Listening under difficult conditions: An activation likelihood estimation meta-analysis
Abstract
The brain networks supporting speech identification and comprehension under difficult listening conditions are not well specified. The networks hypothesized to underlie effortful listening include regions responsible for executive control. We conducted meta-analyses of auditory neuroimaging studies to determine whether a common activation pattern of the frontal lobe supports effortful listening under different speech manipulations. Fifty-three functional neuroimaging studies investigating speech perception were divided into three independent Activation Likelihood Estimate analyses based on the type of speech manipulation paradigm used: Speech-in-noise (SIN, 16 studies, involving 224 participants); spectrally degraded speech using filtering techniques (15 studies involving 270 participants); and linguistic complexity (i.e., levels of syntactic, lexical and semantic intricacy/density, 22 studies, involving 348 participants). Meta-analysis of the SIN studies revealed higher effort was associated with activation in left inferior frontal gyrus (IFG), left inferior parietal lobule, and right insula. Studies using spectrally degraded speech demonstrated increased activation of the insula bilaterally and the left superior temporal gyrus (STG). Studies manipulating linguistic complexity showed activation in the left IFG, right middle frontal gyrus, left middle temporal gyrus and bilateral STG. Planned contrasts revealed left IFG activation in linguistic complexity studies, which differed from activation patterns observed in SIN or spectral degradation studies. Although there were no significant overlap in prefrontal activation across these three speech manipulation paradigms, SIN and spectral degradation showed overlapping regions in left and right insula. These findings provide evidence that there is regional specialization within the left IFG and differential executive networks underlie effortful listening.
1 INTRODUCTION
The difficulty of speech identification and comprehension can be manipulated using a variety of methods (for a review, see Mattys, Davis, Bradlow, & Scott, 2012). The three most common methods are amplifying background noise, degrading the spectral quality of the auditory signal using filtering techniques, and presenting unfamiliar or complex linguistic materials. For instance, accuracy in identification of speech sounds decreases as background noise increases (Anderson Gosselin & Gagne, 2011; Gosselin & Gagne, 2011; Zekveld, Kramer, & Festen, 2011). Similarly, in studies manipulating the spectral quality of speech sounds (as in those using noise-vocoded speech stimuli), speech comprehension decreases with decreasing amounts of spectral information (Shannon, Zeng, Kamath, Wygonski, & Ekelid, 1995). Word intelligibility is also reduced when high-frequency speech information is attenuated with a low-pass filter (Bhargava & Başkent, 2012; Eckert et al., 2008; Vaden et al., 2011). In addition, speech comprehension is more effortful with increased linguistic complexity—operationalized as the use of semantically ambiguous words or syntactically complex sentences (Bilenko, Grindrod, Myers, & Blumstein, 2009). These manipulations in perceptual and cognitive difficulty are reflected in behavioral performance (e.g., lower accuracy in speech identification or comprehension, increased response time), and physiological measures in pupil dilation (Koelewijn, Zekveld, Festen, & Kramer, 2012; Kuchinsky et al., 2016; Zekveld, Kramer, & Festen, 2010). Increased pupil diameter and decreased behavioral performance are considered manifestations of mental effort (Kahneman, 1973; McGarrigle et al., 2014; Westbrook & Braver, 2015), and these indices accompany effortful listening when speech stimuli are manipulated using either of the three manipulations described above.
Listening under difficult conditions has been associated with enhanced activity in prefrontal regions implicated in cognitive control, attention, and working memory processes (Du, Buchsbaum, Grady, & Alain, 2016; Erb & Obleser, 2013; Love, Haist, Nicol, & Swinney, 2006; Peelle, Troiani, Wingfield, & Grossman, 2010b; Rodd, Johnsrude, & Davis, 2010a; Vaden, Kuchinsky, Ahlstrom, Dubno, & Eckert, 2015; Vaden et al., 2013; Wong et al., 2009a; Zekveld, Rudner, Johnsrude, Heslenfeld, & Ronnberg, 2012). For instance, evidence from functional magnetic resonance imaging (fMRI) suggests that accurate speech-in-noise (SIN) processing depends on a widely distributed neural network, including the left ventral premotor cortex (PMv) and Broca's area (i.e., the speech motor system), bilateral dorsal prefrontal cortex, superior temporal gyrus (STG), and parietal cortices (Binder, Liebenthal, Possing, Medler, & Ward, 2004; Du, Buchsbaum, Grady, & Alain, 2014; Du et al., 2016; Hervais-Adelman, Pefkou, & Golestani, 2014; Wong et al., 2009a). Similarly, positron emission tomography (Scott, Rosen, Lang, & Wise, 2006) and other fMRI studies (Davis & Johnsrude, 2003; Evans & Davis, 2015; Meyer, Steinhauer, Alter, Friederici, & von Cramon, 2004; Vaden et al., 2011; Wild et al., 2012b) have shown increased activation in left inferior frontal gyrus (IFG), bilateral STG, and middle temporal gyrus (MTG) when hearing noise-vocoded and low-pass filtered speech stimuli. Increased levels of syntactic, lexical, and semantic complexity have also been associated with activation in the left IFG (Bilenko et al., 2009; Friederici, Fiebach, Schlesewsky, Bornkessel, & von Cramon, 2006), left middle frontal gyrus (MFG) (Caplan, Alpert, & Waters, 1999; Meltzer, McArdle, Schafer, & Braun, 2010), and left STG (Grindrod, Bilenko, Myers, & Blumstein, 2008).
