Subcortical roles in lexical task processing: Inferences from thalamic and subthalamic event-related potentials
Abstract
Subcortical functions for language capacities are poorly defined, but may be investigated in the context of deep brain stimulation. Here, we studied event-related potentials recorded from electrodes in the subthalamic nucleus (STN) and the thalamic ventral intermediate nucleus (VIM) together with surface-EEG. Participants completed a lexical decision task (LDT), which required the differentiation of acoustically presented words from pseudo-words by button press. Target stimuli were preceded by prime-words. In recordings from VIM, a slow potential shift apparent at the lower electrode contacts persisted during target stimulus presentation (equally for words and pseudo-words). In contrast, recordings from STN electrodes showed a short local activation on prime-words but not target-stimuli. In both depth-recording regions, further components related to contralateral motor responses to target words were evident. On scalp level, mid-central activations on (pseudo)lexical stimuli were obtained, in line with the expression of N400 potentials. The prolonged activity recorded from VIM, exclusively accompanying the relevant LDT phase, is in line with the idea of thalamic “selective engagement” for supporting the realization of the behavioral focus demanded by the task. In contrast, the phasic prime related activity rather indicates “procedural” STN functions, for example, for trial sequencing or readiness inhibition of prepared target reactions. Hum Brain Mapp 38:370–383, 2017. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
Subcortical nuclei appear to participate in language functions, as suggested by many clinical observations, for example, in patients with thalamic infarction [Bogousslavsky et al., 1986; Bruyn, 1989; Carrera and Bogousslavsky, 2006; De Witte et al., 2011; Kuljic-Obradovic, 2003; Schmahmann, 2003; Van der Werf et al., 2000]. Respective language deficits are sometimes labeled as “thalamic aphasia” and feature word finding difficulties, dysnomia, lexical amnesia, and different types of paraphasias [Crosson, 1999]. Yet, the concept of subcortical aphasia is still relatively vague, and seems to be at odds with the corticocentric view on the functional anatomy of language, inspired by the seminal postmortem findings in aphasic patients reported by Broca and Wernicke [Broca, 1861; Wernicke, 1874]. Although in the meantime [Eling and Whitaker, 2010] more complex biolinguistic models have been elaborated [Hickok and Poeppel, 2007; Mesulam, 1990; Poeppel et al., 2012], many traditional assumptions are still influential. Altogether, they posit a left hemispheric “language system” with an inferior frontal “motor” and posterior temporal “perceptive” section, interconnected by the arcuate fasciculus [Geschwind, 1970; Lichtheim, 1885]. Sensu stricto, subcortical nuclei are not part of this model, or just provide the transsynaptic propagation of signals from corticofugal to corticopetal axons as passive information relays. This view has, however, been challenged in various models suggesting a role of subcortical nuclei in language processing [e.g., Nadeau and Crosson, 1997; Ullman, 2004]. These theoretical frameworks have proposed variable, mostly supportive functions of thalamic and basal ganglia structures such as monitoring, coordination, integration, or selection of cortical language activities. Yet, empirical evidence for these assignments is difficult to gain for different reasons: Correlations between aphasic symptoms and lesion sites are not unequivocal since subcortical lesions are seldom restricted to one particular structure, but mostly extend to adjacent or even remote brain regions. Furthermore, subcortical lesions determined by structural imaging can be paralleled by cortical perfusion deficits accounting for the observed language deficit [Choi et al., 2007; Hillis et al., 2004]. Finally, functional imaging and EEG, as typical research tools, depict cortical activations more easily than activities in circumscribed, small volume subcortical nuclei.
A seldom opportunity for the investigation of subcortical processes arises in the clinical context of deep brain stimulation (DBS) for the treatment of movement disorders. If indicated for disabling essential tremor and Parkinson's disease, the tissues in and around the thalamic ventral intermediate nucleus (VIM) or the subthalamic nucleus (STN) receive high frequency electrical pulses from bilateral electrodes, implanted in either structure and connected to a subcutaneous battery device [for review see Miocinovic et al., 2013]. This has beneficial effects with respect to the clinical symptoms of the underlying movement disorder at comparably low risks for severe cognitive sequels [e.g., Kalia et al., 2013]. However, a frequently reported DBS side effect is a decline in verbal fluency in respective standard tasks [Ardouin et al., 1999; De Gaspari et al., 2006; Fields et al., 2003; Funkiewiez et al., 2004; Lefaucheur et al., 2012; Mikos et al., 2011; Moretti et al., 2003; Morrison et al., 2004; Pillon et al., 2000; Schuurman et al., 2002; Troster et al., 1998; Witt et al., 2013; Woods et al., 2003; Zangaglia et al., 2012]. Whether this relies on a perturbation of proper lexical operations or other factors, such as the disruption of executive functions, cannot be answered unambiguously. Previous studies have suggested that the cause of verbal fluency decline in DBS patients may depend on the specific target structure involved: Regarding STN DBS, low verbal fluency is mostly considered a permanent postoperative sequel from brain lesions along the electrode trajectory, regardless of the actual stimulation status [Ehlen et al., 2014; Lefaucheur et al., 2012; Mikos et al., 2011; Morrison et al., 2004; Okun et al., 2009; Pillon et al., 2000; Witt et al., 2013]. It was thought to reflect a superordinate executive dysfunction, leading to reduced procedural speed, instead of a specific lexical impairment [Ehlen et al., 2014]. In contrast to this, patients with VIM DBS showed a marked stimulation-dependent VF decline, indicative of its relatively local causation [Ehlen et al., 2014]. These findings are consistent with a proposed thalamic involvement in the processing of syntactic and semantic phrase information as suggested on the basis of language-related potentials simultaneously recorded from VIM DBS and surface EEG electrodes, whereas no such activations were obtained from basal ganglia electrodes [Wahl et al., 2008]. Further, in patients with VIM DBS, performance in a primed lexical decision task (LDT) and word-related scalp potentials were found reduced as a function of the proper stimulation, which was not the case in patients with STN DBS [Krugel et al., 2014]. This frequently investigated task requires participants to discriminate words from pseudo-words that are preceded by either related or unrelated prime words. Behavioral responses on related word-pairs, for example, BREAD-BUTTER, are typically faster than on words without a categorial association, for example, BREAD-DOCTOR, [Meyer and Schvaneveldt, 1971]. Accelerated word recognition for primed targets has been interpreted as facilitated retrieval through spreading activation within the semantic network [Collins and Loftus, 1975]. Similar priming effects are also observed for phonemically related word-pairs and are most consistent if their final phonemes overlap, for example, RAMP-LAMP [Radeau et al., 1995], example from [Norris et al., 2002]. In contrast to semantic priming effects involving the mental lexicon, the facilitated processing of target words that rhyme with preceding primes has been explained by repeated activation of prelexical or sublexical representations (e.g., syllables) [Dufour, 2008]. In electrophysiological investigations, semantic as well as phonemic priming effects are accompanied by modulations of the N400-component [e.g., Bentin et al., 1985; Radeau et al., 1998]. This negative event-related potential (ERP) peaking around 400 ms poststimulus onset is sensitive to various stimuli and tasks; regarding word recognition the N400 seems to reflect categorization (e.g., of lexicality during word/pseudo-word classification) paralleled by attempts to access meaning [for review see Kutas and Federmeier, 2011]. Therefore, a combination of ERPs and the LDT allows studying behavioral and electrophysiological measures related to word recognition and sublexical processes. Furthermore, potentially different response patterns on lexical stimuli either as primes or as targets may help to distinguish processing of lexico-semantic versus executive task demands.
