Impaired rich club and increased local connectivity in children with traumatic brain injury: Local support for the rich?
Funding information: Special Research Fund (BOF) from the University of Ghent, Grant/Award Number: 01N00214
Abstract
Recent evidence has shown the presence of a “rich club” in the brain, which constitutes a core network of highly interconnected and spatially distributed brain regions, important for high-order cognitive processes. This study aimed to map the rich club organization in 17 young patients with moderate to severe TBI (15.71 ± 1.75 years) in the chronic stage of recovery and 17 age- and gender-matched controls. Probabilistic tractography was performed on diffusion weighted imaging data to construct the edges of the structural connectomes using number of streamlines as edge weight. In addition, the whole-brain network was divided into a rich club network, a local network and a feeder network connecting the latter two. Functional outcome was measured with a parent questionnaire for executive functioning. Our results revealed a significantly decreased rich club organization (p values < .05) and impaired executive functioning (p < .001) in young patients with TBI compared with controls. Specifically, we observed reduced density values in all three subnetworks (p values < .005) and a reduced mean strength in the rich club network (p = .013) together with an increased mean strength in the local network (p = .002) in patients with TBI. This study provides new insights into the nature of TBI-induced brain network alterations and supports the hypothesis that the local subnetwork tries to compensate for the biologically costly subnetwork of rich club nodes after TBI.
1 INTRODUCTION
It is widely accepted that there are a few central and highly connected regions in the brain, called hubs, which play a crucial role in the global network topology of the brain (Hagmann et al., 2008; Sporns, Honey, & Kotter, 2007; van den Heuvel, Stam, Boersma, & Hulshoff Pol, 2008). The anatomical locations of hubs might vary slightly according to the employed methods, such as the parcellation scheme, MRI modality, and the definition of a hub. However, hubs are often found in the following anatomical areas: superior parietal gyrus, superior frontal gyrus, precuneus, putamen, hippocampus, thalamus, cingulate cortex, insula, or occipital modules (Hagmann et al., 2008; van den Heuvel & Sporns, 2011). In addition, it has been shown that these network hubs are densely interconnected, reflecting a core network or so-called “rich club organization” (van den Heuvel & Sporns, 2011). These rich club hubs enable efficient information processing between brain regions across the network and are thus essential in adaptive behavior (van den Heuvel, Kahn, Goni, & Sporns, 2012). A study of Baggio et al. (2015) in healthy older participants, for example, found an association between rich club connectivity and general cognitive functioning, underscoring the importance of this highly connected rich club.
A drawback of this rich clubness is the biological cost because hubs are metabolically demanding, and are spatially distant from each other requiring extensive long-distance white matter fibers to interconnect them (Collin, Sporns, Mandl, & van den Heuvel, 2014b). Crossley et al. (2014) showed that because of this high value yet high cost, hubs are more likely to be affected compared to other brain regions in patients with brain disorders. Their evidence is strengthened by studies in different clinical populations, for example, in multiple sclerosis (Stellmann et al., 2017), schizophrenia (van den Heuvel et al., 2013), and attention-deficit hyperactivity disorder (Ray et al., 2014), all showing reduced rich club connectivity.
Long-distance white matter connections are often affected in patients with traumatic brain injury (TBI) and these impairments are commonly the result of diffuse axonal injuries (DAI) (Hayes, Bigler, & Verfaellie, 2016; Sharp, Scott, & Leech, 2014). The disruption of long-range white matter fibers leads to a wide array of deficits of higher order functions (such as executive functioning) that reside on the integration of widespread brain areas (Castellanos et al., 2011; Sharp et al., 2014). Given the high wiring cost and high metabolic activity of the rich club, it is suggested that the rich club network is also vulnerable in TBI (Crossley et al., 2014).
A handful of studies have employed a structural connectomic framework exploring hubs in patients with TBI. Results from these studies have revealed that patients with TBI (adults and children) exhibit similar hubs as identified in healthy controls (Caeyenberghs et al., 2012, 2014; Caeyenberghs, Leemans, Leunissen, Michiels, & Swinnen, 2013; Yuan, Wade, & Babcock, 2015). In addition, previous research has demonstrated decreased connectivity in hubs in children with TBI (Yuan et al., 2015). Fagerholm, Hellyer, Scott, Leech, and Sharp (2015) revealed a reduced connectivity of network hubs in adult patients with moderate to severe TBI compared to healthy controls, especially in patients with pronounced DAI. Finally, a recent study by Solmaz et al. (2017) in 40 adults with moderate to severe TBI in the subacute phase of recovery found that the most affected connections are those that link highly central brain regions and hubs, suggesting a more pronounced effect of TBI on the core network. These studies all investigated the existence of hubs; however they did not assess the rich club organization or interconnectedness of these network hubs.
To date, only two studies have explored the rich club organization using graph theoretical analysis in adults with TBI (Antonakakis, Dimitriadis, Zervakis, Papanicolaou, & Zouridakis, 2017; Hillary et al., 2014), albeit studying functional connectomes (using functional MRI). The results of these studies show increased functional connectivity in the rich club of patients with TBI. However, whether rich club organization is affected in structural connectomes of young TBI patients remains unclear.
