Evidence from pupillometry and fMRI indicates reduced neural response during vicarious social pain but not physical pain in autism
Abstract
Autism spectrum disorder (ASD) is characterized by substantial social deficits. The notion that dysfunctions in neural circuits involved in sharing another's affect explain these deficits is appealing, but has received only modest experimental support. Here we evaluated a complex paradigm on the vicarious social pain of embarrassment to probe social deficits in ASD as to whether it is more potent than paradigms currently in use. To do so we acquired pupillometry and fMRI in young adults with ASD and matched healthy controls. During a simple vicarious physical pain task no differences emerged between groups in behavior, pupillometry, and neural activation of the anterior insula (AIC) and anterior cingulate cortex (ACC). In contrast, processing complex vicarious social pain yielded reduced responses in ASD on all physiological measures of sharing another's affect. The reduced activity within the AIC was thereby explained by the severity of autistic symptoms in the social and affective domain. Additionally, behavioral responses lacked correspondence with the anterior cingulate and anterior insula cortex activity found in controls. Instead, behavioral responses in ASD were associated with hippocampal activity. The observed dissociation echoes the clinical observations that deficits in ASD are most pronounced in complex social situations and simple tasks may not probe the dysfunctions in neural pathways involved in sharing affect. Our results are highly relevant because individuals with ASD may have preserved abilities to share another's physical pain but still have problems with the vicarious representation of more complex emotions that matter in life. Hum Brain Mapp, 2015. © 2015 Wiley Periodicals, Inc.
Abbreviations
-
- ACC
-
- anterior cingulate cortex
-
- AIC
-
- anterior insula
-
- ASD
-
- autism spectrum disorder
-
- fMRI
-
- functional magnetic resonance imaging
-
- FWE
-
- family-wise error
-
- PP
-
- physical pain
-
- ROI
-
- regions of interest
-
- SP
-
- social pain
INTRODUCTION
One of the core symptoms in high-functioning individuals with autism spectrum disorder (ASD) is their reduced capacity to intuitively represent their own and others’ mental states [Levy et al., 2009; Lombardo et al., 2007, 2010]. This affects many aspects of life, but predominantly alters experience and behavior within complex social situations [American Psychiatric Association, 2013; Klin et al., 2007]. A frequent explanation for this impairment is based on the patients' often described deficit in embodying another's affective states [Gallese et al., 2013; Hill et al., 2004]. The deficient embodied representation has been attributed to malfunctions of the interoceptive cortex [Craig, 2002, 2003], a network comprising the anterior insula cortex (AIC) and the anterior cingulate cortex (ACC) [Bird et al., 2010; Silani et al., 2008]. This network, and specifically the AIC, provides a salient representation of one's own bodily states and has been suggested to be a correlate of one's conscious experience of emotion [Gu et al., 2013]. The observation that the same regions are activated while witnessing the affect of other individuals led to the idea that the representation of another's affect is reached through the embodied simulation of our own states [Gallese and Sinigaglia, 2011; Keysers and Gazzola, 2009]. Such embodied representations of emotions are thought to underlie and motivate socially competent behavior [Eisenberg and Miller, 1987; Hein et al., 2010]. Emotional resonance can occur for others’ primary emotions such as physical pain [Singer et al., 2004] or disgust [Wicker et al., 2003], as well as for more complex social emotions such as rejection and embarrassment [Beeney et al., 2011; Krach et al., 2011; Paulus et al., 2014].
Embarrassment on behalf of others’ mishaps (e.g., observing a waiter in a fully occupied restaurant stumbling and dropping dishes to the floor) constitutes a form of vicarious social pain [Krach et al., 2011; Müller-Pinzler et al., 2012; Paulus et al., 2013a, 2014]1. The experience of vicarious embarrassment from the perceiver's perspective thereby has a conceptual and neurobiological overlap with vicarious physical pain (henceforth PP). The negative affect induced by witnessing another person's PP is thought to provide strong signals for the injuries of another's bodily integrity [Singer et al., 2004]. Likewise, the experience of vicarious social pain (henceforth SP) — and particularly embarrassment — is thought to serve a similar function, thereby signaling threats to another's social integrity [Macdonald and Leary, 2005; Krach et al., 2011; Paulus et al., 2014]. Vicarious responses for SP result in cortical activation comparable to those associated with vicarious PP [Immordino-Yang et al., 2009; Krach et al., 2011; Masten et al., 2011], and are coupled with increased autonomic activation [Müller-Pinzler et al., 2012].
Most of the previous neuroimaging studies have framed social pain rather narrow and used paradigms of social exclusion [Eisenberger, 2012a, 2012b; Eisenberger and Lieberman, 2004]. However, the concept of social pain has been conceptualized much broader, also including negative affective states such as shame, guilt, or embarrassment because they provide signs that one might not live up to others’ standards [Macdonald and Leary, 2005]. In the aforementioned example, the waiter attracts the attention of the audience in a situation which is severely unfavorable for his social image. The situation poses a threat to his social integrity and might potentially result in devaluation by desired partners or groups. The conceptualization of embarrassment as a form of social pain has been discussed in several earlier studies [Müller-Pinzler et al., 2015; Paulus et al., 2103a, 2014]. Although the emotion of embarrassment is conceptually different from the experience of social exclusion, both share the underlying threat to one's social integrity which justifies integrating embarrassment in the broader construct of social pain.
To generate the vicarious emotional response for SP, however, contextual demands such as knowledge about norms and values, expectations of the social environment, and appraisals of the social target need to be dynamically integrated [Paulus et al., 2013b, 2014]. In contrast, contextual demands are less complex for understanding and experiencing others’ PP. This distinction is supported by the faster neural response of the AIC for PP as compared to SP [Immordino-Yang et al., 2009]. Basically, while witnessing others’ PP, the individual's own body provides sufficient input to build an adequate model of another's affect. Thus, contrasting SP with PP offers a unique opportunity to test the hypothesis of domain-general deficiencies of embodied representation in ASD. If domain-general deficiencies in embodied representations exist in ASD, these should affect PP and SP alike. In contrast, if it is the contextual demand of the social situation that defines whether individuals with ASD are able to rely on conscious access to their emotional representation, then alterations should specifically surface in SP. If difficulties in embodying SP exist in ASD, we would expect them to manifest within the circuit involved in such an embodiment, in particular in the AIC and ACC [Gallese and Sinigaglia, 2011; Keysers and Gazzola, 2009].