Here, we analyzed evoked potentials recorded from VIM and STN depth electrodes during a primed auditory LDT. The obtained responses were compared to simultaneously recorded, foremost cortically generated scalp-ERP. Based on the aforementioned clinical observations, conceptual considerations as well as DBS-induced changes in task-performance and ERP expression, we aimed to identify subcortical roles for word and task-related processing. We expected thalamic contributions to content-related operations as opposed to an STN involvement in executive task processing.
MATERIALS AND METHODS
Patients and Implantation of DBS-Leads
Ten subjects (three female, seven male; mean age 54.9 ± 12.6 years) with Parkinson's disease and seven subjects (three female, four male; mean age 61.9 ± 17.2 years) with essential tremor took part in the study. All were native German speakers. The mean disease duration in the Parkinson's disease group was 8.7 ± 7.2 years (range 2–27 years), and 16.9 ± 4.2 years (range 11–23 years) in the essential tremor group. None of the participants had significant cognitive impairment or relevant psychiatric symptoms such as psychosis or apathy based on a detailed neuropsychological and psychiatric assessment performed during clinical evaluation for DBS and at study inclusion. Demographic and clinical data of the participants are summarized in Table 1. The study protocol was approved by the institutional research Ethics Board (protocol number EA2/047/10); all participants gave their informed written consent according to the Declaration of Helsinki.
| Age, gender | Hand used for motor response | Disease duration (years) | Electrode positions | |
|---|---|---|---|---|
| Left | Right | |||
| STN | ||||
| 69, m | Left | 4 | 0-/1-/2+/3- | 0-/1+/2-/3- |
| 31, f | Right | 4 | 0+/1+/2+/3+ | 0+/1+/2+/3+ |
| 53, m | Right* | 9 | 0+/1+/2+/3+ | 0+/1+/2+/3+ |
| 47, f | Left* | 4 | 0+/1+/2+/3- | 0+/1+/2+/3+ |
| 77, f | Right | 2 | 0-/1+/2+/3+ | 0+/1+/2+/3- |
| 49, m | Left* | 5 | 0+/1+/2+/3- | 0+/1+/2-/3- |
| 51, m | Left | 12 | 0+/1+/2+/3+ | 0+/1+/2+/3+ |
| 61, m | Right | 10 | 0+/1+/2+/3+ | 0+/1+/2+/3+ |
| 52, m | Right | 10 | 0+/1+/2+/3+ | 0+/1+/2+/3+ |
| 59, m | Right | 27 | 0+/1+/2+/3- | 0+/1+/2+/3+ |
| VIM | ||||
| 73, m | Left* | 18 | 0-/1-/2+/3+ | 0-/1+/2+/3+ |
| 75, m | Right | 11 | 0-/1-/2+/3- | 0-/1-/2+/3+ |
| 70, f | Right | 19 | 0-/1-/2+/3+ | 0-/1-/2+/3+ |
| 58, f | Right | 14 | 0-/1 -/2-/3-(1.1) | 0-/1-/2-(1.9)/3- |
| 67, m | Right | 20 | 0-/1-/2+/3+ | 0-/1-/2+/3+ |
| 25, f | Right | 13 | 0-/1-/2-(1.1)/3- | 0-/1-/2-(1.8)/3- |
| 65, m | Right | 19 | 0-/1-/2-/3+ | 0-/1-/2+/3- |
- In the STN-group, patients had Parkinson's disease, in the VIM-group essential tremor. Patients using their nondominant hand for the button press due to disabling symptoms are marked with an asterisk. Electrode contacts localized within (≤ 1.0 mm) the designated target nucleus are denoted with (+) or (-) if outside. If all electrode-contacts were localized at a distance of > 1.0 mm from the target voxel, the distance between the contact closest to the target structure is given in brackets.
