Reproducible network and regional topographies of abnormal glucose metabolism associated with progressive supranuclear palsy: Multivariate and univariate analyses in American and Chinese patient cohorts
Funding information: National Natural Science Foundation of China, Grant/Award Numbers: 81361120393, 81571232, 81171189, 81671239, 81371413, and 81401135; Shanghai Sailing Program from Shanghai Science and Technology Committee, Grant/Award Number: 18YF1403100; National Institute of Neurological Disorders and Stroke (NINDS), Grant/Award Numbers: R01 NS 083490 and P50 NS 071675
This work was performed at Feinstein Institute for Medical Research in the USA and Shanghai Huashan Hospital in China.
Abstract
Progressive supranuclear palsy (PSP) is a rare movement disorder and often difficult to distinguish clinically from Parkinson's disease (PD) and multiple system atrophy (MSA) in early phases. In this study, we report reproducible disease-related topographies of brain network and regional glucose metabolism associated with PSP in clinically-confirmed independent cohorts of PSP, MSA, and PD patients and healthy controls in the USA and China. Using 18F-FDG PET images from PSP and healthy subjects, we applied spatial covariance analysis with bootstrapping to identify a PSP-related pattern (PSPRP) and estimate its reliability, and evaluated the ability of network scores for differential diagnosis. We also detected regional metabolic differences using statistical parametric mapping analysis. We produced a highly reliable PSPRP characterized by relative metabolic decreases in the middle prefrontal cortex/cingulate, ventrolateral prefrontal cortex, striatum, thalamus and midbrain, covarying with relative metabolic increases in the hippocampus, insula and parieto-temporal regions. PSPRP network scores correlated positively with PSP duration and accurately discriminated between healthy, PSP, MSA and PD groups in two separate cohorts of parkinsonian patients at both early and advanced stages. Moreover, PSP patients shared many overlapping areas with abnormal metabolism in the same cortical and subcortical regions as in the PSPRP. With rigorous cross-validation, this study demonstrated highly comparable and reproducible PSP-related metabolic topographies at network and regional levels across different patient populations and PET scanners. Metabolic brain network activity may serve as a reliable and objective marker of PSP, although cross-validation applying recent diagnostic criteria and classification is warranted.
1 INTRODUCTION
Progressive supranuclear palsy (PSP) is one of the most common forms of atypical parkinsonism characterized by gait dysfunction, gaze palsy, cognitive impairment, early dysphagia and dysarthria. At early disease stages, PSP remains difficult to diagnose because of its substantial overlap of clinical symptoms with Parkinson's disease (PD), multiple system atrophy (MSA), corticobasal degeneration and dementia with Lewy bodies. Despite progressive improvement in clinical diagnostic criteria for parkinsonian syndromes in the past few decades, it has been reported that approximately 20%–47% of patients with PSP are still misdiagnosed in life (Joutsa, Gardberg, Roytta, & Kaasinen, 2014; Respondek et al., 2013). Given that disease prognoses and treatment options can differ considerably, a correct diagnosis of PSP from other types of parkinsonism is of great importance in clinical practice as well as in research trials of novel therapies.
Neuroimaging with positron emission tomography (PET) has been used extensively to provide increased accuracy of clinical diagnosis for PSP in vivo. So far, reduced striatal binding in dopaminergic imaging has demonstrated high sensitivity for detecting PSP and other forms of parkinsonism, but it has not been sufficiently accurate for discrimination among these diseases (Jin et al., 2013). Although cholinergic deficiency in cortical and subcortical regions may be suggestive of PSP, this marker has shown neither sensitivity nor specificity, especially at early stages of the disease (Gilman et al., 2010; Hirano et al., 2010). The application of functional imaging of neuropathology (i.e., tau fibrillar aggregates and β-amyloid deposition) is still in its infancy, thereby also being of limited value in early differential diagnosis of parkinsonism (Broski et al., 2014; Kepe et al., 2013).
Brain metabolic activity measured with 18F-fluorodeoxyglucose (18F-FDG) PET has proven to be valuable for studying parkinsonian disorders (Eckert et al., 2005; Eckert et al., 2008; Niethammer & Eidelberg, 2012; Teune et al., 2010; Teune et al., 2013; Wu et al., 2013; Wu et al., 2014). In the past decade, a variety of voxel-based techniques have been successfully applied to 18F-FDG PET images to describe characteristic patterns of regional glucose metabolism in patients with PSP. To date, disease-specific metabolic markers associated with PSP have been reported in several independent patient cohorts to assist in early differential diagnosis on a single-case basis. This prospective application was facilitated qualitatively by visually inspecting the similarity of each 18F-FDG PET image to PSP-related metabolic templates (Eckert et al., 2005; Teune et al., 2010) or quantitatively by assessing the activity of PSP-related metabolic networks (Eckert et al., 2008; Teune et al., 2013). These studies utilized two complementary (i.e., univariate or multivariate) analytical approaches but were limited by the lack of objective comparisons between the patterns across independent imaging centers. Before the PSP-related metabolic marker can be used as a reference standard to help routine clinical diagnosis, it is necessary to assess its reproducibility on a whole-brain basis in a direct comparison of different patient cohorts using the same analytical techniques.
In this study, we used multivariate brain network analysis to identify, characterize and cross-validate PSP-related metabolic patterns (PSPRP) together and separately in an American cohort and a comparable Chinese cohort of both healthy control subjects and patients with parkinsonism. We also evaluated similarities and differences in abnormal regional metabolism in these 18F-FDG PET images using univariate brain mapping analysis. Moreover, we examined the reproducibility of PSP-specific metabolic characteristics and network biomarkers across study populations, PET instruments and analytical approaches.
2 MATERIAL AND METHODS
2.1 Subjects
This study included two different cohorts of healthy subjects and parkinsonian patients. The first cohort were recruited from both American and Chinese populations resulting in a total of 100 participants: 20 normal controls along with 20 PSP, 20 MSA, and 40 PD patients. The American cohort was comprised of 10 healthy subjects and 10 PSP, 10 MSA, and 20 PD patients recruited at North Shore University Hospital (NY, USA) and used originally for the identification and internal evaluation of disease-related metabolic patterns associated with parkinsonism (i.e., PSPRP, MSARP, and PDRP; Eckert et al., 2008; Ma, Tang, Spetsieris, Dhawan, & Eidelberg, 2007). These patterns had played a critical role in the development and validation of novel classification algorithms for early differential diagnosis of parkinsonism in independent populations from different countries (Ko, Lee, & Eidelberg, 2017; Niethammer et al., 2014; Tang et al., 2010; Tripathi et al., 2016). To match the sample size, the Chinese cohort consisted of the same numbers of healthy subjects and parkinsonian patients who were prospectively enrolled at Huashan Hospital (Shanghai, China) between January 2014 and September 2015. Demographic and clinical data for the healthy and patient groups are provided in Table 1. In the combined cohort there was a trend effect of age among four subject groups (ANOVA: F[3,96] = 2.46, p = .067) with the PSP patients marginally older than the healthy controls (p = .068). The patients with PSP and MSA were highly similar in their clinical characteristics but had more severe motor symptoms and shorter disease duration than the PD patients. These individuals underwent 18F-FDG PET imaging at the American and Chinese sites respectively.
