Desynchronization of autonomic response and central autonomic network connectivity in posttraumatic stress disorder
Abstract
Objectives
Although dysfunctional emotion regulatory capacities are increasingly recognized as contributing to posttraumatic stress disorder (PTSD), little work has sought to identify biological markers of this vulnerability. Heart rate variability (HRV) is a promising biomarker that, together with neuroimaging, may assist in gaining a deeper understanding of emotion dysregulation in PTSD. The objective of the present study was, therefore, to characterize autonomic response patterns, and their related neuronal patterns in individuals with PTSD at rest.
Methods
PTSD patients (N = 57) and healthy controls (N = 41) underwent resting-state fMRI. Connectivity patterns of key regions within the central autonomic network (CAN)—including the ventromedial prefrontal cortex (vmPFC), amygdala, and periaqueductal gray (PAG)—were examined using a seed-based approach. Observed connectivity patterns were then correlated to resting HRV.
Results
In contrast to controls, individuals with PTSD exhibited lower HRV. In addition, whereas controls engaged a localized connectivity pattern of CAN-related brain regions, in PTSD, key CAN regions were associated with widespread connectivity patterns in regions related to emotional reactivity (vmPFC and amygdala to insular cortex and lentiform nucleus; PAG to insula) and motor readiness (vmPFC and amygdala to precentral gyrus; PAG to precentral gyrus and cerebellum). Critically, whereas CAN connectivity in controls was strongly related to higher HRV (insula, mPFC, superior frontal cortex, thalamus), HRV covariation was absent in PTSD subjects.
Conclusions
This study provides the first evidence for a specific psychophysiological–neuronal profile in PTSD individuals characterized by lower resting HRV and a lack of HRV covariation with CAN-related brain connectivity. Hum Brain Mapp 38:27–40, 2017. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
As evidenced by the newly added symptoms of “negative alterations in cognition and mood” and the “inability to experience positive emotions” in the DSM-5 (2013), posttraumatic stress disorder (PTSD) is increasingly acknowledged as involving persistent negative emotional states. The integration of heightened negative states and the decreased ability to experience positive emotions into these diagnostic guidelines highlights an emerging focus on emotion regulatory capacities in PTSD [Friedman et al., 2011; Lanius et al., 2011, 2015; Nawijn et al., 2015; Powers et al., 2015a, 2015b; Resick et al., 2008; Sadeh et al., 2015; Taylor, 2015; van Wingen et al., 2011]. Accordingly, numerous studies have begun to characterize emotional dysregulation associated with contrasting states of emotional under- and over-modulation. These opposing states have been linked to the experience of heightened or depressed emotionality, including re-experiencing symptoms and a detachment from emotional experience that occurs during emotional numbing and states of depersonalization and derealization, respectively [Etkin and Wager, 2007; Lanius et al., 2010; Nicholson et al., 2015; Reinders et al., 2014; Wolf et al., 2014].
Heart rate variability (HRV) is one promising psychophysiological indicator that may assist in gaining a deeper understanding of emotion regulatory capacity in PTSD. HRV reflects vagal activity that indexes the state of the autonomic nervous system (ANS), enabling the organism to calibrate bodily, emotional, and cognitive reactions to contextual demands. The ANS is separated into two branches, normally behaving in a balanced fashion: the parasympathetic nervous system (PNS), associated with restorative and vegetative functioning, and the sympathetic nervous system, linked to energy mobilization. Imbalance of these two branches is associated with blunted flexibility to react to environmental changes, increased vulnerability to somatic diseases, and has been associated with mental disorders [Carney and Freedland, 2009; Stein et al., 2000; Thayer and Brosschot, 2005]. Thus, HRV is thought to guide the individual`s ability to organize emotional, cognitive, and behavioral responses, and higher levels of HRV to be a physiological index of the ability to respond in a context appropriate manner [Gillie and Thayer, 2014; Melzig et al., 2009; Thayer and Lane, 2000].
To date, HRV investigations in PTSD have revealed that indices of high-frequency heart rate variability (HF-HRV) and root mean squared successive differences (RMSSD) of the inter-beat interval time series at rest, both indexing activity of the PNS, are reduced as compared to both trauma and non-trauma exposed healthy control subjects [for meta-analyses see Chalmers et al., 2014; Sammito et al., 2015; but see Agorastos et al., 2013; Keary et al., 2009 for null findings]. Fewer studies have addressed cardiac response to stressful tasks, revealing heterogeneous findings [Cohen et al., 2000, 1998; Hauschildt et al., 2011; Keary et al., 2009; Norte et al., 2013]. Whereas three studies failed to identify differential effects of affective cues or stressful tasks on HRV response in PTSD as compared to control subjects [Cohen et al., 2000, 1998; Hauschildt et al., 2011], two studies reported a greater decrease of HRV in response to stressful tasks or trauma script exposure [Keary et al., 2009; Norte et al., 2013].
A growing number of studies have investigated the relation between HRV and neural activation, where the central autonomic network (CAN) is thought to serve as a critical link between the brain and the ANS [for meta-analyses see Beissner et al., 2013; Thayer et al., 2012]. The CAN is comprised of the ventromedial prefrontal cortex (vmPFC), anterior cingulate (ACC), insular cortex, amygdala, hypothalamus, periaqueductal gray (PAG), parabrachial complex, nucleus of the tractus solitaris, and the ventrolateral medulla [Benarroch, 1993; Cersosimo and Benarroch, 2013; Palkovits, 1999; Thayer and Brosschot, 2005]. These components are reciprocally interconnected, with higher-order cortical functions associated with cognitive functioning regulating the response of subcortical structures, which in turn regulate autonomic input to the heart and allows for the complex variability that characterizes the healthy HR time series [Thayer and Brosschot, 2005]. Despite this knowledge, to date, no studies to date have examined connectivity in CAN regions in association with HRV in PTSD.
