Periods of rest in fMRI contain individual spontaneous events which are related to slowly fluctuating spontaneous activity
Abstract
fMRI studies of brain activity at rest study slow (<0.1 Hz) intrinsic fluctuations in the blood-oxygenation-level-dependent (BOLD) signal that are observed in a temporal scale of several minutes. The origin of these fluctuations is not clear but has previously been associated with slow changes in rhythmic neuronal activity resulting from changes in cortical excitability or neuronal synchronization. In this work, we show that individual spontaneous BOLD events occur during rest, in addition to slow fluctuations. Individual spontaneous BOLD events were identified by deconvolving the hemodynamic impulse response function for each time point in the fMRI time series, thus requiring no information on timing or a-priori spatial information of events. The patterns of activation detected were related to the motor, visual, default-mode, and dorsal attention networks. The correspondence between spontaneous events and slow fluctuations in these networks was assessed using a sliding window, seed-correlation analysis, where seed regions were selected based on the individual spontaneous event BOLD activity maps. We showed that the correlation varied considerably over time, peaking at the time of spontaneous events in these networks. By regressing spontaneous events out of the fMRI signal, we showed that both the correlation strength and the power in spectral frequencies <0.1 Hz decreased, indicating that spontaneous activation events contribute to low-frequency fluctuations observed in resting state networks with fMRI. This work provides new insights into the origin of signals detected in fMRI studies of functional connectivity. Hum Brain Mapp, 2013. © 2012 Wiley Periodicals, Inc.
INTRODUCTION
Since the initial reports of temporal correlations of fMRI signal fluctuations across neuroanatomically related brain regions in the absence of a specific task (resting state) [Biswal et al., 1995], a new fMRI field has emerged aimed at understanding the biological and functional significance of brain activity at rest. To date, numerous studies have shown that when apparently at rest the brain remains active and is organized into functional networks that are consistent across subjects [Beckmann et al., 2005; De Luca et al., 2006; Damoiseaux et al., 2006; Fox and Raichle, 2007; Smith et al., 2009; Biswal et al., 2010]. Such activity of the brain when apparently at rest is observed not only in fMRI but also by other imaging modalities based on electrophysiology [Laufs et al., 2003; Leopold et al., 2003; Martini et al., 2007; He et al., 2008; Liu et al., 2010]. The term “at rest” generally describes the fact that no stimuli have been applied to, and no responses have been recorded from, the central nervous system; it does not exclude the occurrence of spontaneous efferent signals or spontaneous activity arising from within the central nervous system [Fox and Raichle, 2007]. Henceforth, we refer to this apparent resting state activity as spontaneous activity.
A key feature of the fMRI signal at rest is slow (<0.1 Hz) intrinsic fluctuations in the blood-oxygenation-level-dependent (BOLD) signal [Biswal et al., 1995; Cordes et al., 2001; Lowe et al., 1998; Hampson et al., 2002; Beckmann et al., 2005; De Luca et al., 2006; Fox and Raichle, 2007; Cole et al., 2010]. The neurophysiologic origin of these fluctuations is not completely understood but studies indicate that slow changes in the power of rhythmic neuronal activity across several frequency bands probably contribute [Leopold et al., 2003; Martini et al., 2007; He et al., 2008; Nir et al., 2008; Liu et al., 2010]. Given the predominance of these slow fluctuations at rest as well as their relative ease of detection at a temporal scale of several minutes, the majority of resting-state fMRI research has to-date focused on signal patterns occurring throughout the duration of an fMRI acquisition (≥5 min), implicitly assuming temporal stationarity (see Fox and Raichle, 2007 for review).