The above studies suggest that the prefrontal cortex is ubiquitously involved in supporting effortful listening when speech sounds are masked by background noise, or spectrally degraded, or when linguistic information is unfamiliar or complex. That is, when the auditory cortex in the STG cannot effectively process speech sounds, executive functions such as attentional, working memory, and speech-motoric processes in the prefrontal regions may be recruited to compensate for speech comprehension (Du et al., 2014, 2016; Pichora-Fuller et al., 2016; Ronnberg et al., 2016; Skipper, Devlin, & Lametti, 2017; Van Engen & Peelle, 2014). According to sensorimotor integration theories of speech perception, predictions generated from the frontal articulatory network, including Broca's area and PMv, impose phonological constraints to auditory representations in sensorimotor interface areas, for example, the Sylvian-parietal-temporal area (Spt) in the posterior planum temporale (Hickok, 2009; Hickok & Poeppel, 2007; Rauschecker & Scott, 2009). This kind of sensorimotor integration is proposed to facilitate speech perception, especially in adverse listening environments. For instance, Du et al. (2014) found greater specificity of phoneme representations in the left PMv and Broca's area than in bilateral auditory cortices during syllable identification in high background noise. Older adults also showed greater specificity of phoneme representations in frontal articulatory regions than auditory regions (Du et al., 2016). Thus, upregulated activity in prefrontal speech motor regions may provide a means of compensation for decoding impoverished speech representations.
Together, this indicates that effortful listening under increased background noise, poor spectral details or increased linguistic complexity likely requires greater recruitment of the brain networks underlying speech comprehension (described above). What remains unclear, however, is the extent to which increased prefrontal activations during effortful speech comprehension reflects: (a) the recruitment of the speech motor system; (b) a common anterior attention network needed to successfully identify speech stimuli; and/or (c) distinct patterns of prefrontal activations as a function of the listening challenges. For instance, increasing linguistic complexity may engage different brain regions within the left IFG from those recruited when the signal is degraded or masked by noise because linguistic complexity presents higher level cognitive and semantic challenge in deciphering the meaning of speech sounds, rather than lower level sensory and perceptual challenge. Similarly, effortful listening when the speech signal is spectrally degraded versus when masked by background noise might engage different neural networks.
There is some evidence suggesting that increasing background noise and spectrally degrading the signal yield similar changes in brain activity (Davis & Johnsrude, 2003), but that finding should be interpreted cautiously because it is based on a small sample of only 12 participants. Different patterns of activations may have been missed or undetected even if the net effect of both manipulations on the speech intelligibility was similar. SIN tasks require individuals to suppress competing auditory inputs, whereas no such suppression is needed while processing spectrally degraded speech. Consequently, SIN tasks may require greater executive control needed to focus attention on task-relevant speech sounds. Moreover, SIN tasks require segregation of concurrent sound objects which are presented in their entirety. By contrast, in spectrally degraded speech paradigms, parts of the speech signal are missing, so listeners must “fill in” the missing parts. Therefore, even though SIN, spectrally degraded speech, and linguistic complexity all require effortful listening, these three speech manipulation paradigms may recruit different networks and elicit different subpatterns of activation.
Meta-analyses of published neuroimaging studies provide a means of identifying brain regions that are reliably recruited across multiple studies and/or in different laboratories. Several such meta-analyses have been conducted on speech perception and production (Adank, 2012; Adank, Nuttall, Banks, & Kennedy-Higgins, 2015; DeWitt & Rauschecker, 2012; Rodd, Vitello, Woollams, & Adank, 2015; Samson et al., 2001), but none have directly compared activation patterns elicited between distinct methods of speech manipulation. For instance, Adank's (2012) meta-analysis grouped studies using different types of speech distortions (e.g., accented speech, background noise, compressed speech, and noise-vocoded speech). In another meta-analysis, Rodd et al. (2015) found differences in patterns of activation for semantic versus syntactic processing of spoken and written words in medial prefrontal regions. However, they also observed substantial overlap between semantic and syntax processing in the left IFG, even with more than 50 studies included in the meta-analysis. This suggests that semantic and syntactic processing in prefrontal cortex may share common resources, yet it remains unknown whether the patterns of activations observed for increasing linguistic complexity would differ from those reported in SIN studies and studies manipulating the spectral quality of speech sounds.
The aims of this study are to (a) determine patterns of brain activation that support effortful listening when there is background noise, when the auditory signal is spectrally degraded, and when linguistic information is unfamiliar or complex and (b) identify regions of overlap (if any) among these three different effortful listening situations. We focus on three particular paradigms of speech manipulation: SIN comprehension, spectrally distorted speech, and linguistic complexity—because those are the most commonly used paradigms and thus have a sufficiently large number of neuroimaging studies to assess patterns of brain activation (Eickhoff, Bzdok, Laird, Kurth, & Fox, 2012; Eickhoff, Laird, Fox, Lancaster, & Fox, 2017; Eickhoff et al., 2016). Of course, there are many other methods of speech manipulation techniques used to increase listening effort (e.g., increase speech rate or use accented speech); however, the number of studies published to date using any of these other methods is insufficient to obtain reliable estimates. In this study, the linguistic complexity category grouped together auditory studies that manipulated syntactic, lexical, and semantic ambiguity because the number of studies within these subgroups was too small to stand alone. Importantly for our study objectives, none of the studies included in linguistic complexity involved manipulation of the signal-to-noise ratio, providing a valid comparison of challenging listening situations without overlapping manipulation methods.
We sought to identify which (if any) brain areas are uniquely recruited during listening in SIN, spectral degradation, and linguistic complexity scenarios. To accomplish this, we used Activation Likelihood Estimation software (Eickhoff et al., 2009, 2012; Turkeltaub et al., 2012) to analyze activation results from relevant fMRI and PET studies. We anticipated that all three manipulations would yield patterns of prefrontal and temporal activations. We also performed contrast analyses to compare the patterns of activations within the prefrontal cortex and within the STG across different manipulations. The regions that are consistently recruited with increasing listening effort can be identified from the conjunction analyses between the three manipulations and from the omnibus analysis that comprises all studies regardless of the manipulation used to increase listening effort. If the analyses reveal significant overlapping prefrontal regions, then this will indicate that effortful listening recruits nonspecific neural networks regardless of the particular conditions that make it effortful. Conversely, if different manipulation paradigms elicit different patterns within the prefrontal cortex, this will indicate that listening effort involves brain regions which are differentiated as a function of listening conditions.
