Attachment models affect brain responses in areas related to emotions and empathy in nulliparous women
Abstract
Background: The attachment model, as assessed by means of the Adult Attachment Interview (AAI), is crucial for understanding emotion regulation and feelings of security in human interactions as well as for the construction of the caregiving system. The caregiving system is a set of representations about affiliative behaviors, guided by sensitivity and empathy, and is fully mature in young-adulthood. Here, we examine how different attachment models influence brain responses in areas related to empathy and emotions in young-adult subjects with secure and dismissing attachment models. Methods: By means of AAI, we selected 11 nulliparous young-adult females with a secure model and 12 with a dismissing model. Subjects underwent functional magnetic resonance, whereas imitating or observing and empathizing with infant facial expressions. Subjects were tested for alexithymia and reflective functioning. Results: Dismissing subjects activated motor, mirror, and limbic brain areas to a significantly greater extent, but deactivated the medial orbitofrontal cortex (mOFC) and the perigenual anterior cingulated cortex (pACC). During emotional faces, increased activity in dismissing women was seen in the right temporal pole. Furthermore, greater alexithymia was correlated with greater activity in the entorhinal cortex and greater deactivation in the pACC/mOFC. Conclusions: These findings provide evidence of how the attachment model influences brain responses during a task eliciting attachment. In particular, hyperactivation of limbic and mirror areas may reflect emotional dysregulation of infantile experiences of rejection and lack of protection, whereas increased deactivation of fronto-medial areas may be the expression of the inhibition of attachment behaviors, which is a typical aspect of dismissing attachment. Hum Brain Mapp, 2013. © 2012 Wiley Periodicals, Inc.
INTRODUCTION
The attachment theory defines the organization of a variety of attachment behaviors within the individual in response to internal and external cues, thus serving the aim of maintaining and acquiring a feeling of security [Bowlby, 1969; Sroufe and Waters, 1977]. According to Bowlby [1979], emotions are strongly associated with attachment: “many of the most intense emotions arise during the formation, the maintenance, the disruption and the renewal of attachment relationships.” Early attachment relationships are basically emotional, because they are characterized by immediate propensity of infants and parents to be attracted to and seek contact with one another, thereby facilitating their interaction [Parsons et al., 2010].
The most fundamental aspect of attachment theory is its focus on the biological basis of the attachment behavior [Ainsworth, 1967; Bowlby, 1969, 1988]. The attachment behaviors have been evolutionarily selected because they increased the likelihood of child-mother proximity, which in turn increased the likelihood of protection and provided survival advantage [Cassidy and Shaver, 2008].
Connected to the attachment organization is the caregiving system, a subset of parental behaviors designed to promote proximity and comfort when the parent perceives that the child is in real or potential danger [George and Solomon, 1996, 1999]. In women, this system remains immature until late adolescence. During puberty, hormonal and neurobiological changes interact with environmental stimuli and prior attachment experiences [Ammaniti et al., 2000; Grossmann et al., 2005] to form a sensitive period that pushes the caregiving system toward maturity. By virtue of such transformations, late adolescents and young-adulthood females show thoughtfulness regarding mothering and begin to represent themselves as future parents [George and Solomon, 1996, 1999].
A mother's capacity to regulate her infant's fear and distress is crucial to that child's ultimate feeling of security [Ainsworth et al., 1978; Lyons-Ruth and Spielman, 2004]. In the course of interactions with the mothers, the infant develops an internal working model of attachment, which can be regarded as generalized representations of “lived experiences” [Bretherton, 1987; Bretherton et al., 1986]. Attachment models remain fairly stable across the lifespan, guiding the individual functioning and the construction of significant relationships, particularly parental love [Bowlby, 1988; Cassidy and Shaver, 2008; Shaver and Mikulincer, 2002].
The assessment of attachment in adults is conducted by means of the AAI [Main and Goldwyn, 1997], which classifies individuals as secure/free autonomous, dismissing, preoccupied, unresolved/disorganized, and cannot classify. A meta-analysis conducted on more than 10,000 AAI classifications [Bakermans-Kranenburg and Van IJzendoorn, 2009] indicated that secure and dismissing models are the most represented in nonclinical populations.
Secure people (58% of the normal population) [Bakermans-Kranenburg and Van IJzendoorn, 2009] have had infantile experiences with their parents, who guaranteed protection and emotional availability toward their attachment needs. They have worked out childhood relationships with their parents and recognize a relevant value of these relationships for their own personal history and their present mental state. They have stable and long-lasting relationships, cope well with stress, and feel comfortable with intimacy and independence. Dismissing subjects (23% of the normal population) [Bakermans-Kranenburg and Van IJzendoorn, 2009], on the other hand, have had infantile experiences of refusal toward emotional needs and, as adults, seem incapable of valuing their attachment relationships. As mechanisms of defence, they do not show overt affective responses to their memory of early and painful situations. They feel uncomfortable with intimacy, avoid close relationships, and tend to suppress their feelings. They have difficulty in regulating affective states (especially negative ones) and show increased reactivity to stress [Feeney and Kirkpatrick, 1996; Heim and Nemeroff, 1999; Powers et al., 2006]. Therefore, dismissing individuals are more likely to develop emotional disorders and psychopathology in general [Berry et al., 2007; Liotti, 2006].
The current attachment research is expanding in a new direction, with the emphasis shifting from mechanisms of physical proximity and protection to mechanisms of intersubjective exchange [Lyons-Ruth, 2006]. In humans, there has been an evolutionary shift to an intersubjective basis for attachment regulation, which allows for far more subtlety and variety in the quality of relatedness between the parent and infant than occurs in other primates. Although previous approaches have viewed the attachment motivational system as being activated by fear-arousing situations and as terminated by closeness to the caregiver, the human infant's new capacities for continuous intersubjective exchanges allow the regulation of fearful arousal to occur in the intersubjective context.
Empathy provides a comprehensive account of intersubjective intercourses, enabling individuals to establish a meaningful connection with the others' emotions. Such competencies are modulated by individual affective regulatory strategies, which vary according to the differences in attachment models [Mikulincer et al., 2005; Thompson and Gullone, 2008].
The experience of empathy has been conceptualized as the result of the dynamic interaction between three major functional components [Decety and Jackson, 2004].