A longstanding theory in autism research suggests that from the time of their early childhood onwards, patients with ASD try to compensate for their lack of social intuition by adhering to learned social rules and conventions – often in an inflexible or stereotyped manner [Baron-Cohen et al., 2003; Klin et al., 2003]. Using this strategy, patients with ASD learn to navigate the complex social world being less dependent on social intuition. If this is truly the case, we would, in turn, expect activity in regions involved in such processes to be more pronounced in ASD individuals. Neuropsychological data, imaging and lesion studies show that the hippocampus is highly implicated in memory formation [Squire, 1992; Squire and Zola-Morgan, 1991]. Similar to tasks that require different elements to be remembered as a pair, such as a name and a face [Squire et al., 2004], social norms, conventions, and etiquettes are culturally learned connections of certain behaviors with certain social occasions (e.g., wearing a black suit at a funeral). We therefore hypothesize hippocampal involvement in the experience of SP, specifically in ASD individuals, and tested these hypotheses using combined pupillometry and functional magnetic resonance imaging (fMRI) within a sample of 16 young, male adults with ASD and 16 matched controls (see Table 1).
| ASD | HC | P < | |||||
|---|---|---|---|---|---|---|---|
| ADOS-SA | 9.47 ± 4.5 | (9.36 ± 4.8) | |||||
| ADOS-RRB | 1.80 ± 1.4 | (1.64 ± 1.3) | |||||
| ADOS comparison score | 6.27 ± 2.9 | (6.18 ± 3.1) | |||||
| ADI-R COM | 14.27 ± 4.4 | (12.88 ± 3.9) | |||||
| ADI-R SOC | 18.00 ± 5.2 | (18.00 ± 6.1) | |||||
| ADI-R STEREO | 5.82 ± 2.8 | (4.63 ± 1.6) | |||||
| Age | 21.50 ± 2.9 | (20.90 ± 1.8) | 24.31 ± 2.9 | (24.27 ± 3.3) | 0.012 | (0.008) | |
| Age at diagnosis | 14.76 ± 3.1 | (14.07 ± 2.3) | |||||
| Verbal IQ | 117.5 ± 14.4 | (116.8 ± 15.9) | 113.3 ± 10.7 | (113.5 ± 9.1) | 0.368 | (0.550) | |
| AQ | 30.06 ± 8.8 | (28.64 ± 9.0) | 11.91 ± 5.7 | (11.28 ± 5.7) | < 0.001 | ( < 0.001) | |
| TAS-20 | 55.53 ± 14.3 | (56.80 ± 14.1) | 44.93 ± 10.02 | (44.56 ± 12.0) | 0.030 | (0.059) | |
| Rating | PN | 1.29 ± 0.3 | (1.18 ± 0.2) | 1.23 ± 0.3 | (1.26 ± 0.4) | 0.603 | (0.565) |
| PP | 3.68 ± 0.7 | (3.67 ± 0.6) | 3.56 ± 0.4 | (3.69 ± 0.4) | 0.531 | (0.937) | |
| SN | 1.14 ± 0.2 | (1.14 ± 0.3) | 1.04 ± 0.7 | (1.04 ± 0.1) | 0.120 | (0.258) | |
| SP | 2.82 ± 0.8 | (2.83 ± 0.8) | 2.99 ± 0.6 | (3.00 ± 0.7) | 0.502 | (0.607) | |
| RT | PN | 753.5 ± 233.2 | (729.6 ± 124.2) | 597.6 ± 125.1 | (590.0 ± 122.7) | 0.025 | (0.015) |
| PP | 851.7 ± 297.2 | (871.5 ± 302.6) | 787.0 ± 144.2 | (760.3 ± 156.5) | 0.440 | (0.331) | |
| SN | 807.8 ± 274.2 | (752.3 ± 195.0) | 755.5 ± 194.8 | (725.4 ± 143.0) | 0.539 | (0.715) | |
| SP | 1016.6 ± 416.1 | (941.4 ± 298.3) | 954.4 ± 259.6 | (931.9 ± 230.0) | 0.616 | (0.934) | |
| Pupil dilation | PN | (0.69 ± 0.60) | (0.55 ± 0.31) | (0.498) | |||
| PP | (1.06 ± 0.52) | (1.11 ± 0.34) | (0.255) | ||||
| SN | (−0.09 ± 0.65) | (−0.05 ± 0.43) | (0.849) | ||||
| SP | (0.04 ± 0.63) | (0.50 ± 0.41) | (0.055) | ||||
- Abbreviations: ASD, autism spectrum disorder; HC, healthy controls; ADOS, autism diagnostic observational schedule [Lord et al., 2000; Rühl et al., 2004]; ADOS-SA, ADOS social affect; ADOS- RRB, ADOS restricted and repetetive beahviors; ADI-R, autism diagnostic interview-revised [Bölte and Poustka, 2001; Lord et al., 1994]; ADI-R COM, communication and language; ADI-R SOC, reciprocal social interaction; ADI-R STEREO, patterns of behavior; PN, physical neutral; PP, physical pain; SN, social neutral; SP, social pain; RT, reaction time in ms.
- Verbal IQ was assessed using the German Wechsler Adult Intelligence Scale [Tewes, 1991]. The German version of the Autism Spectrum-Quotient questionnaire (AQ) [Freitag et al., 2007] was used to assess autistic symptoms and the Toronto Alexithymia Scale (TAS-20) to assess symptoms of alexithymia [Taylor et al., 1997]. Numbers indicate means and standard deviations for the whole group. For the subgroup of participants (N = 11) for which valid eye-tracking data was available, numbers are indicated in parentheses. P-values result from two-sample t-tests for mean differences.
MATERIALS AND METHODS
Participants
Patients (N = 16) were older than 18 years, male, and had a verbal IQ > 85; matched the DSM IV criteria for ASD; had a confirmed ICD-10 diagnosis of autism (n = 1), Asperger syndrome (n = 14), or atypical autism (n = 1); had undergone standardized diagnostic procedures with either Module 3 or Module 4 of the Autism Diagnostic Observation Schedule (ADOS) [Lord et al., 2000, 2012; Rühl et al., 2004, Supporting Information Methods for further details], the Wechsler Intelligence Scale for Children [Wechsler, 2003], and, if parental informants were available (n = 11), the Autism Diagnostic Interview-Revised (ADI-R) [Bölte and Poustka, 2001; Lord et al., 1994]. Healthy controls (HC; N = 16) were matched for sex, age (ASD: M = 21.5 years; HC: M = 24.3 years; t(30) = 2.68; P = 0.012), and verbal IQ (ASD: M = 117.4; HC: M = 113.3; t(28) = 0.93; P = 0.368) using the Wechsler Adult Intelligence Scale [Tewes, 1991] (see Table 1).