However, the concept that the brain can exhibit a dynamic nonstationary behavior during apparent rest has been considered previously. A recent study using the wavelet transform coherence [Chang and Glover, 2010] demonstrated modulation in the amplitude and/or coherence of slow intrinsic fluctuations across the default-mode and its “anticorrelated” network [Fox et al., 2005] within the temporal scale of an fMRI acquisition (<5 min). The dynamic element of these fluctuations has also been probed by task-based studies, for example, by investigating differences in low-frequency dynamics and/or connectivity metrics before and after task performance [Greicius et al., 2003; Waites et al., 2005; Peltier et al., 2005; Duff et al., 2008; Barnes et al., 2009], or between behavioral states [Harrison et al., 2008; Bianciardi et al., 2009, Donahue et al., 2011]. It is also highly likely that subjects will perform various mental “tasks” during rest-periods in fMRI paradigms that will modulate the fMRI baseline signal [Stark and Squire, 2001; Mason et al., 2007]. Furthermore, recent elegant work combining concurrent EEG and fMRI measurements at rest showed that very short neuronal events (∼100 ms) detected on the scalp, termed EEG microstates, correlate with BOLD signal changes across reported resting state networks [Britz et al., 2010; Musso et al., 2010]. Subsequent work showed that EEG microstates have scale-free temporal patterns that extend over periods of up to several seconds [van de Ville et al., 2010]. MEG evidence also points to the dynamic alteration of resting-state fluctuations on the order of seconds [de Pasquale et al., 2010]. The above suggests that the fMRI signal at rest may reflect, at least in part, transient neuronal activations which might be observable as individual spontaneous BOLD events.
This work demonstrates the existence of spontaneous BOLD events in the fMRI signal at rest, and relates these to intrinsic slow fluctuations. Spontaneous events were identified using a paradigm free mapping (PFM) technique that requires no prior information on timing of events [Caballero-Gaudes et al., 2010, 2011], and no a-priori spatial assumptions. Electromyography (EMG) measurements were recorded concurrently with the fMRI data to assist in the interpretation of the fMRI results. The patterns of activation detected were related to known cortical networks [motor network (MN), visual network (VN), default mode network (DMN), and dorsal attention network (DAN)]. The spatial and temporal correspondence between spontaneous activation events and slow fluctuations in these networks was assessed with a sliding window, seed-correlation analysis, where seed regions were selected based on individual spontaneous activity BOLD maps. We predicted that (1) the sliding-window approach would reveal temporal variations in an active network and (2) if a spontaneous event dominated the correlation in a particular network then the correlation would diminish when that event was excluded from the window.
METHODS
The data used here were obtained as part of a previous study [Caballero-Gaudes et al., 2010]. This paradigm included a finger-tapping task that assisted in validating the detection of BOLD activity with PFM. In this work, we directed our investigation toward task-unrelated activity not previously examined in detail. The data acquisition is briefly described in the following section.
fMRI Data Acquisition
Imaging
Six normal volunteers (age 24–32 years) participated in the study after providing informed consent under the approval of the University of Nottingham ethics committee. fMRI data were acquired on a 7T Philips scanner (Best, Netherlands) using a 16-channel head coil (Nova Medical, MA). Subjects' heads were secured in place using foam pads inside the head coil. Data were acquired using a single-shot gradient-echo echo planar imaging (EPI) sequence with 2-mm isotropic resolution, sensitivity encoding (SENSE) factor = 1.5, echo time (TE) = 30 ms, repetition time (TR) = 2 s, and flip angle = 80° and consisted of 342 dynamic repeats. Twenty oblique slices were acquired at ∼+15° to the cantomeatal line above the corpus callosum from superior frontal to occipital cortices (Fig. 2F). An additional data set was acquired at TR = 400 ms but not used in this study due to the limited spatial coverage. A 1-mm isotropic resolution T2*-weighted 3D spoiled FLASH scan was also acquired as an anatomical reference image. Cardiac and respiratory data were recorded using a respiratory belt and a pulse oximeter to allow physiological noise correction of the data. EMG measurements were acquired from the left and right extensor and right flexor muscles of both hands [Caballero et al., 2010].
Paradigm
The fMRI run lasted 684 s, and started with an initial rest period of 140 s, followed by two trials of visually cued finger tapping at 140 and 180 s (trial duration: 4 s) and followed by a second rest period from 184 to 384 s. At 384 s, a message (TAP at will) was projected onto a screen instructing subjects to carry out two additional trials of 4 s finger tapping at a time of their choosing (no visual cue). Thus, the final 300 s of the run duration included a rest period of variable length (in the range of 164 and 224 s) depending on each subject's performance. During rest periods, subjects were asked to fixate on a cross. The visual instructions were projected from an LCD projector via angled mirrors onto a screen located inside the scanner room, viewed through prism glasses. Subjects were instructed about the paradigm before the scanning session.