The first component is the affective sharing between the self and the other, which may be conceptualized as the ability to detect and resonate with the immediate affective state of another person [Trevarthen and Aitken, 2001]. This ability is based on mechanisms of perception/action coupling that lead to shared representations between the self and the other. In social neuroscience, mirror neurons provide evidence of this perception/action coupling, because they map observed and executed actions, observed and personally experienced emotions or sensations within the same neural substrate [Gallese, 2001, 2006; Rizzolatti and Craighero, 2005].
Second, there is the self-other awareness, without which the only affective sharing would lead to the phenomenon of emotional contagion, that is, the “total identification without discrimination between one's feelings and those of the other” [DeWaal, 1996]. It has been noted that as the level of emotional self-awareness increases, the differentiation of self from other increases [Lane and Schwartz, 1987]. A specific deficit in emotional self-awareness is observed in alexithymia. Alexithymia is characterized by individuals' difficulty in recognizing and describing emotions in themselves and in differentiating mental states from bodily sensations [Taylor, 2000; Taylor and Bagby, 1988]. It has been demonstrated that alexithymic subjects have difficulty even in describing the emotional experiences of others in hypothetical situations [Bydlowski et al., 2005]. As a personality trait associated with impairments in affective regulation [Taylor et al., 1997], alexithymia has been hypothesized to correlate with dismissing attachment model [Taylor, 2000; Verhaeghe, 2004].
The last component of empathy is the mental flexibility to adopt the subjective point of view of the other. This ability is linked to reflective functioning (RF), which allows individuals to ascribe to the others mental states (i.e., feelings, wishes, thoughts, intentions, and desires) and to interpret them in a meaningful way [Fonagy et al., 1995, 2001]. RF is a developmental acquisition linked above all to secure infantile attachment relationships, because it emerges from the infant's experiences of “feeling felt” [Siegel, 2001, 2006] by a mother who is able to recognize and make sense of the child's mental states [Slade, 2002]. RF seems interconnected in particular to the integrity of self-other awareness. Indeed, it has been demonstrated that alexithymic subjects present difficulties in mentalizing, associated with an impairment to take the perspective of others [Moriguchi et al., 2006].
Numerous functional magnetic resonance (fMRI) studies have recently shown that empathy may rely on several brain areas, including some areas containing mirror neurons (i.e., motor, premotor areas, posterior parietal cortex, inferior frontal gyrus, posterior temporal cortex), as well as limbic and para-limbic structures [Singer, 2006a, b], which are active both when empathizing and imitating adult emotional faces [Carr et al., 2003]. A recent study showed that infant emotional faces elicit brain activity in the same network in a group of mothers [Lenzi et al., 2009]. The peculiar configuration of infants' faces (characterized by a large head, big eyes, high and protruding forehead, chubby cheeks, small nose and mouth) act as powerful motivators of parental caregiving behaviors [Darwin, 1872; Eibl-Eibesfeldt, 1989; Lorenz, 1943, 1971; Sprengelmeyer et al., 2009]. This response of attraction to infants is also present in adults who are not yet parents [Glocker et al., 2009a, b; Parsons et al., 2010; Stern, 1977] and may be linked to evolutionary mechanisms ensuring survival of the species.
Although Bowlby suggested that the attachment organization has a neurobiological basis, research in this area is still very difficult and limited [Buchheim et al., 2006; Lemche et al., 2006; Strathearn et al., 2009; Vrticka et al., 2008]. Research suggests that networks of highly conserved hypothalamic–midbrain–limbic–paralimbic–cortical circuits modulate parental brain responses to infants [for a review see Swain, 2011]. It has been shown that the activity of the frontolimbic system intervenes in modulating social and emotional behaviors and affect-regulating functions that are specifically involved in the attachment system [Schore, 2001]. Furthermore, the role of the right orbitofrontal cortex in attachment processes and nurturing behaviors has been stressed [Henry, 1993; Horton, 1995; Nitschke et al., 2004; Schore, 2001, 2003].
- 1
During fMRI, dismissing subjects, when compared with secure ones, have lower brain activations in areas related to emotions, empathy, and attachment when the caregiving attitude is stimulated by the images of infants with varying emotional expressions;
- 2
Dismissing subjects, when compared with secure ones, present an alexithymic profile (due to an impairment in emotional self-other awareness) as well as lower RF levels (due to a difficulty in adopting the subjective point of view of the other), and that these measures correlate with brain activation.
METHODS
Twenty-three young-adult nulliparous right-handed females were enrolled. Subjects were divided in two groups according to their state of mind with respect to attachment [Main and Goldwyn, 1997]: 11 secure subjects (F) and 12 dismissing subjects (Ds).
The secure and dismissing subjects were aged from 20 to 28 years with a mean of 23.4 years and 23.5 years, respectively. Exclusion criteria were: (i) history of major medical and/or psychopathological disorders; (ii) ongoing medical therapy; (iii) present or past pregnancy; and (iv) MRI contraindications.
To enroll the two groups, we screened 157 female students who completed the Symptom Checklist-90-revised (SCL-90-R) [Derogatis, 1983] and the Attachment Style Questionnaire (ASQ) [Feeney et al., 1994]. The SCL-90-R is a 90-item self-report symptom inventory designed to reflect psychological symptom patterns; the ASQ is a 40-item self-report measure which captures the general orientation (namely style) of an individual's attachment, on the basis of five subscales that explore a set of attitudes, beliefs, and behaviors in interpersonal relationships that are thought to stem from attachment experiences (see Supporting Information). Subjects reporting no psychopathological condition on the SCL-90-R and with scores ≥75th percentile of the normative distribution [Fossati et al., 2003] on the ASQ, respectively, reflecting secure (confidence in self and others) and dismissing attachment style (discomfort with closeness and relationships as secondary) were contacted for the administration of the AAI [Main and Goldwyn, 1997], until the completion of the two groups. Nine subjects were excluded, because they reported neither a secure attachment model nor a dismissing one.
-
“I would like you to imagine that you have a 1-year-old child, and I wonder how you think you might respond, in terms of feelings, if you had to separate from this child? Do you think you would ever feel worried about this child?”
-
“Now I would like you to continue to imagine that you have a 1-year-old child for just another minute. This time, I would like to ask, if you had three wishes for your child twenty years from now, what would they be? I am thinking partly of the kind of future you would like to see for your imagined child. I will give you a minute or two to think about this one.”
-
“We have been focusing a lot on the past in this interview, but I had like to end up looking quite a ways into the future. We have just talked about what you think you may have learned from your own childhood experiences. I had like to end by asking you what you would hope your imagined child might have learned from his/her experiences of being parented by you?”
