The retinal ganglion cell layer predicts normal-appearing white matter tract integrity in multiple sclerosis: A combined diffusion tensor imaging and optical coherence tomography approach
Funding information: BIGDATIMAGE; CENTRO-01-0145-FEDER-000016; Centro 2020 FEDER; COMPETE; Portuguese Foundation for Science and Technology, PAC – MEDPERSYST; POCI-01- 0145-FEDER-016428; POCI-01-0145-FEDER-007440; FCT-UID/NEU/04539/2013; UID/NEU/04539/2016; Biogen; Portuguese Foundation for Science and Technology, Grant Number: SFRH/BD/76013/2011.
Abstract
We investigated the relationship between retinal layers and normal-appearing white matter (WM) integrity in the brain of patients with relapsing-remitting multiple sclerosis (MS), using a combined diffusion tensor imaging and high resolution optical coherence tomography approach. Fifty patients and 62 controls were recruited. The patients were divided into two groups according to presence (n = 18) or absence (n = 32) of optic neuritis. Diffusion tensor data were analyzed with a voxel-wise whole brain analysis of diffusion metrics in WM with tract-based spatial statistics. Thickness measurements were obtained for each individual retinal layer. Partial correlation and multivariate regression analyses were performed, assessing the association between individual retinal layers and diffusion metrics across all groups. Region-based analysis was performed, by focusing on tracts associated with the visual system. Receiver operating characteristic (ROC) curves were computed to compare the biomarker potential for the diagnosis of MS, using the thickness of each retinal layer and diffusion metrics. In patients without optic neuritis, both ganglion cell layer (GCL) and inner plexiform layer thickness correlated with the diffusion metrics within and outside the visual system. GCL thickness was a significant predictor of diffusion metrics in the whole WM skeleton, unlike other layers. No association was observed for either controls or patients with a history of optic neuritis. ROC analysis showed that the biomarker potential for the diagnosis of MS based on the GCL was high when compared to other layers. We conclude that GCL integrity is a predictor of whole-brain WM disruption in MS patients without optic neuritis.
1 INTRODUCTION
Multiple sclerosis (MS) is the most prevalent inflammatory demyelinating disease of the CNS, affecting over two million people worldwide (Costello, 2013). Being a very complex pathology, different disease mechanisms may uniquely combine in each patient. Finding reliable biomarkers that reflect independent pathogenic processes in MS is therefore of the utmost importance (Katsavos & Anagnostouli, 2013).
The retina has been previously described as a potential “window” to the brain in major neurodegenerative diseases, and MS is no exception (London, Benhar, & Schwartz, 2013). Patients are often afflicted by damage in the visual pathway, in particular with optic neuritis (ON). Previous studies have assessed the relationship between optical coherence tomography (OCT) and magnetic resonance imaging (MRI) in MS. A relationship between both grey and white matter (WM) volumes and retinal nerve fibre layer (RNFL) thickness has been consistently observed in this disease (Oakley, Meyer, Frohman, Newsome, & Ratchford, 2013; Pfueller et al., 2011; Siger et al., 2008; Young et al., 2013; Zimmermann et al., 2012). A similar observation seems to hold true in a few studies addressing overall ganglion cell layer-inner plexiform layer (GCL-IPL) thickness (without explicit segmentation of each layer) (Oakley et al., 2013; Zimmermann et al., 2012).
However, studies regarding the association between WM tract integrity in MS and OCT are scarce. Diffusion tensor imaging (DTI) can be used to calculate diffusion metrics—fractional anisotropy (FA) and mean diffusivity (MD)—based on the three diffusion eigenvectors. These indices are indirect and sensitive markers of WM integrity. MD is higher in damaged tissues as a result of increased free diffusion, while FA decreases due to the loss of coherence in the main preferred diffusion direction (Soares, Marques, Alves, & Sousa, 2013).
A few studies assessed the relationship between DTI indices (focusing on the optic nerve, tract and radiation) and a single layer, the RNFL, using low resolution time-domain OCT (Dasenbrock et al., 2011; Kolbe et al., 2012; Reich et al., 2009; Smith et al., 2011). Recently, a study suggested that peripapillary RNFL thinning indicates WM damage even beyond the visual pathway (Scheel et al., 2014).