| N | Gender (M/F) | Age (years) | Hoehn and Yahr Scale | UPDRS (motor) | Duration (years) | |
|---|---|---|---|---|---|---|
| Combined cohort | ||||||
| Healthy Control | 20 | 10/10 | 59.0 ± 11.4 | N/A | N/A | N/A |
| PSP | 20 | 11/9 | 65.3 ± 8.3 | 3.9 ± 0.7a | 35.7 ± 11.7 | 3.1 ± 1.7a |
| MSA | 20 | 9/11 | 62.6 ± 7.1 | 4.4 ± 0.5a | 47.8 ± 13.7b | 3.2 ± 2.3a |
| PD | 40 | 26/14 | 61.0 ± 5.2 | 2.7 ± 1.3 | 31.3 ± 18.3 | 6.8 ± 4.6 |
| American cohort | ||||||
| Healthy control | 10 | 3/7 | 64.3 ± 7.6c | N/A | N/A | N/A |
| PSP | 10 | 5/5 | 67.9 ± 5.7 | 3.5 ± 1.0 | 27.9 ± 14.9 | 2.5 ± 1.2 |
| MSA | 10 | 3/7 | 63.7 ± 8.7 | 4.6 ± 0.5 | 43.3 ± 17.3 | 3.5 ± 2.2 |
| PD | 20 | 14/6 | 60.1 ± 5.4 | 3.1 ± 1.6 | 29.6 ± 18.8 | 9.3 ± 4.5d |
| Chinese Cohort | ||||||
| Healthy Control | 10 | 7/3 | 53.6 ± 12.5 | N/A | N/A | N/A |
| PSP | 10 | 6/4 | 62.8 ± 9.9 | 4.1 ± 0.6 | 39.5 ± 8.5 | 3.6 ± 2.0 |
| MSA | 10 | 6/4 | 61.5 ± 5.4 | 4.3 ± 0.5 | 51.4 ± 9.7 | 2.9 ± 2.6 |
| PD | 20 | 12/8 | 61.8 ± 5.1 | 2.5 ± 1.1 | 32.6 ± 18.3 | 4.9 ± 3.8 |
- Data are given as mean ± SD.
- a p < .001.
- b p < .005 compared to the PD group in the combined cohort (post-hoc Bonferroni tests).
- c p < .05.
- d p < .005 compared to the corresponding group in the chinese cohort (two-sample t tests).
- UPDRS = the unified Parkinson's disease rating scale; PSP = progressive supranuclear palsy; MSA = multiple system atrophy; PD = Parkinson's disease.
Before taking part in this study, all patients were followed by two specialists of movement disorders at each institution for at least one year. The clinical diagnosis was further ascertained through post-imaging follow-up by the clinicians who were blinded to the imaging results. All patients with PSP met the consensus diagnostic criteria for “probable” PSP (NINDS-SPSP; Litvan et al., 1996; Respondek et al., 2013) and showed vertical supranuclear palsy and prominent postural instability with tendency to fall in the first year of disease onset. All patients with MSA and PD satisfied the criteria of the Second Consensus Statement on the Diagnosis of MSA (Gilman et al., 2008) and the UK Brain Bank (Gibb & Lees, 1988), respectively. These were clinically-confirmed patients at relatively advanced stages in order to identify and cross-validate disease-specific patterns.
However, most patients referred to scanning in clinical routine are more likely to be suspected of having parkinsonism at earlier stages. To more realistically assess differential diagnosis of parkinsonism, we included a second independent Chinese cohort with a total of 68 participants: 20 normal controls and 14 PSP, 14 MSA and 20 PD patients (Table 2). These patients were initially scanned with 18F-FDG PET at the Chinese site as clinically uncertain cases, and then followed up for several years until a clinical diagnosis was made unequivocally by their neurologists. The subjects in this early patient cohort were at very early clinical stages (p < .05) compared to those in the combined American and Chinese cohort (Table 2) with less severe motor symptoms (PSP: p = .33; MSA or PD: p < .005) and shorter disease duration (p < .005). Normal control and PSP subjects were closed matched in age (p ≥ .25) but MSA and PD patients were younger (p < .001) compared to those in the combined cohort. No patients showed brain structural abnormalities (e.g., mass lesions and ischemia) on magnetic resonance imaging (MRI). All healthy controls participating in this study underwent a neurological examination by a senior movement disorders specialist to rule out history of neurologic or psychiatric disorders. None of the patients and healthy volunteer subjects had any prior exposure to neuroleptic agents or drug use.
| N | Gender (M/F) | Age (years) | Hoehn and Yahr Scale | UPDRS (motor) | Duration (years) | |
|---|---|---|---|---|---|---|
| Early patient cohort | ||||||
| Healthy Control | 20 | 4/16 | 62.5 ± 6.7a, d | N/A | N/A | N/A |
| PSP | 14 | 9/5 | 65.6 ± 8.2a, e | 3.2 ± 1.1a | 29.6 ± 20.2c | 1.5 ± 0.6 |
| MSA | 14 | 8/6 | 52.1 ± 7.2 | 2.6 ± 0.9b | 33.3 ± 12.7b | 1.4 ± 0.5 |
| PD | 20 | 14/6 | 50.9 ± 11.2 | 1.5 ± 0.7 | 16.2 ± 7.9 | 1.1 ± 0.5 |
- Data are given as mean ± SD.
- a p < .0001.
- b p < .005.
- c p < .05 compared to the PD group (post-hoc Bonferroni tests).
- d p < .01.
- e p < .001 compared to the MSA group (post-hoc Bonferroni tests).
- UPDRS = the unified Parkinson's disease rating scale; PSP = progressive supranuclear palsy; MSA = multiple system atrophy; PD = Parkinson's disease.
Ethical permission for the study procedures was obtained from the Institutional Review Boards at North Shore University Hospital and Huashan Hospital. This study was done in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and the standards established by the author's local Institutional Review Boards and granting agencies. Written consent was obtained at each institution from each subject following detailed explanation of the scanning procedures. Authors had access to information that could identify individual participants during or after data collection.
2.2 18F-FDG PET imaging
All subjects were asked to fast for at least 6 hr but had free access to water prior to imaging. All patients underwent 18F-FDG PET at least 12 hr after the cessation of oral antiparkinsonian medications. The American subjects were scanned on a GE Advance Tomograph (General Electric, Milwaukee, WI) in three-dimensional (3D) mode (Ma et al., 2007). Following a transmission scan, a PET scan was acquired over 35–45 min after an intravenous injection of 18F-FDG (5–10 mCi) and reconstructed with the 3D reprojection method. The Chinese subjects were scanned with a Siemens Biograph 64 PET/CT (Siemens, Munich, Germany) in 3D mode (Wu et al., 2013). After a CT transmission scan for attenuation correction, a PET scan was acquired over 45–55 min post-injection and reconstructed with the ordered subset expectation maximization method. All studies in patients and normal subjects were performed in a resting state in a quiet and dimly lit room. Only relative brain glucose metabolism was measured without arterial blood sampling as in any routine clinical imaging protocol.
2.3 Image processing
PET images were preprocessed using Statistical Parametric Mapping software (SPM5; Wellcome Department of Imaging Neuroscience, Institute of Neurology, London, UK) implemented in Matlab 7.4.0 (MathworksInc, Sherborn, MA). Scans were spatially normalized into Montreal Neurological Institute (MNI) brain space with linear and nonlinear 3D transformations. They were then smoothed by a Gaussian filter of 10 mm width over a 3D space to increase signal to noise ratio for statistical analysis.