AAI transcripts were coded by three certified coders and were also used to score subjects' RF by means of the RF Scale [Fonagy et al., 1998]. Higher scores on this scale identify individuals' ability to represent themselves and others in terms of mental states.
Subjects also completed the Toronto Alexithymia Scale (TAS-20) [Bressi et al., 1996; Taylor et al., 1992] (see also Supporting Information), to identify alexithymia, that is, the difficulty in identifying and describing emotions, and minimizing emotional experience by focusing attention externally. TAS-20 assesses alexithymia on the basis of a total score and of the following factors: difficulty in identifying feelings (TAS-F1); difficulty in describing feelings (TAS-F2); externally oriented thinking (TAS-F3). Higher total scores (TAS-Tot) identify subjects with possible or certain alexithymia.
fMRI stimuli were 72 pictures of children aged from 6 to 12 months (6 children, 3 females) selected from a previous study [Lenzi et al., 2009]. Each baby was videotaped during a face-to-face interaction with her/his mother and color photographs of their faces, with eye gaze in the centre, were selected. We identified three main facial expressions (joy-j-, distress-d-, and neutral-n-) according to precise criteria [Izard et al., 1983; Oster et al., 1992; Sullivan and Lewis, 2003], with every expression being represented by 4 pictures of each child (i.e., 24 pictures/expression; 4 pictures/child/expression).
Subjects underwent 6 fMRI sessions (on the same day). During each session they were instructed either to “watch and imitate the children's expressions without moving the head” (imi) or to “observe and try to empathize with the children's expressions, without moving either the face or the head” (emp; three sessions per task, counterbalanced within groups). During each session, stimuli were presented in a random and unpredictable order. Each face was shown for 2,300 m/s, with an inter-trial interval of 750 ms (±250 ms, jittered). These short inter-trial intervals were chosen to maximize the design efficiency for differential effects between conditions [Friston et al., 1999]. Nonetheless, each fMRI run also included 16 randomly interspersed “null-events” (fixation cross), which allowed us to measure fMRI activation versus rest (r). Neuroimaging data were obtained on a 3 T scanner (Magnetom Allegra, Siemens). For the fMRI tasks, echo planar T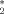 -weighted imaging was used (repetition time (TR) = 2,080 ms, echo time (TE) = 30 ms, 32 axial slices, slice thickness = 2.5 mm, voxel size = 3 × 3 mm, flip angle = 70 degree, matrix size 64 × 64, field of view (FOV) = 192 mm). For each run, 160 whole brain volumes were collected.
-weighted imaging was used (repetition time (TR) = 2,080 ms, echo time (TE) = 30 ms, 32 axial slices, slice thickness = 2.5 mm, voxel size = 3 × 3 mm, flip angle = 70 degree, matrix size 64 × 64, field of view (FOV) = 192 mm). For each run, 160 whole brain volumes were collected.
Data were analyzed using SPM5 (Statistical Parametrical Mapping, http://www.fil.ion.ucl.ac.uk). Preprocessing and first-level analysis was separated for imitation and empathizing sessions. Preprocessing consisted of rigid realignment and slice timing correction (middle slice as reference). The images were normalized to the Montreal Neurological Institute space [affine regularization to the international consortium for brain mapping (ICBM)—space template], using the mean of the functional volumes and smoothed (Gaussian filter of 8-mm full-width at half maximum). Statistical inference was based on a random effect approach [Holmes and Friston, 1998].
Single subject first level analysis considered the onset of each event (duration = 0), convolved with the SPM5 haemodynamic response function (comprising 2 gamma functions, one modeling the peak and the other the undershoot). The movement parameters (translation and rotation) resulting from motion correction were included as regressors in the model.
For each subject and task, we calculated the following contrasts: single faces (d > r; j > r; n > r), emotional faces (d\j > n), all faces (d\j\n > r), to be used for the analysis of variance (ANOVAs) and correlation analyses (see below).
The second level analyses consisted of separate ANOVAs for the empathizing and the imitation tasks. For each task, the corresponding ANOVA included the three single faces contrasts, modeled separately for the two groups. We used these models to investigate the overall effect of empathizing and imitation of faces (irrespective of emotion), testing both within-group effects and between-group differences. The statistical threshold was set at PFWE = 0.05 corrected for multiple comparisons at the cluster level (cluster extent estimated at Punc = 0.001).
Next, we explored the effects of emotion using the emotional faces contrast (see above). To increase the statistical power, a single ANOVA included the contrasts of the two groups, during both the imitation and empathizing tasks. We then applied a mask created by the common activation during imitation and empathizing of emotional faces (thresholded at the cluster level corrected P < 0.05) to explore the between-group differences, and considered as significant those voxels, which survived a correction of multiple comparison at the voxel level family wise error (FWE), P < 0.05 [Worsley et al., 1996].
Correlation analyses were performed between brain activity for all faces and emotional faces and subjects' psychological scores (TAS-Tot/F1/F2). The statistical models (separate for empathizing and imitation tasks) included the main effect of groups and two covariates corresponding to the psychological scores of each group separately. Correlations were considered significant when corrected for multiple comparisons at the cluster level P < 0.05, with the cluster extent estimated at Punc = 0.001.
Moreover, because we found between-group differences in the main ANOVA of the empathizing task, that is, some areas more active in Ds than F and one area (ACC/OFC; see Fig. 2) deactivated in Ds though not in F, we also explored the possible relationship between these two findings by means of a post hoc correlation analysis. For each subject, we extracted the effect of all faces in the ACC/OFC, and we built a new statistical model that included the main effect of group and two covariates corresponding to ACC/OFC-effect of all faces in two groups. The effect of all faces was extracted from the ACC/OFC cluster resulting from the contrast F > Ds (see above, and Fig. 2). We then assessed whether the ACC/OFC-signal correlated with the signal in areas of increased activation using a mask derived from the between-group ANOVA (i.e., volume of interest for the regression analysis defined with the Ds > F contrast, see also Fig. 2, threshold set at voxel level PFWE-corrected = 0.05).
Finally, to further explore the possible physiological role of these medial areas, we then considered only activity in these regions of interest for correlation analysis with the psychological scores (TAS-Tot,/F1/F2). Even in these post hoc analyses, we considered as significant those voxels which survived a voxel level correction FWE P < 0.05.