In this study, we asked whether the integrity of individualized retinal layers can be taken as a window to WM tract integrity in the MS brain. We investigated to what extent retinal thickness of well-defined retinal layers can be used as biomarkers for WM dysfunction in this context. This study makes use of tract-based spatial statistics (TBSS), a quantitative data-driven approach to evaluate whole-brain diffusion metrics, and OCT to measure retinal layers' thickness, using state-of-the-art spectral-domain OCT and a recent algorithm for semi-automated segmentation of all retinal layers (Abràmoff, Garvin, & Sonka, 2011; Garvin et al., 2009; Li, Wu, Chen, & Sonka, 2006).
2 MATERIALS AND METHODS
2.1 Participants
Relapsing-remitting MS (RRMS) is the most common subtype of MS (85% of all cases; Costello, 2013) and, consequently, the most extensively studied. Therefore, and for homogeneity purposes, 50 RRMS patients and 62 healthy controls (HC), age and gender matched, were enrolled in this study (Table 1). Patients were periodically followed in the Neurology Department of the Coimbra Clinical and Academic Centre (CHUC-UC) and were enrolled consecutively, while controls were recruited from the community. All participants in the study gave their written informed consent, approved by the Ethics Committee of the University of Coimbra.
|
HC (n = 62) |
RRMS (n = 50) | p-valuea | ||
|---|---|---|---|---|
| MSON (n = 18) | MSNON (n = 32) | |||
| Age ± SD | 36.5 ± 9.64 | 37.6 ± 4.96 | 36.1 ± 7.13 | ns |
| Female/Male (ratio) | 41/21 (1.95) | 12/6 (2.00) | 19/13 (1.46) | ns |
| EDSS median (IQR) | – | 2.0 (1.13) | 2.0 (1.00) | ns |
| Disease duration | – | 10.8 ± 1.61 | 9.6 ± 1.13 | ns |
| Whole skeleton lesion load (%) | – | 1.56 ± 1.64 | 3.31 ± 3.15 | ns |
| Visual acuity | 11.6/10 | 9.5/10 | 10.0/10 | .060 |
- a As a p value close to the level of significance was found for visual acuity (p = .060), its value is displayed, although acuities were near normal for all groups (>9.5).
- Abbreviations: HC = healthy controls; RRMS = relapsing-remitting multiple sclerosis; MSON = patients with history of optic neuritis; MSNON = patients with no history of optic neuritis; SD = standard-deviation; IQR = interquartile range; ns = not significant.
The included patients were aged between 18 and 55 years old, had a definite diagnosis of MS according to the 2010 McDonald criteria (Polman et al., 2011) and relapsing-remitting disease course. Exclusion criteria for all participants were a history of neurological (other than MS in the patient group) or systemic disease, a significant visual impairment or other ocular or medical conditions with known effects on the retina. For patients, a relapse or steroid treatment within 8 weeks preceding evaluation were also considered as exclusion conditions. All patients were under treatment with disease-modifying drugs.
Excessive tissue injury following MS associated ON may alter the underlying retinal-brain relationships in MS (Siger et al., 2008; Zimmermann et al., 2012) and a potential interference effect of ON-eyes on the fellow no ON-eyes has been previously reported (Klistorner et al., 2014). Thus, our patients' cohort was divided on the subject level in patients with a history of ON (MSON) in one or both eyes, and with no history of ON (MSNON) in either eye, and the average of both eyes' clinical and OCT data were considered in all analyses.
2.2 Clinical assessment
A full medical history was obtained for all patients. The collected clinical and demographic data included age, gender, disease duration and history of ON (which had to be determined by existing medical records). Physical disability was evaluated using the detailed Kurtzke Expanded Disability Status Scale (EDSS) (Kurtzke, 1983). Medical history of healthy controls was obtained by an interview preceding assessment. Visual acuity testing was performed monocularly in all participants, using the Snellen acuity chart. Lesion load percentage was calculated for every patient, as the percentage of the considered WM tracts affected by T2-hyperintense lesions.
2.3 MRI acquisition
All MRI acquisitions were performed on a 3 Tesla Siemens Magnetom TrioTim scanner (Erlangen, Germany) at the Institute of Nuclear Sciences Applied to Health (ICNAS) using a 12-channel birdcage head coil. Scans were prescribed in an axial-oblique orientation, parallel to the sub-callosal line.