2 MATERIALS AND METHODS
2.1 Search strategy
The PubMed (www.pubmed.org) and Psychlib (hosted by the Department of Psychology at University of Toronto) database were searched using the following terms: “speech intelligibility,” “speech in noise,” “SIN,” “vocoded speech,” “distorted speech,” “degraded speech,” “listening effort,” “semantic ambiguity,” “linguistic complexity,” crossed with “fMRI,” “PET,” “positron emission tomography,” and “neuroimaging.” All relevant review articles retrieved in the primary search were then hand-searched for articles not previously identified. The search included studies published in peer-reviewed journals and in English as of October 2017.
2.2 Screening process
Each record was screened based on its title and abstract against the eligibility criteria outlined in Figure 1, which depicts the complete screening process. Full texts of potentially eligible articles were retrieved and screened, and any disagreements were settled by consensus among all authors.
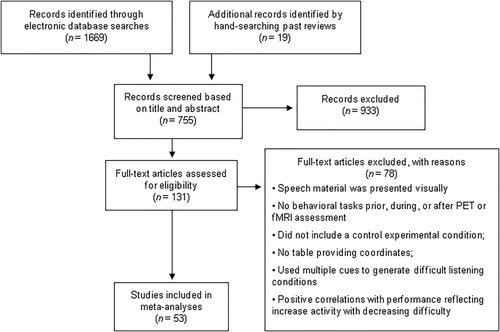
Screening process for studies included in the meta-analysis
Articles were included if they met the following criteria: (a) speech materials were presented aurally; (b) the study included a behavioral task performed prior to, during, or after scanning; (c) the study included a control experimental condition; (d) whole-brain analysis from fMRI or PET on 3D coordinates in either Talairach (Talairach & Tournoux, 1988) or Montreal Neurological Institute (MNI) standardized space were reported; (e) the study reported higher activity associated with increased listening difficulty; and (f) participants were young adults, without any hearing problems, psychiatric or neurological disorders, or brain abnormalities. Studies which combined data from healthy and patient participants were excluded. Studies meeting these selection criteria use a variety of stimuli and tasks, such as identification of isolated phonemes and comprehension of continuous speech. Studies were included whether or not there was behavioral difference between experimental conditions because comparable task performance does not always mean that listening effort is comparable (Alain, McDonald, Ostroff, & Schneider, 2004). That is, in more difficult listening conditions, participants may invest extra attention to maintain a comparable level of behavioral performance to that of easier listening conditions. Studies without behavioral data were included if participants were instructed to perform a task during scanning such as mental addition of numbers presented in noise or repeating aloud the last word of a sentence in noise.
2.3 Activation likelihood estimate (ALE)
Coordinate-based quantitative meta-analyses of neuroimaging results were performed using the GingerALE software (version 2.3.6) available on the BrainMap website (http://brainmap.org/ale/index.html). The MNI coordinates were converted to Talairach space using the Lancaster transformation (Lancaster et al., 2007) before being entered into the analysis. This software generates a brain activation map based on coordinates provided in the included articles and uses a permutation test to determine whether the group mean activation is statistically reliable or not. It calculates above-chance clustering maps between experiments by modeling a three-dimensional Gaussian probability distribution centered on each focus reported in a study (weighted by the number of participants) and combining the probabilities of activation for each voxel. It then calculates voxel-wise scores representing convergence in similar brain locations across experiments. We used the smaller mask size and the random effect Turkeltaub nonadditive method, which minimizes both within-experiment and within-group effects by limiting probability values of neighboring foci from the same experiment (Turkeltaub et al., 2012). Cluster-level inference was used to identify brain areas consistently recruited during SIN, spectrally degraded speech, and linguistic complexity. We used 1,000 permutations to test for statistical significance. We also used an uncorrected p value of .001 for “cluster-forming threshold” and .05 for cluster-level inference as being statistically significant (Eickhoff et al., 2012, 2016, 2017).
Coordinates were selected for inclusion if they reflected: (a) activations from a direct comparison of the task of interest with a control task (e.g., speech-in-noise versus speech without noise) or (b) a correlation between brain activation and signal-to-noise ratio (SNR) when studies manipulated SNR for speech sounds. Separate analyses were performed for SIN, spectrally degraded speech, and linguistic complexity.
Three sets of follow-up contrast analyses were performed to determine whether different effortful listening situations yielded a different pattern of brain activation. These contrasts revealed the similarity (conjunction image) between data sets and contrast images created by directly subtracting one condition from the other. Pairwise contrast and conjunction analyses were performed between (a) SIN and spectrally degraded speech, (b) SIN and linguistic complexity, and (c) spectrally degraded speech and linguistic complexity. In GingerALE, contrasts are calculated using a voxel-wise minimum statistic (Nichols, Brett, Andersson, Wager, & Poline, 2005), which ascertains the intersection between the individually thresholded meta-analysis results and produces a new thresholded (p < .05) ALE image and cluster analysis. For contrast, we used uncorrected p value of .05, with 10,000 permutation and minimum volume of 100 mm3.
For visualization, BrainNet software was used to display foci (Xia, Wang, & He, 2013). The ALE-statistic maps were projected onto a cortical inflated surface template using SUrface MApping (SUMA) with Analysis of Functional Neuroimages software (Cox, 1996, 2012).
3 RESULTS
Fifty-three studies met inclusion criteria (SIN: 16 studies, involving 224 participants; spectrally degraded speech: 15 studies, involving 270 participants; linguistic complexity: 22 studies, involving 368 participants; Table 1).