Surgery and Electrode-Localization
During surgery, the DBS target structures were identified on the basis of typical discharge patterns in microelectrode recordings and verified by macrostimulation effects. Postoperatively, the exact position of DBS-electrodes in relation to the respective target structures was determined based on the preoperative and postoperative T2-weighted MRI-scans. MRI-data were spatially normalized into the standardized Montreal Neurological Institute (MNI) stereotactic space. This was done by use of a nonlinear unified segmentation algorithm in the Statistical Parametrical Mapping (SPM) software version 12, corresponding to the “New Segment” algorithm in the previous version (SPM 8). The LEAD DBS toolbox [Horn and Kuhn, 2014, see also http://www.lead DBS.org] was used for semiautomatic reconstruction of electrode contacts which were superimposed with a digitized and normalized to MNI-space version of the Morel atlas [Jakab et al., 2012; Krauth et al., 2010; Morel et al., 1997]. Based on the Euclidean distances between DBS electrode contact centers and the nearest voxel within STN or VIM the electrode contacts were categorized as within or outside the respective target structures using a distance cutoff of 1 mm.
Experimental Procedure
The experiments were conducted within the first days (range 1–5; M = 3.1 ± 1.1) after implantation of the DBS electrodes while the leads were still externalized before implantation of and connection with the permanent stimulation device. In this short period, test stimulations and MRI are performed to verify the planned electrode position when potentially necessary surgical revisions are still possible at relatively low costs.
In the LDT, each trial comprised the acoustic presentation of a German word (noun) as “prime” followed by the target stimulus, being either a word or a pseudo-word. Target words were either semantically or phonemically related (each n = 15) or unrelated (n = 15) to the preceding prime. Pseudo-words were phonemically related (n = 15) or unrelated (n = 30 to match the number of trials in the “word condition”). Thus, each session included 90 prime and 90 target stimuli (the latter comprising 45 words and 45 pseudo-words in randomized order). Words and pseudo-words were monosyllabic or disyllabic with a mean duration of 745 ± 111 ms (prime-words), 746 ± 100 ms (target words), and 755 ± 111 ms (target pseudo-words). All real words were matched for word-frequency (according to “Online-Wortschatz-Informationssystem Deutsch”/“Online-Vocabulary-Information-System German”; http://www.owid.de; mean frequency layer 8.36). Pseudo-words were matched to real words from the semantically related category, in that they had the same number of syllables and of vowels. They resembled real German words by containing combinations of letters typically found in the German language; the frequency of the contained letters, digraphs and trigraphs was matched to that of real German words, and the first syllable was stressed so that altogether they were pronounceable easily. Disyllabic items were usually composed of two existing German syllables. Inter-stimulus intervals between prime-words and target words/pseudo-words were 100 ms. Primes were preceded by a fixation cross for 750 ms on a computer screen to minimize eye and head movements. Participants were instructed to indicate real target words by key press, whereas no response was required on pseudo-words. Therefore, wrong button presses following prime-stimuli or pseudo-words and omitted responses to real words amongst target-stimuli were defined as errors. Stimuli were presented at individually adjusted volume levels (semi-open earphones; Beyerdynamic™, DT-880). We used Presentation™ (Software Version 15.0, Neurobehavioral Systems) for task presentation and result recording.
Recording of Depth- and Scalp-EEG
EEG was recorded from depth electrodes and simultaneously from 12 scalp electrodes (Fz, F3, F4, Cz, C3, C4, Pz, P3, P4, T7, T8, and Oz; impedances were < 5 kΩ). Because of postoperative scalp wounds, edema, burr holes, and head bandages, the full array was, however, possible in a subset of patients only (see under Results). Each DBS electrode had four circular contacts numbered 0–3 from the lowest (0) to the uppermost (3) contact. Distances between each contact were 0.5 mm in STN DBS and 1.5 mm in VIM DBS (DBS Lead Models 3389 and 3387; Medtronic, Minneapolis, MN). The recordings were referenced to linked mastoid electrodes. Off-line, different bipolar channels were built along the DBS electrodes. EEG-data was continuously sampled at a rate of 2 kHz using high and low-frequency band-pass filters at 0.05 and 500 Hz (amplifier: Neuroscan SynAmps2, software: Acquire 4.5; Neuroscan, Charlotte, NC). For correction of eye-blink artefacts, vertical and horizontal electrooculograms were additionally recorded.
Data Analysis
The EEG-data was bandpass filtered from 0.1 to 20 Hz (12 dB/oct) using the software Vision Analyzer Version 2.0 (Brain Products GmbH, Germany). The baseline was defined as the 500 ms segment before presentation of prime-words. With respect to the depth-recordings, bipolar derivations were built by subtracting values between adjacent electrode-contacts, resulting in three channels (0-1/1-2/2-3). This was done to identify local near-field activity in either DBS target region for which the monopolar electrode array is generally less suited, for example, due to contamination by cortical activity spread [Wennberg and Lozano, 2003; Wennberg et al., 2002]. In the bipolar montage, particularly phase reversals and steep amplitude gradients are suggestive of activity generated within or close to the recording structure, whereas far-field activity is expected to occur similarly across all adjacent channels.
The responses were averaged per subject, target stimulus class (words vs. pseudo-words), and subcategory (unrelated words/pseudo-words, phonemically related words/pseudo-words, semantically related words). Averages of amplitudes and latencies evoked by prime words were analyzed in the same manner. In addition to these “forward analyses,” response-locked backward averages were built over all trials that contained motor responses to the correctly classified words.
By including all LDT phases into our analysis instead of focusing on relatedness effects we sought to identify different response patterns, which may allow to further characterize the respective recording site during lexical, procedural and motor-related processing. In case of identifiable ERPs, we were primarily interested in their dynamic course. In particular, the comparison of ERP onset and peak latency obtained from the different recording levels were considered as informative regarding processing differences between subthalamic, thalamic, and cortical regions. The ERP onset was determined as the moment from which onwards none of the subsequent sampling points reached the polarity opposite to that of the ERP peak. Signal magnitude differences between the distinct DBS sites and brain levels were not considered as meaningful, since ERP amplitude values might reasonably vary between STN versus VIM versus scalp recordings due to different volumes, neuronal densities, and in the case of the depths structures unknown electrical field properties as well as different electrode models used for VIM and STN, respectively.