2.4 Network analysis
Brain network analysis and related computing procedures were performed using ScanVP software, Version 5.9.1 (http://www.feinsteinneuroscience.org at Center for Neuroscience, the Feinstein Institute for Medical Research, Manhasset, NY). Disease-related brain network was generated using 18F-FDG PET data from patients and healthy subjects by applying a voxel-based spatial covariance mapping algorithm known as Scaled Subprofile Modeling based on principal component analysis (SSM/PCA). This algorithm and procedures have been described in detail previously (Ma et al., 2007; Spetsieris & Eidelberg, 2011). Briefly, the method executed PCA on covariance matrix of group-wise image data within a brain mask of gray matter for identifying a disease-related spatial covariance pattern with greater expression in patients than in control subjects. The pattern was defined by a linear combination of select principal components (PCs) whose expression could best discriminate the patients from the controls, as determined by the lowest Akaike Information Criterion (AIC) value in a logistical regression model. Subject scores of this pattern were computed prospectively in any other scans using a voxel-based topographic profile rating (TPR) algorithm. This entailed multiplying each individual image with that of the pattern (i.e., dot product between the two images) and summing the result over the brain mask. The resulting scores were then Z-transformed by using subject scores of the healthy controls involved in the pattern identification, that is, Z-score = (score – control mean)/control standard deviation.
2.4.1 Pattern identification and disease discrimination
Specific metabolic pattern associated with PSP (PSPRP) were identified using 18F-FDG PET data from the PSP and healthy subjects in the combined American and Chinese cohort. The reliability of this pattern was estimated at each voxel by means of an iterative bootstrapping scheme (Habeck et al., 2008; Peng et al., 2014). This step produced a map of inverse coefficient of variation (ICV) to assess a brain-wide statistic. Network scores of PSPRP were then computed for all scans in the combined cohort as well as in the early patient cohort and Z-scored using subject scores of the healthy controls in the identification cohort of PSPRP.
2.4.2 Cross-validation of topographies and pattern expression between the USA and China
We next identified a separate PSPRP using 18F-FDG PET scans of PSP and healthy subjects in the American and Chinese cohorts respectively and quantitatively evaluated their similarities in terms of topographic distribution and network expression. The topographies were assessed by voxel-based correlation of regional weights in a set of significant voxels over the whole brain. Only the voxels with absolute values ≥1.0 were used to calculate the correlation between the two patterns. Subject scores of each pattern were calculated for the normal, PSP, MSA, and PD groups in each cohort and cross-validated by computing network scores of the PSPRP identified in the USA for the subjects in China or vice versa. Group discrimination was examined using the subject scores within each site and across the two sites. Moreover, the subject scores for the PSPRP identified in the USA were also correlated with those for the prospective validation of the PSPRP derived in China or vice versa.
2.5 Analysis of regional metabolic activity
Differences in regional metabolism between patients and normal controls were analyzed using SPM5 as described previously (Zuo et al., 2013). For the combined or separate cohort, we used a two-sample t test according to the general linear model to characterize metabolic activity in PSP patients compared with controls. Mean signal differences over the whole brain were modeled by ANCOVA per subject. The contrasts for the decreased and increased metabolism were set as [1 −1] and [−1 1]. Using SPM5 we also utilized a flexible factorial design to localize brain regions with common or different abnormal metabolism between the American and Chinese cohorts (Asanuma et al., 2006), with site and group added as two main effects and global values entered as a covariate to eliminate the potential confound. The contrasts for the commonality and the differences were detected separately using conjunction analysis [1, −1, 1, −1]/[−1, 1, −1, 1] and interaction analysis [1, −1, −1, 1]/[−1, 1, 1, −1].
To evaluate significant differences, we set the voxel threshold at p < .001 (uncorrected) over whole-brain with an extent threshold empirically chosen to be more than 2–3 times of the expected voxels per cluster estimated in each SPM run and searched for clusters that survived at family-wide error (FWE)-corrected p < .05. Significant regions were localized by Talairach Daemon software (Research Imaging Center, University of Texas Health Science Center, San Antonio, TX) after applying an MNI-to-Talairach conversion. SPM maps of t-statistic were overlaid on a standard T1-weighted MRI brain template in stereotaxic space. To quantify metabolic changes in specific regions, we constructed a 4-mm radius spherical volume of interest (VOI) in the image space, centered at the peak voxel of clusters that were significant in each SPM analysis. We then calculated the relative cerebral glucose metabolic values (i.e., globally normalized) in patients and normal controls with ScanVP software.
2.6 Statistical analysis
Differences in PSPRP scores between patients and normal controls were assessed using two-sample t tests in the derivation cohort of each pattern. Changes in PSPRP score over multiple groups in one cohort or between the cohorts were evaluated using one-way analysis of variance (ANOVA) with differences between groups examined post-hoc with Bonferroni tests. Discrimination power was determined by receiver operating characteristic (ROC) analysis. Relationships between PSPRP scores and clinical measures in patients or between two PSPRP scores in the same groups were determined by computing Pearson correlation coefficients. Group differences in regional metabolic values were assessed using two-sample t tests in a single cohort and one-way ANOVA across the two cohorts. All analyses used SPSS software (SPSS Inc., Chicago, IL) and were considered significant for p < .05.
3 RESULTS
3.1 Metabolic brain network in the combined cohort
3.1.1 Pattern identification and disease discrimination
Spatial covariance analysis of 18F-FDG PET scans in the PSP and healthy subjects from the USA and China examined the first 5 PCs that accounted for 54.5% of subject × voxel variance. A PSPRP was selected from a linear combination of PC1, PC2, PC3, and PC5 (16.8% variance accounted for [VAF]) whose expression yielded the lowest AIC value for group separation in the logistic regression model. This pattern was characterized bilaterally by relative metabolic decreases in the middle prefrontal/cingulate and ventrolateral prefrontal cortices, striatum, thalamus and midbrain, associated with metabolic increases in the hippocampus or parahippocampus, insula and parieto-temporal cortices (Figure 1a) and reliable (Figure 1b; p < .001, bootstrap estimation: 1,000 iterations). Subject scores of PSPRP measured in all subjects in this combined cohort showed an effect of group (ANOVA: F[3,96] = 138.1, p < .0001; Figure 1c) with elevation in the PSP/MSA patients (p < .0001, post-hoc tests) but not in the PD patients (p = .18) compared to the normal (NL) groups. Network scores were also greater in the PSP patients (p < .0001) than in the MSA patients and correlated with disease duration in the PSP patients (r = .49, p = .028) but not in the MSA or PD patients (|r| ≤ .21, p ≥ .22). Network scores did not correlate with the unified Parkinson's disease rating scale (UPDRS) motor ratings in any patient groups (p ≥ .22). PSPRP scores exhibited increased accuracy in discrimination of the PSP patients from the MSA, PD and NL groups (area under the curve [AUC] ≥ .928 ± .049) and the MSA patients from the PD and NL groups (AUC ≥ .938 ± .031) according to the ROC analysis (Figure 1d; Supporting Information Table S1). Furthermore, PSPRP scores did not differ (p ≥ .17) between the two cohorts in the NL, PSP, and MSA subjects but were greater (p < .01) in the American patients with PD.
