatic PD, corresponding to Braak pathologic stages 3–4 (Braak et al., 2003). The additional subgroup analyses from the present study suggest that the rate of microstructural change within these regions is greatest in early clinical disease stages (HY < 2), and declines with disease duration. Furthermore, both studies demonstrated subtle differences in the rate of microstructural changes involving more extensive subcortical regions, suggesting that progression to mesocortical and neocortical disease (Braak stages 4–6) is underway.
Whether these microstructural changes are a primary or secondary phenomenon in the disease process is uncertain. MRI-based microstructural indices are affected by a number of pathologic and nonpathologic processes, including ischemia, edema/inflammation, demyelination, and axonal damage (Alexander et al., 2007; Jones et al., 2013). Of interest, microglial activation involving brainstem, basal ganglia, and frontal cortex has been demonstrated using 11C-PK11195 PET in PD (Gerhard et al., 2006), is limited to the midbrain in early PD (Ouchi et al., 2005), and remains stable with disease progression (Gerhard et al., 2006; Ouchi et al., 2005). In addition, subtle brainstem DWI differences have been described in REM Sleep Behavior disorder, a REM parasomnia associated with a strong risk for the eventual development of PD (Unger et al., 2010). As such, an accelerated rate of DWI change in the brainstem might even be detectable during preclinical disease and may taper off at meso- and neo-cortical stages of disease progression; however, longitudinal studies in at-risk populations would be needed to evaluate this hypothesis.
Although the present study was focused on WM, other longitudinal studies (Loane et al., 2016; Ofori et al., 2015) have investigated rates of DWI change within gray matter structures in PD, reporting contradictory results. Loane et al. (2016) reported progressive FA decline and MD increase in the SN in PD; however, the control group was not studied longitudinally. Ofori et al. (2015), in contrast, observed no difference in the rate of change in nigral FA or MD between PD and control subjects over 1 year, however, measures of free-water fraction increased more within the posterior SN in PD subjects relative to controls. Based on the known pathology of PD, diffusion changes within the SN are expected, while pathologic involvement of cerebral WM is more controversial.
The present study also detected a greater rate of change in diffusion measures within the cerebellar peduncles over 18 months in unilateral PD patients relative to controls. While cerebellar circuits are involved in the pathogenesis of PD symptoms, pathological changes in the cerebellum have not been widely described. In PD, disruption of striatal networks is thought to lead to changes in the activity of cerebellar networks (Cao, Xu, Zhao, Long, & Zhang, 2011; Ma, Tang, Spetsieris, Dhawan, & Eidelberg, 2007; Tomše et al., 2017), including both decreased resting blood flow and increased activation with tasks. Changes in network activity are known to influence myelination, which may also be a reason for the observed DWI changes (Demerens et al., 1996; Pajevic, Basser, & Fields, 2014). The cerebellar findings may be further supported by Zhang et al. (2011), who reported an association between cerebellar WM MD and FA and olfactory identification scores in PD.
4.1 Limitations
This study was not without its limitations. The sample size was relatively small, especially for the PD subgroups, limiting the statistical power of the analyses—thus the lack of detectable difference in the rate of DWI change between the bilateral PD subgroup and control group may result from small sample size. In addition, due to patient withdrawal (health or personal complications), participant attrition between study visits was greater in the PD group than the control group, which may have limited the representation of PD patients with more rapid motor progression or cognitive decline over the study interval. This also may account for the lack of observed differences in DWI change rate between control and more advanced PD subject groups.
5 CONCLUSIONS
In conclusion, our results support an accelerated rate of loss of WM microstructural integrity in nondemented PD patients in comparison to age-matched controls and provides support for the Braak hypothesis of predominant brainstem involvement at pathologic stage 3 and 4 PD (Braak et al., 2003). Future studies of at-risk participants and newly diagnosed PD patients should be done to determine whether subtle differences in the rate of WM microstructural change can be used to predict PD.
ACKNOWLEDGMENTS
This work was supported by Merit Review Award 101CX000555 (Gallagher, PI) from the United States Department of Veterans Affairs Clinical Sciences Research and Development. The authors would also like to acknowledge the support provided by the University of Wisconsin Alzheimer's Disease Research Center Clinical Core (NIH grant P50 AG033514; Asthana, PI) for subject recruitment and the Neuroimaging Core for study design and data analysis as well as the facilities and resources at the Geriatric Research, Education, and Clinical Centers (GRECC) of the William S. Middleton Memorial Veterans Hospital, Madison, WI. The content is solely the responsibility of the authors and does neither represent the official views of the US Department of Veteran Affairs, nor the US Government, nor the National Institutes of Health. Importantly, the authors wish to thank our volunteers, without whom this work would not be possible.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DISCLOSURE OF INTERESTS
Authors Vincent Pozorski, Jennifer M. Oh, Nagesh Adluru, Andrew Merluzzi, Frances Theisen, Ozioma Okonkwo, Amy Barzgari, Stephanie Krislov, Jitka Sojkova, Sterling Johnson, Andrew Alexander, and Catherine L. Gallagher declare no conflicts of interest.