RESULTS
Psychological Data
AAI transcripts of our subjects show that F subjects can easily think about an imagined child. They express preoccupation about the hypothetical separation from the imagined child and stress the importance of affective proximity with him/her. The wishes for the future of the imagined child concern affective balance, love and the capacity to build rewarding relationships with others. On the contrary, Ds subjects show difficulty in thinking about themselves as mothers; when they try to imagine having a child, they stress the importance of his/her independence, affective autonomy, economic stability, professional realization, and physical health.
Two-samples t-test revealed significant between-group differences in relation to TAS-20 Tot score (t = −2.60, P < 0.05), with F presenting no condition of alexithymia (mean: 40.6 ± 10.61; range: 27–64) and Ds fitting in the “possible alexithymia” category (mean: 52.1 ± 10.51; range: 33–70).
Significant differences were also found for the TAS-F1 subscale (difficulty in identifying feelings) (t = −2.55, P < 0.05) and TAS-F2 subscale (difficulty in describing feelings) (t = −2.20, P < 0.05), with F presenting lower scores both in TAS-F1 (mean: 14.6 ± 3.80; range: 9–21) and in TAS-F2 (mean: 11.7 ± 4.67; range: 7–23), than Ds (TAS-F1: mean: 19.3 ± 5.05; range: 9–26. TAS-F2: mean: 15.4 ± 3.32; range: 10–23). These results show that Ds have a greater difficulty in both identifying and describing feelings than F.
Further, two-Sample t-test disclosed significant differences between groups with regard to the RF Scale (t = 4.27, P < 0.001), with F presenting “middle levels” of RF, which demonstrate the sufficiently articulated comprehension of their own and of others' mental states (mean: 4.7 ± 1.35; range: 2–6), and Ds presenting “low levels” of RF, which point to the use of superficial levels of reflective abilities (mean: 2.8 ± 0.62; range: 2–4).
fMRI
All Faces
Empathizing
Within-group effects
Empathizing of children facial expressions (all faces) induced a common pattern of activation in the two groups in the expected areas, that is, in motor [bilateral sensory-motor cortex (SMC), ventral and dorsal premotor cortex (vPMC and dPMC), presupplementary cortex (pre-SMA) and SMA, basal ganglia, thalami, and cerebellum], mirror [bilateral inferior frontal gyrus (IFG), PMC, pre-SMA, posterior parietal cortex (PPC), superior temporal sulcus (STS)], limbic areas [bilateral amygdale, hippocampus, cingulum, basal ganglia, thalami, and temporal poles (TP)], as well as in the visual system [bilateral fusiform gyrus (FuG) and occipital cortex (OcC); results corrected for multiple comparison at the cluster level, P < 0.05, see Fig. 1 and Table I].
Imitation and empathizing, within group effects: maps of activation for the two tasks (empathizing and imitation, within-group analysis) projected onto 3D rendering of the standard SPM template. For each task, we report the pattern of activation in F (Top) and in Ds (Bottom). All statistical maps are projected at a threshold of P < 0.001 uncorrected, corrected at the cluster level P < 0.05. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
| Region | Emp | Imi | ||||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | T | x | y | z | T | |
| L SMC | −54 | −12 | 52 | 5.2 | −46 | −24 | 42 | 9.4 |
| R SMC | 44 | −12 | 40 | 4.7 | 50 | −16 | 30 | 8.9 |
| L dPMC | −32 | −6 | 52 | 8.3 | −22 | −6 | 56 | 10.9 |
| R dPMC | 42 | 2 | 54 | 4.7 | 28 | 0 | 62 | 7.6 |
| L vPMC | −52 | 0 | 42 | 12.5 | −56 | 2 | 36 | 21.6 |
| R vPMC | 42 | 10 | 26 | 11.2 | 58 | 0 | 34 | 18.3 |
| L IFG | −52 | 20 | 24 | 8.2 | −60 | 4 | 24 | 13.0 |
| R IFG | 44 | 28 | 20 | 6.8 | 58 | 8 | 10 | 6.8 |
| L pre-SMA | −4 | 10 | 56 | 12.6 | −2 | 4 | 60 | 14.3 |
| R pre-SMA | 10 | 10 | 64 | 8.7 | 4 | 4 | 58 | 11.5 |
| L SMA | 0 | 0 | 68 | 15.6 | −2 | −6 | 64 | 6.9 |
| R SMA | 6 | −8 | 68 | 5.8 | 4 | −4 | 62 | 6.7 |
| L ACC | −4 | 18 | 40 | 5.8 | −8 | 14 | 38 | 6.8 |
| R ACC | 14 | −10 | 38 | 3.8 | 10 | 12 | 38 | 5.7 |
| R STS | 50 | −40 | 6 | 6.7 | 50 | −38 | 8 | 4.7 |
| L STS | −48 | −60 | 6 | 6.7 | – | – | – | – |
| R TP | 40 | 0 | −36 | 4.4 | – | – | – | – |
| L PPC | −24 | −64 | 44 | 9.8 | −38 | −38 | 44 | 10.5 |
| R PPC | 30 | −60 | 48 | 8.0 | 36 | −36 | 46 | 8.0 |
| L Striatum | −22 | 6 | −2 | 6.9 | −22 | 6 | 6 | 12.9 |
| R Striatum | 22 | 10 | 4 | 7.1 | 24 | 2 | 2 | 11.9 |
| L Thalamus | −8 | −16 | 0 | 5.1 | −12 | −18 | 2 | 5.7 |
| R Thalamus | 12 | −18 | 2 | 4.0 | 12 | 14 | 0 | 6.8 |
| L Pulvinar | −6 | −32 | −4 | 7.1 | −6 | −30 | −6 | 6.5 |
| R Pulvinar | 10 | −32 | −4 | 8.3 | 8 | −28 | −6 | 6 |
| L Hippocampus | −20 | −30 | −4 | 15.1 | −22 | −28 | −4 | 7.9 |
| R Hippocampus | 22 | −28 | −6 | 18.0 | 24 | −26 | −4 | 10.3 |
| L Amygdala | −18 | −10 | −22 | 3.2 | −26 | 0 | −12 | 7.1 |
| R Amygdala | 30 | −2 | −28 | 5.4 | 24 | 0 | −14 | 5.31 |
| L FuG | −26 | −82 | −14 | 22.3 | −42 | −48 | −22 | 12.7 |
| R FuG | 38 | −62 | −22 | 21.9 | 40 | −56 | −20 | 16.0 |
| L OcC | −14 | −104 | 4 | 29.0 | −16 | −102 | 4 | 23.3 |
| R OcC | 28 | −86 | −12 | 27.4 | −14 | −100 | −2 | 21.3 |
| L Cerebellum | −36 | −60 | −28 | 18.0 | −36 | −48 | −30 | 10.3 |
| R Cerebellum | 42 | −62 | −30 | 14.2 | 38 | −48 | −30 | 10.1 |
- In the table, significant peaks and t values of areas activated during empathizing and imitation of child faces in all subjects are reported. For each task, results of the contrast all faces (vs. rest) are reported. Peaks are reported in MNI coordinates. We report only peaks of clusters corrected for multiple comparison at cluster level, P < 0.05; Figure 1. EMP = empathizing; IMI = imitation; SMC = sensory-motor cortex; Pre-SMA = presupplementary motor area; SMA = supplementary motor area; vPMC = ventral premotor cortex; dPMC = dorsal premotor cortex; IFG = inferior frontal gyrus; ACC = anterior cingulate cortex; STS = superior temporal sulcus; TP = temporal pole; PPC = posterior parietal cortex; FuG = fusiform gyrus; OcC = occipital cortex; BG = basal ganglia.