Healthy controls had no history of mental or neurological disorders or cases of ASD in first- or second-degree relatives and all participants had normal or corrected-to-normal vision. The study was approved by the local ethics committee and written informed consent was obtained from all participants involved in the study.
Stimuli and Paradigm
In two consecutive experiments we induced SP and PP with stimuli and paradigms that have been previously described [Krach et al., 2011; Paulus et al., 2015]. To induce PP, 56 digital color photographs depicting another person's left or right hand or foot from a first-person perspective in either painful or nonpainful (physical neutral - PN) situations were presented to the participants (Fig. 1a, upper row). PP and PN were matched in number and for semantic content and luminance (t(27) = 0.26, P = 0.790). Participants were instructed to attend to each photograph for 4.5 seconds and to estimate the intensity of physical pain that the depicted protagonist would experience in the respective scenario on a trial-by-trial basis (i.e., 1 = no suffering, 5 = very strong suffering). A fixation cross followed the rating phase (3s) for an average of 6.1s (Fig. 1b, upper row). In total, the experiment lasted 14.48 min.
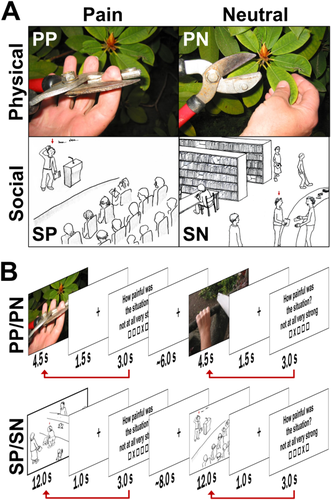
Experimental paradigms to induce physical pain (PP) and social pain (SP). A: The upper row illustrates one example stimulus of the experimental paradigm to induce PP (left) and one of the neutral control condition (PN, right, stimuli were taken from Jackson et al., 2005). The lower row illustrates one example stimulus of the experimental paradigm to induce SP (left) and one of the neutral control condition (SN, right [Krach et al., 2011]. Sketches depict a protagonist, indicated by the red arrow above his/her head, in potentially embarrassing or neutral situations. During the fMRI measurements, each sketch was accompanied by a sentence introducing the current scenario. B: The sequence of events during the functional MRI scanning is exemplified for PP/PN (upper row) and SP/SN (lower row) together with the subsequent rating periods and low-level baselines. The red arrow illustrates the weighting procedure of the PP and SP events with the intensity ratings provided after stimulus presentation. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
To induce SP, 50 validated hand-drawn sketches displaying a protagonist in either socially undesirable (SP; 40 sketches) or neutral public scenarios (socially neutral - SN; 10 sketches) were presented to the participants (Fig. 1a, lower row). SP stimuli consisted of four facets of previously established and validated vicarious social pain situations [Krach et al., 2011]. SP and SN stimuli did not differ regarding their luminance (t(48) = 1.54, P = 0.130). Participants were instructed to attend to each sketch for 12 s and to evaluate the intensity of their vicarious embarrassment experience on a trial-by-trial basis (i.e., 1 = no suffering, 5 = very strong suffering). A blank screen with a fixation cross (1s) was interleaved between the presentation of the sketch and the rating period (3s), which was followed by a 8s low-level baseline separating the trials (see Fig. 1b, lower row). The total experiment lasted 20.28min.
Acquisition and Analyses of Pupil Diameter
Pupil dilation is considered to be a measure of autonomic arousal which provides an additional and directly observable quantitative measure of the temporal pattern of brain reactivity to emotional stimuli [Silk et al., 2007]. Pupil diameter was continuously recorded at 500 Hz using an MRI-compatible Eyelink-1000 device (SR Research, Kanata, ON, Canada) during both fMRI paradigms. Valid pupil measurements were available for eleven ASD patients and eleven healthy controls (see Table 1). To characterize each trial by a single value, we determined the sustained increase in pupil size during the picture presentation of PP and SP (see Supplemental Methods). Trial-specific estimates of pupil dilation were included in the respective fMRI models to test the association of the pupil dilation with the hemodynamic signal during PP and SP (Fig. 1b and below).
Functional MRI Data Acquisition and Analyses
Participants were scanned at 3T (Siemens Trio, Erlangen, Germany) with 36 near-axial slices and a distance factor of 10% providing whole brain coverage. An echo planar imaging (EPI) sequence was used for acquisition of functional volumes (TR = 2.2s, TE = 30 ms, flip angle = 90°, slice thickness = 3 mm, FoV = 192). Overall, we obtained 395 volumes for PP and 553 volumes for SP. The first seven (PP) and four (SP) volumes of each session were discarded from further analyses. To rule out potential anatomical abnormalities, we acquired high resolution images with a T1-weighted 3D FFE sequence (TR = 25 ms, TE = 4.59 ms, NS = 170 (sagittal), ST = 2 mm, IG = 1 mm, FOV = 256 × 256 mm2, voxel size = 1 × 1 × 2 mm3). For two participants of the control group anatomical images were either not available or distorted.
Data were analyzed using SPM8 (www.fil.ion.ucl.ac.uk/spm). For each session, brain volumes were corrected for slice timing, head motion, and spatially normalized to the standard EPI template of the Montreal Neurological Institute (MNI) using linear and nonlinear transformations of the mean EPI images of each time session. The normalized volumes were resliced with a voxel size of 2 × 2 × 2 mm, smoothed with an 8 mm full-width half-maximum isotropic Gaussian kernel and high-pass filtered at 1/192Hz for the PP and 1/256 for the SP task. Group differences in head motion were examined using the translations and rotations for each session to control for potential biases between ASD and HC. For each, the average as well as the variance during SP and PP, a multivariate analysis of variance (MANOVA) was conducted including all six parameters (i.e. three translations and three rotations) as dependent variables. The four MANOVAs revealed no significant differences in subject's head motion between groups both for the averages (SP: F(6,25) = 1.36, P = 0.269; PP: F(6,25) = 1.07, P = 0.407) and the variances (SP: F(6,25) = 2.26, P = 0.070; PP: F(6,25) = 2.01, P = 0.102).
Two separate fixed-effects general linear models (GLMs) were calculated at the within-subject level each for PP and SP (as detailed below) in order to test for (1) activation differences and differences in the correspondence of the ratings with hemodynamics and (2) the association of pupil dilation with the hemodynamic response within ASD and HC.