In this study, the rich club organization of the structural connectome in a young group of chronic moderate to severe TBI patients is investigated. We hypothesized that the rich club organization in these patients would be affected. In addition, we expected that this reduced rich club connectivity would be related to poorer executive functioning, because these functions rely heavily on the integration of distributed brain areas.
2 METHODS
2.1 Participants
Patients were recruited from the Child Rehabilitation Center of the University Hospital at Ghent, Belgium and the Rehabilitation Center for children and adolescents at Pulderbos, Belgium between March 2015 and January 2017. Eligibility criteria for the study were (a) 10–17 years of age at time of injury and <18 years at the start of the study, (b) time since injury 1–5 years (i.e., chronic stage of TBI; Hulkower, Poliak, Rosenbaum, Zimmerman, & Lipton, 2013), (c) classified as moderate to severe TBI based on the Mayo Classification System for TBI severity (Malec et al., 2007), and (d) a diagnosis of diffuse axonal injury based on anatomical scans at time of the study. For every patient, an age and gender matched healthy control participant was recruited via social contacts. All participants met magnetic resonance imaging (MRI) safety criteria (i.e., no metal implants or orthodontic braces) and patients with a recurrent TBI or significant neurological illness were excluded. The study was approved by the Ethics Committee of the Ghent University Hospital (#2014/0540) and informed written consent was obtained from both parents and children. All study procedures were conducted according to the declaration of Helsinki.
2.2 MRI acquisition
Imaging was performed using a Siemens 3.0 T Magnetom Trio whole-body scanner equipped with a 32-channel head coil. A diffusion weighted single-shot echo planar image with a twice-refocused spin echo sequence was acquired (60 contiguous transversal slices, FoV = 240 mm, voxel size = 2.5 mm isotropic, TR = 10,800 ms, TE = 83 ms, TA = 12:36 min). Diffusion gradients were applied along 64 noncollinear directions (b-value = 1,200 s/mm2) and one volume without diffusion weighting was obtained. In addition, a high-resolution 3D T1-weighted image was acquired using magnetization-prepared rapid gradient-echo acquisition (176 contiguous sagittal slices, FoV = 256, voxel size = 1.0 mm isotropic, TR = 1,590 ms, TE= 4.18 ms, flip angle = 9°, TA = 5:14 min). Finally, a susceptibility weighted imaging (SWI) scan (88 contiguous slices, FoV = 230, voxel size = 1.0 × 0.9 × 1.5 mm, TR = 28 ms, TE = 20 ms, flip angle = 15°, TA = 4:56 min) and a 3D fluid-attenuated inversion recovery (FLAIR) were administered (182 contiguous slices, FoV = 250, voxel size = 1 × 1 × 0.9 mm, TR = 6,000 ms, TE = 420 ms, TI = 2,100 ms, TA = 7:14 min). Two board-certified neuroradiologists identified the presence and location of DAI and cortical encephalomalacia based on these two scans. Round- or ovoid-shaped hypointense lesions with a maximum diameter of 10 mm and clear margins on the SWI images were classified as DAI lesions (Moenninghoff et al., 2015). When in case of doubt, the neuroradiologists used the FLAIR sequence to rule out potential DAI mimics (such as flow voids and calcium or iron deposits). The presence and location of DAI lesions was checked in every participant for the following brain areas: frontal, temporal, parietal and occipital lobes, cerebellum, corpus callosum, brainstem, substantia nigra, mesencephalic tegmentum, thalamus, basal ganglia, and internal capsule.
2.2.1 Preprocessing of T1-weighted images
The T1-weighted image of each subject was parcellated into 82 cortical and subcortical regions using FreeSurfer version 5.3.0 (Fischl et al., 2004) according to the Desikan-Killiany atlas (Desikan et al., 2006). The MRI preprocessing pipeline is illustrated in Figure 1. To ensure accuracy of the cortical surface reconstruction by FreeSurfer, the grey matter–white matter boundary and pial surface of each subject was visually inspected.
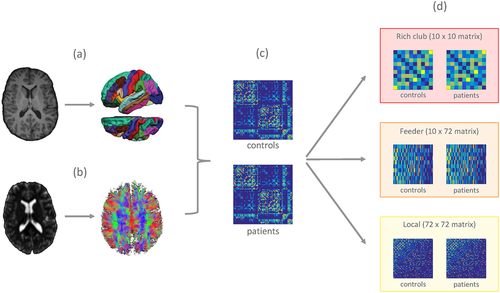
Reconstruction of the structural connectomes. For every subject, the following steps were performed: (a) T1-weighted image was segmented using FreeSurfer into 82 cortical and subcortical regions; (b) preprocessing of the DWI data and whole brain probabilistic tractography was performed using FSL and MRtrix; (c) T1 parcellation and diffusion tractogram were combined to reconstruct the structural connectome resulting in a weighted adjacency matrix; and (d) the structural connectome was divided into a rich club network (interconnecting the hubs), a feeder network (connecting the hubs to the nonhubs), and a local network (interconnecting the nonhubs) [Color figure can be viewed at wileyonlinelibrary.com]
2.2.2 Preprocessing of diffusion-weighted images
MRtrix3 (www.mrtrix.org/; version 0.3.15) was used for the analyses of the DWIs unless specifically stated otherwise. A visual inspection was performed after every step in the processing pipeline to assure the quality of the output. As a first step, raw data were checked for any obvious artefacts by looping through all volumes in different orthogonal views (i.e. axial, sagittal, and coronal).