Between-groups effects
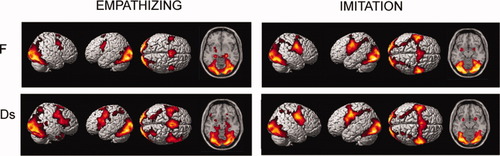
The comparison between the two groups (condition × group interaction) showed that the Ds group significantly activated several brain areas more than the F group, that is, the bilateral SMC, PMC (bilateral dPMC and L vPMC), the bilateral IFG, the R superior frontal gyrus (SFG), the R pre-SMA and SMA, the L middle temporal gyrus (MTG), the L ACC, the R STS, the R hippocampus, the L PPC, the bilateral thalami, the bilateral precuneus, the bilateral OcC, and the R cerebellum. Conversely, the F significantly activated the perigenual anterior cingulated cortex (pACC) and the medial orbitofrontal cortex (mOFC) more than the Ds (see Table II, Fig. 2); indeed, plots of the effects of interest for the pACC/mOFC revealed that Ds deactivated more than F, and that these effects appeared to be driven mainly by distress faces (Fig. 2, in red); signal plots of effect also show that greater activity in the Ds group than in the F group was instead due to hyperactivity in the aforementioned areas (Fig. 2, in green).
Empathizing, all faces (vs. rest), between-group effects: on the SPM T1-WI standard template sections, we report areas that activated differentially in F and Ds. Areas in green are those which are significantly more active during empathizing in Ds than in F (for some of these areas we show the corresponding signal plot and in green the Ds bars). In red, we report the area which is more deactivated in Ds than in F (also in red, the corresponding Ds bars of the signal plot) (ANOVA). The plots show the mean effects in all six conditions (F-d, F-j, F-n, Ds-d, Ds-j, and Ds-n). MNI coordinates are shown in brackets. See results and Table II for details of other areas that are significantly more active in Ds. All statistical maps are projected at a threshold of P < 0.001 uncorrected, corrected at the cluster level P < 0.05. a.u. = arbitrary units, 90% confidence interval (C.I.); d = distress; j = joy; n = neutral; R = right; L = left; pre-SMA = presupplementary motor area; vPMC = ventral premotor cortex; IFG = inferior frontal gyrus; PPC = posterior parietal cortex; pACC/mOFC = pregenual anterior cingulate cortex and medial orbitofrontal cortex. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
| Region | Secure > Dismissing | Dismissing > Secure | |||||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | T | x | y | z | T | ||
| EMP | L SMC | – | – | – | – | −64 | −20 | 30 | 5.5 |
| R SMC | – | – | – | – | 46 | −36 | 56 | 4.5 | |
| L dPMC | – | – | – | – | −32 | 2 | 60 | 6.7 | |
| R dPMC | – | – | – | – | 22 | 8 | 52 | 4.0 | |
| L vPMC | – | – | – | – | −48 | −4 | 44 | 5.4 | |
| L IFG | – | – | – | – | −50 | 22 | 26 | 7.1 | |
| R IFG | – | – | – | – | 50 | 12 | 12 | 6.0 | |
| L pACC | −4 | 28 | −10 | 5.5 | – | – | – | – | |
| L ACC | – | – | – | – | 0 | 24 | 38 | 4.8 | |
| R mOFC | 6 | 30 | −18 | 4.8 | – | – | – | – | |
| R SFG | – | – | – | – | 18 | 50 | 20 | 5.2 | |
| R pre-SMA | – | – | – | 2 | 16 | 66 | 6.9 | ||
| R SMA | – | – | – | – | 0 | −10 | 70 | 6.2 | |
| L Thalamus | – | – | – | – | −16 | −16 | 12 | 5.4 | |
| R Thalamus | – | – | – | – | 8 | −8 | 4 | 5.3 | |
| L PPC | – | – | – | – | −22 | −66 | 54 | 5.1 | |
| L MTG | – | – | – | – | −38 | −54 | −6 | 6.9 | |
| R STS | – | – | – | – | 58 | −52 | 14 | 4.9 | |
| R Hippocampus | – | – | – | – | 38 | −24 | −18 | 6.2 | |
| R FuG | – | – | – | – | 10 | −44 | 2 | 4.0 | |
| L Pcu | – | – | – | – | −8 | −78 | 42 | 5.2 | |
| R Pcu | – | – | – | – | 16 | −66 | 46 | 5.0 | |
| L OcC | – | – | – | – | −14 | −62 | −2 | 5.1 | |
| R OcC | – | – | – | – | 24 | −54 | 0 | 4.4 | |
| R Cerebellum | – | – | – | – | 18 | −82 | −26 | 6.5 | |
| IMI | L IFG | – | – | – | – | −44 | 18 | 24 | 5.6 |
| R MFG | – | – | – | – | 28 | 26 | 38 | 4.7 | |
| R post-STG | – | – | – | – | 58 | −50 | 16 | 5.5 | |
| L OcC | −50 | −78 | −8 | 5.8 | – | – | – | – | |
- In the table, significant peaks and t values of areas which are differently activated in the two groups during empathizing (top) and imitation (bottom) of child faces (Fig. 1) are reported. Peaks are reported in MNI coordinates. EMP = empathizing; IMI = imitation; SMC = sensory-motor cortex; vPMC = ventral premotor cortex; dPMC = dorsal premotor cortex; Pre-SMA = presupplementary motor area; SMA = supplementary motor area; IFG = Inferior Frontal Gyrus; STS = superior temporal sulcus; pACC = perigenual anterior cingulate cortex; mOFC = medial orbitofrontal cortex; PPC= Pcu = precuneus; OcC = occipital cortex.