| Paper | Stimuli and contrast revealing increasing activation | Task and behavioral findings: during, no task, outside scanner | Number of participants (sex and age) | Foci | Coordinate space | Source in paper |
|---|---|---|---|---|---|---|
| Speech in noise | ||||||
| Adank, 2012 | Sentences in speech-shaped noise > sentences in quiet | Indicate if a sentence was true or false, RT for speech in noise > clear speech | 26 (20F; 18–28 years) | 8 | MNI | Table 2 |
| Bee, 2014 | Arithmetic addition in noise > addition in quiet | Perform arithmetic addition, accuracy not reported | 18 (0F; 20–28 years) | 3 | MNI | Table 1 |
| Binder, 2004 | Phonemes in noise: negative correlation with SNR | Two-interval forced-choice procedure, decrease accuracy and increase RT with increasing noise | 18 (10F; 24–47 years) | 3 | Talairach | Table 1 |
| Dos Santos Sequiera, 2010 | Consonant-vowel syllables in babble noise > babble noise | Dichotic listening task, accuracy in quiet > babble conditions | 21 (8F; 19–35 years) | 19 | MNI | Table 4 |
| Du, 2014 | Phonemes in broadband noise: negative correlation with SNR-modulated accuracy | Phoneme identification task, decrease accuracy and increase RT with decreasing SNR | 16 (8F; 21–34 years) | 8 | Talairach | SI, Table 2 |
| Evans, 2016 | Two talker setting vs single talker | Participants were asked to listen to a target speaker, hit rate for clear speech > masked speech | 20 (10F; 19–36 years) | 8 | MNI | Table 1 |
| Golestani, 2013 | Word in noise; negative correlation with SNR-modulated accuracy | Choose between two visual probes, increase RT with decreasing SNR | 9 (6F, not provided) | 6 | MIN | Table 2 |
| Guerreiro, 2016 | Words in low SNR > high SNR | One-back task, accuracy for high SNR > low SNR | 9 (7F; 19–56 years) | 9 | Talairach | Table 3 |
| Hervais-Adelman, 2014 | Word in noise, negative correlations with noise | Choose between two visual probes, increase RT with decreasing SNR | 9 (6F, not provided | 6 | MNI | SI, Table 1 |
| Peelle, 2010 | Sentences in continuous scanning EPI > quiet EPI | Respond to a probe word on a screen, no difference in behavioral performance | 6 (3F; 20–26 years) | 7 | MNI | Table 4 |
| Salvi, 2002 | Sentences in noise > sentences in quiet | Repeat aloud the last sentence word, not reported | 10 (5F; 23–34 years) | 7 | Talairach | Table 1 |
| Scott, 2004 | Sentences in noise masker and speech masker, negative correlation with SNR | Participants were asked to listen to the female speaker for meaning, accuracy for higher SNRs > lower SNRs | 7 (0F; 35–52 years) | 2 | MNI | Figure 4 |
| Sekiyama, 2003 | Syllables at low SNR minus visual control | McGurk effect for lower SNRs > higher SNRs | 8 (1F; 22–46 years) | 9 | Talairach | Table 1 |
| Vaden, 2013 | CVC syllables in multitalker babble: +3 dB > +10 dB SNR | Repeat each word aloud, hit rate for +10 dB > +3 dB SNR | 18 (10F; 20–38 years) | 5 | MNI | Table 1 |
| Wong, 2008 | Words in multitalker babble: −5 dB SNR > quiet | Word identification, longer RT and lower accuracy for SNR −5 dB than SNR 20 dB | 11 (7F; 20–34 years) | 20 | Talairach | Table 1 |
| Zekveld, 2012 | Sentences in long-term average speech spectrum > speech in quiet | Report sentence aloud, accuracy for speech in quiet > in noise | 18 (9F; 20–29 years) | 12 | MNI | Table 2 |
| Spectrally degraded speech | ||||||
| Eckert, 2009 | Filtered words, parametric increased in activity with decreasing intelligibility | Repeat word aloud, word recognition (accuracy) decrease with decreasing high-frequency information | 11 (5F; mean age 32.8 ± 10.9 years) | 7 | MNI | SI, Table 1 |
| Erb, 2013 | Noise-vocoded sentence > clear sentence | Repeat sentence aloud, accuracy for clear > noise-vocoded speech | 30 (15F; 21–31 years) | 5 | MNI | Table 1 |
| Evans, 2015 | Noise-vocoded phoneme > clear phoneme | One back task, d′ scores for clear > noise-vocoded speech | 17 (12F; 18–40 years) | 3 | MNI | Table 1 |
| Hervais-Adelman, 2012 | Noise-vocoded words > clear words | Respond to target sounds, accuracy for clear > noise-vocoded speech | 15 (10F; 18–35 years) | 4 | MNI | Table 2 |
| Hesling, 2004 | Expressive filtered sentences > normal sentences | Shadowing of normal speech > expressive filtered speech | 12 (0F; 24–38 years) | 4 | Talairach | Table 5 |
| Kyong, 2014 | Noise-vocoded sentences: negative correlates of sentence intelligibility | Participants were asked to try to understand what was being said. Clear speech > noise vocoded speech | 19 (18–40 years) | 6 | MNI | Table 5 |
| Lee, 2016 | Noise-vocoded sentences > clear sentences | Indicate gender of the character performing the action, accuracy for clear speech marginally higher than noise vocoded speech | 26 (12F; 20–34 years) | 7 | MNI | Table 4 |
| Meyer, 2002 | Prosodic filtered (PURR-filtering) sentences > normal sentences | Indicate if the sentence was normal or has prosody, accuracy for clear > filtered speech | 14 (6F; mean age 25.2 ± 6 years) | 8 | Talairach | Table 3 |
| Meyer, 2004 | Distorted sentences > normal sentences | Prosody comparison task, no behavioral data provided. | 14 (8F; 18–27 years) | 10 | Talairach | Table 1 |
| Sharp, 2010 | Noise-vocoded words > clear words | Indicate whether two words were related or not, RT for noise-vocoded speech > clear speech | 12 (5F; 35–61 years) | 2 | MNI | Table 2 |
| Takeichi, 2010 | Modulated sentences > nonmodulated sentences | Participants asked to listen to a narrative, accuracy higher for nonmodulated than modulated speech | 23 (12F; 20–38 years) | 2 | MNI | Table 1 |