Vicarious Physical Pain
Hemodynamic response and correspondence with behavior
The first model for PP included three regressors modeling the hemodynamic responses to PP and the corresponding neutral control condition (PN), and the rating period with the aforementioned stimulus durations. The PP events were additionally weighted with the corresponding rating response to examine the relationship between neural activation and behavior. Weighted β-images contrasting PP to PN and the effect of the parametric weights on PP were computed and analyzed at the group level.
Association of hemodynamic response and pupil diameter
The second model tested the association of the pupil dilation with the hemodynamic response. Therefore, the trial specific pupil dilation was entered as an additional parametric weight in the subsample of n = 11 participants for whom valid eye-tracking data were available. β-Images of the parametric modulators of the pupil dilation during the painful and neutral condition were analyzed at the group level.
Group-level analyses of PP
Three random-effects GLMs were computed for PP at the group level. The first model compared PP related neural activation in the ASD and HC group. The second model tested the association of trial-by-trial variability in the pupil slope and the hemodynamic response with a 2 × 2 repeated measures design including the parametric weights within PP and PN as repeated factor. To control for potential confounds due to group differences in pupil dynamics, this model included the intraindividual standard deviations of the pupil slope as a covariate for each group. The third model tested the association of trial-by-trial variability in PP self-reports with hemodynamic responses in both groups. To control for potential confounds because of group differences in the variance of the self-report, this model included the intraindividual standard deviations of ratings as a covariate for each group. All three random-effects group-level models also included the participants’ age as an additional covariate in order to control for potential age-related confounds in the group comparisons.
Vicarious Social Pain
All GLMs for SP were built in an identical fashion with two separate models on the within-subject level and three random-effects GLMs on the group level.
Hemodynamic response and correspondence with behavior
The first GLM for SP included six regressors, with four epoch regressors modeling the hemodynamic responses to the SP facets, one for the neutral control condition (SN), and one for the rating period with the aforementioned stimulus durations. The SP events were additionally weighted with the corresponding rating response to examine the relationship between neural activation and behavior [Paulus et al., 2014]. Weighted β-images contrasting each SP condition to the SN and the effect of the four parametric weights on SP were computed and analyzed at the group level.
Association of hemodynamic response and pupil diameter
The second model tested the association of the pupil dilation with the hemodynamic response. Therefore, the trial-specific pupil dilation was entered as an additional parametric weight for all SP and SN conditions in the subsample of n = 11 participants for whom valid eye-tracking data were available. β-Images of the parametric modulators of the pupil dilation during the painful and neutral condition were analyzed at the group level.
Group-level analyses of SP
Three random-effects GLMs were computed for SP at the group level. The first model compared the SP related neural activation in the ASD and HC group by implementing a 4 × 2 repeated measures ANOVA with the four SP facets as repeated factor. The second model tested the association of trial-by-trial variability in pupil dilation and the hemodynamic response with a 5 × 2 repeated measures design including the parametric weights within the SP facets and SN as repeated factor and the ASD and HC group. To control for potential confounds because of group differences in pupil dynamics, this model included the intraindividual standard deviations of the pupil slope as a covariate for each group. The third model tested the association of trial-by-trial variability in SP self-reports with hemodynamic responses in both groups with a 4 × 2 repeated measures ANOVA with the parametric weights of the four SP facets as repeated factor. To control for potential confounds because of group differences in the variance of the self-report, this model included the intraindividual standard deviations of ratings as a covariate for each group. All three random-effects models also included the participants’ age as an additional covariate in order to control for potential age-related confounds in the group comparisons and average effects were estimated across the four SP facets. All results were family-wise error (FWE)-corrected for multiple comparisons either in whole-brain or regions of interest (ROI) analyses using Gaussian-random field theory as implemented in SPM8.
Regions of Interest Analyses
Previous results stress the AIC and the ACC as key regions in processing PP and SP. Accordingly, two sets of functional ROIs were used in the present study: First, for PP, the bilateral AIC and ACC were defined in an independent sample contrasting PP-PN [Paulus et al., 2015], and second, the left AIC and ACC during SP were derived from another independent sample with the respective contrast SP-SN [Krach et al., 2011] (Supporting Information Fig. S2 for an illustration of the ROI volumes for PP and SP). Additionally, because individuals with ASD were hypothesized to compensate for their social deficits by adhering to learned social rules, we expected regions associated with associative learning and memory retrieval to be involved in processing complex social scenarios. We therefore generated an anatomical mask of the hippocampus including the parahippocampal gyrus — a key region for memory processes [Squire et al., 2004] — as defined by the anatomical labeling atlas with a dilation factor of one [Tzourio-Mazoyer et al., 2002]. All ROI analyses were conducted using the small-volume correction as implemented in SPM8.
To examine the relationship between brain activity and autistic symptoms we extracted the mean parameter estimates within the AIC ROIs for SP-SN and PP-PN for each individual in the ASD group. The activation parameters for each condition were then correlated with the two ADOS subscales for social affect (ADOS-SA) and restricted and repetitive behaviors (ADOS-RRB). We calculated Spearman's rho as a nonparametric measure for the association since the sample size for this group was rather small (n = 15). All analyses and the statistical analyses of the pupil dilation and behavioral data were conducted using IBM SPSS Statistics 22 (Armonk, NY: IBM Corp. Released 2013).
RESULTS
Vicarious Physical Pain
On the behavioral level, PP was successfully induced in both groups, as indicated by a significant main effect of Condition (PP: M = 3.62, SD = 0.57; PN: M = 1.26, SD = 0.34; F(1,30) = 401.06; P < 0.001; repeated measures ANOVA) and nonsignificant effects of Group (F(1,30) = 0.66; P = 0.422) and Group × Condition (F(1,30) = 0.075; P = 0.787, see Table 1) on the pain ratings. Additionally, there was a significant main effect for increase in pupil diameter, indicating arousal-related sympathetic activation [Bradley et al., 2008; Paulus et al., 2015] during PP compared to PN in both groups (F(1,20) = 32.65; P < 0.001; repeated measures ANOVA, see Table 1) and no significant effects of Group (F(1,20) = 0.68; P = 0.797) or Group × Condition interaction (F(1,20) = 1.21; P = 0.284). Furthermore, whole brain analyses indicated PP to induce strong and consistent activations of brain regions typically associated with the processing of one's own or others’ pain in ASD and HC [Lamm et al., 2011] (Fig. 2a and Table 2). This multimodal evidence for preserved affective reactivity in the ASD group was supported by a trial-specific correspondence of increase in pupil diameter and hemodynamic changes in the ROIs of the AIC (right: t(39) = 4.33; PFWE = 0.003; left: t(39) = 3.53; PFWE = 0.032) and ACC (t(39) = 4.42; PFWE = .0.008) as indicated by a conjunction of the effects in both groups (Supporting Information Fig. S1 and Table 3 for results on the average effect), thus integrating both measures for arousal related activation [see also Paulus et al., 2015].