The aim of the present study was, therefore, to investigate the functional neural correlates of autonomic activity in individuals with PTSD as compared to controls. As studies in PTSD have suggested not only lower HRV in PTSD but also alterations in top–down modulatory processes on a neuronal level [Etkin and Wager, 2007; Lanius et al., 2010; Nicholson et al., 2015; Reinders et al., 2014; Wolf et al., 2014], combining resting HRV and neural activation will facilitate a greater understanding of altered emotion regulatory processes in this disorder. Specifically, we investigated resting state neural connectivity patterns using fMRI and their HRV-related associations with three seed regions that comprise key nodes of the CAN: (1) vmPFC; (2) amygdala; and (3) the PAG [Beissner et al., 2013; Thayer et al., 2012; Thayer and Lane, 2000]. Consistent with prior findings indicating impaired emotion regulation capacity, we hypothesized that individuals with PTSD would exhibit diminished HRV [Chalmers et al., 2014; Sammito et al., 2015] and show reduced correlations between HRV scores and connectivity between key structures of the CAN as compared to healthy controls [Beissner et al., 2013; Thayer, 2006].
METHODS AND MATERIALS
Sample Description
Ninety-eight participants were included in the current study: 57 individuals with a primary diagnosis of PTSD and 41 healthy control subjects. Subjects were recruited between 2009 and 2015 from the Department of Psychiatry London Health Services Center (LHSC), through family physician, mental health professionals, psychology/psychiatric clinics, community programs for traumatic-stress survivors, and posters/advertisements, all within the London, ON community. PTSD diagnosis was confirmed via the Clinician-Administered PTSD scale, with a cut-off score of 50 for moderate PTSD [Blake et al., 1995]. Co-morbid Axis I disorders were diagnosed with the Structured Clinical Interview for DSM-IV Axis I Disorders [First, 1997]. Childhood trauma history was assessed with the Childhood Trauma Questionnaire (CTQ) [Bernstein et al., 2003], as well as depressive symptoms with the Beck Depression Inventory (BDI) [Beck et al., 1997]. Subjects also completed the Multiscale Dissociation Inventory (MDI) [Briere et al., 2005], capturing dissociative experiences. Immediately after the resting state scanning procedure, subjects were instructed to rate their state anxiety (3 items of the State-Trait Anxiety Inventory: STAI-S) [Kvaal et al., 2005] and their level of state dissociation, re-experiencing, avoidance, and hyperarousal symptoms by means of the Response to Script Driven Imagery (RSDI) Scale [Hopper et al., 2007a]. Clinical and socio-demographic variables are presented in Table 1.
| PTSD N = 57 | HC N = 41 | F/χ value | P | |||
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age mean (SD) | 33.98 | (11.57) | 37.11 | (12.80) | 0.96 | 0.33 |
| Gender (N) | ||||||
| Female | 18 | 26 | 0.27 | 0.61 | ||
| Male | 39 | 15 | ||||
| Clinical Characteristics | ||||||
| CAPS Re-experiencing mean (SD) | 21.96 | (6.73) | — | — | — | |
| CAPS Avoidance/ Numbing mean (SD) | 26.22 | (7.00) | — | — | — | |
| CAPS Hyperarousal mean (SD) | 22.41 | (5.99) | — | — | — | |
| CAPS Total mean (SD) | 69.16 | (15.47) | — | — | — | |
| MDI Depersonalization mean (SD) | 8.11 | (3.59) | 5.24 | (0.69) | 22.45 | <0.001 |
| MDI Derealization mean (SD) | 9.89 | (4.12) | 5.27 | (0.66) | 47.18 | <0.001 |
| MDI Total mean (SD) | 61.96 | (20.07) | 34.15 | (4.04) | 64.31 | <0.001 |
| CTQ mean (SD) | 62.47 | (23.29) | 31.28 | (8.37) | 65.61 | <0.001 |
| BDI mean (SD) | 26.73 | (9.79) | 1.54 | (2.60) | 234.37 | <0.001 |
| State Characteristics | ||||||
| STAI-S (SD) | 1.97 | (0.76) | 1.22 | (0.42) | 32.66 | <0.001 |
| RSDI Dissociation (SD) | 1.51 | (0.61) | 1.14 | (0.21) | 17.60 | <0.001 |
| RSDI Re-Experiencing (SD) | 1.46 | (0.67) | 1.06 | (0.19) | 16.77 | <0.001 |
| RSDI Avoidance (SD) | 2.04 | (1.06) | 1.35 | (0.76) | 14.33 | <0.001 |
| RSDI Hyperarousal (SD) | 1.67 | (0.56) | 1.18 | (0.26) | 34.95 | <0.001 |
| Comorbidities (%) | ||||||
| Alcohol dependence | 0 | 0 | ||||
| MDD | 18 | 0 | ||||
| Panic Disorder w/wo Agoraphobia | 11 | 0 | ||||
| Social Phobia | 7 | 0 | ||||
| Specific Phobia | 3 | 0 | ||||
| Generalized Anxiety Disorder | 0 | 0 | ||||
| Obsessive Compulsive Disorder | 2 | 0 | ||||
| Somatization Disorder | 5 | 0 | ||||
| Somatoform Disorder | 8 | 0 | ||||
| Eating Disorder | 1 | 0 | ||||
| HRV characteristics | ||||||
| LN RMSDD mean (SD) | 3.76 | (0.09) | 4.06 | (0.11) | 4.79 | 0.031 |
| LN LF power value mean (SD) | 6.05 | (0.20) | 6.91 | (0.23) | 8.34 | 0.005 |
| LN HF power value mean (SD) | 6.35 | (0.19) | 6.93 | (0.25) | 4.14 | 0.045 |
- Significance threshold P < 0.05.