| Tuennerhoff, 2016 | Filtered sentences > normal sentences | No difference in target detection between filtered and normal speech, unprimed filtered speech less intelligible than normal speech | 20 (10F; median age 24.05 years) | 12 | MNI | Table 1 |
| Wild, 2012a | Noise-vocoded sentences vs clear sentences, main effect of intelligibility, noise-elevated response | Repeat the sentence aloud, accuracy for clear > noise-vocoded speech | 21 (13F; 19–27 years) | 4 | MNI | Table 1 |
| Wild, 2012b | Noise-vocoded sentences > clear speech and rotated noise-vocoded sentences | Participants were asked to listen attentively | 19 (11F; 18–26 years) | 1 | MNI | Table 1 |
| Zekveld, 2014 | Noise-vocoded sentences > clear sentences | Indicate whether a visual probe were part of the sentence, accuracy for clear > noise-vocoded speech | 17 (9F; 19–33 years) | 4 | MNI | Table 4 |
| Linguistic complexity | ||||||
| Bekinschtein, 2011 | Semantically ambiguous > unambiguous sentence | Rate sentences as funny or not, no significant difference in performance between ambiguous and unambiguous sentences | 12 (not specified) | 2 | MNI | Table 1 |
| Bilenko, 2009 | Semantically ambiguous > unambiguous words | Speeded lexical decision task, slower RT for ambiguous than unambiguous words | 16 (9F; 18–21 years) | 2 | Talairach | Table 3 |
| Buchweitz, 2014 | Unfamiliar > familiar sentences | True–false question that probe comprehension, RT for unfamiliar > familiar passages | 9 (3F; 18–25 years) | 6 | MNI | Table 3 |
| Caplan, 1999 | Semantically implausible > plausible sentences | Plausibility judgment task, RT for implausible > plausible sentences | 16 (8F; 22–34 years) | 3 | Talairach | Table 2 |
| Davis, 2007 | High ambiguity > low ambiguity sentences | Participants listened passively | 12 (3F; 29–42 years) | 7 | MNI | Supporting Information, Table 3 |
| Friederici, 2006 | Increase activity with increasing grammatical complexity of sentences | Rate acceptability of the sentence, RT increase with increasing complexity | 13 (7F; mean age 23.1 years) | 2 | Talairach | Figure 4 |
| Friederici, 2010 | Syntactically incorrect > correct sentences | Participants were asked to listen attentively | 17 (9F; 20–30 years) | 5 | MNI | Table 1 |
| Grindrod, 2008 | Sequences of semantically disconcordant > neutral words | Lexical decision task, RT for discordant > concordant or neutral words | 15 (8F; 19–29 years) | 1 | Talairach | Table 3 |
| Guediche, 2013 | Semantically ambiguous > unambiguous sentences | Press a button to target word, longer RT, and lower accuracy in ambiguous than unambiguous target | 17 (8F; 24.5 ± 3.6 years) | 3 | Talairach | Table 3 |
| Herrmann, 2012 | Syntactically incorrect > correct sentences | Grammatically judgment task, accuracy for correct > incorrect grammar | 25 (12F; 22–32 years) | 5 | MNI | Table 2 |
| Lopes, 2016 | Semantically complex > easy semantic decision task | Semantic decision task, accuracy for easy > complex semantic decision | 24 (15F; 20–31 years) | 6 | MNI | Table 2 |
| Meltzer, 2010 | Main effect of syntactic sentence complexity | Match heard spoken sentence with visual probes, RT for reversible > irreversible sentence | 24 (12F; 22–37 years) | 1 | Talairach | Table 3 |
| Obleser, 2011 | Positive correlation with syntactic complexity | Participants were asked to listen attentively, no difference in accuracy | 14 (8F; 23.4 ± 2.1 years) | 1 | MNI | Table 2 |
| Peelle, 2004 | Object-relative > subject-relative sentences | Indicate the gender of the character performing the action, longer RT, and lower accuracy for object related than subject-related sentences | 8 (4F; 19–27 years) | 2 | Talairach | Table 1 |
| Rissman, 2003 | Semantically unrelated > related words | Lexical decision task, longer RT, and lower accuracy for unrelated than related words | 15 (8F; 18–44 years) | 5 | Talairach | Table 2 |
| Rodd, 2005 | High semantic ambiguity > low ambiguity sentences | Relatedness judgment task, no difference in performance between high and low ambiguity | 15 (10F; 18–40 years) | 4 | MNI | Table 4 |
| Rodd, 2010 | High > low semantic ambiguity words | Indicate whether the incoming word was related or not to the sentence meaning | 14 (19–37 years) | 1 | MNI | Table 3 |
| Ruff, 2008 | Semantic unrelated > related words | Relatedness judgment task, RT for unrelated > related words | 15 (8F; 19–31 years) | 9 | Talairach | Table 2 |
| Tyler, 2005 | Regular vs irregular verb | Same or different judgment task, RT regular > irregular verb, no difference in error rate | 18 (8F, mean age 24 ± 7) | 9 | MNI | Table 2 |
| Tyler, 2011 | Control group: syntactically ambiguous > unambiguous sentences | Listen to spoken sentence. Longer RT and lower accuracy for ambiguous than unambiguous sentences | 15 (8F; 46–74 years) | 3 | MNI | Table 3 |
| Vitello, 2014 | Semantic ambiguous > unambiguous semantic sentences | Participant indicated whether a probe was related or unrelated to the sentence they just heard | 20 (11F; 18–35 years) | 3 | MNI | Table 4 |
| Wright, 2010 | Complex > simple words, main effect of complexity | Lexical decision task, RT for nonwords > real words, RT for complex > simple words | 14 (sex not reported; 19–34 years) | 1 | MNI | Table 3 |
3.1 Neural substrates of SIN, degraded speech, and linguistic complexity
The coordinates of the cluster-level brain areas consistently activated in SIN, spectrally degraded speech, or linguistic complexity studies are shown in Table 2. Figure 2 shows the individual foci used in the meta-analyses in each listening condition. Figure 3 displays the ALE-statistic maps for regions of statistically significant concordance for each of the three different listening conditions and the results from the omnibus analysis that included all studies.