Time Series Processing
Time series data were inspected and corrected for motion, which resulted in the data from one subject being discarded due to excessive motion during the scan. The remaining datasets were corrected for physiological noise using retrospective correction of physiological motion effects (RETROICOR) [Glover et al., 2000], and detrended by deconvolving the voxel time series with sine and cosine waveforms with one cycle over the scan duration and up to fourth-order Legendre polynomials, thus removing frequencies below ∼0.005 Hz. These steps were performed using Analysis of Functional NeuroImages (AFNI) [Cox, 1996].
Subsequently, the effects of changes in CO2 level relating to changes in respiration volume over time (RVT) and cardiac rhythm (CR) on the data were investigated [Birn et al., 2006; Shmueli et al., 2007; Bianciardi et al., 2009]. RVT and CR time courses were computed from the pneumatic belt and pulse oximeter waveforms [Birn et al., 2006; Shmueli et al., 2007] and then correlated against the time series data for all voxels in the brain for a range of time lags from −16 to 16 s with a step of 1 s, by performing a linear regression for each time shift separately (total 33 regressions). If significant RVT or CR effects were found (P < 10−6 uncorrected, equivalent to approximately P < 0.05 after Bonferroni correction across voxels, not taking into account the multiple comparisons across lags [Birn et al., 2006]), the time series data were corrected by deconvolution of two RVT and/or CR regressors chosen at the time shifts where the maximum positive and minimum negative T-values were obtained [Bianciardi et al., 2009], and the regression analysis against the corrected time series data was repeated across all time lags to check for adequate removal of RVT and/or CR effects (Supporting Information Fig. 1).
Detection of Activity
The resulting time series data were analyzed using a sparse PFM (SPFM) approach [Caballero-Gaudes et al., 2010, 2011]. Single BOLD events were detected based on a linear deconvolution of the canonical hemodynamic impulse response model [Caballero-Gaudes et al., 2010, 2011; Gitelman et al., 2003] with the Dantzig Selector [Candes and Tao, 2007; Caballero-Gaudes et al., 2011]. The Dantzig Selector estimates BOLD responses in each fMRI voxel time series by solving an L1-norm regularized problem which simultaneously minimizes the L1-norm of the estimate coefficients, i.e., promoting more sparse estimates with less nonzero coefficients, and bounds the correlation between the residuals and regressors of the model, which here are defined using the canonical hemodynamic response function. The Bayesian Information Criterion (BIC) was used to choose the regularization parameter that balances the trade-off between sparsity and fitting. This analysis is based on sparse regression techniques, and it allows a spatiotemporal detection of the BOLD responses associated with cortical events without prior knowledge of their timing, no assumptions on their spatial distribution, and without the need of thresholding [Yamashita et al., 2008]. The output of the analysis was a time-series of maps depicting the activation events (nonzero coefficients representing the onset of a BOLD response estimated by the Dantzig selector). An activation time series (ATS) was then created to compress this 4D data set into a 1D plot (Fig. 1B). The amplitude of the ATS is given by the number of voxels that had nonzero coefficient in the deconvolved signal at each time point, of either positive or negative signal change. The time of each peak in the ATS indicates the onset time when widespread coordinated activity was detected across the cortex, according to the deconvolution process.
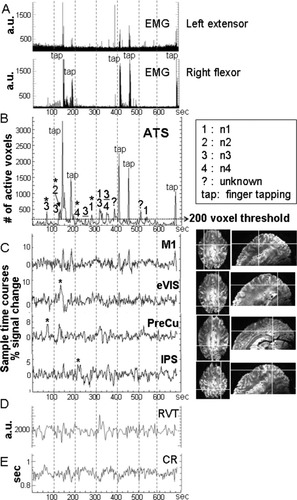
ATS for one example subject (S1) (B), with detected events and the associated networks indicated on the graph (blue numerals mark negative signal changes). A shows the EMG trace for both hands. C illustrates single voxel time courses in the hand area of the primary motor cortex (M1) activated by the finger tapping task, and in extrastriate visual (eVIS), precuneus (PreCu), and IPS regions that were found spontaneously active. The voxel locations are marked by crosshairs on the images on the right (axial view: EPI image, sagittal view: T2*w anatomical image). Maps for events corresponding to the peaks marked by (*) in B and C, are shown in Figure 2. Panels D and E show the changes in RVT and CR over time respectively. The event at 386 s coincides with the visual cue ‘tap at will’ though the activation map obtained for that event did not show activity in a particular network and the network is thus marked as unknown.