Two high-resolution T1-weighted three-dimensional Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE; repetition time: 2,530 ms, echo time: 3.42 ms, inversion time: 1.1 s, flip angle: 7°, field of view: 256 × 256 mm2, 176 slices, voxel size: 1 × 1 × 1 mm3) were acquired. The protocol also included a sagittal three-dimensional Fluid Attenuated Inversion Recovery image (FLAIR; repetition time: 5 s, echo time: 388 ms, inversion time: 1.8 s, field of view: 250 × 250 mm2, 160 slices, voxel size: 1 × 1 × 1 mm3) for lesion detection and a DTI sequence (repetition time: 7,800 ms, echo time: 90 ms, number of excitations: 1, matrix: 96 × 96 × 63 contiguous axial slices; isotropic voxel resolution: 2 × 2 × 2 mm3; bandwidth: 1,628 Hz/pixel, echo spacing: .72 ms, 63 non-collinear directions, b value = 1,000 s/mm2).
2.4 MRI analysis
FLAIR images were segmented using the lesion growth algorithm (Schmidt et al., 2012) as implemented in the Lesion Segmentation Toolbox (http://www.applied-statistics.de/lst, version 1.2.3) for SPM, to evaluate T2-hyperintense WM lesion load. Binary lesion masks were created with the toolbox, followed by visual inspection and manual correction when necessary, using the MRIcron software (www.mricro.com/mricron).
DTI images were processed using Oxford University's FMRIB Software Library (FSL, http://www.fmrib.ox.ac.uk/fsl) version 5.0.9, on a Linux-based platform, following the TBSS pipeline. TBSS is a whole-brain, automated and observer-independent method that allows for statistically powerful analyses (Smith et al., 2006).
Eddy current distortions and motion artefacts were corrected, and extra-cerebral tissue was removed during the pre-processing stage. The diffusion tensors were fitted to each voxel, and quantitative measures of FA and MD were derived voxel-wise for each participant.
The FA images were then non-linearly registered to a 1 × 1 × 1 mm3 standard space image (FMRIB58_FA) and averaged. A threshold of FA > .2 was applied on the resulting mean FA image when creating the thinned mean FA skeleton, to exclude non-WM voxels. By creating a representation of the center of every participant's fiber tracts, TBSS minimizes inter-subject variability (Smith et al., 2006). The MD data were processed similarly, using the nonlinear warps and skeleton projections previously obtained in the FA processing steps.
2.5 OCT acquisition and analysis
All participants underwent retinal imaging with spectral domain OCT (Cirrus HD-OCT, Carl Zeiss Meditec, Dublin, CA). We found it important to go beyond the classical RNFL and overall (non-segmented) GCL-IPL comparisons in MS (González-López et al., 2014; Saidha et al., 2011), assessing the macular GCL and IPL thickness individually and by investigating the role of the remaining retinal layers. Furthermore, considering the better signal-to-noise ratio (SNR) in macular scans compared to optic nerve head scans, our analyses were restricted to the macular area.
Macular measurements were performed using either the “Macular cube 200 × 200” or “Macular cube 512 × 128” settings, to obtain a 6 x 6 mm cube around the fovea centralis. OCT scans were performed by two experienced, trained and certified technicians and were later individually visually assessed on their quality when evaluating the quality of the segmentation.
The macular scans were analyzed using the Iowa Reference Algorithm software, version 4.0.0 (Retinal Image Analysis Lab, Iowa Institute for Biomedical Imaging, Iowa City, IA) (Abràmoff et al., 2011; Garvin et al., 2009; Li et al., 2006). This software allows for reliable segmentation of the following retinal layers: RNFL, GCL, IPL, inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), inner segment/outer segment junction (IS/OS), outer segment (OS), outer photoreceptor (OPR), subretinal virtual space and retinal pigment epithelium (RPE). In this study, we focused on the individual layers from RNFL to ONL (Figure 1).
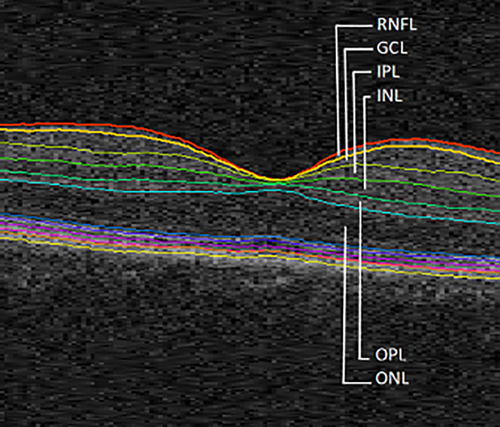
Macular image segmentation results, identifying the layers considered for this study. RNFL, retinal nerve fiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer [Color figure can be viewed at wileyonlinelibrary.com]
Following the segmentation process, all scans and layers were verified by one of the authors (CA). Manual corrections were made whenever a clear algorithm failure was identified. The thickness of each layer was calculated as the average from the whole macular area.