Preprocessing of the DWIs was performed using the following multistep-procedure: (a) denoising the data to enhance signal-to-noise ratio using random matrix theory (Veraart, Fieremans, & Novikov, 2016), (b) motion and eddy currents correction using FSL EDDY (Andersson & Sotiropoulos, 2016), (c) B1-field inhomogeneity correction (Zhang, Brady, & Smith, 2001), and (d) global DWI intensity normalization across subjects using the median white matter b = 0 value as a reference (Raffelt et al., 2012).
Subsequently, the anatomical T1-weighted images were linearly registered to diffusion space using FSL FLIRT's epi_reg (Jenkinson & Smith, 2001), and segmented to generate partial volume maps with FSL FAST (Zhang et al., 2001) to be used during tractography (as described in the next section). An average response function was computed across the subjects of both groups with an iterative algorithm for single-fiber voxel selection and response function estimation (Tournier, Calamante, & Connelly, 2013). Constrained spherical deconvolution was used to estimate the fiber orientation distribution (Tournier, Calamante, & Connelly, 2007, 2012) with default settings and a maximum spherical harmonic order of 8.
2.2.3 White-matter tractogram reconstruction
The second-order integration over fiber orientation distributions (iFOD2) algorithm (Tournier, Calamante, & Connelly, 2010) was used to reconstruct the tractograms (step size = 1.25 mm, maximum curvature = 45° per step, length = 5–125 mm). For each participant, 107 streamlines were generated by seeding from the grey matter–white matter boundary within the anatomically constrained probabilistic streamline tractography (ACT) framework with the “back-tracking mechanism” (Smith, Tournier, Calamante, & Connelly, 2012). Finally, spherical-deconvolution informed filtering of tractograms (SIFT) was implemented to decrease reconstruction biases by filtering the number of streamlines from 107 to 106, which has shown to improve biological plausibility (Smith, Tournier, Calamante, & Connelly, 2013).
2.2.4 Connectome reconstruction
For each subject, the individual parcellation of the T1-weighted image and whole brain tractography output was combined to reconstruct the structural connectome. The nodes were represented by 82 distinct regions, and for each possible node pair, interregional connectivity was defined as the number of reconstructed streamlines (NOS), representing the edges of the connectome. This resulted in a weighted adjacency matrix for each subject.
To minimize the presence of spurious fibers, an edge was set to zero if the NOS was fewer than three as a first thresholding step (Collin, Kahn, de Reus, Cahn, & van den Heuvel, 2014a; Galantucci et al., 2017). In addition, a group consensus threshold of 60% was employed to provide a good balance between eliminating false positives and false negatives (de Reus & van den Heuvel, 2013). Since edges that are “real” might not be present in our TBI patients as a result of their underlying pathology (i.e., diffuse axonal injury) we might reduce the sensitivity in our analyses using a threshold based on the entire sample. Therefore, we only retained those edges in all participants that were present in at least 60% of the participants in the control group for subsequent analyses (McColgan et al., 2015). To check the robustness of the results, analyses were repeated across a range of thresholds from 30% to 90% with a step size of 10.
2.3 Network analyses
2.3.1 Global network topology measures
Four global graph metrics were calculated to investigate group differences in overall brain topology using the Brain Connectivity Toolbox (BCT) (Rubinov & Sporns, 2010), including (a) network density (defined as the number of existing edges divided by the total number of all possible edges), (b) total network strength S (calculated as the sum of all reconstructed streamlines in the network), (c) weighted clustering coefficient C (which is a measure of the connectedness of a node to its neighboring nodes, tapping into network segregation and specialization), and (d) weighted average shortest path length L (computed as the average shortest path to go from one node to any other node in the network, reflecting network integration). The latter two network measures were normalized by comparing these to the mean C and mean L of 1,000 random networks (Maslov & Sneppen, 2002), providing the normalized clustering coefficient γ and normalized path length λ respectively. In addition, nodal degree (number of edges connected to a node) was calculated for subsequent analyses of rich club organization (see below).