The ATS was also correlated (Spearman's correlation) against the RVT and CR waveforms as an additional check for these effects, for a range of time lags from −16 to 16 s [Birn et al., 2006; Shmueli et al., 2007].
Identification of Spontaneous Activation Events
By thresholding the ATS, peaks in the ATS involving more than 200 voxels (corresponding to >∼0.4% of brain voxels, >1.75 × interquartile range (IQR) of the ATS, and ∼10% of the maximum value in the ATS on average across subjects—indicated on Fig. 1B) were identified and selected for further analysis. Peaks corresponding to the visual cues and the task-related finger tapping events were identified and confirmed by the EMG (Fig. 1A,B). Statistical activation maps were generated for each spontaneous peak that was above threshold (Table II, row 1) based on a conventional general linear model (GLM) regression analysis in AFNI [Cox, 1996]. To do this, these peaks were used to form two regressors (canonical HRF plus temporal derivative) where the onset times of activations were taken as the times of the peak maximum in the ATS due to the deconvolution process inherent in SPFM. The task-related peaks were included as separate regressors to ensure accurate separation of task-related activity. Three additional regressors were constructed at arbitrary onset times in periods where no peak was present in the ATS and were also included in the GLM analysis as covariates of interest. These three regressors were used to show that the detection of significant activity was not a random occurrence. Six regressors consisting of the motion parameter estimates (translations and rotations) were also included as covariates of no interest. The GLM T-statistic maps were False Discovery Rate (FDR) corrected at a P value <0.05 and clustered at a neighborhood of five voxels. Finally, task-independent hand movement was identified from the EMG (Fig. 1A) as peaks above the noise floor of the EMG traces. The noise floor was computed as four times the standard deviation of the EMG trace, excluding the periods of task-related tapping.
Identification of Cortical Networks Associated With Spontaneous BOLD Events
The GLM T-maps corresponding to each spontaneous event were visually inspected, and each active area was classified according to an anatomical brain atlas [Mai et al., 2008]. Thus, each event was associated with a set of anatomically defined activated regions, and if possible, each of these sets was assigned to a particular function based on the similarity of the associated spatial pattern to known functional networks described on the literature. This resulted in the identification of four cortical networks consistently observed across subjects as shown in Table I (Fig. 2A–D and Supporting Information Fig. 2). The spatial extent of activity, however, varied between different events that were assigned to a given function (Fig. 2A–D and Supporting Information Fig. 2), and in certain cases, an event encompassed more than one functional network. Identification of a functional network was contingent on more than half of the relevant cortical regions included in the imaged slices (Fig. 2F) being active. If no corresponding function could be related to the cortical regions involved in any given event then the cortical network was marked as unknown.
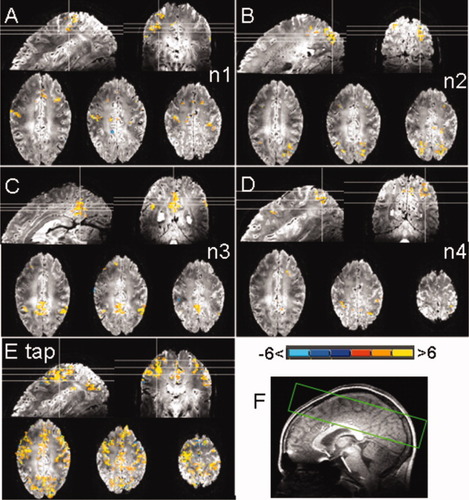
GLM T-statistic maps (P < 0.05, FDR corrected) for the ATS peaks marked by (*) in Figure 1B illustrating brain regions associated with networks n2–n4 (B–D), and network n1 (A) (Table I). Panel E illustrates the activity map for the first finger tapping trial as a reference. Panel F shows the slice positioning used for the fMRI acquisition. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
| Label | Cortical regions | Associated function |
|---|---|---|
| n1 | Precentral, postcentral, and central gyrus, medial frontal cortex (SMA) | Sensorimotor (MN)a |
| n2 | Striate cortex, occipital gyri (extrastriate cortex), cuneus | Visual (VN)b |
| n3 | Posterior cingulate, precuneus, medial frontal cortex, inferior parietal cortex (supramarginal, angular gyri) | Episodic memory, self-referential processing, default-mode network (DMN)c |
| n4 | Superior parietal lobule, intraparietal sulcus, postcentral, precentral/superior frontal gyrus, posteromedial parietal cortex (cingulate gyrus, paracentral lobule) | Working memory, dorsal attention network (DAN), “task-positive” network, imagery.d |
- Classification of spontaneous BOLD events to known functional networks. Middle column shows the cortical regions corresponding to the spatial pattern of an event. Right column shows the associated function based on the similarity of the spatial pattern to known functional networks reported in the literature (footnotes). The functional networks identified were labelled n1–n4 (left column).