- Abbreviations: PTSD = posttraumatic stress disorder group; HC = healthy control group; CAPS= Clinician-Administered PTSD scale; MDI = Multiscale Dissociation Inventory; CTQ =Childhood Trauma Questionnaire; BDI = Beck Depression Inventory; STAI-S = State-Trait Anxiety Inventar ; RSDI = Response to Script Driven Imagery Scale; MDD = major depressive disorder; RMSSD = root-mean square differences of successive R-R intervals; LF-HRV = low frequency heart rate variability, HF-HRV = high frequency heart rate variability, ln = natural logarithm.
Exclusion criteria for all participants comprised implants or metals that do not comply with 3T fMRI safety standards for research, a history of head injury with any loss of consciousness, significant untreated medical illness, a history of neurological disorders, history of any pervasive developmental disorders, pregnancy, and current use of any psychotropic or cardiovascular medications within one month prior to study (except 1 subject). Further exclusion criteria for individuals with PTSD were a history of bipolar disorder or schizophrenia, alcohol, or substance abuse/dependence not in sustained full remission within 6 month prior to participation of the study, while healthy control subjects had to be free of any lifetime Axis-I mental disorder. Scanning took place either at the Robarts Research Institute's Center for Functional and Metabolic Mapping or at the Lawson Health Research Institute for Imaging in London, ON, Canada. The study was approved by the research ethics board at Western University of Canada. All subjects provided written informed consent.
fMRI Data Acquisition
All images were collected using a 3.0, whole-body MRI scanner (Magnetom Tim Trio, Siemens Medical Solutions, Erlangen, Germany) with a manufacturer's 32-channel phased array head coil. BOLD fMRI images were obtained with the standard gradient-echo EPI pulse sequence. EPI volumes were acquired with 2 mm isotropic resolution with the following parameters: FOV = 192 mm × 192 mm × 128 mm (94 × 94 matrix, 64 slices), TR/TE = 3,000 ms/20 ms, flip angle = 90°, 120 volumes. Participants were instructed to close their eyes, relax, and let their minds wander during the 6-min resting scan.
Statistical Analyses
HRV analysis
Pulse data were recorded using a finger-tip pulse oximeter (Powerlab 8/35, LabChart 7 Pro) during the 6-min resting functional MRI scanning procedure and sampled at 200 Hz (Robarts Research Institute) or 40 Hz (Lawson Health Science Institute). Pulse data acquired at 40 Hz were re-sampled at 200 Hz by linear interpolation. In-house software provided by Stefanie Lis was applied for peak detection and visual inspection of the pulse signal. The inter-beat time series were saved as text files and imported to the KUBIOS Heart Rate Variability Software Package (Kuopio, Finland; version 2.2) [Tarvainen et al., 2014]. Data were then re-sampled at 4 Hz and common time domain measures were computed (RMSSD). Frequency domain estimates of HF-HRV and LF-HRV were calculated following the Task Force Guidelines (1996). Spectral analysis using a Fast Fourier transform algorithm, with a window width of 256 s and a window overlap of 50% was applied to generate heart period power spectrum. Frequency bands were set to 0.04–0.15 for the LF component and 0.15–0.4 for the HF component, respectively (Kuopio, Finland; version 2.2) [Tarvainen et al., 2014]. Raw measurements, including RMSSD, HF-HRV, and LF-HRV were natural log transformed before the analysis to normalize their distributions. Further analyses were performed using SPSS (version 22; SPSS). Group differences in HRV measurements were assessed with multivariate analyses of variance.
fMRI analysis
Image preprocessing and statistical analyses of the BOLD signal were conducted using Statistical Parametric Mapping (SPM 8, Wellcome Trust Center of Neuroimaging, London, UK: http://www.fil.ion.ucl.ac.uk/spm) implemented in Matlab 8.3 and 11b (MathWorks).
Preprocessing
For each subject, all functional images were realigned to the first image in their series, re-sliced and the mean functional image created. ART software (Gabrieli Lab; McGovern Institute for Brain Research, Cambridge, MA) was used to compute motion outlier regressors, which were applied within the 1st level analysis as a covariate of no interest. Given that an increasing body of literature points to an influence of head movement on functional connectivity measurements [Power et al., 2012, 2014; Pujol et al., 2014; Satterthwaite et al., 2013, 2012; Van Dijk et al., 2012], we wanted to further ensure that groups did not differ with regard to their frequency of defined outliers. We, therefore, coded whether the ART software detected any outliers within each individual scan and subsequently ran a Fisher's exact test to examine potential group differences. Here, group differences were not observed (P = 0.520). The images were further spatially normalized and the mean image was co-registered to the SPM EPI template. The resulting deformation matrix was applied to the functional images. All images were then smoothed using a 6 mm full-width-half-maximum isotropic Gaussian filter and band-pass filtered within a 0.012–0.1 Hz range with in house software by Jean Théberge.