Statistical analysis was conducted in IBM SPSS Statistics (software version 22), using nonparametric tests (Wilcoxon signed-rank test for within group comparisons and Wilcoxon rank-sum test for comparisons across groups).
RESULTS
Localization of DBS Leads and Clinical Outcome
In most participants, at least one of the electrode contacts was localized within the target structure (see Table 1). MNI-coordinates of electrode-contacts across individuals can be found in Table 2; see Figure 1 for a visualization of electrode-localizations. With particular respect to each target structure, the upper contacts (2 and 3) of VIM DBS electrodes were within (or closest to) the planned target structure, whereas contacts 0 and 1 were below as well as slightly medial and posterior to VIM in all patients. In the STN group, contacts 1 or 2 were within the designated target structure bilaterally or in one hemisphere in all patients. In those patients, where none of the contacts was localized within the designed targets (i.e., within a distance of ≤ 1 mm), the closest distance to the target nucleus was smaller than 2 mm. According to presumed propagation spread of DBS into surrounding tissue, all positions could be considered as clinically acceptable. This was confirmed by the clinical outcomes assessed in follow-up visits over 1 year after DBS surgery. Clinical benefit from DBS was documented in all patients with significant improvement of motor symptoms. Relevant side effects were unsteady gait and dysarthria in two cases, but repeated adjustments of DBS stimulation-parameters eventually provided sufficient therapeutic gain. In one patient DBS was temporarily discontinued due to manic symptoms with suicidal tendencies, which finally resolved under changed stimulation parameters and concomitant medication.
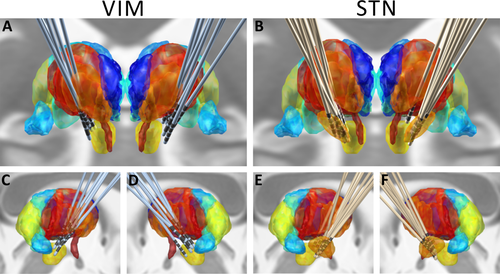
Electrode localizations. Views of DBS-electrode localizations in both groups from anterior (A, B) and lateral (C–F) directions. The anatomical structures were reconstructed based on the Morel atlas, normalized to MNI-space [Jakab et al., 2012; Krauth et al., 2010; Morel et al., 1997]. The medial thalamus is depicted in dark blue, anterior and lateral thalamus in red/dark orange, the pulvinar in green and light blue; the STN is shown in orange (B, E and F only). Below both thalami, the nucleus ruber (yellow) and mamillothalamic tract (dark orange) can be seen. The visualization of DBS-electrodes was created by using the LEAD-DBS toolbox in MATLAB [Horn and Kuhn 2014]; see text for details. [Color figure can be viewed at wileyonlinelibrary.com.]
| R-0 | R-1 | R-2 | R-3 | L-0 | L-1 | L-2 | L-3 | |
|---|---|---|---|---|---|---|---|---|
| VIM | ||||||||
| X | 12,1 (±0,8) | 13,0 (±0,7) | 13,9 (±0,6) | 14,8 (±0,6) | −12,4 (±0,6) | −13,3 (±0,5) | −14,2 (±0,5) | −15,0 (±0,5) |
| Y | −17,9 (±1,3) | −16,2 (±1,1) | −14,6 (±1,1) | −13,0 (±1,2) | −17,4 (±1,1) | −15,6 (±1,0) | −13,8 (±1,0) | −11,9 (±1,1) |
| Z | −7,0 (±0,7) | −4,0 (±0,9) | −0,9 (±1,1) | 2,1 (±1,4) | −6,3 (±0,9) | −3,4 (±1,1) | −0,6 (±0,8) | 2,3 (±1,7) |
| STN | ||||||||
| X | 11,6 (±1,2) | 12,1 (±1,2) | 12,7 (±1,1) | 13,2 (±1,1) | −10,4 (±0,9) | −11,0 (±0,9) | −11,7 (±1,0) | −12,3 (±1,0) |
| Y | −14,9 (±1,1) | −14,0 (±1,1) | −13,1 (±1,2) | −12,2 (±1,2) | −14,0 (±0,8) | −13,0 (±0,8) | −11,9 (±0,7) | −10,9 (±0,8) |
| Z | −9,1 (±1,4) | −7,2 (±1,2) | −5,4 (±1,1) | −3,5 (±1,0) | −8,6 (±1,3) | −6,9 (±1,1) | −5,1 (±1,1) | −3,4 (±1,1) |
- Electrode localizations in the MNI stereotactic space (averages ± standard deviation across all individuals per group). The X-axis describes medio-lateral, Y-axis antero-posterior, and the Z-axis rostro-caudal planes.
Behavioral Results
Mean reaction times (RTs), that is, the time-interval from onset of target-words until button press on correctly identified words, were similar in both groups with 1,172 ± 200 ms in patients with STN DBS and 1,125 ± 54 ms with VIM DBS. The mean error rate (omissions of button press on real words and wrong button presses following pseudo-words) in the STN DBS group was 8.1 ± 4.7% and 6.7 ± 5.8% for patients with VIM DBS. Neither RTs nor error rates differed between the two patient groups. The mean RT was significantly shorter on related (1,100 ± 167 ms) than unrelated word-pairs (1,265 ± 159 ms; P = 0.001). This pattern was maintained when comparing unrelated word pairs to both subcategories of relatedness, that is, to semantically and phonemically (both: P = 0.001) related word pairs as well as when analyzing both groups separately. In VIM patients, the priming effect was somewhat greater for phonemic than semantic relatedness (mean RTs: 1,040 ± 42 ms vs. 1,092 ± 64 ms; P = 0.018). The latter difference was not found in STN patients (mean RTs: 1,133 ± 215 ms vs. 1,116 ± 228 ms; P = 0.87).