- a Hanakawa et al., 2003.
- b DeYoe et al., 1996, Wandell et al., 2007, Nir et al., 2006.
- c Cabeza and Nyberg, 2000, Gusnard et al., 2001, Raichle et al., 2001, Grecius et al., 2003, Shannon and Buckner, 2004, Cabeza et al., 2008, Cavanna and Trimble, 2006.
- d Cabeza and Nyberg, 2000, Corbetta et al., 2002, Shannon and Buckner, 2004, Fox et al., 2005.
Correspondence of Spontaneous Activation Events and Slow Fluctuations
It has previously been established that the MN, VN, DMN, and DAN shown in Table I exhibit slowly fluctuating patterns of activity when apparently at rest. The relationship between the spontaneous activation events and slow fluctuations in these networks was examined using seed-voxel Pearson's correlation analysis. Seeds were selected by visual inspection in regions found spontaneously active in T-statistic maps, in extrastriate visual area (eVIS) for VN, precuneus (PreCu) for DMN, and intraparietal sulcus (IPS) for DAN (Fig. 1C). A seed in the hand area of the primary motor cortex (M1, Fig. 1C) was also selected, assuming that task-related activity would dominate the correlation observed in the relevant sensorimotor regions. Seed time courses consisted of the averaged signal time course from seven adjacent voxels, subsequently smoothed in time with a 3-point median filter.
Correlation analysis was then performed in two steps. First, the spatial extent of each network was identified by correlating the entire seed time course with all voxels in the brain (including periods of task-related activity). The resulting correlation coefficient maps were thresholded at P < 10−7 (uncorrected), revealing a set of commonly fluctuating regions, corresponding to distinct cortical networks according to the seed used for all subjects (Fig. 3 and Supporting Information Fig. 3). Next, to study temporal fluctuations in the strength of correlation across the time series, the correlation between the voxels of each network [those that showed positive correlation across the whole time series above threshold (Table III)] and the seed voxel was computed on a sliding-window basis (window length of 50 s and window step of 4 s). Window lengths of 40, 50, 60, 120, and 180 s were examined but a window of 50 s (0.02 Hz) was selected as being sufficiently long to capture both slow and fast variations in correlation. The resulting correlation time courses were averaged across the correlated voxels for display (Fig. 4). Finally, using the same seed regions and correlated voxels, the sliding window correlation analysis was repeated on the residuals of the GLM regression which excluded the detected task-related and spontaneous events.