2.3.2 Rich club organization
A brain network is thought to have a rich club organization if nodes with a high degree are more densely and stronger interconnected than would be expected by chance (van den Heuvel & Sporns, 2011). The presence of a rich club organization was examined by calculating the weighted rich club coefficient using BCT (Rubinov & Sporns, 2010). More specifically, the weighted rich club coefficient φw(k) was determined for every degree level k present in the network (Opsahl, Colizza, Panzarasa, & Ramasco, 2008). For this, first a subgraph was created with only the nodes having a degree larger than k. Then, the number of edges n connecting the nodes in the subgraph was counted and their weights were summed resulting in the strength of the subgraph. Next, the edges of the total network were ranked according to their weight and the weights of the n strongest edges were summed. The weighted rich club coefficient was calculated by dividing the strength of the subgraph by the strength of the n strongest edges of the total network. Similar to the γ and λ, the φw(k) was normalized by comparing it to the mean weighted rich club coefficient of 1,000 random networks (Maslov & Sneppen, 2002). A rich club organization is present when the normalized rich club coefficient is larger than 1 over a range of degree levels (Opsahl et al., 2008).
2.3.3 Rich club nodes
To investigate strength and density in subnetworks of the total network, rich club nodes were determined based on the entire sample. Nodes were ranked according to their degree for every subject. The 10 nodes (12%) that appeared most often in the top 10 nodes of all subjects were selected as the rich club. A similar procedure has been used in previous studies regarding rich club organization (Collin et al., 2014b) and this workflow has shown to result in consistent results (Collin et al., 2014b; van den Heuvel & Sporns, 2011). In addition to the group-based rich club selection, rich club nodes were also determined on single-subject level to account for possible individual differences. Specifically, for each subject, the top 10 highest degree nodes were selected as their rich club nodes.
2.3.4 Edge categories: Rich club, feeder, and local network
We further identified categories of edges, as follows: (a) edges can link two rich club nodes (rich club edges); (b) edges can link a non-rich club node with a rich club node (feeder edges); or (c) edges can link two non-rich club nodes (local edges) (see Figure 2 for a graphical representation of the different edge categories). Both total strength and density were calculated for every subject and for every subnetwork (local, feeder, and rich club). Because total strength is strongly influenced by density, the mean connectivity strength within the three subnetworks was also computed (as the sum of weights of the present edges divided by the number of present edges).
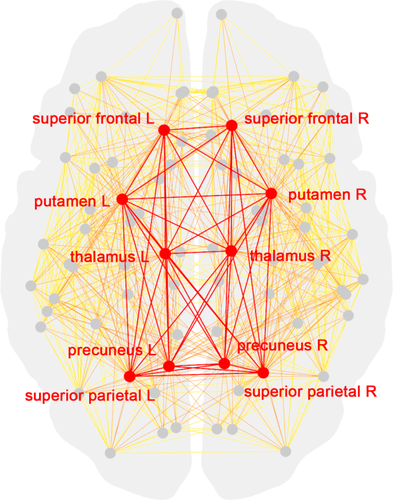
Schematic representation of a group-averaged structural connectome. Red nodes denote rich club nodes and grey nodes reflect nonrich club nodes. Edges are color coded (red = rich club edges; orange = feeder edges; and yellow = local edges) [Color figure can be viewed at wileyonlinelibrary.com]
2.4 Measurement of executive functioning
Executive functioning in daily life was assessed with the Dutch version of the Behavior Rating Inventory of Executive Function (BRIEF) (Huizinga & Smidts, 2009). For this study, we used the parent report form, consisting of 86 items. The BRIEF comprises two summary index score, metacognition index (MCI) and behavioral regulation index (BRI), and an overall general index score (Huizinga & Smidts, 2009). The questionnaire has a good psychometric integrity with high internal consistency, adequate reliability features and good face validity. The two-factor structure proves to be consistent and the two indices have convergent and discriminant validity (Gioia & Isquith, 2004). For this study, we decided to focus on the MCI because the subscales of this index (Initiate, Working Memory, Plan–Organize, Organization of Materials, and Monitor) are all aspects of executive functioning which depends on whole-brain coordination.
2.5 Statistical analyses
First, we compared global network topology measures of the TBI children with the control group using permutation testing. We opted for a nonparametric permutation approach (with 5,000 permutations) due to the non-normal distribution of some dependent variables (i.e., Shapiro–Wilks normality test (p < .05)). To determine if a rich club organization was present in the groups, a one sample t test was performed to examine whether the normalized rich club coefficients statistically exceeded 1 over a range of degree levels in each group separately. Next, group differences in rich club organization between TBI patients and controls was assessed using the permutation approach whereby the normalized φw(k) was compared between groups across the degree levels wherein the normalized φw(k) was significantly >1 in either group. To investigate group differences in density, strength, and mean strength in the rich club, feeder, and local networks, again a permutation testing approach was employed. Executive functioning of the TBI children was compared with the control group using the permutation method. Finally, the relationship between rich club connectivity (rich club density, rich club total strength and rich club mean strength) and executive functioning (MCI scores) in patients with TBI was examined via a partial Spearman rank–order correlation analyses, taking age into account. Tests were two-tailed with a significance level of p < .05 and a false discovery rate (FDR) to 5% was applied in all subanalyses to protect against type I errors when performing multiple comparisons (Benjamini, 2010).