Surface-EEG
For scalp wounds, edema and burr holes, signal quality of the surface EEG was comparably poor and scalp recordings could only be performed and analyzed in five out of seven VIM DBS and seven out of ten STN DBS patients. Given these limitations, group-wise grand averages of the scalp EEG were rather considered as reference signals to which the depth recordings were compared than as informative on a single subject level. In grand averages, the ERP pattern showed slow negativities most prominent over mid central recording sites (Cz) on both prime and target stimuli. The polarity and time course of the slow components were compatible with the sequential expression of N400 potentials to either stimulus class (see Figs. 2 and 5), peaking at 515 ± 51 ms (primes) and 561 ± 82 ms (targets) when averaged across all subjects. The N400-component was preceded by the N1-P2 complex, a sequence of a negative peak occurring at 166 (± 18 ms for primes, ± 14 ms for targets) followed by a positive ERP with a mean latency of 254 ± 16 ms (primes) and 257 ± 20 ms (targets). Neither ERP amplitudes nor latencies showed any differences between groups, stimulus classes, or subcategories.
Depth-Recordings: Stimulus-Locked ERPs
Recordings from VIM electrodes
Thalamic recordings from both sides showed slow deflections on target stimuli, beginning shortly after the prime-words present in the lowest channel 0-1. Overall, the expression of this sustained, slow activity on targets was not clearly bilateral: A left-thalamic preponderance was found in four as opposed to predominantly right-thalamic activations in three patients. Therefore, we analyzed the “dominant” ERPs, be they left or right hemispheric in a given subject, instead of an analysis performed for each hemisphere separately. This was done under the premise that close-to-midline ERP sources could spread distinctly into nearby DBS electrodes (e.g., due to slightly different angulations, placements, or anatomical asymmetries), so that potential ERP modulations (only showing up on the prominent side) were not missed (see also Discussion).
There were no thalamic ERPs elicited during the duration of prime-words with the mentioned response onset occurring at 827 ± 254 ms with a peak at 1,728 ± 94 ms (see Fig. 2). Given the mean duration of prime-words (745 ± 112 ms) and interstimulus intervals of 100 ms, this suggests that activity started with or slightly prior to the onset of target stimuli (words and pseudo-words). Accordingly, when referencing ERPs to target words and pseudo-words, the slow negative deflection was again seen in the lower channel 0-1 (see Fig. 3). Overall, an ERP pattern with an amplitude loss toward the upper bipolar channels was evident in six out of seven VIM DBS patients. The chronometric analysis for target-word elicited ERPs revealed a mean onset-to-peak latency of 678 ± 122 ms, beginning 154 ± 74 ms before stimulus-onset. With respect to pseudo-words, similar values were obtained with mean peak latencies of 748 ± 156 ms and a start 96 ± 179 ms before target presentation. Comparisons of any of these measures or amplitudes between conditions did not reach significance.
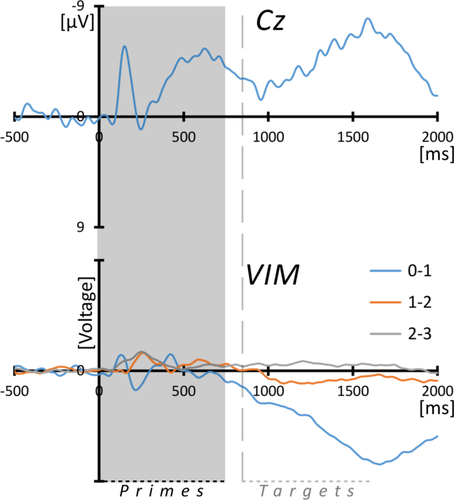
Depth- and surface-ERPs during prime-words processing (VIM). Grand averaged ERPs from surface-EEG at electrode Cz (top) and from thalamic DBS-leads in bipolar montages in the right (n = 3) or left (n = 4) hemisphere, according to the strongest activation (see text for details). Due to the large variations of amplitude between individuals and the small number of samples, the amplitudes were normalized by setting the maximum amplitude to a standard value and adjusting all other derivations by the respective factor. The gray box marks the mean duration of word primes (745 ms) and the vertical line the (approximated) onset of target stimuli (i.e., words and pseudo-words) after interstimulus intervals of 100 ms. Note that scalp-EEG is shown with negative values up. [Color figure can be viewed at wileyonlinelibrary.com.]

Depth-ERPs during word/pseudo-words processing (VIM). Grand averaged ERPs from thalamic DBS-leads in bipolar montages in the right (n=3) or left (n=4) hemisphere, according to the strongest activation (see text for details). For the process of normalized amplitudes across individuals, see legend for Figure 2. The gray box marks the mean duration of target stimuli, that is, words and pseudo-words (751 ms), “X” and the vertical gray line indicate the average RT until button press on correctly identified words (1,125 ms). [Color figure can be viewed at wileyonlinelibrary.com.]
The analysis of relatedness on ERPs on word/pseudo-word subcategories (see Fig. 4) did reveal differences between target stimuli related or unrelated to preceding primes: ERPs on unrelated words peaked later than ERPs on phonemically related words (mean latencies: 919 ± 79 ms vs. 760 ± 106 ms; P = 0.028) and (although this not being statistically significant) on semantically related words (mean latency: 827 ± 165 ms). Similarly, phonemically related pseudo-words also yielded shorter mean peak-latencies than unrelated pseudo-words (703 ± 67 ms vs. 888 ± 99 ms; P = 0.018). On the level of ERP amplitude, no comparable effects were identified.