Connectivity analysis
For each subject, the mean signal intensity time course was extracted from seeds defined in WFU PickAtlas (Functional MRI Laboratory, Wake Forest University School of Medicine) using in-house software by co-author Jean Théberge for each seed region, including the vmPFC [2 22 −8] [Thayer et al., 2012], right and left amygdala (WFU PickAtlas), and PAG [−2 −27 −3] [Napadow et al., 2008]. All seed regions were based on previous publications and defined as 6 mm spheres for each reported coordinate. Extracted time courses were then used as a regressor within a first level multiple regression model for each seed region. Connectivity was thus indicative of a correlation between each seed and other brain areas, where both positive correlations and anti-correlations were examined.
To explore connectivity patterns within groups, one-sample t-tests were conducted for each group separately. To control for the effects of a current major depression, we re-ran the within-group PTSD analyses by applying a multivariate analysis of covariance and including the comorbid diagnosis as a covariate of no interest. Two-sample t-tests were used to compare PTSD with healthy control subjects for each seed region. Statistical significance was set at a P-value of < 0.001 corrected for whole brain FDR and an extent threshold of k = 10 voxels.
Correlations between HRV and BOLD functional connectivity
To examine associations between HRV and CAN-related connectivity patterns, separate multiple regression analyses for RMSSD, LF-HRV, and HF-HRV scores were examined; each of these scores revealed significant differences between groups. PAG seed correlations were masked with a CAN mask. The CAN mask was created in WFU PickAtlas based on 10 mm spheres on prominent coordinates published in previous literature [Beissner et al., 2013; Thayer et al., 2012], that is, bilateral amygdala ([−20 −6 −18] and [20 −6 −18]) [Beissner et al., 2013], right medial prefrontal cortex [10 54 18; Thayer et al., 2012], right dorsal cingulate [2 10 40] [Beissner et al., 2013]; left thalamus [−4 −16 8] [Beissner et al., 2013]. Two additional masks were created, comprising the bilateral insula from the WFU PickAtlas applied to the vmPFC seed correlation, as well as a PAG mask [Napadow et al., 2008] applied to the right and left amygdala seed correlations, each with a 10-mm radius. A second level multiple regression model was established for each seed in combination with each HRV score, respectively. Simple multiple regression models examining both positive and negative associations between scores were conducted and the results masked with the CAN, PAG, and insula mask. Two-samples t-tests were used to compare PTSD with healthy controls for each seed region and each HRV score respectively, with a covariate (HRV score), and a term that computes the interaction between the covariate and factor group. The interaction effect indicates regions for which the functional connectivity with the seed region is greater in people that have greater HRV scores in controls than individuals with PTSD (and vice versa). Significance was set to P < 0.05, ROI FDR corrected and an extent threshold of k = 10 voxels.
RESULTS
Cardiovascular Responding (HRV)
As compared to controls, individuals with PTSD showed lower RMSSD values (F(1,96) =4.79, P = 0.031, η2 = 0.048), lower LF power (F(1,96) =8.34, P = 0.005, η2 = 0.080) and lower HF power (F(1,96) =4.14, P = 0.045, η2 = 0.041) (see also Fig. 1, Table 1).
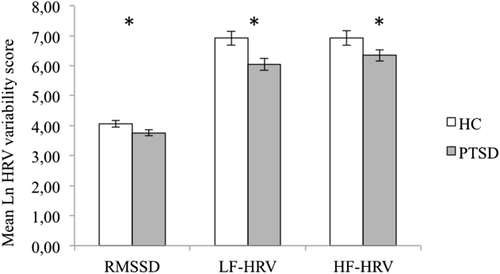
Between group differences in HRV. Figure represents significant lower Mean Natural Log RMSSD, LF-HRV, and HF-HRV in PTSD as compared to the control group (all F(1,96)'s > 4.14, all P's < 0.048). Statistical threshold P < 0.05. Abbreviations: PTSD = posttraumatic stress disorder group; HC = healthy control group; RMSSD = root-mean square differences of successive R-R intervals; LF-HRV = low frequency heart rate variability, HF-HRV = high frequency heart rate variability, ln = natural logarithm.
Seed-Based Functional Connectivity
Ventromedial prefrontal cortex
Resting-state activity in the vmPFC predicted a localized connectivity pattern within control subjects. In contrast, vmPFC activity predicted widespread connectivity to multiple cortical and subcortical regions in PTSD. Adding current major depression as a covariate did not change the results (for details on each seed regions see Supporting Information Table I, Supporting Information Figure I).
Between-group comparisons indicated that HC individuals did not exhibit any significantly greater connectivity to the vmPFC as compared to PTSD individuals. By contrast, PTSD subjects exhibited greater functional connectivity between the vmPFC and cingulate cortex, frontal cortex, bilateral precentral gyrus, left insula, left parahippocampal gyrus, bilateral lentiform nuclei, and left thalamus (see also Fig. 2A, Supporting Information Table IV).