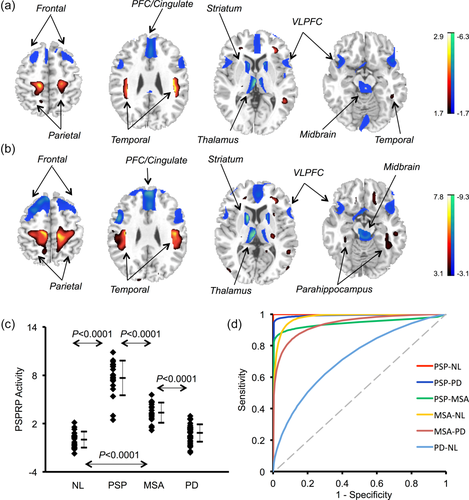
PSP-related pattern (PSPRP) identified by spatial covariance analysis of 18F-FDG PET scans from PSP patients and healthy controls in both American and Chinese cohorts. (a) This pattern was characterized by decreased (blue) metabolic activity in the bilateral middle prefrontal cortex (PFC)/cingulate, ventrolateral prefrontal cortex (VLPFC), striatum, thalamus and midbrain, covarying with increased (red) metabolic activity in the hippocampus/parahippocampus and parieto-temporal cortices. The overlays are depicted in neurological orientation. The thresholds of the color bars represent Z-values. (b) The topography of PSPRP was reliable as estimated by a voxelwise bootstrapping algorithm in conjunction with spatial covariance analysis in the combined cohort. The displays represent the maps of inverse coefficient of variation (ICV) thesholded at p < .001 following 1,000 iterations over the whole brain. (c) The expression of PSPRP discriminated the PSP patients from the healthy controls (i.e., NL) as well as from the MSA and PD patients (p < .0001; post-hoc tests). Network scores were also elevated in the MSA patients compared with the healthy controls and PD patients, but much lower than those in the PSP patients (p < .0001). The error bars represent standard deviations. (d) Receiver operating characteristic (ROC) analysis revealed different levels of group discrimination by subject scores of PSPRP. The graph displays the fitted ROC curves for improved visualization
3.1.2 Disease discrimination by pattern expression in early stage patients
There was also an effect of groups in PSPRP scores in Cohort 2 with parkinsonian patients at early stages (ANOVA: F[3,64] = 48.1, p < .0001; Figure 2a); Network scores were elevated in the PSP patients (p < .0001, post-hoc tests) compared to the NL, MSA, and PD subjects but did not differ among the latter three groups (p = 1.0). Likewise, the ROC analysis disclosed increasing accuracy for discriminating the PSP patients from the MSA, PD, and NL groups (AUC ≥ .964 ± .030; Figure 2b; Supporting Information Table S2).
Differential diagnosis by subject scores of PSPRP in an independent Chinese cohort of healthy controls and parkinsonian patients at early stages. (a) The expression of PSPRP discriminated the PSP patients from the healthy controls (i.e., NL) as well as from the MSA and PD patients (p < .0001; post-hoc tests). There were no differences in network scores between the MSA and PD patients or compared with the healthy controls. (b) Receiver operating characteristic (ROC) analysis revealed different levels of group discrimination by subject scores of PSPRP. The graph displayed the fitted ROC curves for improved visualization. The error bars represented standard deviations [Color figure can be viewed at wileyonlinelibrary.com]
3.1.3 Differences in relative regional metabolism
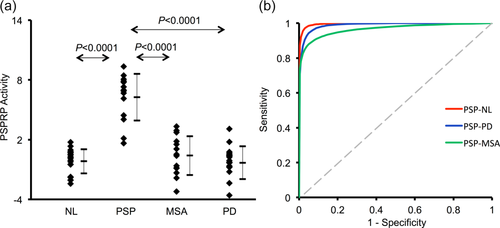
SPM analysis of 18F-FDG PET scans from the USA and China revealed bilaterally distributed abnormal metabolism in the PSP patients compared to the healthy subjects. Relative regional metabolism decreased in the middle prefrontal/cingulate and ventrolateral prefrontal cortices, striatum, thalamus and midbrain, but increased in the hippocampus/parahippocampus, insula, cerebellum and parieto-temporal regions (Figure 3a; Table 3, p < .001; none survived at FWE-corrected p < .05). This was also seen by relative metabolism measured post-hoc in several primary regions showing reductions in the frontal lobe, striatum and thalamus but increases in the parahippocampus and paracentral gyrus in the patients relative to the controls (p < .001; Figure 3b–g).
Brain regions with significant metabolic differences in PSP patients compared with healthy controls detected by SPM analysis of 18F-FDG PET scans in both American and Chinese cohorts. (a) Normalized glucose metabolism in the PSP patients decreased (blue) in the middle prefrontal cortex (PFC)/cingulate, ventrolateral prefrontal cortex, striatum, thalamus and midbrain, but increased (red) in the hippocampus/parahippocampus and parieto-temporal regions compared to normal (NL) subjects (p < .001). The overlays are depicted in neurological orientation. The thresholds of the color bars represent t values. (b–g) Group differences in relative metabolic values in select cortical and subcortical regions, obtained post-hoc in spherical volumes of interest (4 mm radius) centered at the peak coordinates of clusters deemed significant from the prior SPM analysis on a voxel-by-voxel basis. The metabolic values in the healthy controls or the PSP patients were separated into different sites so as to visualize their reproducibility across both cohorts. The error bars represent standard deviations [Color figure can be viewed at wileyonlinelibrary.com]
| Structure | Brodmann area | Hemisphere | MNI coordinatesa | Z max | Cluster size (mm3) | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Decreased metabolismb | |||||||
| Middle frontal gyrus | BA 6 | L | −46 | 4 | 56 | 3.84 | 6,008 |
| Inferior frontal gyrus | BA 44 | L | −56 | 16 | 20 | 4.55 | |
| Inferior frontal gyrus | BA 47 | R | 44 | 20 | −2 | 3.72 | 3,256 |
| Putamen | L | −18 | 4 | 12 | 4.54 | 44,160 | |
| Thalamus (extending to midbrain) | L | −8 | −18 | 10 | 4.46 | ||
| Increased metabolismb | |||||||
| Paracentral gyrus | BA 4 | L | −8 | −40 | 72 | 4.10 | 38,784 |
| Precentral gyrus | BA 4 | R | 66 | −10 | 26 | 3.71 | 8,640 |
| Insula | BA 13 | L | −36 | −20 | 28 | 3.99 | 5,824 |
| Middle temporal gyrus | BA21 | R | 38 | 2 | −34 | 3.84 | 1,880 |
| Parahippocampus | L | −36 | −2 | −26 | 3.93 | 5,184 | |
| Hippocampus | R | 34 | −30 | 16 | 3.64 | 5,888 | |
| Anterior cingulate | BA 32 | L | −20 | 42 | 0 | 3.85 | 2,184 |
| Cerebellar culmen | L | −12 | −52 | −28 | 3.32 | 6,304 | |
| Cerebellar dentate | R | 14 | −52 | −30 | 3.34 | 7,088 | |
- a Montreal Neurological Institute (MNI) standard space.
- b Survived at uncorrected p < .001, extent threshold = 110 voxels (880 mm3). Nothing survived at FWE p < .05.