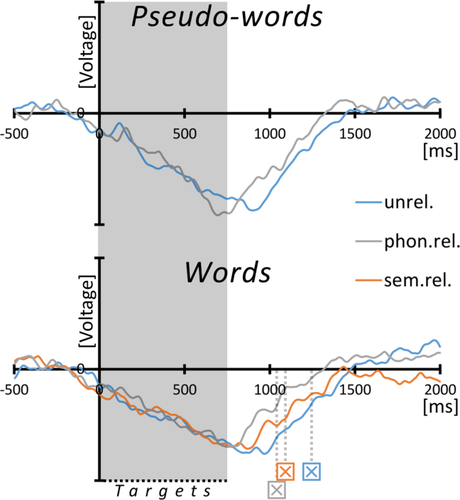
Depth-ERPs on word/pseudo-word subcategories (VIM). Grand averaged ERPs on target stimulus subcategories from thalamic DBS-leads in bipolar montage between the lowest electrode-contacts (0-1) in the right (n=3) or left (n=4) hemisphere, according to the strongest activation (see text for details). For the process of normalized amplitudes across individuals, see legend for Figure 2. The gray box marks the mean duration of target stimuli, that is, words and pseudo-words combined (751 ms), “X” indicates the mean RTs on correctly identified words in the respective subcategories according to color of the graphs. Abbreviations: unrel, unrelated; sem.rel, semantically related; phon.rel, phonemically related. [Color figure can be viewed at wileyonlinelibrary.com.]
Recordings from STN electrodes
Prime-words elicited a short, bilateral, and monophasic ERP. It was—by inspection—slightly left-lateralized and occurred mainly in lower and middle bipolar channels (electrode-pairs 0-1 and 1-2) with phase reversals (in eight out of ten patients) between lower and middle toward the upper electrode sections as well as between middle and lower channels (see Fig. 5). The component peaked at 318 ± 75 ms (left) and 362 ± 91 ms (right) without significant hemispheric differences. Target-stimuli were not associated with any apparent ERP component; baseline variations around 1,000 ms following target words were seen more contralateral to the motor-response.
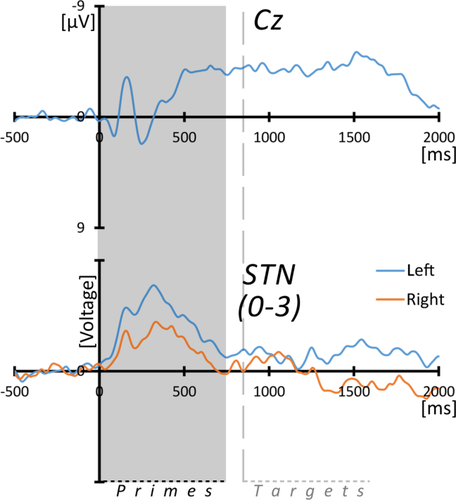
Depth- and surface-ERPs during prime-words processing (STN). Grand averaged ERPs from surface-EEG at electrode Cz (top) and from subthalamic DBS-leads in bipolar montages as indicated. The largest bipolar grid 0-3 was chosen for the grand-average due to variable phase-reversal patterns between adjacent bipolar channels across individuals, which would result in phase cancellations at group level. In the largest bipolar channel, all patients showed a positive polarity of the early peaking component. For the process of normalized amplitudes across individuals, see legend for Figure 2. The gray box marks the mean duration of word primes (745 ms) and the vertical line the (approximated) onset of target stimuli (i.e., words and pseudo-words) after interstimulus intervals of 100 ms. Note that scalp-EEG is shown with negative values up. [Color figure can be viewed at wileyonlinelibrary.com.]
Depth-Recordings: Response-Locked ERPs
Recordings from VIM electrodes
A short-latency ERP around the key-press was seen in VIM recordings contralateral to the hand used for the motor response with frequent phase reversals between upper (1-2 and 2-3) and lower (0-1) electrode channels (see Fig. 6). Additionally, the mentioned slow activity shift (as observed for the target-stimuli in stimulus-locked ERPs) was discernible markedly prior to the motor response in lower electrode-pairs (0-1). There were no consistent phase-reversals for this slow component, but again a steep amplitude gradient toward the upper electrode-pairs. Precisely, in lower electrodes (0-1), contralateral to the movement, the slow component started 1,285 ± 337 ms and peaked 218 ± 141 ms before the button press. In recordings from ipsilateral VIM-electrodes (bipolar channel 0-1), this slow component was also present with a similar time-course (starting at 1,389 ± 268 ms and peaking 156 ± 134 ms before button press). ERPs obtained from upper bipolar channels (values obtained from bipolar montage between electrodes 1-3) contralateral to the motor response showed a peak maximum occurring at 100 ± 95 ms with an onset—276 ± 142 ms with respect to response time. Upper derivations of VIM electrodes ipsilateral to the motor reaction did not contain any apparent activity.
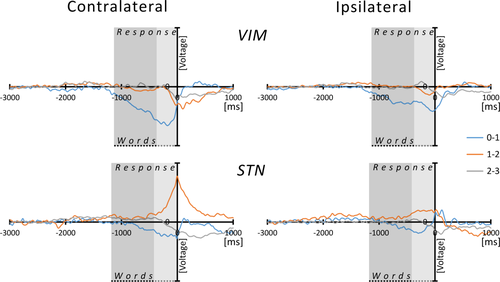
Response-related ERPs. Grand averaged, response-related ERPs from thalamic and subthalamic DBS-leads in bipolar montages as indicated. For the process of normalized amplitudes across individuals, see legend for Figure 2. Instead of left and right hemispheres, ERPs were calculated for the hemisphere contra- and ipsilateral to the hand used for the motor response. Time “0” relates to button-press; the gray box marks the lexical decision time as the sum of mean RTs (mean duration of word stimuli 746 ms, darker gray) and mean RTs (VIM: 1,125 ms; STN: 1,172 ms, lighter gray). [Color figure can be viewed at wileyonlinelibrary.com.]
Recordings from STN electrodes
In STN recordings contralateral to the button press, the middle bipolar channels (1-2) and less frequently the lower channel (0-1) contained the strongest signal related to the motor response (see Fig. 6), except maximum activity in the upper channel (2-3) in one patient. This ERP component started 459 ± 210 ms before and peaked 17 ± 66 ms after the button press. Phase reversals could be observed between middle and either lower (0-1) or cranial (2-3) derivations in eight out of ten subjects.