2.6 Statistical analysis
Group comparisons were performed using the t test for unpaired samples or the nonparametric Mann–Whitney test, when comparing between two groups, and the one-way ANOVA or the Kruskal–Wallis test when comparing between three groups, when appropriate. Post-hoc comparisons were performed using a Bonferroni correction for the one-way ANOVA and Dunn's correction for the Kruskal–Wallis test. A chi-square test was used for comparing categorical variables. Normality was previously assessed using the Kolmogorov–Smirnov test. Statistical testing was performed using SPSS version 19.0 (SPSS Inc., Chicago, Ill). The tests were performed two-tailed and p < .05 was used as a threshold for statistical significance, after applying Bonferroni corrections to account for multiple comparisons.
For analysis of the TBSS results, voxel-wise statistics for each mean FA skeleton voxel were calculated using FSL's randomise, which combines permutation test theory and a general linear model design matrix. Partial correlation analyses were thus performed between retinal layers' thickness and diffusion metrics for the MSON, MSNON, and HC groups, treating age as a covariate of no interest (Inano, Takao, Hayashi, Abe, & Ohtomo, 2011). Lesion maps were also taken into consideration as voxel-wise covariates, after being transformed to the same standard space as the FA images, to model out their effect and focus on changes in normal-appearing WM.
A total of 5,000 permutations were used with Threshold-Free Cluster Enhancement fully corrected for multiple comparisons by controlling for family-wise error (FWE) rates (Smith & Nichols, 2009). We added an additional correction for multiple comparisons, by also considering the number of layers being tested.
The skeletal regions with significant results were labeled anatomically by mapping the TBSS FWE-corrected statistical maps to the JHU-ICBM-DTI-81 WM atlas, containing a total of 48 WM tract labels (Oishi et al., 2009). Masks of the optic radiation (OR) and splenium of the corpus callosum (SCC), as WM tracts associated with the visual system, and the non-visual skeleton, as the remainder of the WM skeleton excluding the OR and SCC, were derived from the atlas and analysed. The tracts were dilated by using the “tbss_fill” command in FSL to enhance visualisation of the results.
Afterwards, we performed a stepwise multiple linear regression analysis to determine the variables potentially predictive of WM integrity, using the retinal layers' thickness, age and lesion load percentage per tract as predictors for the FA and MD metrics in the OR, SCC and remainder of the WM skeleton, using SPSS. Mean FA and MD values per tract were obtained from the whole-brain WM skeletonized map, before the voxel-wise analysis.
A receiver operating characteristic (ROC) curve analysis was also performed in SPSS, to compare the biomarker potential of the retinal layers and the diffusion metrics for the diagnosis of MS.
3 RESULTS
3.1 Group differences in demographic, clinical, DTI and OCT data
Regarding demographic and clinical data (Table 1), age and gender were matched between all groups. Disease duration, EDSS and whole-skeleton lesion load did not differ between RRMS subgroups. Visual acuity was nearly normal in all groups (≥9.5/10, in spite of the observed statistical trend for a small difference).
Table 2 shows the mean absolute thickness of all considered retinal layers for HC, MSON and MSNON groups. Regarding the thickness of each of the outer layers' (OPL and ONL) and INL, there were no differences between any of the groups. However, the three innermost layers (RNFL, GCL, and IPL) showed statistically significant differences between groups. Post-hoc tests with Bonferroni correction revealed atrophy in GCL and IPL between both RRMS subgroups and HC, and also between MSON and MSNON (p < .01 in all comparisons). RNFL thinning was observed for MSON and MSNON compared to HC (p < .001 for both), however the same does not hold true when comparing between RRMS subgroups (marginal significance level, p = .056).
| Layer |
HC (n = 62) |
RRMS (n = 50) | p-valuea | |
|---|---|---|---|---|
| MSON (n = 18) | MSNON (n = 32) | |||
| RNFL | 30.36 ± .43 | 23.69 ± .89 | 28.38 ± .81 | <.001a |
| GCL | 38.87 ± .39 | 30.14 ± 1.02 | 34.17 ± .98 | <.001a |
| IPL | 36.14 ± .35 | 30.43 ± .78 | 33.55 ± .76 | <.001a |
| INL | 34.79 ± .39 | 33.87 ± .41 | 34.10 ± .61 | ns |
| OPL | 26.76 ± .30 | 26.44 ± .43 | 27.17 ± .43 | ns |
| ONL | 81.79 ± .85 | 80.45 ± .85 | 81.75 ± 1.32 | ns |
- a p-values <.05 were considered statistically significant (Bonferroni corrected).