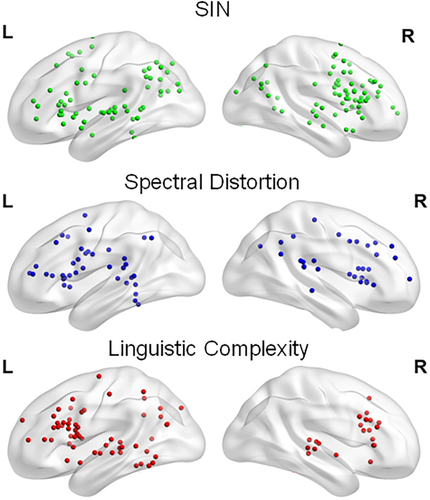
Foci from the speech in noise (SIN), spectrally degraded speech, and linguistic complexity studies
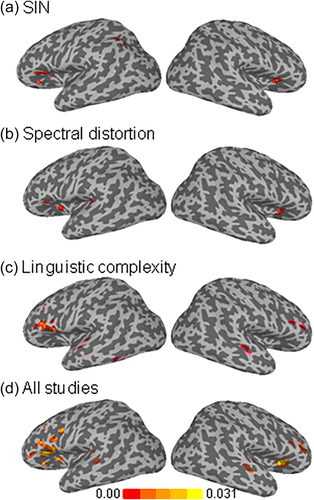
ALE-statistic maps for regions of significant concordance in neuroimaging studies: (a) speech in noise (SIN), (b) spectral distortion, (c) linguistic complexity studies, and (d) all studies together
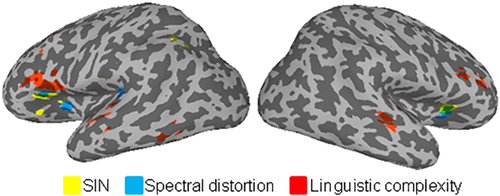
Overlay of ALE-statistic maps for all three tasks. Blue = SIN; yellow = spectral distortion; red = linguistic complexity; and green = significant overlap between spectral distortion and SIN
| Studies | Brain region, BA | Talairach. coordinates (x, y, z) | Cluster size (mm3) | #Studies/cluster |
|---|---|---|---|---|
| Speech in noise | ||||
| Left inferior frontal gyrus, 45 | −36, 19, 8 | 1,616 | 6 | |
| Right insula, 13 | 31, 18, 12 | 1,248 | 5 | |
| Left inferior parietal lobule, 40 | −35, −50, 36 | 800 | 3 | |
| Spectrally degraded speech | ||||
| Right insula, 13 | 28, 18, 7 | 1,704 | 6 | |
| Left insula, 13 | −34, 16, 5 | 1,384 | 5 | |
| Left superior temporal gyrus, 13 | −37, −29, 10 | 1,144 | 3 | |
| Linguistic complexity | ||||
| Left inferior frontal gyrus, 44 | −49, 13, 19 | 5,544 | 15 | |
| Right middle frontal gyrus, 46 | 43, 22, 23 | 1,896 | 7 | |
| Left middle temporal gyrus, 37 | −45, −48, −11 | 1,088 | 4 | |
| Right superior temporal gyrus | 51, −25, 4 | 952 | 3 | |
| Left superior temporal gyrus, 22 | −55, −16, 3 | 928 | 5 | |
| All studies combined | ||||
| Left insula, 13 | −43, 13, 16 | 9,400 | 29 | |
| Right insula, 13 | 35, 20, 14 | 5,392 | 19 | |
| Left superior temporal gyrus | −42, −29, 9 | 1,376 | 5 | |
| Right superior temporal gyrus | 52, −22, 4 | 920 | 4 | |
3.1.1 Speech in noise
The total number of foci from SIN manipulations was 129, with 61 of them located in the left hemisphere. The left and right foci were primarily distributed in prefrontal and posterior temporal and parietal regions (Figure 2). Processing SIN was associated with peak activations in the left IFG, right insula, and left inferior parietal lobule (Table 2 and Figure 3).
3.1.2 Spectrally degraded speech
Studies that manipulated the spectral quality of the speech sounds were associated with 79 foci, 40 of which were located in the left hemisphere. The analysis of foci from studies using spectrally degraded speech yielded peak activity in the right and left insula and left STG (Table 2 and Figure 3). There was no common area of activity in prefrontal regions, which could be accounted for by the spread of foci within prefrontal cortex (Table 2 and Figure 2).
3.1.3 Linguistic complexity
Linguistic complexity manipulations yielded 81 foci, with 60 of them in the left hemisphere (Figure 2). These were primarily distributed within the prefrontal cortex. Studies that focused on linguistic complexity consistently showed increased activation of the left IFG and right MFG, left MTG, and bilateral STG (Table 2 and Figure 3).
3.1.4 Networks involved in effortful listening independent of manipulation
This meta-analysis aimed to identify brain areas that are consistently recruited during effortful listening by including all studies regardless of manipulation (i.e., all 53 studies) into a single analysis. Figure 3 shows the brain areas that were consistently activated across studies. These include the insula and STG bilaterally (Table 2). It also included the anterior and dorsal portion of the IFG bilaterally.
3.2 Contrast between patterns of activations
In three separate contrast analyses, we tested whether the spatial patterns of activations observed in one task differed with those from another task. The brain areas that were significantly different between the pairwise subtractions are listed in Table 3. The contrast between SIN and spectrally degraded speech revealed greater left STG activation in SIN than in spectral degradation. Moreover, SIN studies yielded greater activation in the left IFG, right insula, and left IPL activation than studies that manipulated linguistic complexity. Studies that manipulated spectral quality of the speech signal showed greater activation in the insula bilaterally and in the left transverse temporal gyrus than studies that manipulated linguistic complexity. Last, linguistic complexity studies generated greater left IFG activation than studies manipulating SIN or spectral quality.