When comparing the time-course of the motor-related thalamic and subthalamic ERPs, signals obtained from upper VIM-electrode contacts (1-3) and STN (1-2) did not differ in either latency or signal-onset. The comparison of the slow deflections obtained from lower VIM-electrodes (0-1) with the motor-related ERP recorded from the STN at electrodes 1-2 (both contralateral to moving hand) yielded a significant difference of both latency (P = 0.001) and onset (P = 0.001) with an earlier activation in thalamic recordings.
DISCUSSION
The identified ERP patterns differed markedly in recordings from STN versus VIM, suggestive of distinct functions of either DBS target region for processing the task demands. With respect to the STN, two ERP components were evident: First, a bilateral phasic activity peaking 300–400 ms after prime word onset, best discernible in the wide grid of the DBS electrode and, second, a component rising shortly before and decaying right after the motor reaction on target words in the hemisphere contralateral to the response hand, predominantly in the central derivation channel. In contrast to this, in the derivations from the lower VIM channels a slow potential shift during the period of target presentation was seen bihemispherically without clear lateralization. This activation was similarly observed on all target subcategories with shorter peak-latencies for related than for unrelated words as well as for pseudo-words, that is, occurring irrespective of any subsequent motor-response, in line with analogue behavioral priming effects. In analogy to STN-recorded activity, a further component contralateral to the motor reaction was observed. Unlike the preceding thalamic component, this ERP was most prominent in the recordings from the upper portion of the VIM electrode. These results shall be discussed in terms of functional anatomy and concepts of subcortical contributions to cognitive processing.
Origin of Thalamic and Subthalamic ERPs
All components mentioned above appeared to be generated either in or close to the recording structure. Not surprisingly, in view of nuclei mostly associated with different aspects of motor processing, motor activity most likely accounted for activity occurring before and during button press on correctly identified real words in the contralateral hemisphere. An origin of these components within the DBS target structures is suggested by frequent phase reversals between adjacent bipolar channels in STN and VIM. Furthermore, in both groups these bipolar channels encompassed those electrodes, which were located within the designated target structures. Similar motor-related activations in STN and VIM recordings have been reported previously during self-paced and externally cued movements [Paradiso et al., 2003, 2004; Purzner et al., 2007].
Beyond these motor-related potentials in both STN and VIM recordings, bihemispheric components were identified without clear side preponderance. Concerning the early phasic component in STN recordings on prime-words, the steep amplitude gradients from middle and lower to upper electrode-pairs (located within the STN) as well as the occurrence of phase reversals suggest a local origin of this signal similar to that presumed for the motor-related ERP. In contrast, the slow potential shift obtained from VIM electrodes during target stimulus presentation was confined to the lower channel spanning from contact 0 to 1. Since remote activity should spread almost equally into the narrow-spaced longitudinal channel arrangement, the signal loss in upper and central channels is suggestive of a thalamic source adjacent to instead of within VIM. According to MRI-based electrode localization, the lowest contacts lay just outside VIM in all patients and the electrode trajectory was mostly vertical with a slight angulation toward near-midline structures as candidate generators of the thalamic nonmotor signal. In particular, the midline, mediodorsal, and intralaminar (comprising the centromedian and parafascicular) thalamic nuclei in the caudo-medial vicinity of the DBS target have been implicated in a wide scope of executive and language-related operations [Carreiras et al., 2009; Crosson, 1999; Ketteler et al., 2008; Liebermann et al., 2013; Rumsey et al., 1997; Van der Werf et al., 2000; Vannest et al., 2011; Ye et al., 2011; Zoppelt et al., 2003; for a review on the “higher-order” thalamus see Saalmann, 2014].
Thalamus
As aforementioned, thalamic activity evolved close to the onset of target stimuli in both hemispheres. This slow ERP component was identical in trials with words and pseudo-words, that is, regardless of the presence or absence of a motor response. Of note, pseudo-words in our task resembled real words rather than nonwords or plain sounds, suggesting that they engaged similar semantic and phonemic networks [Raettig and Kotz, 2008]. The preceding prime words were not accompanied by a comparable activation which, however, seemed to be the case at cortical levels. These properties suggest that the thalamic signal in question does not reflect the primary lexical processing, since then it should have occurred similarly on word stimuli, be they targets or primes. In this context, the absence of any left-hemispheric ERP preponderance might also be noted. Aside from a suggested, although ambiguous role of both hemispheres in, for example, speech comprehension or bilingualism [Federmeier et al., 2008; Hickok and Poeppel, 2007; Hull and Vaid, 2007], this finding is consistent with results from fMRI experiments: In a recent meta-analysis thalamic activations during language tasks were close to midline and mostly bilateral [Llano, 2013]. This may in the present case have caused variable spread into bithalamic DBS electrodes, for example, due to slightly different trajectories and placements or simply individual anatomical asymmetries, possibly underlying the variable lateralization observed across individuals.
Under the perspective of chronometry, thalamic nonmotor ERP began to build up in the short interval between the end of prime-words and the beginning of target stimulus presentation. Note that thalamic responses were absent during prime words, which were of no overt task relevance. Activation only started—and was maintained until the next prime presentation—when the explicit demand to categorize stimuli as real or pseudo-words was initiated by the imminent presentation of target words. In contrast to this, the grand averages from scalp recordings showed an ERP evolution already in the course of prime words outlasting target presentation, compatible with the sequential expression of N400 components. This ERP peaks 400 to 800 ms after stimulus onset and is thought to reflect semantic processing of (pseudo)lexical information in left temporal (posterior and middle) and frontal (inferior) gyri [Lau et al., 2008; Van Petten and Luka, 2006].