- Abbreviations: HC = healthy controls; RRMS = relapsing-remitting multiple sclerosis; MSON = patients with history of optic neuritis; MSNON = patients with no history of optic neuritis; RNFL = retinal nerve fibre layer; GCL = ganglion cell layer; IPL = inner plexiform layer; INL = inner nuclear layer; OPL = outer plexiform layer; ONL = outer nuclear layer; ns = not significant.
Regarding the diffusion metrics analysis, statistically significant differences were observed between all groups, for both diffusion metrics and every considered tract (OR, SCC, and non-visual skeleton). Post-hoc comparisons (Table 3) revealed a decrease of FA and increase of MD values in both the visual and non-visual WM tracts in both RRMS patients' subgroups when compared to HC. However, no differences were observed between RRMS subgroups.
| RRMS (n = 50) | p-value | |||||
|---|---|---|---|---|---|---|
| DTI metric | HC (n = 62) | MSON(n = 18) | MSNON(n = 32) | HC versusMSON | HC versusMSNON | MSON versusMSNON |
| OR | ||||||
| FA | .185 ± .001 | .159 ± .005 | .166 ± .003 | <.001a | <.001a | ns |
| MD (×103 mm2/s) | .226 ± .005 | .254 ± .005 | .257 ± .003 | <.001a | <.001a | ns |
| SCC | ||||||
| FA | .151 ± .001 | .146 ± .002 | .145 ± .001 | .007a | <.001a | ns |
| MD (×103 mm2/s) | .136 ± .003 | .154 ± .003 | .151 ± .002 | <.001a | <.001a | ns |
| Non-visual skeleton | ||||||
| FA | .127 ± .001 | .121 ± .002 | .122 ± .001 | <.001a | <.001a | ns |
| MD (×103 mm2/s) | .168 ± .004 | .182 ± .002 | .184 ± .001 | <.001a | <.001a | ns |
- a p-values <.05 were considered statistically significant (post-hoc Bonferroni/Dunn corrected).
- Abbreviations: OR = optic radiation; SCC = splenium of the corpus callosum; FA = fractional anisotropy; MD = mean diffusivity; HC = healthy controls; RRMS = relapsing-remitting multiple sclerosis; MSON = patients with history of optic neuritis; MSNON = patients with no history of optic neuritis; ns = not significant.
3.2 Partial correlations between diffusion metrics and thickness of retinal layers
To fully assess the relationship between the retina and WM tract integrity in the brain, we first conducted partial correlation analyses for each group, between OCT and DTI data. Considering the MSON group, no relation could be found between the diffusion metrics and the thickness of retinal layers (data not shown).
However, regarding the MSNON group, TBSS analysis showed a positive association between the thickness of GCL and IPL layers and FA, as well as a negative correlation between MD and both these layers. These correlations were observed both in the tracts associated with the visual system and outside (Figure 2, Table 4) although MD measures in the OR only displayed a trend towards significance for GCL. No association could be found between the OCT and DTI data in the control group (data not shown).
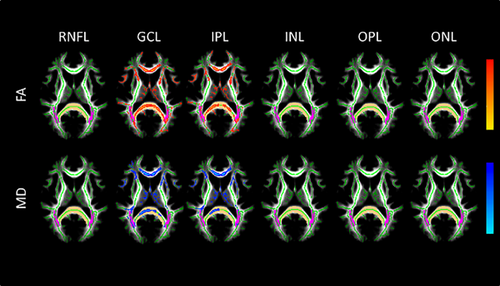
Axial images of TBSS results for the correlation analysis between both FA and MD and thickness of retinal layers in the MSNON group. The results shown are for statistical testing of a positive correlation between FA and retinal layers thickness measurements (top), and negative correlation for MD (bottom). Regions with significant correlation (with cluster-based thresholding corrected for multiple comparisons as well as Bonferroni correction for the number of retinal layers analyzed) are displayed in red to yellow (positive) and blue to light-blue (negative). Results are overlaid on the mean WM skeleton (green) and mean FA image (gray). Both the OR and SCC are highlighted, in pink and light-brown colors, respectively. White matter voxels with lesions were excluded from the analysis. Thus, only NAWM was considered. The significant portion of the tracts was dilated by using the “tbss_fill” command in FSL to enhance visualization. FA, fractional anisotropy; MD, mean diffusivity; OR, optic radiation; SCC, splenium of the corpus callosum; NAWM, normal-appearing white matter; RNFL, retinal nerve fiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer [Color figure can be viewed at wileyonlinelibrary.com]