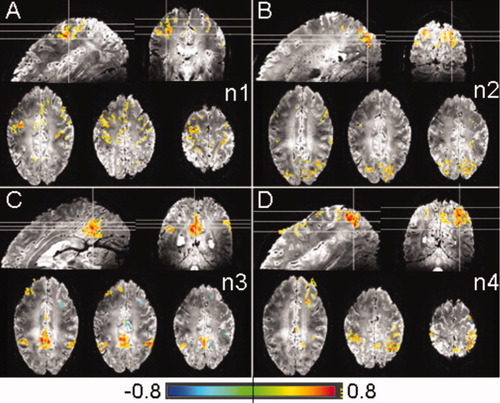
Correlation coefficient maps (P < 10−7, uncorrected) between seed time courses in M1 (A), PreCu (B), eVIS (C), and IPS (D) and all voxels in the brain for subject S1. Maps correspond to the motor system (activated by the task) (A), and the VN (B; n2), DMN (C; n3), and DAN (D; n4) networks respectively. The same slices as Figure 2 are shown and the seed locations are shown in Figure 1C. The seed time courses are shown in Figure 4. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
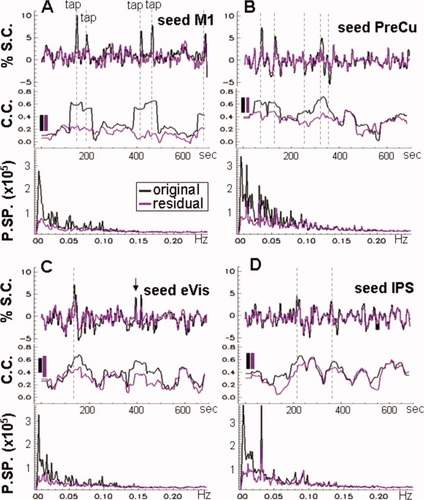
Sliding window correlation over time between the seed and the correlated voxels for subject S1, for the seeds in M1 (A), PreCu (B; DMN), eVIS (C; VN), and IPS (D; DAN). Upper graphs for each panel show the seed time course obtained from the original data (black lines) and the residuals of the GLM regression (purple lines), in percent signal change (%SC). Dashed vertical lines mark detected events. Note that the event marked by the arrow in (C) coincided with the visual cue (“tap at will”). Second row of graphs shows the sliding window correlation coefficient (CC) over time between the original seed and data (black) and between the residual seed and the residual time courses from the GLM regression (purple). The CC time courses are the average across the correlated voxels; solid bars indicate the standard deviation across voxels which was approximately equal for each sliding step. Third row of graphs per panel shows the power spectra (PSP) computed from the original (black) and residual (purple) time course data, and then averaged across the correlated voxels for each network. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
RESULTS
Following RETROICOR correction and detrending, for subjects S1, S2, S4, and S5 no significant RVT and CR effects (P < 10−6, uncorrected) were found in the signal time course (Supporting Information Fig. 1). The proportion of the fMRI voxel time series variance explained by RVT for all voxels in the brain, expressed as the coefficient of determination, R2, multiplied by 100 (%R2) [Biancardi et al., 2009], was <1% on average, for all time lags. Significant RVT effects were found for subject S3 (P < 10−6, uncorrected), but these were removed by correction for RVT effects, and the efficacy of this correction was demonstrated by repeating the correlation between the signal time course and the RVT (Supporting Information Fig. 1).
No significant correlation (P < 0.001) was found between RVT, CR, and the ATS (Fig. 1B,D,E) for all subjects (including subject S3 for whom RVT correction had been performed).
Spontaneous Activation Events
Several spontaneous events were detected for all subjects. Table II (row 1) shows the total number of events detected per subject, identified as peaks in the ATS (Fig. 1B). Figure 1 shows results for one example subject (S1); the spontaneous events detected on the ATS were categorized by a numeral (1–4), where each numeral corresponds to a different cortical network involved in the event (n1–n4, Table I), or by “?” when the network could not be identified. Events corresponding to task-related activity were confirmed by the EMG (Fig. 1A,B).
| S1 | S2 | S3 | S4 | S5 | ||
|---|---|---|---|---|---|---|
| Total number of spontaneous events | 13 | 15 | 15 | 8 | 11 | |
| Number of events per network | n1 | 3 (1) | 3 | 9 (6) | 2 | 5 |
| n2 | 1 | 3 | 9 (7) | 5 | 7 | |
| n3 | 5 (4) | 2 | 2 (1) | 1 | 3 | |
| n4 | 2 (1) | 5 (1) | 4 (1) | 4 (1) | 4 | |
| Unknown | 2 (1) | 4 | 0 | 1 | 1 | |
- First row: Number of spontaneous events detected for each subject (S1–S5), i.e., the number of peaks in the ATS consisting of more then 200 voxels (as shown in Fig. 1B), excluding task-related activity. Rows 2–5: Number of times each of the four functional networks (n1–n4) were observed per subject, excluding task-related activity. The number of events associated with EMG activity is indicated in parenthesis. Few events could not be associated with a particular function, and are assigned as unknown.