| Contrast analysis | Brain region, BA | Talairach coordinates (x, y, z) | Cluster size (mm3) |
|---|---|---|---|
| SIN vs spectrally degraded speech | Left superior temporal gyrus, 39 | −36, −51, 34 | 440 |
| Spectrally degraded speech vs SIN | Right lentiform nucleus | 26, 17, 2 | 144 |
| SIN vs linguistic complexity | Left inferior frontal gyrus, 13 | −35, 19, 8 | 1,392 |
| Right insula, 13 | 30, 17, 13 | 904 | |
| Left inferior parietal lobule, 40 | −35, −50, 36 | 800 | |
| Linguistic complexity vs SIN | Left inferior frontal gyrus, 44 | −50, 12, 18 | 4,032 |
| Spectrally degraded speech vs linguistic complexity | Right insula, 13 | 28, 17, 7 | 1,328 |
| Left insula, 13 | −31, 17, 2 | 592 | |
| Left transverse temporal gyrus, 41 | −34, −32, 15 | 288 | |
| Right insula, 13 | 55, −32, 19 | 272 | |
| Linguistic complexity vs spectrally degraded speech | Left inferior frontal gyrus, 44 | −50, 17, 20 | 3,100 |
| Right middle frontal gyrus, 46 | 45, 18, 22 | 400 |
| Conjunction analysis | Brain region, BA | Talairach coordinates (x, y, z) | Cluster size (mm3) |
|---|---|---|---|
| SIN ∧ spectrally degraded speech | |||
| Right insula, 13 | 29, 19, 11 | 592 | |
| Left insula, 13 | −36, 18, 8 | 456 | |
| SIN ∧ linguistic complexity | |||
| No significant cluster | |||
| Spectrally degraded speech ∧ linguistic complexity | |||
| No significant cluster | |||
3.3 Overlap between patterns of activations
The three types of speech manipulation paradigms included in this meta-analysis are associated with changes in accuracy and/or response time, reflecting changes in the difficulty of speech identification and comprehension. In three separate conjunction analyses, we tested whether the spatial patterns of activation observed in one task overlap with those from another task. The conjunction analyses between SIN and spectrally degraded speech revealed significant bilateral overlap in the insula. There was no significant overlap in prefrontal regions. Neither the conjunction analysis between SIN and linguistic complexity nor the conjunction analysis between spectrally degraded speech and linguistic complexity yielded any significant overlapping regions in either the STG or in prefrontal cortices.
4 DISCUSSION
This study aimed to identify unique and overlapping activation patterns across three types of effortful listening conditions.
4.1 Speech in noise
Comprehending speech in noise is challenging. Several speech-motor networks have been proposed to compensate for challenging listening conditions. Our results revealed a left fronto-parietal network that includes the IFG (Broadmann area 45) and IPL. Brodmann area 45, known as the Pars Triangularis of Broca's area, is believed to be involved in language perception and production (Hickok, 2009; Rauschecker & Scott, 2009), and has a role in the cognitive control of memory (Badre, 2008; Badre, Poldrack, Pare-Blagoev, Insler, & Wagner, 2005; Grady, Yu, & Alain, 2008). The observed activation in left IFG is consistent with findings from a prior meta-analysis (Adank, 2012) and provides converging evidence supporting sensorimotor accounts of speech processing (Hickok & Poeppel, 2007; Rauschecker & Scott, 2009). This account posits that the speech motor system generates internal models which predict sensory consequences of articulatory gestures under consideration, and such forward predictions are matched with acoustic representations in sensorimotor interface areas located in the left pSTG or IPL to constrain perception. Prefrontal speech motor-based predictive coding is believed to be especially useful for disambiguating phonological information under adverse listening conditions thus increasing the probability of extracting target signals from noise (Du et al., 2014, 2016).
The observed activity in the left IFG may also reflect compensatory mechanisms or increased attentional demands needed to segregate and identify speech sounds. The decline-compensation hypothesis posits that deficits in sensory processing regions can be mitigated by recruitment of more general executive control areas in the prefrontal cortex (Cabeza, Anderson, Locantore, & McIntosh, 2002; Wong, Perrachione, & Margulis, 2009b). That is, when peripheral and central auditory systems cannot effectively process speech sounds, the relative contributions of sensory, prefrontal, and parietal cortices may change, and the nature of that change depends on the task. Prefrontal activity may reflect engagement of predictive processes based on lexical and grammatical knowledges to offset the impoverished encoding and representations of speech sounds in auditory short-term memory. However, such knowledge-based predictive processes may not be of much use for identifying isolated syllables or phonemes in noise. In that context, participants may rely on articulatory representations of speech stimuli held in working memory, which could act as templates against which an incoming sound of syllable or phoneme could be compared.
4.2 Spectrally degraded speech
Studies that manipulate the spectral quality of incoming speech stimuli using noise-vocoded speech (e.g., Davis, Johnsrude, Hervais-Adelman, Taylor, & McGettigan, 2005; Shannon et al., 1995) or low-pass filtered speech (Bhargava & Başkent, 2012; Eckert et al., 2008; Vaden et al., 2011) have shown reduced speech intelligibility with decreased speech spectral details. The present meta-analysis results showed that decreasing speech intelligibility with spectral-filtering techniques consistently yields increased activation in the insula bilaterally and left STG.
These areas are part of the auditory ventral stream, which is thought to be particularly important for auditory object formation and sound identification. The observed insula activation is also consistent with the idea that, when presented with a degraded signal, acoustical details need to be analyzed to a greater extent than in normal conditions—because the level of available acoustic information might not be sufficient for lexical access. Although 13 out of 15 studies reported foci in the frontal lobes, the analysis indicated that there was no significant overlap in prefrontal activation across studies. That is, no prefrontal cortex areas were consistently recruited in studies using spectrally degraded stimuli. This was unexpected considering that most studies were fairly similar in methodology and stimuli. This is also surprising given the evidence from transcranial direct current stimulation (tDCS) studies suggesting that stimulation of the left IFG can improve identification of degraded speech (Sehm et al., 2013). The lack of a common pattern of activation in prefrontal cortex suggests that activation within the prefrontal cortex is widely distributed during tasks involving spectrally degraded stimuli. This spread of activation may be related to differences in task instructions, with a third of the studies included having participants listen to the stimuli without having to generate a response during scanning. In fact, there was higher proportion of spectral degradation studies without behavioral measures during scanning compared to SIN or linguistic complexity studies. The absence of behavioral measurements during scanning makes it difficult to determine participants’ attention and engagement in processing the speech stimuli and could likely contribute to variability in prefrontal activation. Further research is needed to better understand the source of this variability in prefrontal activation within studies using spectrally degraded speech stimuli.