| Retinal Layer | |||||||
|---|---|---|---|---|---|---|---|
| Tract | Statistic | RNFL | GCL | IPL | INL | OPL | ONL |
| FA | |||||||
| OR | p-value | ns | .018a | .042a | ns | ns | ns |
| Partial r | .299 | .350 | .323 | .137 | .093 | –.045 | |
| SCC | p-value | ns | .012a | .024a | ns | ns | ns |
| Partial r | .280 | .367 | .363 | .196 | .081 | –.016 | |
| Non-visualskeleton | p-value | ns | .024a | .048a | ns | ns | ns |
| Partial r | .305 | .362 | .354 | .335 | .047 | –.003 | |
| MD | |||||||
| OR | p-value | ns | .066 | ns | ns | ns | ns |
| Partial r | –.301 | –.297 | –.299 | –.368 | –.074 | .070 | |
| SCC | p-value | ns | .024a | .030a | ns | ns | ns |
| Partial r | −.311 | −.402 | −.393 | −.320 | −.141 | .051 | |
| Non-visualskeleton | p-value | ns | .048a | .042a | ns | ns | ns |
| Partial r | −.316 | −.354 | −.362 | −.331 | −.060 | −.019 | |
- a p-values <.05 were considered statistically significant (Bonferroni corrected). As a p value close to the level of significance was attained when correlating GCL with MD in the SCC (p = .066), its value is displayed.
- Abbreviations: OR = optic radiation; SCC = splenium of the corpus callosum; FA = fractional anisotropy; RNFL = retinal nerve fibre layer; GCL = ganglion cell layer; IPL = inner plexiform layer; INL = inner nuclear layer; OPL = outer plexiform layer; ONL = outer nuclear layer; ns = not significant.
3.3 Multiple linear regression analysis
To further assess the association between retinal layers' thickness and the diffusion metrics in the whole skeleton (within the visual system and otherwise), we performed a multiple linear regression analysis, using age, lesion load percentage per tract and the considered layers' thickness as predictors for the diffusion metrics (Table 5).
| Coefficients | Model Performance | |||||
|---|---|---|---|---|---|---|
| Tract | Predicting: | Significant predictors | Standardized β | Significance | F (p < .001) | R2 |
| DTI metrics | ||||||
| OR | FA | OR lesion load | –.540 | <.001 | F (2,29) = 35.029 | .707 |
| GCL | .514 | <.001 | ||||
| MD | OR lesion load | .546 | <.001 | F (3,28) = 20.067 | .683 | |
| GCL | –.292 | .016 | ||||
| Age | .271 | .026 | ||||
| SCC | FA | GCL | .669 | <.001 | F (1,30) = 24.366 | .448 |
| MD | GCL | –.731 | <.001 | F (1,30) = 34.461 | .535 | |
| Non-visual skeleton | FA | Non-visual skeleton lesion load | –.425 | .009 | F (2,29) = 20.032 | .580 |
| GCL | .425 | .009 | ||||
| MD | Non-visual skeleton lesion load | .465 | .003 | F (2,29) = 23.835 | .622 | |
| GCL | –.416 | .007 | ||||
| Whole-brain | FA | Whole skeleton lesion load | –.502 | <.001 | F (2,29) = 32.751 | .693 |
| GCL | .436 | .002 | ||||
| MD | Whole skeleton lesion load | .512 | .001 | F (2,29) = 25.901 | .641 | |
| GCL | –.389 | .008 | ||||
- Abbreviations: OR = optic radiation; SCC = splenium of the corpus callosum; DTI = diffusion tensor imaging; FA = fractional anisotropy; MD = mean diffusivity; GCL = ganglion cell layer.
Using the step model, we found that GCL thickness was the only retinal layer to independently explain a very significant amount of the variance in FA and MD in the whole WM skeleton of MSNON patients (Figure 3). Lesion load percentage per tract and age also significantly and independently predicted the diffusion values in some of the considered tracts.
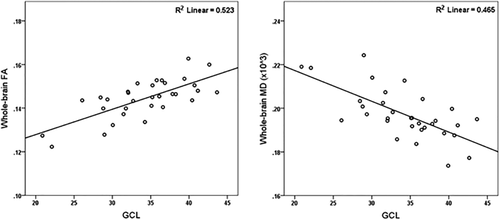
Scatterplot demonstrating the relationship between GCL thickness (main retinal predictor) and whole-brain FA (left) and MD (right). GCL, ganglion cell layer; FA, fractional anisotropy; MD, mean diffusivity
Regarding the MSON group, lesion load percentage per tract was the sole predictor of FA and MD variance in the whole WM skeleton, while the HC group had no significant predictors to report (data not shown).