Imitation
Within-group effects
As expected, activations during imitation are almost identical to those during empathizing. In particular, during the imitation task (all faces), the two groups activated common brain motor and mirror regions (bilateral SMC, PMC, pre-SMA and SMA, basal ganglia, IFG, bilateral PPC, thalamus, and cerebellum), as well brain areas belonging to the limbic system (bilateral ACC, hippocampus, and amygdala), to the visual system (bilateral FuG and OcC) and to the pulvinar (results corrected for multiple comparison at the cluster level, P < 0.05, see Table I and Fig. 1).
Between-groups effects
The direct comparison between the two groups (condition × group interaction) showed that F activated the left inferior occipital gyrus more than Ds. Conversely, Ds activated the right MFG, the L IFG (pars opercolaris) and the R STS more than F (see Table II).
Emotions
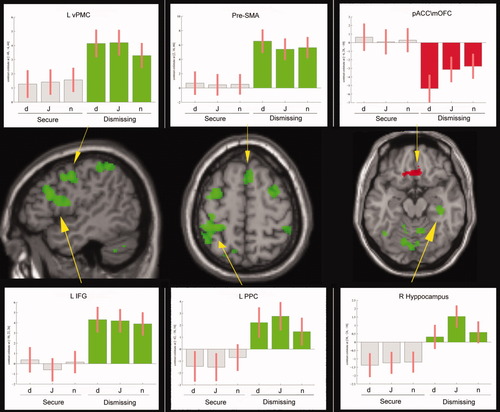
Emotional faces (j/d > n) elicited common (for group and for task, see methods) activity in the bilateral SMC, R vPMC, R pre-SMA, R STS, bilateral post-MTG, insula, thalamus, striatum, amygdala, TP, L ACC, and R cerebellum (Fig. 3, Table III).
Empathizing and imitation of emotional face: on the SPM T1-WI standard template sections, we report areas commonly activated in the two groups during both the empathizing and the imitation of the emotional faces (in blue, R striatum and R amygdala, plots from voxel located in x,y,z: 24, 2, −6; 34, −2, −22, respectively) as well as the area which is more activated in Ds than in F (TP, in red, plot from voxel in x,y,z: 38, 14, −40), with the corresponding plots. MNI coordinates are shown in brackets. See results and Table III for more details. All statistical maps are projected at a threshold of P < 0.001 uncorrected, corrected at the cluster level P < 0.05. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
| Emotions | ||||
|---|---|---|---|---|
| Region | x | y | z | T |
| L SMC | −42 | −16 | 34 | 6.1 |
| R SMC | 42 | −10 | 34 | 6.7 |
| R vPMC | 64 | −2 | 16 | 5.0 |
| R pre-SMA | −4 | −2 | 54 | 3.3 |
| L post MTG | −46 | −64 | −6 | 4.2 |
| R post MTG | 50 | −60 | 6 | 4.5 |
| R STS | 44 | −56 | 4 | 4.9 |
| L insula | −40 | 2 | 8 | 4.2 |
| R insula | 38 | −10 | −2 | 4.6 |
| L ACC | −2 | 24 | 22 | 4.5 |
| L thalamus | −6 | −12 | −8 | 4.7 |
| R thalamus | 8 | −10 | −4 | 4.8 |
| L striatum | −24 | 8 | 0 | 6.1 |
| R striatum | 24 | 2 | −6 | 6.2 |
| L TP | −42 | 14 | −38 | 5.7 |
| R TP | 38 | 14 | −40 | 6.2 |
| L amygdala | −28 | −6 | −14 | 6.5 |
| R amygdala | 34 | −2 | −22 | 6.4 |
| R cerebellum | 18 | −60 | −26 | 4.8 |
- In the table, significant peaks and t values of areas activated during empathizing and imitation (pooled in a single ANOVA) of emotional faces in all subjects are reported. For each task, results of the contrast emotions (vs. neutral faces) are reported. Peaks are reported in MNI coordinates (Fig. 3). In bold is reported the area which results to be more active in Ds when compared to F. SMC = sensory-motor cortex; vPMC = ventral premotor cortex; Pre-SMA = presupplementary motor area; STS = superior temporal sulcus; MTG = middle temporal gyrus; ACC = anterior cingulate cortex; TP= temporal pole.
Between-group analysis showed that Ds activated the right temporal pole more than F (Fig. 3, Table III). Analysis of single emotions (j > d, d > j) did not reveal any significant differences between groups.
Correlations
During the empathizing of all faces, we found that the L entorhinal cortex (EC; x,y,z = −20, −44, −12, T = 7.8, P < 0.001, corrected for multiple comparison at cluster level) and the L cerebellum (x,y,z = −8, −86, −20, T = 6.5, Pcorr < 0.034, corrected for multiple comparison at cluster level) were directly correlated with the TAS-Tot only in Ds (within group effect), which thus indicates that the more severe the alexithymia, the greater the activity within these regions.
Within the same contrast (all faces, within group effect) in the Ds, activity in the L EC (x,y,z = −32, −42, −16, T = 5,7, P < 0.001) was directly correlated with the TAS-F1, which thus indicates that the greater the activity within this area, the greater the difficulty in identifying feelings. RF did not correlate significantly with subjects' brain activity. We did not find any correlation between brain activity in the F group and psychological scores, nor did we find any correlations differences between the two groups.
Post Hoc Analysis
Correlation analyses between brain activation in both groups during empathizing of all faces restricted to the pACC/mOFC and psychological tests revealed that activity in this area correlated inversely with scores TAS-F2, which thus indicates that the greater the difficulty in describing feelings, the greater the deactivation in this area (x,y,z = 4, 30, 2, T = 4, Pcorr = 0.02; −2, 23, −2, T = 4.1, Pcorr = 0.03). Among the areas previously shown to be more active in Ds than in F during empathizing of all faces, we found a nearly significant inverse correlation between signal changes in the pre-SMA and in the pACC/mOFC (x,y,z = 8, 14, 66, T = 5.2, Pcorr = 0.08).