3 RESULTS
3.1 Participant characteristics
Data were collected from 17 children (10 boys, 7 girls; mean age 15.71 ± 1.75 years) with moderate to severe TBI as a result of traffic accidents or sports injuries in the chronic stage of recovery (mean time since injury 2.36 ± 1.08 years). All TBI children had distributed DAI in the cerebrum, most frequently located in the grey–white matter junctions of the frontal (n = 14) and temporal lobes (n = 15), but also in parietal (n = 10), and occipital lobes (n = 9) and in the cerebellum (n = 5). In addition, encephalomalacia was found in the majority of the patients (n = 11) and was most present in the temporal (n = 10) and frontal (n = 8) lobes. Demographic characteristics and lesion locations of the patients can be found in Table 1. Seventeen matched healthy control subjects (10 boys, 7 girls; mean age 15.62 ± 1.79 years) participated in this study. There was no group difference in gender (χ2(1) = 0, p = 1) or age (t(32) = −0.136, p = .89).
| Age/gender/TSI | Cause accident | GCS/coma information from medical records | Lesion location/pathology (MRI scan at time of study) |
|---|---|---|---|
| 16.38/M/3.83 | Traffic accident | “stuporous” | DAI in right FL, TL, PL, OL, C and in left FL, TL, PL. EM in bilateral TL. |
| 16.70/M/3.15 | Traffic accident | GCS = 3 | DAI in right TL and in left FL, TL, OL, C and in CC and in left basal ganglia |
| 16.67/M/4.55 | Traffic accident | “subcomatose” | DAI in CC |
| 17.54/M/2.24 | Ski accident | Coma >24 h | DAI in right FL, TL, PL, OL and in left FL, TL PL and in CC. EM in bilateral FL |
| 15.57/F/3.15 | Traffic accident | Coma of 7 weeks | DAI in right FL. EM in bilateral FL, TL and right PL |
| 14.47/F/1.29 | Traffic accident | GCS = 5 | DAI in right FL, TL, PL, OL, C and in left FL. EM in right bilateral FL and left TL |
| 15.54/M/1.67 | Traffic accident | Coma >24 h | DAI in right FL, TL, PL, OL and in left FL, TL, PL, OL and in CC. EM in bilateral FL, right TL |
| 17.26/M/1.35 | Ski accident | GCS = 7 | DAI in right FL, TL, PL, OL, C and in left FL, TL, PL |
| 15.99/M/2.79 | Traffic accident | GCS = 7 | DAI in right FL, TL, PL and in left FL, TL, PL and in CC, right basal ganglia and right internal capsule |
| 11.14/F/1.18 | Traffic accident | N/A | DAI in right PL and in left TL. EM in left TL. |
| 15.48/M/1.89 | Plane accident | GCS = 3 | DAI in right FL, TL, PL, OL and in left FL, TL, PL, OL and in left basal ganglia. EM in right FL, TL and in left FL. |
| 17.23/F/2.80 | Traffic accident | N/A | DAI in right FL, TL and in left FL, TL. EM in bilateral FL, TL. |
| 12.94/F/1.58 | Traffic accident | GCS = 3 | DAI in right FL, TL and in left FL, TL and in CC, bilateral substantia nigra, right mesencephalic tegmentum and left thalamus |
| 16.03/F/1.25 | Traffic accident | Coma > 24 h | DAI in right FL, TL, PL and in left FL, TL and in CC and bilateral mesencephalic tegmentum. EM in bilateral FL. |
| 13.74/M/1.37 | Traffic accident | GCS = 3 | DAI in right TL, C and in left C and in CC, right thalamus and bilateral basal ganglia |
| 17.46/F/1.92 | Horse accident | GCS = 5 | DAI in right FL, TL, PL, OL and in left FL, TL, PL, OL and in CC, brain stem, bilateral substantia nigra and bilateral mesencephalic tegmentum. EM in right FL, TL and in left FL. |
| 16.88/M/4.05 | Traffic accident | LOC = 20 m | DAI in right FL, OL and in left FL, TL, OL and in CC. EM in left TL |
- Note. Abbreviations: C = cerebellum; CC = corpus callosum; EM = encephalomalacia; F = female; FL = frontal lobe; GCS = Glasgow Coma Scale; LOC = loss of consciousness; DAI = diffuse axonal injury; M = male; OL = occipital lobe; PL = parietal lobe; TL = temporal lobe; TSI = time since injury (in years).
3.2 Global network topology measures
Overall network density was found to be reduced in patients with TBI (p < .001) when compared to healthy controls (for results, see Table 2). Both normalized clustering coefficient (p < .001) and normalized characteristic path length (p < .001) were significantly increased in the TBI group compared to the controls. In contrast, the total strength of the network was similar in both groups (p = .601). Results for other consensus threshold levels were consistent with the reported 60% threshold (Supporting Information).