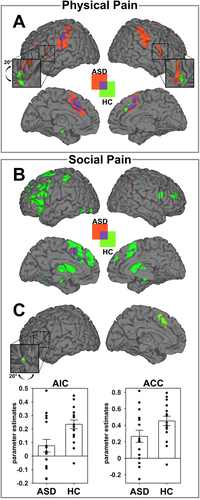
Brain activation during physical pain (PP) and social pain (SP). A: Brain activation for main effect of task (PP-PN) for patients with autism spectrum disorders (ASD) and healthy controls (HC). B: Brain activation for main effect of task (SP-SN) for patients with ASD and HCs. C: Parameter estimates of the left anterior insula cortex (AIC) and the anterior cingulate cortex (ACC) show reduction of SP-related activation in the ASD group. Parameter estimates are plotted together with standard errors at the peak voxel and illustrate the contrast of SP-SN for each group within the left AIC and the ACC (see also Supporting Information Fig. S2 and Table II). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Vicarious Social Pain
The SP task revealed a very different picture. On the behavioral level, SP was successfully induced in both groups, as indicated by a significant main effect of Condition (SP: M = 2.90, SD = 0.73; SN: M = 1.09, SD = 0.18; F(1,30) = 216.78; P < 0.001; repeated measures ANOVA) and no significant effects of Group (F(1,30) = 0.07; P = 0.791) or Group × Condition interaction (F(1,30) = 1.25; P = 0.272, see Table 1). However, the groups differed markedly on all neurobiological parameters. First, while there was a significant main effect of Condition on pupil dilation (F(1,20) = 16.05; P < 0.001) there was no main effect of Group (F(1,20) = 1.41; P = 0.250). Importantly, the Group × Condition interaction was significant (F(1,20) = 6.05; P = 0.023). Direct comparisons of the conditions within groups showed greater pupil dilation during SP in the HC group (t(10) = 5.15; P < 0.001), which was not evident in ASD individuals (t(10) = 0.99; P = 0.344, Table 1).
In accordance with the results of the pupillometry, in HCs, whole brain analyses revealed SP to elicit significant cortical activations of the ACC, AIC, thalamus, cerebellum, inferior frontal, and temporal gyrus [Krach et al., 2011; Paulus et al., 2014] (Table 2 and Fig. 2b). In patients with ASD brain activation was overall less pronounced (Fig. 2b) and ROI analyses showed a specific decrease compared to HCs within the left AIC (x = −28, y = 24, z = 2; t(118) = 2.74; PFWE = 0.032) and the ACC at trend level (x = −4, y = 22, z = 48; t(118) = 2.71; Puncorrected = 0.004, Fig. 2c). To ensure that the obtained results were not affected by potential outliers, we examined the parameter estimates for each individual and also computed robust regressions using Huber's M-estimator on the extracted parameters. Visual inspection of the parameter estimates revealed no influential data points in the direction of our hypothesis (Fig. 2c) and the robust regression showed stable t-values, specifically in the AIC (t(30) = 2.78; P = 0.005) with a less substantial trend in the ACC (t(30) = 1.99; P = 0.028). Notably, the trial-by-trial variability in the pupil dilation again corresponded to the dynamics in hemodynamic response in the ROIs of the left AIC (x = −34, y = 26, z = 0; t(99) = 2.56; PFWE = 0.040) and the ACC (x = −10, y = 18, z = 38; t(99) = 3.13; PFWE = 0.041) in the HC group (Supporting Information Fig. S1 and Table 3 for the results of the average effect). These data thus provide multimodal evidence for reduced embodied representation of SP in ASD.
| Anatomical region | Cyto area | Side | Cluster size | MNI coordinates | T | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||||
| Physical pain | ||||||||||
| HC: PP > PN | ||||||||||
| Cingulate/SMA | L/R | 1,495 | < 0.001 | |||||||
| SMA | −2 | 22 | 44 | 8.25 | ||||||
| Middle cingulate | 2 | 26 | 34 | 6.56 | ||||||
| SMA | 2 | 12 | 56 | 6.51 | ||||||
| Somatosensory | L | 783 | < 0.001 | |||||||
| Supramarginal gyrus | PFt | −56 | −26 | 38 | 6.75 | |||||
| Postcentral gyrus | PFop | −58 | −20 | 22 | 5.62 | |||||
| Postcentral gyrus | Area 1 | −54 | −34 | 54 | 4.78 | |||||
| Anterior insula | L | 296 | 0.005 | |||||||
| Insula lobe | −36 | 16 | 6 | 6.01 | ||||||
| Insula lobe | −30 | 26 | 0 | 3.89 | ||||||
| Anterior insula | R | 310 | 0.004 | |||||||
| Insula lobe | 34 | 20 | −4 | 5.71 | ||||||
| Insula lobe | 44 | 16 | −2 | 4.74 | ||||||
| Insula lobe | 32 | 20 | 6 | 4.47 | ||||||
| ASD: PP > PN | ||||||||||
| Somatosensory | L | 1,580 | < 0.001 | |||||||
| Postcentral gyrus | PFt | −58 | −22 | 32 | 7.64 | |||||
| Supramarginal gyrus | PFt | −62 | −28 | 38 | 6.86 | |||||
| Inferior parietal lobe | PFt | −46 | −34 | 42 | 5.72 | |||||
| Cingulate/SMA | L/R | 1,548 | < 0.001 | |||||||
| Superior medial gyrus | −2 | 20 | 42 | 6.89 | ||||||
| Posterio-medial frontal | 0 | 12 | 56 | 6.11 | ||||||
| Middle cingulate cortex | −6 | 24 | 34 | 5.76 | ||||||
| Somatosensory | R | 1,267 | < 0.001 | |||||||
| Postcentral gyrus | PFt | 52 | −26 | 40 | 6.67 | |||||
| Supramarginal gyrus | PFop | 64 | −18 | 28 | 6.06 | |||||