4.3 Linguistic complexity
Linguistic complexity is operationalized as situations in which the acoustic quality of the incoming signal is kept constant while the semantic, lexical and grammatical aspects of the speech materials are manipulated using more semantically or syntactically ambiguous words or sentences. Results showed that there were consistent activations of the left IFG (Broadmann area 44), right MFG (Broadmann area 46), and bilateral STG when processing more ambiguous or complex sentences. The observed common pattern of activations in the left IFG is consistent with that of an earlier meta-analysis showing consistent left IFG activity during semantic and syntactic processing (Rodd et al., 2015). Brodmann area 44 in the left hemisphere, also known as the Pars Opercularis of Broca's area, is recognized as one of the main language areas. It is important for inner speech (Kuhn et al., 2013), perception and expression of prosodic and emotional information (Merrill et al., 2012), and auditory working memory (Alain, He, & Grady, 2008; Buchsbaum et al., 2005; Grady et al., 2008). Brodmann area 46, the MFG, which is a part of the dorsolateral prefrontal cortex, is involved in motor planning, organization, regulation, and working memory. The pattern of prefrontal activation may reflect enhanced verbal working memory needed to process meaning with increasing syntactic, lexical and semantic intricacy. It may also reflect attention to internal memory representations, that is, “listening” back in time to what a person just said (Backer & Alain, 2012, 2014; Backer, Binns, & Alain, 2015). Indeed, to string spoken phonemes into words and words into sentences, the acoustic representations must be maintained in auditory short-term memory, retrospectively processed, and bound with incoming acoustic signals (Alain & Bernstein, 2008). For effective comprehension, the semantics of these sentences must also be grouped together in a meaningful way. Hence, reflective attention to internal sound representations is crucial for understanding what another person is saying, especially in the midst of other concurrent conversations. In this study, different types of linguistic complexity tasks were grouped together under one single paradigm and provided a comparison group against the two other manipulations affecting the acoustic quality of the speech signals. Further research is needed to determine whether the effort exerted into different linguistic complexity tasks also recruit distinct areas (for further discussion of this issue, see Rodd et al., 2015).
4.4 Patterns of activation during difficult listening conditions
This meta-analysis aimed to determine if there are overlapping activation patterns across speech identification and comprehension studies, which varied either in background noise level, spectral quality of the incoming speech sounds, or in linguistic complexity. The results revealed that brain activation patterns associated with increasing listening difficulty differed across the manipulation paradigms. Both SIN and linguistic complexity studies yielded consistent activations in left the IFG, but spectrally degraded speech sounds did not. Importantly, contrast analyses revealed differential involvement: activation of the Broca's areas (i.e., Brodmann area (BA) 45) was more associated with SIN whereas linguistic complexity recruited more dorsal prefrontal areas (BA 44 and 46). This distinction along the dorsal–ventral axis of prefrontal cortex could reflect the differences in the stimuli used—SIN studies more often used phoneme and single word stimuli, whereas linguistic complexity studies tended to use sentences. In SIN studies, effortful listening may recruit speech motor representations and inhibitory control processes within the left IFG, whereas linguistic complexity may place more demand on verbal working memory processes by recruiting distinct prefrontal regions. Evidence indeed suggests that working memory capacity is correlated with comprehension of complex sentences (Andrews, Birney, & Halford, 2006). Taken together, these results suggest that listening during difficult conditions is not a unitary concept, but rather depends on the specific acoustic and semantic characteristics of the speech signals. Hence, in effortful listening situations, different tasks and stimuli lead to differential recruitment of processes relying on the left IFG.
It is not surprising that right and left insula were consistently activated in both SIN and spectrally degraded speech studies as they directly manipulate acoustic quality of speech. However, the meta-analysis also revealed specific differences in the pattern of activation in the STG across manipulation paradigms, and this suggests that the recruitment of distinct processes is needed for successful identification of speech sounds in different effortful conditions. For instance, SIN requires participants to segregate and identify phonemes, words, or sentences against either broadband noise (energetic masking) or multitalker babble (informational masking). Unlike spectrally degraded speech, speech stimuli embedded in noise are not directly distorted; instead, the main challenge is to separate task-relevant speech signals from irrelevant background noise (figure-ground perception). This may explain the difference observed in the STG, with spectrally degraded speech consistently showing increased activation in the anterior portion of STG, that is, the planum polare, which is important for speech and sound object identification (Alain, Arnott, Hevenor, Graham, & Grady, 2001; Alain, Reinke, He, Wang, & Lobaugh, 2005; DeWitt & Rauschecker, 2012; Rauschecker & Scott, 2009).
5 LIMITATIONS
Meta-analyses of neuroimaging studies enable identification of core brain regions that support task performance independent of differences in methodology (e.g., sample size, materials used, and scanner type). However, one needs to consider the number of studies, the type of studies, and the methodology and statistical thresholds used because these variables may influence the interpretation. We followed recent guidelines for meta-analyses using ALE software. The number of studies included in each meta-analysis was sufficient to yield reliable patterns of activation. Nevertheless, it is possible that these patterns of activation may change as additional studies are published and can be included in each condition.
6 CONCLUDING REMARKS
Processing requirements in speech comprehension are higher when the physical quality of the incoming speech sounds declines or when the speech materials are more linguistically complex. These challenges usually imply an increase in listening effort, which places greater demands on cognitive resources to resolve perceptual or semantic ambiguity. This study indicates that different neural networks are used to resolve this ambiguity depending on the specific type of perceptual or semantic difficulty introduced. These results reveal that functional specialization within the left IFG supports effective listening under effortful conditions. The concept of listening effort is broad, and we recommend that future research studies continue to investigate subtypes within the three speech manipulation paradigms examined here and others that are less commonly explored, with an emphasis on listening resources rather than effort. Ultimately, listening under difficult conditions recruits different pools of resources to support perceptual and cognitive processes during verbal communication.
ACKNOWLEDGMENTS
This research was supported by grants from the Canadian Institutes of Health Research (MOP 106619) and the Natural Sciences and Engineering Research Council of Canada (NSERC) to CA.
ADDITIONAL INFORMATION
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
CA, YD, LJB, and KB designed the study. TB and CA were involved in data collection. CA analyzed the data. CA, YD, LJB, and KB interpreted data and wrote the manuscript.