| Controls (n = 17) | Patients (n = 17) | Group difference | |||
|---|---|---|---|---|---|
| Mean | SE | Mean | SE | p valuea | |
| Global topology measures | |||||
| Density | 0.311 | 0.001 | 0.2994 | 0.003 | <.001* |
| Total strength | 298193 | 3862 | 295363 | 3747 | 0.601 |
| normalized clustering coefficient | 2.209 | 0.014 | 2.376 | 0.042 | <.001* |
| normalized characteristic path length | 1.434 | 0.021 | 1.642 | 0.059 | <.001* |
| Density subnetworks | |||||
| Density—rich club | 0.928 | 0.007 | 0.889 | 0.011 | .005* |
| Density—feeder | 0.529 | 0.003 | 0.508 | 0.006 | .002* |
| Density—local | 0.239 | 0.001 | 0.230 | 0.002 | <.001* |
| Total strength subnetworks | |||||
| Total strength—rich club | 14241 | 479 | 12291 | 245 | <.001* |
| Total strength—feeder | 89649 | 1353 | 83974 | 1575 | 0.010* |
| Total strength—local | 194303 | 2678 | 199098 | 2709 | 0.228 |
| Mean strength subnetworks | |||||
| Mean strength—rich club | 170.534 | 5.651 | 153.828 | 3.109 | .013* |
| Mean strength—feeder | 117.826 | 1.825 | 114.752 | 1.474 | .206 |
| Mean strength—local | 159.098 | 2.112 | 169.231 | 2.294 | .002* |
| Executive functioning | |||||
| Metacognition index score | 42.750 | 5.927 | 60.235 | 9.270 | <.001* |
- Note. Abbreviation: SE = standard error.
- a p value based on permutation testing using 5,000 permutations.
- *p < .05 for two-tailed and FDR corrected.
3.3 Rich club organization
Figure 3 depicts the average normalized rich club coefficients for both groups. Normalized rich club coefficients were statistically >1 for a range of degree values (all p values ≤ .002), both in the control group (range k = 26–40) and the TBI group (range k = 26–39), indicating a rich club organization of the structural networks in both groups. Comparing the normalized φw(k) between controls and TBI patients, patients showed a significantly reduced rich club organization in the range k = 35–40 (p values all <.05, FDR-corrected).
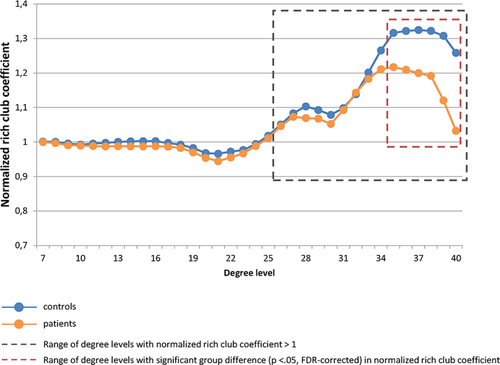
Group-averaged rich club curves of normalized weighted rich club coefficients [Color figure can be viewed at wileyonlinelibrary.com]
3.3.1 Rich club nodes
The 10 rich club nodes that were identified based on the entire sample include the following regions: superior frontal gyrus, precuneus, superior parietal gyrus, thalamus, and putamen, all bilateral (as indicated in red on Figure 2). Similar results were obtained at the individual level, apart from three patients, whereby we identified one other rich club node.
3.3.2 Density, total strength, and mean strength of rich club, feeder, and local subnetworks
We demonstrated decreased density in patients with TBI when compared to healthy controls across the three subnetworks (rich club: p = .005; feeder: p = .002; and local: p <.001). Similar results were obtained when the edges were classified based on the individual determined hubs (rich club: p = .009; feeder: p = .002; and local: p <.001). Moreover, results were consistent over a range of threshold levels (Supporting Information).
A reduction of total connectivity strength was observed in both rich club subnetwork (p < .001) and feeder subnetwork (p = .010) in patients with TBI compared to healthy controls. Local subnetwork strength was similar in both groups (p = .228). Classification of the edges based on the individual hubs resulted in comparable results (rich club: p = <.001; feeder: p = .007; and local: p = .166). Here too, results were similar over a range of threshold levels (Supporting Information).
Investigating group differences in mean connectivity strength for the three subnetworks, different results were obtained. A significant reduction of mean strength was observed in the rich club subnetwork (p = .013) in patients with TBI compared to healthy controls. Whereas, an increase in local mean strength (p = .002) was found in TBI patients (Figure 4). There were no significant group differences for feeder mean strength (p = .206). Classification of the edges based on the individual hubs resulted in comparable results (rich club: p = .003; feeder: p = .135; and local: p = .002). Similar results were obtained across a range of threshold levels (Supporting Information).
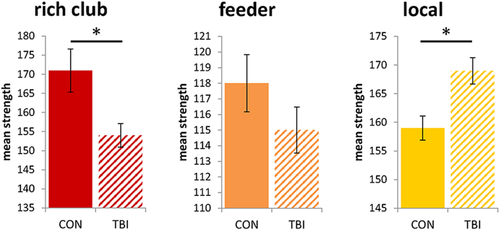
Mean strength of the rich club (red), feeder (orange), and local (yellow) subnetworks, error bars represent standard error * = p values < .05, FDR-corrected [Color figure can be viewed at wileyonlinelibrary.com]
3.3.3 Executive functioning and the relationship with rich club connectivity
Patients with TBI had significantly poorer executive functioning as reported by their parents on the MCI compared to healthy controls (p < .001). Decreases in rich club density were moderately but nonsignificantly associated with worse executive functioning (r = −.45, p = .078) in patients with TBI (Table 3). Furthermore, we found no significant correlation between MCI score and rich club mean strength (r = .23, p = .391) nor with rich club total strength (r = −.12, p = .673).
| RC density | RC total strength | RC mean strength | ||||
|---|---|---|---|---|---|---|
| R value | p value | R value | p value | R value | p value | |
| BRIEF MCI parent score | −.454 | .078 | −.115 | .673 | .230 | .391 |
- Note. Abbreviations: MCI = metacognition index; RC = rich club.