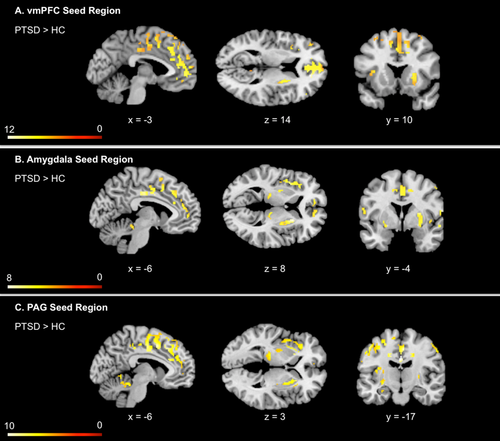
Between group differences in key CAN seed resting state functional connectivity, including vmPFC, amygdala, PAG. Illustration represents greater connectivity between key CAN seed regions, including the vmPFC, amygdala, and PAG with multiple cortical and subcortical regions in PTSD as compared to healthy controls. By contrast, HC individuals did not exhibit any significantly greater connectivity to any of the CAN seed regions as compared to PTSD individuals. Statistical threshold P < 0.001 corrected for whole brain FDR, k = 10 for all two-sample t-tests. Abbreviations: PTSD = posttraumatic stress disorder group; HC = healthy control group; vmPFC = ventromedial prefrontal cortex; PAG = periaqueductal gray; FDR = false discovery rate. [Color figure can be viewed at wileyonlinelibrary.com.]
Amygdala
Resting state connectivity with the left and right amygdala predicted a restricted connectivity pattern in controls. In contrast, among individuals with PTSD, left and right amygdala activity predicted widespread connectivity patterns in multiple cortical and subcortical regions. Adding current major depression as a covariate did not change the results (for details on each seed regions see Supporting Information Table II, Supporting Information Figure I).
Between-group comparisons indicated that HCs did not exhibit significantly greater connectivity to either the left or the right amygdala as compared to individuals with PTSD. By contrast, PTSD individuals exhibited greater functional connectivity between the left amygdala and the cingulate cortex, frontal cortex, bilateral precentral gyrus, bilateral insula, bilateral parahippocampal gyrus, bilateral thalamus, and bilateral lentiform nuclei (see also Fig. 2B, Supporting Information Table VA). Similarly, individuals with PTSD showed greater functional connectivity between the right amygdala and the cingulate cortex, frontal cortex, bilateral precentral gyrus, right parahippocampal gyrus, bilateral insula, and bilateral lentiform nuclei (see also Fig. 2B, Supporting Information Table VB).
Periaqueductal gray
Resting-state activity in the PAG predicted a localized connectivity pattern in control individuals. In contrast, PAG activity predicted widespread connectivity in multiple cortical and subcortical regions in PTSD subjects. Adding current major depression as a covariate did not change the results (for details on each seed regions see Supporting Information Table III, Supporting Information Figure I).
HC subjects did not show any greater connectivity to the PAG as compared to PTSD subjects. In contrast, individuals with PTSD showed greater functional connectivity between the PAG seed and the bilateral mid cingulate cortex, left dorsal cingulate cortex, bilateral medial frontal gyrus, left superior frontal gyrus, left precentral gyrus, left insula, bilateral putamen, left thalamus, hippocampus, and right cerebellar vermis (see also Fig. 2C, Supporting Information Table VI).
HRV Correlations to CAN Related Seed-Based Functional Connectivity
Ventromedial prefrontal cortex
In control subjects, higher RMSSD, LF-HRV, and HF-HRV values predicted increased functional connectivity between the vmPFC and the right insula (see also Fig. 3A, Table 2). In contrast, none of the HRV scores were related to increased or decreased connectivity between the vmPFC and CAN-related brain regions among individuals with PTSD (Table 2).
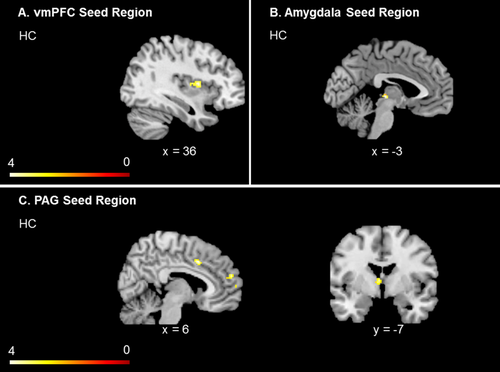
HRV correlations related connectivity to key CAN seed regions within healthy control subjects, including vmPFC, amygdala, PAG. Illustration represents increased functional connectivity patterns to all CAN regions in controls, which is predicted by HRV. In PTSD, analyses did not reveal significant clusters; only healthy control subjects are, therefore, presented. Significance threshold P < 0.05, ROI FDR corrected, k = 10 voxels for all one-sample t-tests. Abbreviations: PTSD = posttraumatic stress disorder group; HC = healthy control group; vmPFC = ventromedial prefrontal cortex; PAG = periaqueductal gray; ROI = region of interest analysis. [Color figure can be viewed at wileyonlinelibrary.com.]