DISCUSSION
fMRI data show that empathizing and imitating all faces in both groups of young nulliparous women activated mirror, motor (SMC, PMC, SMA, pre-SMA, IFG, STS, PPC, striatum, and cerebellum) and limbic areas (hippocampus, amygdala, ACC, and striatum), which are critical for imitation, empathy and emotions, and the visual system (OcG and FuG). Emotions (vs. neutral faces, i.e., emotional faces) also activated limbic (striatum, amygdala, TP and L ACC), motor and mirror areas (SMC, R vPMC, R pre-SMA, R STS, bilateral L postMTG, insula, and R cerebellum). These results are in line with data from a previous study on young mothers by our group based on the same tasks and stimuli [Lenzi et al., 2009], suggesting that similar circuits are engaged by nulliparae and mothers when interacting with infant stimuli. It also has been shown that both imitation and empathizing with adult faces activate these areas [Carr et al., 2003; Lenzi et al., 2009]. According to various researchers, the parieto-frontal cortical circuit that is active during action observation is the circuit with mirror properties that “mirrors” the behavior of others (for example a facial expression) and, by interacting with the limbic system, decodes the emotional content of the actions to empathize with others [Carr et al., 2003; Gallese and Goldman, 1998; Lenzi et al., 2009; Mukamel et al., 2010; Rizzolatti and Craighero, 2004; Rizzolatti and Sinigaglia, 2010]. Nevertheless, other areas have been found to be related to empathy, such as the temporal pole, a widespread system that is crucial for many functions, the most important being learning and empathizing [Gallese and Goldman, 1998; Iacoboni, 2009; Iacoboni et al., 1999; Singer, 2006b].
Our analysis reveals that brain activations in dismissing subjects differ from those of secure subjects. Contrary to what we hypothesized, while empathizing, dismissing subjects activate several areas to a greater extent than secure subjects, including the mirror and limbic systems. On the other hand, in keeping with our hypotheses, dismissing subjects deactivate fronto-medial areas, that is, the pACC and the mOFC. Within this context, hyperactivations of limbic and mirror areas may reflect an implicit and unmodulated emotional involvement, whereas deactivations of the mOFC/pACC may reflect the emotional disinvestment toward attachment relationships, which is typical of dismissing subjects and is an expression of a more cognitive level which compensates for the nonmodulated emotional involvement.
The emotional dysregulation, operating at an implicit level, also emerges from the neuropsychological data (TAS-20, RF). These measures reveal, in the dismissing group, an impairment in self-other differentiation (expressed by a greater difficulty in both identifying and describing feelings), and in the capacity to ascribe the others mental states (naming feelings, wishes, thoughts, intentions, and desires) and to interpret them in a meaningful way [Fonagy et al., 1998, 2001]. These results are in keeping with those of previous studies that reported a connection between dismissing attachment and alexithymia [Taylor, 2000; Verhaeghe, 2004] and between dismissing attachment and RF [Slade, 2002; Slade et al., 2005].
Dismissing Attachment: Increased Activations
Our results, which were obtained with no a priori regions of interests (ROI analysis), show that women with dismissing attachment, compared with those with secure attachment, showed increased activation not only in motor, mirror, and limbic areas (Table II, Fig. 2) but also in other areas, which have only recently been found to contain mirror neurons in humans, that is, SMA, pre-SMA, SMC, medial temporal lobe, and in the cerebellum [Carr et al., 2003; Gallese and Goldman, 1998; Juliana and John, 2010; Keysers, 2009; Lenzi et al., 2009; Mukamel et al., 2010; Rizzolatti and Craighero, 2004]. These data suggest that the attachment model influences processing of emotions and of empathy, and in particular that dismissing women are more emotionally reactive to baby stimuli than secure ones. These results are apparently in contrast to research reporting that secure mothers display more helpful and supportive responses to their children [Crowell and Feldman, 1988, 1991] and that adult security is significantly associated with increased empathy and with responsiveness to others' needs [Carnelley et al., 1996; Collins and Feeney, 2000; Mikulincer et al., 2001; Rholes et al., 1999, 2007].
We may hypothesize that the greater activation in dismissing subjects of mirror areas is not connected to an empathic resonance toward the images of infants expressing different emotions: it may instead be the expression of an affective dysregulation due to the reactivation of infantile memories of parental rejection toward their own attachment needs. In this regard, it has been demonstrated that neurons in the medial temporal lobe are reactivated during autobiographical memory retrieval [Greenberg et al., 2005; Piefke et al., 2003].
This observation lends support to the functional significance of the mirror mechanism, which varies according to the location of mirror neurons in different brain areas [Fabbri-Destro and Rizzolatti, 2008]. These findings are in keeping with the supposition that there may be a variety of mirror neurons [Hamilton, 2007], one of which is more closely associated with a tendency toward personal distress or emotional contagion, conceptualized as an automatic affective response that occurs without discrimination between one's feelings and those of the other [DeWaal, 1996; Hatfield et al., 1994], rather than with perspective-taking, which in turn allows individuals to adopt the subjective point of view of the others [Batson et al., 2003]. It is important to stress how personal distress or emotional contagion can be avoided when the immediate detection of the affective state of another person (leading to neural shared representations of the self and the other, through mirror neurons) dynamically interact with reflective abilities, enabling individuals to be aware of the differentiation between their own and others' mental states and to adopt the subjective perspective of others [Decety and Jackson, 2004]. In this regard, our results found more superficial levels of RF in dismissing subjects than in secure ones, with the latter displaying a relatively more articulated comprehension of their own and of others' mental states.
Our findings are in line with those by other researchers, who have also shown that individuals with alexithymia (dismissing subjects are more alexithymic than secure ones) have a lower perspective-taking ability and higher self-oriented personal distress scores [Moriguchi et al., 2006]. Alexithymic individuals have a reduced perception of others pain despite greater personal distress and activations in several brain areas (IFG, ACC, STS, insula, lateral prefrontal cortex, and cerebellum) if compared with individuals without alexithymia [Moriguchi et al., 2007]. Moriguchi and colleagues, who also studied the mirror areas in adults with or without alexithymia, reported a greater mirror area activation in the first group correlated with the severity of alexithymia itself [Moriguchi et al., 2009].
Correlation analyses provided further evidence of emotional dysregulation in dismissing subjects. Indeed, activity within the entorhinal cortex (EC) was directly correlated with the severity of alexithymia in dismissing subjects. The EC is a well-known memory centre in the brain [Coutureau and Di Scala, 2009]. Even more interestingly, this area has recently found to contain mirror neurons. These neurons may match the sight of actions of others with the memory of the same actions performed by the observer [Mukamel et al., 2010]. Findings by Mukamel et al. (2010) in fact suggest that multiple systems in humans may be endowed with neural mechanisms of mirroring for both the integration and differentiation of perceptual and motor aspects of actions performed by self and others. Again, this result may be the neural correlate of dismissing subjects relating to infant stimuli as a powerful autobiographical recall of rejection during infancy.