3.2 Cross-validation of metabolic brain network in the two cohorts
3.2.1 Comparison of topographic patterns and disease discrimination
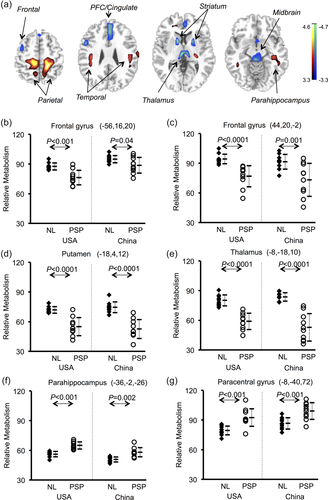
Analogous analysis in the American or Chinese cohort interrogated the first 4 PCs accounting for 58.9% or 53.5% of subject × voxel variance. A PSPRP was selected by a linear combination of PC1 and PC4 (29.5% VAF) or PC1, PC2 and PC3 (29.0% VAF). Both PSP-related patterns showed highly similar topographies (Figure 4a; Figure 5a; c.f., Figure 1a) with their regional weights correlating strongly on voxel-based analysis within the brain (r = .932, p < .0001). PSPRP scores revealed an effect of group among all subjects in the American or Chinese cohort (ANOVA: F[3,46] = 59.2/79.8, p = .0001; Supporting Information Figure S1). Network scores of each PSPRP were abnormally elevated in the PSP (p < .0001) and MSA (p = .038/.003) patients, and also variably in the PD patients (USA: p = .362; China: p < .001) compared to the normal controls. These scores were also greater in the PSP patients (p < .0001) than those in the MSA patients.
PSP-related pattern (PSPRP) identified by spatial covariance analysis of 18F-FDG PET scans from PSP patients and healthy controls in the American cohort. (a) This pattern was characterized bilaterally by decreased (blue) metabolic activity in the middle prefrontal cortex (PFC)/cingulate, ventrolateral prefrontal cortex (VLPFC), striatum, thalamus and midbrain, along with increased (red) metabolic activity in the hippocampus/parahippocampus and parieto-temporal cortices. The overlays are depicted in neurological orientation. The thresholds of the color bars represent Z-values. (b) The expression of PSPRP discriminated the PSP patients from the healthy controls (i.e., NL) in both American and Chinese cohorts (p < .0001; post-hoc tests). The error bars represent standard deviations. (c) Individual scores in the same cohort correlated highly across both healthy controls and PSP patients used for the identification of PSPRP in the USA and for the validation of PSPRP in China
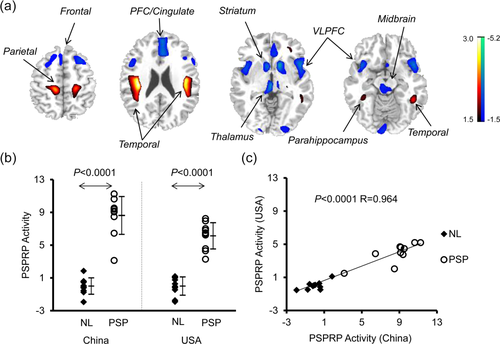
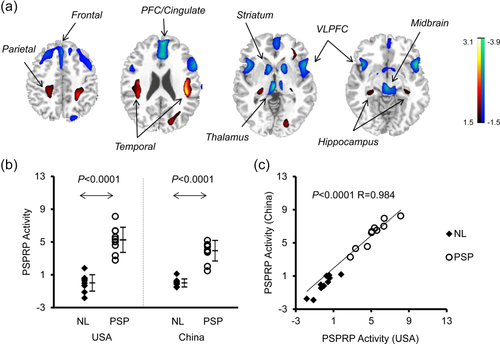
PSP-related pattern (PSPRP) identified by spatial covariance analysis of 18F-FDG PET scans from PSP patients and healthy controls in the Chinese cohort. (a) This pattern was characterized bilaterally by decreased (blue) metabolic activity in the middle prefrontal cortex (PFC)/cingulate, ventrolateral prefrontal cortex (VLPFC), striatum, thalamus and midbrain, associated with increased (red) metabolic activity in the hippocampus/parahippocampus and parieto-temporal cortices. The overlays are depicted in neurological orientation. The thresholds of the color bars represent Z-values. (b) The expression of PSPRP discriminated the PSP patients from the healthy controls (i.e., NL) in both American and Chinese cohorts (p < .0001; post-hoc tests). The error bars represent standard deviations. (c) Individual scores in the same cohort correlated highly across both healthy controls and PSP patients used for the identification of PSPRP in China and for the validation of PSPRP in the USA
3.2.2 Comparison of pattern expression
Subject scores of both PSPRPs for the four relevant groups are provided in Table 4. ANOVA revealed an effect of group for the expressions of PSPRP in the American cohort and those in the Chinese cohort (F[3,36] = 56.8, p < .0001; Figure 4b) or vice versa (F[3,36] = 75.6, p < .0001; Figure 5b). PSPRP scores increased in the PSP patients relative to the healthy controls in both cohorts (p < .0001, post-hoc tests), were similar between the two normal groups (p = 1.0) but differed between the two PSP groups (p = .06/.007). Subject scores in American patients and controls for the identification of PSPRP in the USA and the validation of PSPRP in China correlated highly (both groups: r = .984, p < .001; controls: r = .906, p < .001; PSP: r = .938, p < .001; Figure 4c). Likewise, Chinese subject scores for the identification of PSPRP in China and the validation of PSPRP in the USA also correlated (both groups: r = .964, p < .001; controls: r = .707, p < .02; PSP: r = .799, p < .005; Figure 5c).
| US cohort | Chinese cohort | ||||
|---|---|---|---|---|---|
| Healthy controls | Patients | Healthy controls | Patients | F values | |
| Network analysis (SSM/PCA) | |||||
| PSPRP activity (USA) | 0.00 ± 1.00 | 5.25 ± 1.53 | 0.00 ± 0.48 | 3.93 ± 1.25 | 56.8 |
| PSPRP activity (China) | 0.00 ± 1.12 | 6.13 ± 1.60 | 0.00 ± 1.00 | 8.62 ± 2.31 | 75.6 |
| Conjunction analysis (SPM) | |||||
| Frontal gyrus (50, 16, −6)a | 80.38 ± 5.77 | 58.15 ± 10.26 | 71.78 ± 11.19 | 50.51 ± 13.98 | 15.43 |
| Caudate (14, 12, 2) | 88.72 ± 3.12 | 74.62 ± 4.68 | 89.16 ± 4.68 | 72.15 ± 5.63 | 38.28 |
| Putamen (−16, 2, 12) | 69.51 ± 4.03 | 50.06 ± 8.50 | 70.26 ± 6.28 | 47.18 ± 10.28 | 26.08 |
| Thalamus (−6, −20, 10) | 74.51 ± 5.49 | 52.51 ± 6.79 | 75.88 ± 4.90 | 45.23 ± 12.64 | 36.93 |
| Hippocampus (28, −26, 4) | 65.89 ± 4.06 | 73.96 ± 4.09 | 64.27 ± 3.19 | 70.88 ± 4.34 | 12.85 |
| Paracentral gyrus (16, −32, 56) | 64.44 ± 4.63 | 77.32 ± 4.90 | 61.33 ± 4.07 | 75.66 ± 5.77 | 26.79 |
| Interaction analysis (SPM) | |||||
| Cuneus (12, −80, 18) | 93.63 ± 4.79 | 112.12 ± 14.54 | 107.83 ± 9.16 | 105.16 ± 9.98 | 5.99 |
- Subject scores and regional metabolic values are presented as mean ± SD. PSPRP activity values marked bold indicated subject scores in the original derivation cohort with the other values computed prospectively in the validation cohort. The regional metabolic values were computed post-hoc following the SPM analysis as shown in Figure 6b–g and Figure 7b. F values came from analysis of variance for each measure across the four independent subject groups.
- a The peak coordinates in the Montreal Neurological Institute (MNI) standard space.
- SPM = statistical parametric mapping; SSM/PCA = scaled subprofile modeling based on principal component analysis.