| Postcentral gyrus | Area 2 | 46 | −32 | 52 | 5.80 | |||||
| Anterior insula | R | 554 | < 0.001 | |||||||
| Insula lobe | 40 | 4 | 14 | 5.40 | ||||||
| Pars opercularis | Area 44 | 58 | 10 | 16 | 4.76 | |||||
| Inferior frontal | L | 177 | 0.035 | |||||||
| Pars triangularis | −34 | 20 | 10 | 4.99 | ||||||
| Pars orbitalis | −36 | 22 | −10 | 4.15 | ||||||
| Anterior insula | L | 326 | 0.003 | |||||||
| Insula lobe | −38 | −2 | 16 | 4.82 | ||||||
| Pars opercularis | −50 | 8 | 4 | 4.55 | ||||||
| Social pain | ||||||||||
| HC: SP > SN | ||||||||||
| Cingulate/insula | L/R | 7,787 | < 0.001 | |||||||
| Posterior-medial frontal | −4 | 20 | 48 | 8.81 | ||||||
| Middle frontal gyrus | −50 | 14 | 36 | 6.40 | ||||||
| Insula lobe | −26 | 26 | 2 | 6.16 | ||||||
| Caudate/thalamus | L | 2,058 | < 0.001 | |||||||
| Caudate nucleus | −14 | 6 | 12 | 5.78 | ||||||
| Thalamus | Temporal | −4 | −10 | 8 | 5.08 | |||||
| Cerebellum | R | 180 | 0.043 | |||||||
| Cerebellum | Lobule VI | 30 | −60 | −28 | 5.39 | |||||
| Cerebellum | Lobule VI | 26 | −74 | −24 | 3.99 | |||||
| Inferior temporal gyrus | R | 459 | < 0.001 | |||||||
| Inferior temporal gyrus | −46 | −58 | −14 | 5.03 | ||||||
| Inferior temporal gyrus | −44 | −44 | −18 | 4.15 | ||||||
| Somatosensory | L | 655 | < 0.001 | |||||||
| Postcentral gyrus | Area 2 | −38 | −38 | 54 | 4.85 | |||||
| Inferior parietal lobe | Area 1 | −52 | −30 | 50 | 4.39 | |||||
| Inferior parietal lobe | −32 | −36 | 42 | 4.16 | ||||||
| Inferior frontal gyrus | R | 181 | 0.043 | |||||||
| Pars opercularis | Area 44 | 56 | 10 | 20 | 4.62 | |||||
| Pars triangularis | Area 45 | 62 | 16 | 22 | 4.53 | |||||
| Occipital cortex | L | 240 | 0.018 | |||||||
| Middle occipital gyrus | hOc2 | −12 | −102 | 6 | 4.24 | |||||
| Calcarine gyrus | hOc1 | −14 | −102 | −4 | 4.14 | |||||
| Lingual gyrus | hOc4v | −32 | −90 | −14 | 3.53 | |||||
| ASD: SP > SN | ||||||||||
| Posterior-medial frontal | R/L | 288 | −6 | 12 | 52 | 5.71 | 0.008 | |||
| Interaction: [HC > ASD] × [SP > SN] | ||||||||||
| Anterior insula | L | 13 | −28 | 24 | 2 | 2.74 | 0.032 | |||
- Abbreviations: ASD, autism spectrum disorder; HC, healthy controls; PP, physical pain; PN, physical neutral; SP, social pain; SN, social neutral; all statistics for the PP/SP > PN/SN effects are family-wise error (FWE) corrected for whole-brain analyses at cluster level. The interaction effect was examined in an anterior insula region of interest (ROI) which was derived from an independent study on SP [Krach et al., 2011].
- All P values represent the corresponding FWE corrected value. The ̀Cyto Areá column indicates the assigned cytoarchitectonical area as indicated by the SPM ANATOMY [Eickhoff et al., 2005] toolbox v2.1 if available. Anatomical labels were derived respectively.
| Anatomical Region | Cyto Area | Side | Cluster size | MNI coordinates | T | P | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Physical pain | ||||||||
| Cingulate/SMA | L/R | 3,641 | < 0.001 | |||||
| Posterior-medial frontal | −2 | 14 | 44 | 7.50 | ||||
| Anterior cingulate | Area 33 | 6 | 14 | 28 | 6.93 | |||
| Middle cingulate | 8 | 22 | 34 | 6.64 | ||||
| Insula | R/L | 7,180 | < 0.001 | |||||
| Insula lobe | 34 | 22 | −4 | 7.44 | ||||
| Insula lobe | −34 | 16 | 6 | 6.72 | ||||
| Insula lobe | 40 | 18 | 2 | 6.69 | ||||
| VLPFC | L | 357 | 0.004 | |||||
| Inferior frontal gyrus | −40 | 42 | 6 | 5.45 | ||||
| Middle frontal gyrus | −36 | 52 | 6 | 4.90 | ||||
| Somatosensory | R | 567 | < 0.001 | |||||
| Supramarginal gyrus | PFt | 48 | −32 | 38 | 5.14 | |||
| Supramarginal gyrus | PFt | 56 | −28 | 36 | 4.77 | |||
| Postcentral gyrus | Area 3b | 30 | −20 | 376 | 4.09 | |||
| Precuneus | R/L | 2,098 | < 0.001 | |||||
| Cuneus | Area 7M | 8 | −84 | 40 | 5.13 | |||
| Precuneus | Area 7A | 6 | −66 | 60 | 4.74 | |||
| Cuneus | Area 7M | −2 | 80 | 40 | 4.74 | |||
| Superior frontal | L | 300 | < 0.008 | |||||
| Superior frontal gyrus | −14 | 2 | 74 | 4.89 | ||||
| Superior frontal gyrus | −20 | −6 | 70 | 4.00 | ||||
| Superior frontal gyrus | −28 | −10 | 66 | 3.89 | ||||
| Precentral gyrus | Area 6 | L | 194 | −48 | 2 | 44 | 4.30 | < 0.038 |
| Social pain | ||||||||
| Anterior insula | L | 38 | −32 | 26 | −4 | 2.56 | 0.046 | |
- Effects represent the average effect of the parametric weights for physical (PP and PN) and social pain (SP and SN) across the ASD and HC group. All PP/PN effects are family-wise error (FWE) corrected at the cluster level for whole brain analyses. The SP/SN effect was examined in the anterior insula region of interest (ROI) which was derived from an independent sample on SP [Krach et al., 2011]. All P values represent the corresponding FWE corrected value. The ̀Cyto Areá column indicates the assigned cytoarchitectonical area as indicated by the SPM ANATOMY [Eickhoff et al., 2005] toolbox v2.1 if available. Anatomical labels were derived respectively.