4 DISCUSSION
This study is the first to address the rich club organization of the structural connectome in young TBI patients. First of all, we were interested if we could replicate findings of previous research showing global network topology alterations in chronic TBI children compared to healthy controls. Second, we wanted to examine if the rich club organization was affected in patients with TBI and if rich club connectivity of children with TBI could be related to executive functioning. Finally, we compared connectivity strength and density between patients and healthy controls across three subnetworks.
Investigating the brain structural network as a whole, we demonstrated alterations in graph measures in patients with TBI. First, we found a reduced density and an increased normalized characteristic path length in TBI patients compared with healthy controls. This finding concurs with previous research reporting reduced density and/or increased path length in both children with TBI (Caeyenberghs et al., 2012; Konigs et al., 2017; Yuan, Treble-Barna, Sohlberg, Harn, & Wade, 2017) and adults with TBI (Caeyenberghs et al., 2014; Kim et al., 2014) in the chronic stage of recovery. Furthermore, our findings suggest an increase in normalized clustering coefficient in children with TBI. Again, this result is in agreement with previously reported increases in network segregation after acute childhood TBI (Yuan et al., 2015). In contrast, Königs et al. (2017) failed to find a significant alteration in segregation in a pediatric sample of chronic moderate to severe TBI that was comparable to ours. A possible explanation for these conflicting results could be the difference in connectome construction. We used number of streamlines (NOS) as edge weight in our structural connectomes, whereas Königs et al. based their connectomes on the probability of connectivity which was subsequently weighted for fractional anisotropy (FA). A study by Yeh et al. (2016) demonstrated the effects of bias correction strategies on graph measures and underscores the need for advanced corrections such as SIFT and ACT which were employed in the present study (Smith et al., 2012, 2013). Important to note, inspection of the Supplemental Material of the Königs study revealed that this study also found increased segregation in patients with TBI when using NOS only as weight. The brain is a complex network, where a balance is needed between local specialization and global integration of information processing. In other words, an efficient brain exhibits high clustering with segregated areas for specialization in combination with a short path length enabling efficient integration of information from distributed brain areas (Bassett & Bullmore, 2006). Based on our results, the structural connectomes of pediatric patients with TBI appear to be more segregated into local clusters of connections compared to the networks of healthy controls. There seems to be a shift in balance in the brain of patients with TBI, supporting local segregation at the expense of global integration. Interestingly, total connectivity strength (i.e., the sum of all weights in the network) was comparable in both groups. This suggests that although patients have fewer connections, the connections (or at least some of the connections) are stronger in patients than in controls. Mixed results were reported in the literature for this graph metric, with a similar strength in chronic adult TBI patients compared to controls in a study of Caeyenberghs et al. (2013), and a decrease in strength in other studies examining patients in the acute or subacute phase of TBI (Dall'Acqua et al., 2016; Mitra et al., 2016). A possible explanation for these conflicting results is the time since injury. While strength reduction might be part of the acute phase of TBI, it is possible that connectivity strength normalizes or even increases over time.
Second, our results suggest that the structural brain network of both controls and patients with TBI show a rich club organization. In other words, patients with TBI have a subset of highly connected nodes that are more interconnected than would be expected by chance (van den Heuvel & Sporns, 2011). Notwithstanding the presence of a rich club organization, TBI patients show a significant reduction in rich club interconnectivity compared to healthy controls. Reduced rich club connectivity in patients with TBI might indicate that it is difficult for these patients to maintain or repair the core network given the biological cost. These results are in conflict with the increased rich club connectivity that was observed in studies examining rich club organization in patients with TBI based on functional MRI data (Antonakakis et al., 2017; Hillary et al., 2014). In contrast to reported converging results in healthy adults when directly comparing functional and structural networks (Honey et al., 2009; Meier et al., 2016; Skudlarski et al., 2008; van den Heuvel, Mandl, Kahn, & Pol, 2009), Caeyenberghs et al. (2013) demonstrated that graph metrics obtained from both functional and diffusion MRI show little agreement in patients with TBI. These results point towards a decoupling of functional and structural connectivity in patients with TBI as a result of neural reorganization after trauma. A decoupling of functional and structural networks has been reported in other clinical groups as well. A study by Reijmer et al. (2015) in older adults with white matter hyperintensities for example demonstrated a clear decoupling. The authors suggested this might be related to different neural abnormalities that determine the white matter hyperintensities since they might have a differential effect on the BOLD signal and the diffusion parameters. These differential effects might also be in play in patients with TBI as the pathology is quite similar. In addition to this decoupling it is important to note that Caeyenberghs et al. (2013) showed that hubs determined using different imaging techniques show no overlap. The examination of rich club organization is highly dependent on hub identification and this complicates comparing results from both scan modalities.