3.2.3 Comparison of relative regional metabolism
Conjunction analysis localized a large number of bilaterally overlapping brain regions of abnormal metabolism between the American and Chinese cohorts. In comparison with healthy controls, PSP patients showed decreased metabolism in the middle prefrontal/cingulate and ventrolateral prefrontal cortices, striatum, thalamus and midbrain, and increased metabolism in hippocampus/parahippocampus, cerebellum and parieto-temporo-occipital regions (Figure 6a; Table 5; p < .001, with some regions survived at FWE-corrected p < .05). This was further seen by relative metabolism measured post-hoc in several primary regions showing an effect of group (ANOVA: F[3,36] ≥ 12.85, p < .0001; Table 4) and decreases in the frontal lobe, striatum and thalamus, but increases in the parahippocampus and paracentral gyrus in the patients relative to the controls across both cohorts (post-hoc tests: p < 0.001; Figure 6b–g); no differences were evident between the control (p ≥ .23) or patient (p ≥ .20) groups.
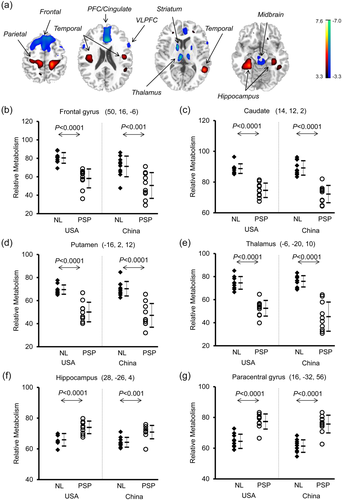
Brain regions with similar abnormal metabolism in PSP patients relative to healthy controls detected by conjunction analysis of 18F-FDG PET scans with SPM across the American and Chinese cohorts. (a) Metabolism in the PSP patients decreased (blue) in the middle prefrontal cortex (PFC)/cingulate, ventrolateral prefrontal cortex (VLPFC), striatum, thalamus and midbrain, but increased (red) in the hippocampus as well as parieto-temporal regions compared to normal (NL) subjects (p < .001). The thresholds of the color bars represent t values. (b–g) Group differences in relative metabolic values in select cortical and subcortical regions, obtained post-hoc in spherical volumes of interest (4 mm radius) centered at the peak coordinates of clusters deemed significant from the prior SPM analysis on a voxel-by-voxel basis. The error bars represent standard deviations [Color figure can be viewed at wileyonlinelibrary.com]
| Structure | Brodmann area | Hemisphere | MNI coordinatesa | Z max | Cluster size (mm3) | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Decreased metabolismb | |||||||
| Medial frontal gyrusc | BA 8 | L | −4 | 46 | 46 | 5.99 | 69,982 |
| Cingulate gyrusc | BA 32 | R | 10 | 20 | 30 | 5.35 | |
| Middle frontal gyrus | BA 8 | R | 54 | 16 | 42 | 4.19 | 12,392 |
| Inferior frontal gyrusc | BA 47 | R | 50 | 16 | −6 | 4.74 | |
| Putamenc | L | −16 | 2 | 12 | 6.00 | 18,360 | |
| Thalamusc | L | −6 | −20 | 10 | 5.81 | ||
| Thalamusc (extending to midbrain) | R | 2 | −20 | 4 | 5.45 | ||
| Increased metabolismb | |||||||
| Precentral gyrusc | BA 4, 6 | L | −36 | −14 | 28 | 4.79 | 19,000 |
| Precentral gyrusc | BA 4, 6 | R | 40 | −12 | 28 | 5.07 | 22,000 |
| Middle temporal gyrus | BA 22 | L | −54 | −38 | 4 | 3.83 | 1,192 |
| Middle temporal gyrusc | BA 21 | L | −36 | 2 | −34 | 6.03 | 22,584 |
| Hippocampusc | L | −30 | −18 | −26 | 5.05 | ||
| Middle temporal gyrusc | BA 21 | R | 38 | 2 | −34 | 4.65 | 13,616 |
| Hippocampus | R | 34 | −24 | −20 | 4.17 | ||
| Middle occipital gyrus | BA 18 | L | −40 | −88 | −2 | 4.37 | 1,840 |
| Cuneus | BA 17 | R | 22 | −82 | 10 | 3.54 | 1,016 |
| Cerebellar fastigium | L | −10 | −58 | −28 | 3.69 | 1,040 | |
| Cerebellar dentate | R | 16 | −56 | −30 | 3.56 | 912 | |
- a Montreal Neurological Institute (MNI) standard space.
- b Survived at uncorrected p < .001, extent threshold = 100 voxels (800 mm3).
- c Survived at FWE p < .05.
Interaction analysis revealed greater PSP-related metabolic increases in the occipital gyrus/cuneus in the American cohort compared to the Chinese cohort (Figure 7a; p < .001 uncorrected and not survived at FWE-corrected p < .05). The post-hoc analysis disclosed an effect of group (ANOVA: F[3,36] = 5.99, p = .002; Table 4) with increased cuneus metabolism in the American cohort but non-significant decline in the Chinese cohort comparing PSP patients to normal controls (Figure 7b). Comparing the Chinese cohort to the American cohort, cuneus metabolism was higher (p = .018) in the controls but decreased not significantly (p = .436) in the PSP patients. More hyperactive regions that differ in abnormal metabolism between both cohorts were detected in anterior cingulate and superior temporal gyrus at a lower threshold (Supporting Information Table S3; p < .01).
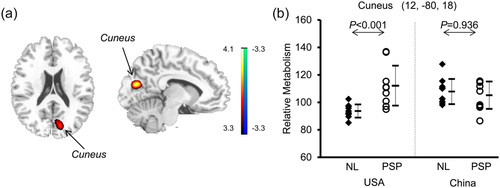
Brain regions with different abnormal metabolism in PSP patients relative to healthy controls detected by interaction analysis of 18F-FDG PET scans with SPM across the American and Chinese cohorts. (a) Metabolism in the PSP patients increased (red) in the middle occipital gyrus/cuneus versus normal subjects (NL) in the American cohort (p < .001) compared to the group difference in the Chinese cohort. The thresholds of the color bars represent t values. (b) Group differences in relative metabolic values in this region, obtained post-hoc in spherical volumes of interest (4 mm radius) centered at the peak coordinates of clusters deemed significant from the prior SPM analysis on a voxel-by-voxel basis. The error bars represent standard deviations [Color figure can be viewed at wileyonlinelibrary.com]
3.2.4 Group differences in relative regional metabolism in each cohort
For the American or Chinese cohort, compared to healthy subjects, PSP patients exhibited metabolic decreases or increases bilaterally in the same regions as revealed in the conjunction analysis described above (Supporting Information Tables S4 and S5 and Figures S2 and S3a). Increased metabolism was more pronounced in the occipital gyrus in the American cohort but in the cerebellar tonsil in the Chinese cohort. Relative metabolism decreased in the frontal lobe, putamen and thalamus but increased in the temporal lobe including parahippocampus in the PSP patients versus the healthy controls (post-hoc: p < .001; Supporting Information Figures S2 and S3b).