| HRV score | Brain regions | BA | Hemisphere | x | y | z | k | T-value | pFDR | pFWE |
|---|---|---|---|---|---|---|---|---|---|---|
| vmPFC | ||||||||||
| HC | ||||||||||
| RMSSD | Insula | 13 | R | 34 | −2 | 14 | 55 | 4.24 | 0.057 | 0.031 |
| HF-HRV | Insula | 13 | R | 34 | −2 | 14 | 116 | 4.12 | 0.022 | 0.023 |
| LF-HRV | Insula | 13 | R | 38 | −12 | 14 | 49 | 4.05 | 0.058 | 0.049 |
| PTSD | ||||||||||
| n.s. | ||||||||||
| Left Amygdala | ||||||||||
| HC | ||||||||||
| RMSSD | PAG, Pulvinar | L | −4 | −26 | 4 | 15 | 4.02 | 0.010 | 0.013 | |
| HF-HRV | PAG, Pulvinar | L | −4 | −28 | 4 | 14 | 4.05 | 0.010 | 0.012 | |
| LF-HRV | PAG, Pulvinar | L | −6 | −28 | 4 | 8 | 3.15 | 0.055 | 0.092 | |
| PTSD | ||||||||||
| n.s. | ||||||||||
| Right Amygdala | ||||||||||
| HC | ||||||||||
| n.s. | ||||||||||
| PTSD | ||||||||||
| n.s. | ||||||||||
| PAG | ||||||||||
| HC | ||||||||||
| RMSSD | Dorsal cingulate cortex | 32 | R | 6 | 8 | 44 | 87 | 4.50 | 0.028 | 0.021 |
| Medial frontal cortex | 9 | R | 4 | 52 | 24 | 55 | 4.33 | 0.028 | 0.033 | |
| Medial frontal cortex | 10 | R | 4 | 56 | 12 | Of55 | 3.25 | 0.038 | 0.346 | |
| Medial frontal cortex | 9 | R | 4 | 46 | 18 | Of55 | 2.85 | 0.044 | 0.616 | |
| Thalamus | md nucl. | L | −4 | −8 | 10 | 18 | 3.97 | 0.028 | 0.080 | |
| Superior frontal cortex | 10 | R | 18 | 58 | 22 | 14 | 3.23 | 0.038 | 0.358 | |
| HF-HRV | Medial frontal cortex | 9 | R | 4 | 52 | 24 | 63 | 4.44 | 0.036 | 0.025 |
| Thalamus | md nucl. | L | −4 | −8 | 10 | 26 | 4.25 | 0.036 | 0.040 | |
| Dorsal cingulate cortex | 32 | R | 6 | 8 | 44 | 32 | 3.59 | 0.038 | 0.181 | |
| Superior frontal cortex | 10 | R | 18 | 58 | 22 | 12 | 3.26 | 0.049 | 0.341 | |
| LF-HRV | n.s. | |||||||||
| PTSD | ||||||||||
| n.s. | ||||||||||
- Significance threshold P < 0.05, ROI FDR corrected, k = 10 voxels for all one-sample t-tests.
- Abbreviations: PTSD = posttraumatic stress disorder group; HC = healthy control group; vmPFC = ventromedial prefrontal cortex; PAG = periaqueductal gray; md nucl = mediodorsal nucleus; FDR = false discovery rate; FWE = family wise error, RMSSD = root-mean square differences of successive R-R intervals; LF-HRV = low frequency heart rate variability, HF – HRV = high frequency heart rate variability; BA = Brodmann Areal.
Direct group comparisons revealed that none of the HRV scores predicted increased functional connectivity between the vmPFC and CAN related brain regions in neither controls as compared to PTSD, nor within the reversed contrast (see also Table 3 for additional information regarding FWE corrected results).
| HRV Score | Brain regions | BA | Hemisphere | x | y | z | k | T-value | pFDR | pFWE |
|---|---|---|---|---|---|---|---|---|---|---|
| vmPFC | ||||||||||
| HC > PTSD | ||||||||||
| RMSSD | Insula | 13 | R | 54 | 2 | −6 | 20 | 3.16 | 0.147 | 0.050 |
| HF-HRV | Insula | 13 | R | 54 | 2 | −6 | 24 | 3.19 | 0.094 | 0.049 |
| LF-HRV | n.s. | |||||||||
| PTSD > HC | ||||||||||
| n.s. | ||||||||||
| Left Amygdala | ||||||||||
| HC > PTSD | ||||||||||
| RMSSD | Insula | 13 | R | 54 | 2 | −6 | 5* | 3.08 | 0.208 | 0.055 |
| HF-HRV | n.s. | |||||||||
| LF-HRV | n.s. | |||||||||
| PTSD > HC | ||||||||||
| n.s. | ||||||||||
| Right Amygdala | ||||||||||
| HC > PTSD | ||||||||||
| RMSSD | Insula | 13 | R | 54 | 2 | −8 | 28 | 3.95 | 0.015 | 0.007 |
| HF-HRV | Insula | 13 | R | 54 | 2 | −8 | 23 | 3.60 | 0.034 | 0.020 |
| LF-HRV | n.s. | |||||||||
| PTSD > HC | ||||||||||
| n.s. | ||||||||||
| PAG | ||||||||||
| HC > PTSD | ||||||||||
| RMSSD | Insula | 13 | R | 48 | 16 | −2 | 20 | 2.85 | 0.212 | 0.049 |
| HF-HRV | Insula | 13 | R | 48 | 16 | −2 | 33 | 3.07 | 0.036 | 0.065 |
| LF-HRV | n.s. | |||||||||
| PTSD > HC | ||||||||||
| n.s. | ||||||||||
- Significance threshold P < 0.05, ROI FDR corrected, k = 10 voxels for all two-sample t-tests. * did not meet k threshold.
- Abbreviations: PTSD = posttraumatic stress disorder group; HC = healthy control group; vmPFC = ventromedial prefrontal cortex; PAG = periaqueductal gray; FDR = false discovery rate; FWE = family wise error, RMSSD = root-mean square differences of successive R-R intervals; LF-HRV = low frequency heart rate variability, HF-HRV = high frequency heart rate variability; BA = Brodmann Areal.
Amygdala
In control subjects, higher RMSSD, as well as higher LF-HRV and HF-HRV values predicted increased connectivity between the left amygdala and the left PAG, while none of the HRV scores were related to the functional connectivity of the right amygdala in controls (see also Fig. 3B, Table 2). In individuals with PTSD, none of the HRV scores were related to increased or decreased connectivity between the left or right amygdala and CAN-related brain regions (Table 2).