The inspection of individual activation parameters showed substantial heterogeneity during the SP task within the ASD group and the interindividual differences of the AIC activation during SP were somewhat larger in individuals with ASD compared with healthy controls (ASD: SD = 0.19, HC: SD = 0.13, Fig. 2c). The observed heterogeneity in the neural response during the SP condition could relate to differences in the severity of autistic symptoms. To test this assumption and explain the heterogeneity of the neural responses in the ASD group, we correlated the two ADOS scales for SA and RRB with the average activation within the AIC ROIs for SP and PP. Nonparametric analyses revealed a significant negative association of the ADOS-SA with SP activation (Spearman's ρ = −0.482; P = 0.034) indicating less SP induced activation with increasing symptom severity in the social affect domain. However, this association was not observed for the PP condition (Spearman's ρ = 0.297; P = 0.141) and the correlations with the ADOS-SA were significantly different between the SP and PP conditions (P = 0.007; Fig. 3 left). Such dissociation was not found for the ADOS-RRB (SP: ρ = −0.007; P = 0.490, PP: ρ = 0.321; P = 0.122, Fig. 3 right) and notably the ADOS-RRB correlation was significantly less pronounced than the association of SP activation with the ADOS-SA domain (P = 0.035).
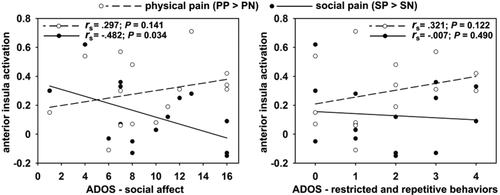
Association of anterior insula (AIC) activation with individual differences in symptom severity as measured with the autism diagnostic observational schedule (ADOS). Spearman's rho correlation coefficients (rs) are depicted together with the slope of the linear fit between symptom severity and AIC activation. The association of symptom severity in the domain of social affect with AIC activation significantly differs between physical and social pain (P = 0.007) and individuals with autism spectrum disorders show less pronounced AIC activation with stronger symptom severity.
Correspondence of Neural Activation and Intensity Ratings
The contradiction between the nonfindings on the behavioral level and the differences on the level of neurobiological parameters, specifically during SP, could be explained by regressing the intensity of the obtained self-reports with hemodynamic responses on a trial-by-trial level. In the HC group, the intensity of the SP self-reports positively correlated with the hemodynamic response in the ROIs of the AIC (t(113) = 3.31; PFWE = 0.007) and ACC (t(113) = 3.36; PFWE = 0.022, see Table 4 and Fig. 4b). This positive association of behavior with neural activation in the AIC was also present during PP in HC (t(27) = 3.68; PFWE = 0.030; see Table 4 and Fig. 4a). In ASD, the correspondence of brain and behavior was not significant for either PP and SP and, compared with the HC group, a significantly smaller association of the hemodynamic response with the intensity of the SP self-report was found in the ROI of the ACC (t(113) = 3.21; PFWE = 0.032, Fig. 4c). In contrast, patients with ASD showed greater correspondence of SP self-reports with activity of the hippocampus (right: x = 20, y = −34, z = −10; t(113) = 3.76; PFWE = 0.031 and left at trend level: x = −28, y = −44, z = −6; t(113) = 3.21; Puncorrected = 0.001, Fig. 4c), which is a key region for memory processes [Squire et al., 2004; Squire and Zola-Morgan, 1991].
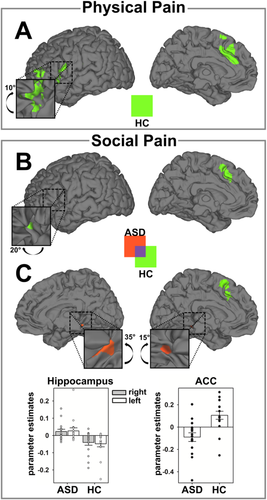
Association of within-subject variability in the intensity of the vicarious physical pain (PP) and social pain (SP) with hemodynamic responses in both groups. A: Neural network comprising the left anterior insula cortex (AIC) and the anterior cingulate cortex (ACC) that was significantly associated with the intensity of the self-report during PP in the healthy control (HC) group. In patients with autism spectrum disorders (ASD) there was no significant correspondence between the intensity of the self-report and the hemodynamic response during PP. B: Neural network comprising the left AIC and the ACC that was significantly associated with the intensity of the self-report during SP in the HC group. In patients with ASD there was no significant correspondence between the intensity of the self-report and the hemodynamic response during SP. C: Direct comparison between ASD and HC in terms of correspondence between SP self-report and hemodynamic response. Stronger associations in ASD are coded in red, stronger associations in HC are coded in green. Parameter estimates of areas showing significant differences between groups are plotted together with standard errors at the peak voxel (Supporting Information Fig. S2 and Table IV). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
| Anatomical region | Side | Cluster size | MNI coordinates | T | P | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Physical pain | |||||||
| HC | |||||||
| Anterior insula | L | 143 | −34 | 30 | 4 | 3.68 | 0.030 |
| Social pain | |||||||
| HC | |||||||
| Anterior insula | L | 62 | −36 | 24 | 0 | 3.31 | 0.007 |
| Anterior cingulate | L/R | 280 | −2 | 10 | 56 | 3.36 | 0.022 |
| −6 | 20 | 38 | 3.15 | 0.038 | |||
| HC > ASD | |||||||
| Anterior cingulate | L/R | 75 | −2 | 18 | 58 | 3.21 | 0.032 |
| ASD > HC | |||||||
| Hippocampus | R | 673 | 20 | −34 | −10 | 3.77 | 0.031 |
- Abbreviations: ASD, autism spectrum disorder; HC, healthy controls; effects represent the effect of the parametric weights for physical (PP) and social pain (SP) within the HC group and the direct comparison between groups for SP.
- No significant effects were found for the main effect of task in the ASD group. Effects were examined in the regions of interests (ROI), which were derived from independent studies on PP [Paulus et al., 2015] and SP [Krach et al., 2011] and the P values represent the corresponding FWE corrected value within the ROIs (for functional ROIs see Supporting Information Fig. S2). The ROI analysis for the hippocampus was conducted within an anatomically defined mask from the anatomical labeling atlas (AAL) [Tzourio-Mazoyer et al., 2002] and the P value represents the corresponding FWE corrected value within the ROI.