4 DISCUSSION
This study reports abnormal cerebral metabolic characteristics in PSP that are highly reproducible across patient populations and tomographs with both multivariate and univariate analytical approaches. Firstly, we used spatial covariance analysis of 18F-FDG PET images to identify specific metabolic networks associated with PSP in the American and Chinese cohorts in combination or singly. This resulted in a highly reliable PSPRP in the combined sample of PSP and healthy subjects and its expression discriminated between the healthy controls, PSP, MSA, and PD patients. We observed a high level of similarities between the two site-specific patterns in terms of the topography and network expression. Indeed, the PSPRP from China accounted for ∼86% of the variation in the region weights for the PSPRP from the USA. The separate analysis in each cohort replicated the differential diagnosis among all subject groups seen in the combined cohort. Both metabolic networks were also comparable by a cross validation with subject scores in prospective cohorts of PSP patients and healthy subjects. This was in accord with a previous study that reported reproducible network expression in multiple American patient groups scanned on the same PET camera (Eckert et al., 2008). Secondly, we localized analogous differences in regional metabolic activity in the same groups of PSP patients and healthy subjects on a whole brain basis with SPM analysis. We found that PSP patients from the two cohorts shared a large number of overlapping areas with abnormal metabolism in both cortical and subcortical regions. Post-hoc VOI analyses of relative metabolic values further confirmed the significant differences in these brain regions between the patient and control groups.
We need to emphasize that this work represented the first cross-validation study to investigate reproducible metabolic abnormalities between truly independent cohorts of patients with PSP using two different analytical approaches. The spatial covariance analysis was aimed to derive a brain network with variance information in glucose metabolism in 18F-FDG PET images from PSP patients and controls. By contrast, the SPM analysis was designed to capture regionally-specific differences in mean glucose metabolism between patients and controls. They actually portrayed different but complementary measures of brain dysfunction. Therefore, the application of network and regional analyses used in this study is helpful in describing the pathophysiology of PSP at both the systems and local levels and may provide more valuable imaging biomarkers for the disease.
The current work demonstrated topographical similarities between the resulting patterns at the systems and local levels in both cohorts (together or individually), in agreement with previous reports in another independent cohort in the Netherlands (Teune et al., 2010; Teune et al., 2013). Comparable distributions of abnormal regional metabolism between both cohorts in this study were responsible for the similarly elevated network scores of the two PSPRPs, given that subject score is a similarity measure between the topography of a covariance pattern and the metabolic distribution of a particular image. The high degree of comparability in the PSPRPs between the Chinese and American medical centers reported here is analogous to that for the Parkinson's disease-related patterns (i.e., PDRPs) from the same two centers (Wu et al., 2013). Our work is part of an international collaborative effort to cross-validate the reproducibility of all PD-related network biomarkers. Indeed, the expression of metabolic brain networks in multiple forms of parkinsonism has proven to be viable biomarkers for early differential diagnosis based on a series of rigorous multi-center validation studies performed prospectively in individual parkinsonian patients (Ko et al., 2017; Niethammer et al., 2014; Tang et al., 2010; Tripathi et al., 2016).
Using both analytical approaches noted above, we observed metabolic decreases in the middle prefrontal/cingulate and ventrolateral prefrontal cortices, striatum, thalamus and midbrain in both cohorts of PSP patients. These findings were compatible with previous imaging studies that described reduced cerebral blood flow or metabolism in patients with PSP (Eckert et al., 2005; Eckert et al., 2008; Kimura et al., 2011; Klein, de Jong, de Vries, & Leenders, 2005; Kobayashi et al., 2013; Stamelou et al., 2009; Teune et al., 2010; Teune et al., 2013). Furthermore, the loss of neurons in relevant regions verified by autopsy studies in PSP patients also provided support to our results (Pahapill & Lozano, 2000; Zweig et al., 1987). The decreased metabolism in these cerebral regions is thought to be correlates of dysfunction in a supraspinal locomotor network (SLN). This network, including both indirect and direct pathways, is considered to be responsible for gait control in human beings (Bohnen & Jahn, 2013; Zwergal et al., 2013). Indeed, the indirect pathway of SLN runs from the frontal cortex via the basal ganglia to the brainstem locomotor centers, allowing for the modulation of gait pattern in response to external demands. By contrast, the direct pathway of SLN goes from the motor cortex to the spinal cord, bypassing the brainstem centers and playing a more important role during undisturbed locomotion. The normal function of the indirect pathway in SLN is largely disturbed in PSP by decreased regional metabolism. Although the direct pathway subsequently overprojects as an early compensatory mechanism, it cannot adequately adapt to environmental demands, causing gait impairment (e.g., akinesia and freezing of gait) in the PSP patients. This assumption is further supported by increased metabolic activity in the cortices surrounding paracentral lobule (including mainly primary motor cortex and supplementary motor area) as observed in the present study, likely reflecting compensation for the direct pathway in SLN.
All PSP patients in this study exhibited upward or downward vertical gaze palsy, a feature with high specificity for differentiating PSP from other parkinsonian syndromes, as was also evident in the discrimination outcome by PSPRP network activity. In accordance with this clinical symptom and network association, we observed a decrease in metabolism in the medial frontal cortex, the area responsible for the control of eye movements in healthy volunteers (Hanakawa, Dimyan, & Hallett, 2008; Paus, Petrides, Evans, & Meyer, 1993). This is compatible with prior imaging studies reporting significant correlation of vertical gaze palsy with decreased metabolism in the same area (Amtage et al., 2014). Given that the frontal cortex is involved with normal function of executive tasks, the early onset of cognitive impairment in PSP may also be related to hypometabolism in this region (Kim, Lee, & Lee, 2014; Lee, Williams, & Anderson, 2016). Pseudobulbar palsy such as dysphagia and dysarthria is another supportive criterion for clinical diagnosis of PSP. This condition is usually caused by damage to the neurons of the brainstem, specifically to the corticobulbar tract. In this vein, we may attribute the decreased metabolism of midbrain observed in this study as an indication of pseudobulbar palsy in PSP. Nevertheless, more studies are needed to provide clearer evidence on this correlation.
This study also revealed increased metabolism in the territories of hippocampus or parahippocampus and temporal cortex in both cohorts of patients with PSP. While abnormal metabolism in hippocampus/parahippocampus was reported in PSP patients (Teune et al., 2010), altered structures in these regions were also noted previously in several anatomical MRI studies (Agosta et al., 2010; Messina et al., 2011; Padovani et al., 2006; Saini et al., 2013). The reasons for such abnormalities still remain unclear, but in all likelihood result from activations by specific circuits in the basal ganglia (Albin, Young, & Penney, 1989) and may be related with early cognitive impairment in PSP patients. Hypermetabolism in temporal lobe has also been described in prior functional imaging studies (Teune et al., 2010), and it is plausible that increased metabolism in this region may reflect elevated sensory control in patients with PSP. We also replicated the previous finding of increased regional metabolism in the insula, middle occipital gyrus and cerebellum (Teune et al., 2010). While there is a possible link between cerebellar abnormality and impaired balance capacity in PSP patients, metabolic changes in insula and occipital gyrus may be explained by deficient hand-eye motor movement and mild cognitive dysfunction in patients (Anderson et al., 1994).