Direct group comparisons revealed that none of the HRV scores predicted increased connectivity between the left amygdala and CAN related brain regions in controls as compared to PTSD, nor within the reversed contrast (see also Table 3 for additional information regarding FWE corrected results). Direct group comparisons revealed that higher RMSSD and HF-HRV values predicted increased connectivity between the right amygdala and the right insula in controls as compared to PTSD. In contrast, none of the HRV scores predicted increased connectivity patterns to the right amygdala in PTSD patients as compared to controls (see also Table 3 for additional information regarding FWE corrected results).
Periaqueductal gray
In control subjects, higher RMSSD and HF-HRV values also predicted increased functional connectivity between the PAG and the right dorsal cingulate cortex, right medial prefrontal cortex, right superior frontal gyrus and left thalamus. In contrast, LF-HRV did not predict functional connectivity between the PAG and CAN-related brain regions in HC subjects, nor did any of the HRV scores predicted increased functional connectivity patterns to the PAG in PTSD (see also see also Fig. 3C, Table 2).
Direct group comparisons revealed that higher HF-HRV values predicted increased functional connectivity between the PAG and the right insula in controls as compared to PTSD. In contrast, none of the HRV scores predicted increased connectivity patterns to the PAG in PTSD patients as compared to controls (see also Table 3 for additional information regarding FWE corrected results).
DISCUSSION
To the best of our knowledge, this is the first study to investigate autonomic response and its relation to resting state central autonomic activity in individuals with PTSD. As hypothesized, as compared to controls, individuals with PTSD showed reduced resting HRV. At the neuronal level, resting-state activity in CAN-associated brain regions, including the vmPFC, amygdala, and PAG predicted a localized and restricted connectivity pattern in control subjects. In stark contrast, individuals with PTSD exhibited widespread functional connectivity to brain regions involved in emotional reactivity [Kirby and Robinson, 2015; White et al., 2015], motor readiness [Herzfeld et al., 2014; Mendoza and Merchant, 2014; Stoodley and Schmahmann, 2010; Stoodley et al., 2012], self-referential processing [Bluhm et al., 2011, 2012; Buckner et al., 2008; Spreng et al., 2009], and stimuli salience detection [Craig, 2009; Sripada et al., 2012; Thome et al., 2014] (emotional reactivity: all seed regions to the insula, vmPFC, and amygdala to the lentiform nucleus; motor readiness: all seed regions to the precentral gyrus, PAG to the cerebellum, self-referential processing: vmPFC and PAG to PCC, dmPFC, amygdala to rostral ACC, salience detection: all seed regions to anterior insula and dorsal ACC). Critically, whereas higher HRV predicted increased functional connectivity within key regions of the CAN, no such correlations were uncovered when examining patients with PTSD, pointing to a relative uncoupling of the ANS from the CAN in this group.
As compared to controls, individuals with PTSD exhibited lower HRV at rest as reflected by all HRV parameters. Indeed, the present findings are consistent with previous cross-sectional studies that reported overall lower resting HRV in PTSD even after controlling for important confounders such as incidence of traumatic brain injury and severity of depression [Minassian et al., 2014; Moon et al., 2013; Norte et al., 2013; Shah et al., 2013; Shaikh al arab et al., 2012; Wahbeh and Oken, 2013], a pattern confirmed by a recent meta-analysis [Chalmers et al., 2014]. Blunted HRV in PTSD is also in line with previous theoretical models, such as the neurovisceral integration model [Thayer et al., 2009], which proposes that prefrontal regions moderate parasympathetic activity and vagal nerve inhibition, and that inadequate vagus nerve regulation is associated with a variety of somatic and mental diseases [Danesh et al., 2000; Duncan et al., 2003; Gao and Hong, 2008]. In this model, whereas healthy functioning is characterized by a high level of adaptive HRV [Thayer et al., 2012, 2009; Thayer and Lane, 2000], lower HRV is thought to reflect decreased vagal output leading to behavioral inflexibility.
Consistent with the neurovisceral integration model, the polyvagal theory [Porges, 2011] further posits that a safe environment promotes an increase of vagal outflow, thereby promoting regeneration and homeostatic functions. It is, therefore, probable that difficulties in detecting safe environments result in an increase in sympathetic tone and concomitant decreased HRV. Indeed, several studies reported a failure of contextual learning in PTSD resulting in an inability to differentiate between threat and safety contexts [Acheson et al., 2015; Levy-Gigi et al., 2016; Steiger et al., 2015; van Wingen et al., 2011]. For example, a recent study demonstrated that individuals with PTSD did not differentiate between threat and safety contexts on a behavioral level (contingency ratings) during the acquisition phase of a differential context and cue conditioning paradigm; this was reflected in increased hippocampal activation in response to both contexts [Steiger et al., 2015]. This decreased capacity for safety perception may, therefore, be associated with sympathetic over reactivity and/or parasympathetic insufficiency, and decreased HRV in PTSD [Tulloch et al., 2014]. However, it is important to note that emerging evidence of blunted HRV and reduced flexibility in PTSD is based primarily on cross-sectional studies. Longitudinal studies are, therefore, urgently needed to determine whether an inflexible ANS represents a vulnerability factor for PTSD or instead emerges as the result of heightened stress states. Interestingly, Minassian et al. [2015] recently demonstrated that, in active-duty marines, higher LF/HF ratios at a pre-deployment visit (1 to 2 months before combat exposure) were positively correlated with the risk of developing PTSD as measured at a post-deployment visit (4 to 6 months after return) [Minassian et al., 2015]. This study points to the fact that ANS functioning may contribute to an individual's proneness, as well as resiliency, in reacting to stress and may, therefore, represent a risk factor for the development of trauma-related disorders [see also Shaikh al arab et al., 2012].