DISCUSSION
The present study analyzed behavioral and neurobiological markers of embodied representations of another's physical and social pain in ASD. Integrating pupillometry with fMRI, in ASD, we found domain-specific decrements in the neurobiological response of vicarious social but not physical pain. The data suggest that in ASD, the behavioral response to SP dissociates from the neurobiological embodiment of others' affect. This evidence provides support for previous findings in patients with ASD that verbal responses may not be sufficient to unravel altered mechanisms in intuitively representing another person's state. This has been documented, for example, in the context of different theory of mind tasks showing that persons with ASD were likely to give correct verbal reports that did not correspond to their spontaneous behavior [Abell et al., 2000; Castelli, 2002; Kana et al., 2009; Klin, 2000] or anticipatory looks [Ruffman et al., 2001; Senju et al., 2009]. Here, we show that persons with ASD use an alternative route to obtain the same behavior and we provide the first evidence for differences in the underlying neurobiological mechanisms that link neural activation with behavior.
In healthy controls, the self-reports on the intensity of their SP experience showed significant coupling with activity of the ACC and AIC, brain regions associated with embodied affects (for a replication of this effect in an independent sample, see Paulus et al., 2014). In contrast, ASD individuals did not base their self-report on the embodied representations of affect in this network, but instead showed a stronger association with the hippocampus, which is a key region for memory processes [Squire, 1992; Squire and Zola-Morgan, 1991]. This echoes with clinical observations that people with ASD compensate for their reduced ability to access embodied signals by means of rigidly learning and memorizing social rules and conventions [Baron-Cohen et al., 2003; Klin et al., 2003]. This gains relevance for the social pain of vicarious embarrassment which, in particular, relies on a conceptual understanding of social norms that can be thought of as culturally learned connections of specific social occasions with specific behaviors. Thus, the present data suggest different pathways to report on one's own vicarious emotions in complex social environments. Individuals with ASD seem to compensate for dysfunctions in the interoceptive route that give access to the embodied representation of others’ affect by retrieving memorized representations of learned rules and conventions.
The lack of reliance on the interoceptive route in ASD also manifests in diminished embodied affect as represented by reduced activation of the ACC and AIC as well as by the pupillary responses. This finding is specific to complex social scenarios in which one has to dynamically integrate intuitive judgments about contextual demands and the expectations of the social environment [Paulus et al., 2013b]. In the context of vicarious physical pain, no peculiarities were found in any of the neurobiological markers of embodied affect. The neural response of the AIC and ACC as well as the pupil response of the ASD group were very similar compared to HC, thereby replicating recent findings on vicarious physical pain in ASD [Bird et al., 2010; Hadjikhani et al., 2014]. This suggests the absence of domain-general disturbances in embodying affect in ASD, but points to a gradient regarding the complexity of social situations. In simple situations, in which one's own body is sufficient to represent the totality of others’ affect, persons with ASD are not compromised. In more complex social situations, however, in which the representation of another's affective experience necessitates the extraction of the gist of the social setting by integrating contextual demands with the expectations of the social environment, patients with ASD lack the intuitive access to the embodied representation of affect. This might help to explain the often observed odd and eccentric behaviors and difficulties during social interactions [Carter et al., 2005] and also suggests that the ability to share another's affect is not fundamentally compromised in ASD [Bird et al., 2010; Keysers and Gazzola, 2014]. Instead, other factors such as the complexity of the situation [Paulus et al., 2013b] and motivational aspects [Chevallier et al., 2012] might impact the vicarious response.
ASD is a highly heterogeneous disorder not only at the level of behavior and the expressed clinical symptoms but also at the underlying neural systems' functional architecture. This is supported by previous studies which reported significant correlations between individual differences in trait markers for social behavior and empathy related brain activity in individuals with ASD [see e.g., Greimel et al., 2010; Schulte-Rüther et al., 2011]. In this line, we observe the neural response during vicarious SP to be related to the ADOS-SA which additionally substantiates the finding for reduced neural responses in more complex social situations. The activation within the AIC was thereby inversely related to autism symptom severity in the domain of social affect, suggesting a central role of embodiment of others' affect for the emotional and social impairments in ASD. Conversely, no such significant correlation was found for restricted and repetitive behaviors. This might be because of the small degree and variance of observed behaviors in this domain or the relatively small sample size of individuals with ASD, but could also indicate that dysfunctions within neural systems that process social cognition specifically relate to clinical symptoms in the similar domain, thus helping to understand the heterogeneity of the ASD phenotype.
The present findings of impaired embodiment of others’ affective states might have implications for therapeutic interventions in ASD. First, interventions focusing on the understanding and sharing of basic emotions in simple social scenarios might not help to improve social intuition and enable the transfer to complex everyday life situations. Second, social skills training [Frith, 2004; Rao et al., 2008] that conveys strategies to avoid maladjusted behavior might reduce potential stigmatization and personal distress of affected individuals, but will not necessarily ease social interactions. Finally, the present data might inspire treatment options to anchor therapeutic interventions in very simply structured social situations and then gradually increase their social complexity. Repeated access to intact embodied measures of affect, and training of neural routes that are not fundamentally impaired, could be transferred to increasingly complex social situations that were previously not directly accessible. As oxytocin has been identified as a significant enhancer of behavioral performance but also neural activity related to empathy and social cognition (Aoki et al., 2014), innovative therapeutic approaches could also aim to combine oxytocin treatment with the training of social skills in order to improve therapeutic outcome.
In conclusion, the present study stresses the relevance for the neurosciences to focus on more refined experimental paradigms to reveal the peculiarities in the neural systems functional architecture of ASD. In line with developments toward using sophisticated tasks to test theory of mind deficits in autism [Baron-Cohen et al., 1997; Happé, 1994; Kaland et al., 2002], more complex paradigms on vicarious emotions are crucially needed to characterize the specific alterations in brain function related to social affect. This is evidenced by the specific association of activation with the symptom severity in the social affect domain of the ADOS only for the more complex vicarious social pain task where the gist of the depicted social situations is more difficult to grasp. The here-presented dissociation between vicarious social and physical pain in ASD on the neural systems level seems particularly valuable, since, to the best of our knowledge, it provides the first neural evidence for long-hypothesized differential strategies in dealing with complex social situations in ASD: while HCs rely on intuitively embodying others' affect, the hippocampal involvement suggests that ASD patients access explicit memory representations of socially appropriate behavior to surpass interoceptive routes.
ACKNOWLEDGMENT
The authors thank two anonymous reviewers for their valuable comments on an earlier draft of this manuscript.