The imaging results reported in this study are generally compatible with demographic and clinical features of subject groups that are closely matched between the American and Chinese cohorts (Table 1). The distribution of these features among different groups is highly representative of sample populations encountered in clinical practice, particularly in the combined cohort. Hence, we used the combined American and Chinese sample to define and characterize the topography of PSPRP and assess the diagnostic performance and clinical correlation with network scores (cf., Figure 1). Comparing the American and Chinese cohorts, PSPRP scores did not differ in the PSP or MSA groups consistent with their similar clinical characteristics; PSPRP scores were higher in the PD group in the American cohort in line with longer disease duration. We further found that without any clinical correlations in the MSA and PD patients, PSPRP scores correlated with disease duration but not UPDRS motor ratings in the PSP patients, even with the use of both early and advanced parkinsonian patients included in this study. The latter could be due to the small sample size in our patients or the use of a clinical rating scale that is not specific to complexity underlying the symptomatology of PSP. Despite age/gender differences in some healthy and patient groups between the different cohorts (Tables 1 and 2), PSPRP scores did not show any differences or correlations with respect to these variables suggesting that age/gender do not play a big role in PSP-related network analysis and have no effect on performance in differential diagnosis.
The patients recruited for the primary analyses in this study were all clinically-confirmed with at least one-year follow-up at the time of 18F-FDG PET imaging. Most patients referred to scanning in clinical routine are more likely to be suspected of having parkinsonism at earlier stages. To this end we found that PSPRP activity can still discriminate PSP from other groups in the second cohort of patients with early, clinically unclassifiable parkinsonism (Figure 2). PSPRP scores were lower in each of these patient groups than those in the combined American and Chinese cohort (see Supporting Information Figure S4). It is expected that diagnostic performance of quantitative metabolic network activity reported in the current study can be further improved in realistic clinical practice with the use of the novel classification algorithm including metabolic brain networks in other forms of parkinsonism (Tang et al., 2010; Tripathi et al., 2016).
Over the last few decades, high resolution MRI scanners combined with advanced quantitative techniques have contributed significantly to measurements of structural and functional changes for the discrimination of PSP from other forms of parkinsonism (Boxer et al., 2017; Whitwell et al., 2017). Currently, magnetic resonance parkinsonism index (MRPI) appears to be one of the most mature MRI biomarkers for assessing subcortical atrophy of PSP, which was defined by multiplying the area ratio of pons to midbrain by the width ratio of middle to superior cerebellar peduncles. It has been recently reported that MRPI could yield very high sensitivity of 100% and specificity of 99.2%–100% for PSP (Nigro et al., 2017) and was also able to predict development of the disease (Whitwell et al., 2017). In response to the great progress in MRI we have initiated a research project to develop and cross-validate PET- and MRI-based brain network biomarkers in parkinsonism using modern multimodality neuroimaging techniques. By computing PSPRP activity in a large cohort with both 18F-FDG PET and perfusion MRI scans we found that network scores from metabolic images are superior compared to blood flow images in accurately differentiating between healthy subjects and moderate-stage patients with PSP, MSA and PD from the preliminary results (Supporting Information Table S6 and Figure S5). A direct comparison of diagnostic performance between PSPRP activity and MRPI in these patient populations with 18F-FDG PET and perfusion/structural MRI) would be warranted given the involvement of brainstem as a common key node in both biomarkers that may help elucidate the unique relationships between metabolic and pathologic substrates underlying the pathogenesis of PSP.
Despite the high degrees of similarity across the two cohorts there remain some inherent discrepancies as a result of inevitable differences in subject characteristics, scanner designs and imaging protocols. We further note that the Chinese PSP patients had more severe symptoms and longer duration than their American counterparts though not significantly different. Albeit not prominent, some differences in PSP-related metabolic characteristics across the patient populations and scanners were reported in this study regardless which analytic approach was used. Indeed, network activity of PSPRP in China was greater but more variable in the Chinese patients than in the American patients, and had lower degrees of correlations per subject group than PSPRP in the USA. Furthermore, metabolic increases were observed in the cerebellum mainly in the Chinese patients with PSP but in the occipital regions predominately in the American patients. We also found that cuneus metabolism in PSP patients was decreased in the Chinese cohort but elevated in the American cohort. The noisier and variable data in the Chinese cohort may be attributed to the PET/CT scanner known to have higher variability than the dedicated PET camera, owe to the use of different attenuation correction methods (CT vs. PET transmission scanning) and reconstruction algorithms (iterative vs. analytical) in the creation of PET emission images. Nonetheless, as these disease-related metabolic characteristics are highly reproducible in major cerebral regions of PSP patients on a whole brain basis and PSPRP activity can accurately differentiate PSP from other forms of parkinsonism, it strongly suggested that such a pattern is well-qualified to offer a specific and reliable imaging marker for clinical diagnosis of PSP.
Several limitations should be noted in this study: (1) in the absence of neuropathological confirmation of PSP in our patient cohorts we relied on the clinical diagnostic accuracy of movement disorders experts at each site. This was a valuable strategy reflecting how PSP is diagnosed in the real-world; (2) the sample sizes of PSP patients and healthy controls were relatively small at both sites so as to conform to the PSP-related metabolic pattern already established in the USA. These limited samples might be justifiable given the more severe metabolic abnormalities inherent in this rapidly degenerative disease. This issue was mostly mitigated by combining all PET images from the two sites in a series of primary analysis reported in this article. The fact that analogous metabolic patterns were identified across two independent cohorts suggest that the clinical diagnosis were accurate and the sample sizes were adequate in this study; (3) the relationship of brain glucose metabolism to clinical measures of disease severity and cognitive dysfunction has not been fully demonstrated due to the limited samples, and despite the observed correlation of network activity with PSP duration; (4) the present study did not exclusively classify patients with subtypes of PSP. Our PSP patients had predominately Richardson's syndrome based on primary clinical features present at follow-up. It will be still necessary to replicate the results reported here in different PSP variants and to identify corresponding clinical correlates with larger sample sizes.
5 CONCLUSION
In this study, we revealed similar topographies in the spatial covariance brain network and distribution of abnormal regional glucose metabolism in PSP by combining 18F-FDG PET data of healthy subjects and patients from American and Chinese medical centers. The results of cross-validation between the two centers confirm the presence of highly reproducible PSP-related metabolic brain networks and regional activity patterns across different patient populations and imaging instrumentation. We also showed that PSP-related brain network is reliable on a voxel basis and its expression in individual subjects can discriminate PSP patients from healthy controls as well as from other parkinsonian patients in independent cohorts at both early and advanced clinical stages of parkinsonism. Network measure from 18F-FDG PET imaging may serve as a reliable and objective marker of PSP in clinical practice and potential research trials. Metabolic brain network generated in a large cohort of healthy controls and pathologically-confirmed patients from a single site or multiple centers can further validate PSPRP going forward.
ACKNOWLEDGMENTS
The core of the analytical work described in this article has been performed by J. G. during her research training at The Feinstein Institute for Medical Research as part of the US–China Biomedical Collaborative Research Program. J. G. is grateful for the great learning experiences provided by the collaborating investigators of this program, in particular S. P. who has shared her extensive expertise in processing and analyzing multimodal neuroimaging data using univariate and multivariate statistical methods. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
FINANCIAL DISCLOSURE/CONFLICT OF INTEREST
D. E. serves on the scientific advisory board for and has received honoraria from the Michael J. Fox Foundation for Parkinson's Research; serves on the editorial board of Annals of Neurology and Journal of Nuclear Medicine and as Associate Editor for The Journal of Neuroscience; and is listed as coinventor of patents re: Markers for use in screening patients for nervous system dysfunction and a method and apparatus for using same, without financial gain; and has received research support from the NIH (NINDS, NIDCD, NIAID), the Dana Foundation, the Bachmann-Strauss Dystonia and Parkinson Foundation, and CHDI Foundation. Y. M. has received research funding from the NIH. All other authors have no disclosures/conflicts to report.