3.4 Receiver operating characteristic curve analysis
As a complementary analysis based on the previous results, and to compare the biomarker potential of retinal layers' thickness, whole skeleton FA and whole skeleton MD in determining the MS diagnosis, a ROC curve was computed (Figure 4).
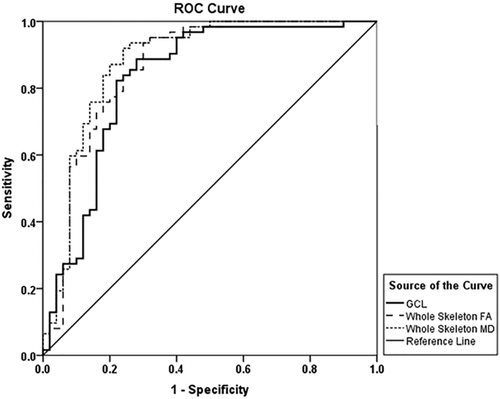
ROC curves for whole skeleton FA, whole skeleton MD and GCL. The reference line represents the random event. All of these areas under the ROC curve were significantly greater than chance (p < .001). GCL, ganglion cell layer; FA, fractional anisotropy; MD, mean diffusivity
While both FA and retinal layers' thickness values decrease in MS patients, there is an increase of MD. To facilitate the comparison, we considered the value 1/MD for the ROC curve analysis.
FA had an area under the curve (AUC) of .86 (95% confidence interval (CI): .79–.94), MD showed an AUC of .88 (95% CI: .81–.95) and GCL an AUC of .83 (95% CI: .75–.91). All three AUCs showed a p value under .001, classifying MS better than chance. These results are graphically presented in Figure 4.
The remaining retinal layers had lower AUC results, some of them being actually at chance level. From highest to lowest: IPL AUC = .77 (95% CI: .68–.86; p value: <.001); RNFL AUC = .75 (95% CI: .65–.85; p value: <.001); INL AUC = .59 (95% CI: .49–.70; p value: ns); ONL AUC = .53 (95% CI: .41–.63; p value: ns) and OPL AUC = .48 (95% CI: .37–.58; p value: ns).
4 DISCUSSION
Previous studies correlating retinal measures with MS biomarkers mainly focused on RNFL derived measures (Oakley et al., 2013; Pfueller et al., 2011; Siger et al., 2008; Young et al., 2013; Zimmermann et al., 2012) but no other explicitly segmented retinal layers. The present cross-sectional study addressed pathology in multiple retinal layers. We found that macular GCL atrophy is the one that most conspicuously predicts whole-brain WM tract pathology associated with RRMS. As such, measuring macular GCL with OCT could have a similar value as compared to DTI as a biomarker for WM integrity assessment in this disease type.
In line with a previous study (Albrecht et al., 2012), which did not segment GCL and IPL individually, retinal atrophy was only identified in the three innermost layers of the retina of RRMS patients although no investigation of cortical tract pathology was performed in that study.
Thinning of the innermost layers of the retina in MSON was more severe than in MSNON, as expected. In MSNON it is possible that retrograde trans-synaptic degeneration occurs (Petzold et al., 2010). Our results in the MSNON subgroup suggest that the extension of retrograde axonal degeneration has the INL level as its boundary. This finding falls in line with a recent OCT study, where the GCL and IPL were considered as a whole, which proposes that neuroplasticity in the INL might act as a barrier for retrograde trans-synaptic degeneration, in long-standing MS (Balk et al., 2014).
As previously stated, diffusion metrics are associated with WM tract integrity. A decrease in FA reflects the loss of WM microstructural integrity, while an MD increase is associated with edema, axonal, and myelin loss (Elshafey, Hassanien, & Khalil, 2014). These observations have been previously reported in MS patients (Liu et al., 2012) and were accordingly observed in the whole-brain WM of the RRMS group in the present study.
To our knowledge, this was the first study to investigate the extent of the association of retinal atrophy and WM tract integrity in the brain as assessed by DTI.