Although numerous studies have investigated HRV in PTSD, here we describe the first study directly linking HRV to brain regions that modulate the variability of HR time series (CAN). While higher HRV scores in healthy controls predicted increased functional connectivity within regions of the CAN (vmPFC to bilateral insula; left amygdala to the left PAG; PAG to right medial and superior PFC dorsal cingulate cortex, and left thalamus; for meta-analyses see also [Beissner et al., 2013; Thayer et al., 2012], measures of HRV within the PTSD group were unrelated with functional connectivity within CAN-related brain regions. The absence of a correlation between HRV and functional connectivity of the CAN within PTSD subjects may reflect an uncoupling of the ANS from the CAN in PTSD where top–down modulation of cardiac function by higher-order brain regions fails to occur. Such a pattern has indeed been reported across a number of different paradigms including emotion-provoking pictures and film scenes, facial processing, gambling tasks, and traumatic script-driven imagery [Cohen et al., 2013; Etkin and Wager, 2007; Felmingham et al., 2008; Hopper et al., 2007b; Lanius et al., 2012, 2015; Mickleborough et al., 2011; Reiser et al., 2014; Shin et al., 2006; van Wingen et al., 2011]. Interestingly, a recent positron emission tomography study in PTSD found an increased correlation between HR and activation in orbitofrontal, precentral and occipital regions in response to traumatic scripts solely among individuals with PTSD [Barkay et al., 2012], suggesting that sensorimotor regions might regulate the stress response induced by traumatic scripts in PTSD. In comparison, the absence of HRV-CAN covariation at rest within the present sample demonstrates a lack of top–down CAN regulation of autonomic responses.
It is important to note that the present sample of individuals with PTSD displayed widespread resting state functional connectivity between CAN-related regions and other brain regions associated with emotional reactivity, motor readiness, self-referential processing, and stimuli salience detection. However, the examined regions appear neither associated with HRV nor associated with anatomical connections to the brain stem, which have been directly related to the vagus nerve. Future studies examining brain stem structures directly are, therefore, warranted.
There is a growing literature suggesting that low resting HRV and related psychopathological states are associated with undifferentiated threat responses to a wide range of conditions and situations [Melzig et al., 2009; Ruiz-Padial and Thayer, 2014; Wendt et al., 2015]. Thus, the present findings of widespread connectivity in PTSD may reflect the neural concomitants of a response pattern, reflecting difficulties in differentiating between threat and safety contexts, which has been observed in other studies [e.g., see Steiger et al., 2015]. However, in contrast, context appropriate activation of localized brain networks has been identified with context appropriate psychophysiological and behavioral responses in persons with high resting HRV, which is associated with a flexibly recruited network of loosely coupled bio-oscillators that has been linked to healthy dynamical systems [Thayer, 2006; Thayer and Lane, 2000]. The widespread connectivity exhibited by PTSD patients in the present study might point to a response pattern that is suboptimal in generating context appropriate responses.
The observed pattern of resting state brain activation in PTSD is also consistent with Panksepp and Biven's [2012] proposal of a basic affective system. Here, the PAG is thought to gate various affective responses or emotional systems through its connections with higher brain structures whose activity contributes to the functioning of all basic emotional systems. Given the increased widespread PAG resting functional connectivity observed in PTSD subjects, it is further probable that this pattern is reflective of a hypersensitive affective system observed during rest. However, future research is needed to more fully investigate this notion.
Several limitations of the current study are worth noting. Due to the cross-sectional design of the present study, these findings provide no indication of whether decreased HRV is caused by traumatic stress or represents a pre-existing vulnerability factor. We did not include trauma-exposed individuals without PTSD in our analyses, as screened individuals matched for trauma severity met exclusion criteria for current or past psychiatric disorders. In addition, given that only one related study links HR to cerebral blood flow during traumatic script exposure [Barkay et al., 2012], future studies using symptom provocation are expected to be useful in identifying potential differential functional connectivity patterns in relation to HRV in PTSD. Here, comparing peripheral physiological and neuronal responses during resting state to those induced during symptom provocation will be necessary to gain insights into emotional regulatory capacity in response to stressors.
In conclusion, the present study represents an important first step in linking peripheral (HRV) and central (CAN) measures of autonomic response in PTSD. HRV was reduced in PTSD, while the CAN evidenced increased functional connectivity across myriad of brain regions, including those associated with emotional processing and motor readiness. Moreover, whereas CAN-related functional connectivity was strongly correlated with HRV in controls, a striking lack of covariation between HRV and CAN-functional connectivity was observed in PTSD. The present investigation, therefore, represents a first step in demonstrating the relative absence of the regulatory capacity of the CAN on the ANS functioning at rest in PTSD, which may partially mediate the negative alterations in cognition and mood observed in this disorder.
ACKNOWLEDGMENTS
Dr. Lanius was the principal investigator of the study and supervised the collection and analysis of data. fMRI data acquisition was supervised by Dr. Thèberge. Mrs. Densmore and Ms. Thome processed and analyzed the data; Ms. Thome prepared the paper, with review and contributions by Dr. Lanius, and the rest of the authors. We thank Stefanie Lis for her support in analyzing the pulse data and providing the software. Conflict of interest/financial disclosure: The following statement covers the last three years: All the authors have no interests to declare generally and in relation to the present study.