Our study was the first to subdivide the network into three subnetworks based on edge classification in a group of patients with TBI. Whereas a similar reduction in density could be observed across the three subnetworks, a differential pattern of change could be demonstrated for connectivity strength. In particular, we showed a decrease in rich club and feeder total strength together with a small (nonsignificant) increase in local total strength. Investigating the mean strength of each subnetwork, we found significant results revealing a differential pattern of mean strength in the subnetworks. Specifically, the mean strength of the rich club network was significantly decreased, while the mean strength of the local network was significantly increased in patients with TBI. As the total strength in the local network is not decreased, the increase in mean strength cannot solely be explained by a loss of weak edges. Taken together, these findings suggest that comparable connectivity strength on a whole-brain level does not automatically imply equal strength values across the subnetworks. This underlines the importance of subdividing the brain in subnetworks and uncovering differential effects of TBI on the subnetworks’ strength. Moreover, the contrasting findings of density and strength demonstrate the relevance of adding strength as a measure of interest in future structural connectome studies in TBI. The increased mean local connectivity strength in chronic TBI might reflect an attempt to restore or compensate reduced rich club connectivity. A longitudinal study by Sidaros et al. (2009) showed that reduced DWI measures can normalize or super-normalize in the chronic phase after TBI, which the authors interpreted as possible axonal regrowth. Moreover, functional improvements have been found to correlate with axonal growth in patients after brain injury (Wieloch & Nikolich, 2006). Another possible biological mechanism for this increased strength is myelin formation or myelin remodeling (Zatorre, Fields, & Johansen-Berg, 2012). The fact that the increased strength in patients with TBI is confined to the local subnetwork could imply that change is confined to connections that are less vulnerable and therefore less affected in TBI. The local network might also have a lower biological cost of connection strength in this network. As mentioned before, the rich club network and to a lesser extent the feeder network are biologically costly, making it harder to maintain, repair or further develop these networks with age (Collin et al., 2014b). Given the cross-sectional nature of our data, our hypothesis remains to be corroborated by prospective studies spanning acute and chronic stages of TBI recovery.
Executive functioning, measured by the metacognition index of the BRIEF parent report version, was significantly reduced in patients with TBI. However, our correlation analyses showed no statistically significant correlations between rich club connectivity (total strength, mean strength, and density) and executive functioning in children with TBI. These results suggest that the relationship between impaired rich club and higher-order cognitive functioning is quite complex. Subnetwork strength and density seem to be sensitive markers for white matter alterations as the result of TBI and the present results may offer a valuable insight into the nature of TBI-induced brain network changes. Future replication studies in larger patient samples might determine the relationship of these subnetwork measures with cognitive, behavioral, and other outcome measures.
There are some important limitations to this study. First of all, the small number of participants in this study limited statistical power. Despite the relatively small sample size, we observed significant group differences. It is hard to recruit large samples of pediatric moderate to severe TBI patients in the chronic stage and future multicenter studies and crowd sourcing initiatives such as ENIGMA TBI (Mohammadi, 2015) are important to accommodate for recruitment difficulties. Second, there was no correction for EPI distortions performed during the preprocessing of the DWI images. At the time of scanning (start scanning date end 2014), we did not acquire a field map or a reverse phase encoded image. This might have impacted the estimation of the structural connectomes, especially in the frontal and temporal areas. However, we believe that EPI distortions are uniformly present in all subjects across both groups, so we do not expect this to influence possible whole brain group differences. In addition, we are not able to conclude that possible compensatory mechanisms after TBI cause increased local strength. Longitudinal studies are required to provide evidence of increases in local connectivity in the chronic phase of TBI and unravel its underlying mechanism. Finally, it is important to note that a developing brain is not equivalent to an adult brain. It is likely that recovery processes are different for children compared to adults, especially given the fact that the rich club connectivity is still in full development (Dennis et al., 2013). Future research in different age groups is needed to determine if similar alterations in strength could be observed in adults with TBI.
5 CONCLUSION
Despite the aforementioned limitations, this is the first study to examine rich club organization in the structural brain network of young patients with TBI in the chronic stage of recovery. Results revealed that children with TBI have a reduced rich club organization compared to healthy controls. We found a specific pattern in group differences of connectivity when we divided the structural network into a rich club network, a local network, and a feeder network connecting these two. More specifically, the children with TBI showed decreased mean rich club strength and an increased mean local strength. In other words, by dividing the whole brain network into subnetworks, we get a valuable insight into the nature of TBI-induced brain network alterations. Future longitudinal studies are needed to determine whether the increased local strength reflects a possible compensation mechanism.
CONFLICTS OF INTEREST
The authors have nothing to disclose.
ACKNOWLEDGMENTS
This research was supported by a grant (#01N00214) from the Special Research Fund (BOF) from the University of Ghent.