Previous studies suggested that peripapillary RNFL and overall GCL-IPL thickness derived from MSON eyes might not correlate with brain volumes of white and grey matter, unlike MSNON measures (Zimmermann et al., 2012). In this study, we observed that none of the analyzed retinal layers from MSON eyes correlated with diffusion metrics in these patients, unlike MSNON. These results in our study might be partially explained due to the smaller sample size of the MSON subgroup (n = 18) compared to the MSNON subgroup (n = 32). Two other explanatory frameworks might explain this effect: disproportionate retinal tissue injury after ON alters the relationship between local retinal atrophy and global WM dysfunction or, given that there is less retinal tissue available due to GCL-IPL thinning resulting from the ON event, its association with WM integrity may become masked (Saidha et al., 2015).
Regarding the MSNON group, it is surprising to note that no association was found between macular RNFL thinning and brain WM damage. This lack of association further highlights the potential value of GCL measures.
OCT measures of GCL and IPL thicknesses were associated with both DTI metrics, thus mirroring WM tract integrity (FA measures), as well as edema and axonal and myelin loss (MD measures). This relationship was found to extend beyond the visual system, reflecting whole-brain WM dysfunction. These findings expand on the previously reported association between peripapillary RNFL thinning and FA decrease in tracts associated with the visual system (Dasenbrock et al., 2011; Kolbe et al., 2012; Reich et al., 2009; Smith et al., 2011) and beyond (Scheel et al., 2014). The lack of such associations in the control group suggests that the relation observed between GCL and IPL and diffusion metrics are explicitly associated with MS pathology.
Multiple linear regression results confirmed that GCL predicts the variance in FA and MD in the whole WM skeleton, and that the outer layers as well as macular RNFL and INL, do not. Moreover, IPL was not able to significantly contribute to the prediction.
These results suggest GCL has an important predictive association with diffusion metrics, mirroring WM dysfunction more accurately than IPL.
The standardized beta coefficients of the significant predictors for the whole-brain FA and MD, GCL, and lesion load, are generally in the same order of magnitude (.389–.512, p < .01) meaning they both have similar contributions to the model.
The influence of MS lesions on TBSS results, although sometimes regarded as minimal due to the skeletal nature of TBSS processing (Scheel et al., 2014) might impact this type of analysis, justifying the implemented correction, and should, therefore, be considered when assessing WM integrity with this method in MS.
Regarding the ROC curve analysis, GCL showed an AUC of .83, depicting a significant biomarker potential for MS diagnosis in patients with the relapsing-remitting subtype. This value was very similar to that of both diffusion metrics, with all three showing classification capabilities well beyond chance, providing further support to the presented hypothesis that GCL could be used as a surrogate for FA and MD when assessing WM integrity. Both RNFL and IPL also displayed AUCs greater than chance, although with somewhat lower performance than GCL (.75 and .77, respectively).
Our results suggest GCL mirrored whole-brain WM tract dysfunction more accurately than IPL. Most importantly, IPL did not contribute to the diffusion metrics prediction in the multiple linear regression analysis, unlike GCL. This is consistent with the observed higher effect size of GCL compared to IPL in the partial correlations analyses and the larger AUC of GCL compared to IPL that was found for the ROC curve analysis.
Thus, further improvements in segmentation algorithms to correctly differentiate both layers could benefit OCT-DTI and potentially OCT-MRI studies in MS.
The present study has some limitations. Being a cross-sectional study, the temporal dynamics of the interplay between retinal changes and central WM tracts dysfunction warrants further investigation in longitudinal studies. Moreover, all patients were on disease-modifying therapies, making it likely that our results underestimate retinal atrophy, which might be higher in untreated MS populations. Future studies should consider homogeneously treated subgroups, for a better assessment of the effects of such therapies on the relation between retinal and WM alterations in the MS brain.
The findings of this study provide evidence of the potential of layer specific GCL measures derived from OCT as complementary to MRI when assessing MS patients, as a cost-effective tool in clinical practice.
ACKNOWLEDGMENTS
The authors would like to thank João Pereira for his contribution to DTI data processing, Luísa Ribeiro for the recruitment of participants, Aldina Reis for the OCT acquisition and João Lemos for verifying and collecting clinical data.
COMPETING INTERESTS
S. Batista has received honoraria for serving on scientific advisory boards of Biogen and Novartis Pharma, and for speaking in scientific meetings of Teva, Merck Serono, Biogen, Genzyme and Novartis Pharma.
L. Sousa has received honoraria for serving on scientific advisory boards or speaking in scientific meetings of Teva, Merck Serono, Bayer, Genzyme, Biogen, and Novartis Pharma.
C. Alves, O. C. d'Almeida, R. Bernardes, L. Cunha and M. Castelo-Branco have no interests to disclose.