However, concerning VIM recordings, the missing responsiveness throughout prime presentation and the “prelexical” onset of activity preceding the beginning of target stimuli in VIM recordings do not necessarily contradict the possibility of lexical processing. In this respect, it is of note that particular aspects of thalamic ERPs were indeed reminiscent of priming effects in word processing, their peak latencies being shorter for related than unrelated prime-target pairs. This—together with the fact that ERPs on lexical stimuli were only evident when they were presented as targets—suggests that the thalamus is involved in the processing of both the presented lexical information and the superordinate task demand. A particularly tight relation of VIM-recorded ERPs to the ongoing behavioral focus has also been reported in other contexts [Marzinzik et al., 2008; Nikulin et al., 2008]. For example, in an oddball paradigm thalamic ERPs were shown to exclusively reflect the status of the eliciting signals as target versus nontarget, whereas aspects irrelevant for the imposed task, for example, the occurrence probability of nontarget stimuli, were only reflected by cortically generated scalp ERPs [Klostermann et al., 2006]. In the present case, the integration of environmental data into the current behavioral set would be the alignment of the known and anticipated task structure with the presented stimuli. Beyond this initial step, thalamic nuclei have been posited to interact continuously with cortical areas serving the monitoring and, consecutively, constellation of the particular cortico-cortical coupling necessary to accomplish with the given task [Hart et al., 2013]. As important prerequisites of such thalamic network orchestration also suggested in the “selective engagement” model [Nadeau and Crosson, 1997], mainly three anatomical and cellular properties deserve mention: (i) the connectivity of the thalamus with virtually all cortical regions, (ii) ubiquitous feed-forward and feed-back projections between both brain levels, and (iii) the capacity of thalamic relay neurons to shut down, amplify and direct the signal flow on the basis of distinct discharge modes [Guillery and Sherman, 2002; Murphy et al., 1999; Sherman, 2012; Steriade, 2000].
STN
Regarding STN recordings, the only consistent nonmotor activation, being a phasic ERP on prime-words, seems compatible with the processing of “procedural” task demands. On a formal level, prime-stimuli open new task trials (regardless of their semantic content). In this sense, they might be treated as “warning cues” allowing for the temporal segmentation of the task structure. This appears in line also with previous derivations from DBS electrodes showing spectral activity changes on stimuli predictive for pending motor responses [Oswal et al., 2012, 2013; Williams et al., 2003]. Furthermore, this is reminiscent of previously suggested STN functions in behavioral switching, that is, preparing for controlled actions with a changing behavioral context and the suppression of automatic responses, allowing for targeted task control [Isoda and Hikosaka, 2008].
Alternatively, the signal on prime-words could indicate an involvement of the STN in response inhibition [Zavala et al., 2015]. In the current task, the general readiness for motor responses, demanded on word target stimuli, had to be temporarily suppressed on prime-words. The STN has indeed been found to be involved in similar demands in Go/No-Go, stop-signal as well as Stroop tasks [for recent reviews see Jahanshahi et al., 2015; Zavala et al., 2015] and, in the context of decision making, was proposed to exert core functions for the suppression of premature actions [Frank, 2006; Wiecki and Frank, 2013]. Further, a very basic and general character of stop signal processing has been described for the STN: subthalamic activity was always found increased on stop signals whether the demanded inhibition took place or not, whereas nigral activity depended on the inhibition success [Schmidt et al., 2013]. Hence, the current prime-related signals could also be interpreted as a correlate of a superordinate STN stop function, beyond the motor domain: Note that pseudo-words required the inhibition of motor responses, but did not elicit an ERP as observed on prime words. Thus, the process induced by prime words does not necessarily refer to the suppression of the motor reaction to target stimuli, but might reflect the inhibition of the overall task demand, being the differentiation of words from pseudo-words.
Limitations
Particular limitations of the study deserve mentions. Given that occasions to record from DBS electrodes arise altogether seldom and are restricted to particular patient groups, it might be asked whether the present results from selected and small cohorts with wide ranges of disease duration were representative of normal task processing. However, we presume that potential differences to physiological conditions are subtle or gradual and should not refer to the overall response pattern. This view is supported, for example, by the neuropsychological evaluations of the study participants which did not show clinically relevant cognitive and, in particular, language deficits. With respect to the STN group, one might note that word processing deficits in PD rather relate to action-verbs than to nouns, as used here [for review see Cardona et al., 2013]. Finally, scalp recordings in freshly operated DBS patients are relatively noisy and therefore might have been somewhat different between groups. In particular, we presumed the scalp data to reflect sequential N400 components on prime and then target stimuli, as discernible in VIM patients. In the STN group, distinct peaks in the prime-related average in PD patients might have been blurred by asynchronous target word onsets together with noisy signals, leading to a sustained negativity. Thus, comparisons with the higher-quality depth signals therefore need to be cautiously interpreted.
CONCLUSIONS
The nonmotor responses obtained in recordings from VIM and STN electrodes indicate roles of neuronal populations in the vicinity of both deep brain regions in the given task context. In view of the thalamus, activity, presumably from caudo-medial generators adjacent to VIM, was present throughout the entire target phase, requiring the differentiation of word from pseudo-word stimuli. Together with the missing activation on prime words, this suggests a functional background, for example, compatible with the idea of “selective engagement” in the recruitment of cortical regions needed for the ongoing challenge. Furthermore, as thalamic ERPs on target stimuli were modulated by their relatedness to the preceding primes (accompanying behavioral priming effects), they additionally appear to reflect aspects of lexical processing in the given task context. In contrast, in the STN nonmotor activation was observed on formally task-irrelevant prime-words, but missing on target stimuli. This could reflect either the processing of the temporal task structure or inhibitory processing for suppressing reactions only required on the upcoming target stimuli. Altogether, the results suggest STN functions for “procedural” aspects of the cognitive demand as compared with a thalamic involvement in the realization of the particular task focus.