So far, neuroimaging studies, which have mainly focused on the neural basis of human attachment security and insecurity, as assessed by brief self-report measures questionnaires [Brennan et al., 1998; Chris Fraley et al., 2006; Griffin and Bartholomew, 1994] designed to explore only a general “orientation” of an individual's attachment [Lemche et al., 2006; Vrticka et al., 2008] found that limbic and temporal areas (amygdala, ACC, PPC, and STS) are directly correlated with insecurity.
Gillath et al. [2005] in particular studied a group of subjects during thinking of negative scenarios and found in their ROI-analysis study that insecurity [Brennan et al., 1998] was directly correlated with activation in emotion-related areas (hippocampus, ACC, and TP).
A pilot fMRI study by Buchheim and collaborators [Buchheim et al., 2006] used the adult attachment projective to explore attachment differences in brain activations, while another study by Strathearn and et al. used the AAI to study the attachment model, and focused on to dopamine-associated brain reward regions [Strathearn et al., 2009].
Attachment research has shown that secure subjects manifest empathy toward others' needs, whereas dismissing subjects ones show avoidant strategies aimed at reducing inner distress in neutral situations [Florian et al., 2001; Mikulincer et al., 2001], but not under threatening contexts. As a result, the reactions of avoidant persons may be considered similar to those of persons who scored high on attachment anxiety, with both reacting with heightened personal distress.
Following this reasoning, two areas in particular caught our attention: the increased activity in dismissing subjects in the hippocampus and in the temporal pole. The hippocampus has been linked with specific arousal-evoking pathways during the processing of emotional autobiographical memories [Kensinger and Corkin, 2004]. Studies in humans and rats [Higley et al., 1991; McGowan et al., 2009; Meaney, 2001] suggest that adverse parental care epigenetically alters the regulation of hippocampal glucocorticoid receptor expression, which in turn leads to an altered response of the hypothalamic-pituitary-adrenal (HPA) axis, the neuromodulatory system that plays an important role in the autonomic regulation of emotion and reaction to stress. The temporal pole, a paralimbic area, is part of the HPA and is involved in emotion processing and empathy [Hein and Singer, 2008; Olson et al., 2007]. Moreover, the TP is well connected to the OFC, to the amygdala and to the hypothalamus.
Dismissing Attachment: Deactivation in the pACC/mOFC
As already mentioned, we also observed that dismissing subjects deactivated the pACC/mOFC to a greater extent than secure subjects when empathizing with all faces. In particular, plots of effects from these areas clearly show this deactivation pattern, which seems to be driven mainly by distress faces (Table II, Fig. 2). On the basis of the connections and architectonics of the OFC, some researchers [Ongur et al., 2003; Ongur and Price, 2000] proposed a fronto-medial functional network that encompasses the pACC and the mOFC as well as the ventral medial prefrontal cortex. The pACC, which is strictly connected to the amygdala, is critical for emotional processing and evidence has suggested that the pACC regulates amygdala response [Quirk and Beer, 2006; Quirk et al., 2003]. The mOFC projects also to the amygdala and massively to the ventral striatum; these projections are thought to subserve the reward or affective value of primary reinforcements including face expression, taste, touch, and texture, and is also involved in the manifestation of positive emotion [Rolls and Grabenhorst, 2008]. This area has been found to be active by positive affect displayed by mothers when viewing pictures of their infants [Nitschke et al., 2004] and viceversa [Minagawa-Kawai et al., 2009]. Thus, the OFC is not only critical for emotion modulation but also has a role in reinforcement and rewarding of positive behaviors such as those during mother-infant interaction, thereby becoming critical for the construction of attachment.
Our results suggest that deactivation of part of this fronto-medial network may represent the neural correlates of attachment avoidance behavior as typically seen in dismissing subjects. Moreover, the alexithymic profile appears to modulate neural activation in response to infants' images.
In particular, activity within medial orbitofrontal areas is directly correlated with activity within the pre-SMA (trend, P = 0.08) and inversely correlated with the TAS-F2, i.e., in dismissing subjects the greater the difficulty in describing feelings, the greater the deactivation in these areas, suggesting a role of these areas in emotional awareness. Furthermore, greater activity in the pre-SMA, which contains mirror neurons [Mukamel et al., 2010; Nakata et al., 2008; Raos et al., 2007; Rizzolatti et al., 1990, 1996], and alexithymia, which is a sign of emotional dysregulation, appear to be correlated with a greater deactivation of brain areas related to attachment and reward (pACC/mOFC).
Two Level Model of Attachment
Contrary to what we hypothesized, our findings revealed that, while empathizing, dismissing subjects activate several areas, including the mirror and limbic systems, to a greater extent than secure subjects, thereby showing higher emotional reactivity to baby stimuli.
These results, which are apparently contradictory, instead define a two-level neural system in dismissing subjects which is perfectly in keeping with the psychological theory according to which dismissing attachment is characterized by multiple (conflicting, incompatible) working models, operating at two different levels of functioning [Main, 1991, 1999; Main et al., 2005]. The implicit level is defined by nonconscious and nonmodulated response to personal affective experience (i.e., emotional dysregulation), due to the recall of infantile experiences of rejection. The explicit level, which is cognitive and overt, is defined by the deactivation of attachment and emotional involvement, in general, and is designed to compensate for the affective dysregulation through mechanisms of defensive exclusion from self-involving affective interactions [Main, 1991, 1999; Main et al., 2005].
The dismissing model has a coherent strategy (in our study defined by the over-activation of some brain areas and by the deactivation of other areas in dismissing subjects), aimed at controlling the emotional reactions toward the caregiving figures and save the relationship with them, avoiding rage and resentment provoked by their rejection of the child's attachment needs. To sum up, this model is characterized by a peculiar “state of mind,” which is designed to maintain a false sense of security by rejecting the affective components of others' experiences that might create anxiety [Main, 1999; Main et al., 2005].
Further studies are warranted to explore brain area function in attachment models other than those considered in this study, especially in the disorganized model, which is more closely connected to the psychopathological outcome, and to investigate whether and, if so, how attachment-based psychotherapy may restore equilibrium of those systems involved in emotion regulation and in attachment avoidance.
Acknowledgements
All the authors disclose any current or potential conflicts of interest.