| S1 | S2 | S3 | S4 | S5 | |
|---|---|---|---|---|---|
| n1 | 2,523 | 4,312 | 5,260 | 2,320 | 3,038 |
| n2 | 2,307 | 2,243 | 4,174 | 4,142 | 2,222 |
| n3 | 1,637 | 3,056 | 2,969 | 1,748 | 1,541 |
| n4 | 2,547 | 3,957 | 3,349 | 4,214 | 3,065 |
- Number of voxels included in the sliding window seed correlation per subject (S1–S5), for each functional network detected. The number of voxels was determined by the correlation between the entire seed time course and every voxel in brain, thresholded at P < 10−7, uncorrected. n1: MN, n2: VN, n3: DMN, n4: DAN.
The amplitude of the spontaneous BOLD signal changes was similar to that for task-dependent BOLD signal changes (Fig. 1C). Spontaneous events consisted of positive and/or negative signal changes (negative signal changes are indicated by underlined numerals in Fig. 1B). Figure 2 shows the activity maps (GLM T-statistic maps, P value < 0.05 FDR corrected) for spontaneous activity events, corresponding to the peaks marked by (*) in Figure 1B, involving networks n1, n2, n3, and n4. Figure 2E shows the activity map for the first finger tapping trial as a reference. The maps (Fig. 2A–D) illustrate the cortical regions associated with each network but the spatial extent of activity varied between events. Similar results were obtained for all subjects (Supporting Information Fig. 2). No significant activation was found for the regressors designed from arbitrarily chosen onset times.
The frequency with which each of the four dominant networks was found to be spontaneously active varied between subjects (Table II, rows 2–5). Some of the events involving the motor system (n1) were related to hand movements detected by the concurrent EMG measurements (Table II, row 2 and Fig. 1A,B). Hand movement coincided with activity in the other networks that were active concurrently with the motor system but also at instances when no activity in the motor system was detected. Few events could not be associated with a particular network (Table II, row 6, Fig. 1B).
Correspondence Between Spontaneous Events and Slow Fluctuations
Correlation between the entire seed time courses and all voxels in the brain revealed cortical networks for all subjects that have been related to the VN, DMN, and DAN resting-state networks [Smith et al., 2009]. Figure 3B–D shows these correlation coefficient maps (P < 10−7, uncorrected) for seed time courses in eVIS, PreCu, and IPS for subject S1. For the MN, a correlation coefficient map was created from the seed in M1 but including spontaneous and task-related activity (Fig. 3A). All seeds were selected in similar regions for all subjects and the correlated cortical regions for each network were similar for all subjects (Supporting Information Fig. 3). Table III shows the number of voxels included in the correlation coefficient map (P < 10−7) per network for each subject.
Sliding window correlation analysis between the seed and the correlated voxel time courses showed slow fluctuations in the strength of correlation with time (Fig. 4). For the sliding window chosen (0.02 Hz), these fluctuations in the correlation occurred at frequencies below 0.01 Hz. When the sliding window included a spontaneous event detected in the ATS (dashed vertical lines on Fig. 4), an increase in correlation was observed for all networks and all subjects. A single exception was one event (Subject S5, network n4) which was observed to precede an increase in correlation strength. However, there were other instances when the correlation strength was elevated but spontaneous events were not present or were not detected above the 200-voxel threshold.
For the MN, regressing out the task-related and spontaneous events resulted in a reduction in correlation strength, averaged over the entire time series, by a factor ranging from 1.4 to 2.2 across subjects as expected (Fig. 4A). Regressing out the spontaneous events detected for VN, DMN, DAN (n2–n4) resulted in a reduction in the correlation strength at that particular time window but the overall pattern of correlation was preserved (Fig. 4B–D). The reduction in the correlation strength, averaged over the entire time series, ranged from 1.15–1.59 (VN), 1.1–1.45 (DMN), and 1.06–1.34 (DAN) across subjects, depending on the number of events detected (Table II). The effect of task-related events on these results was investigated by computing the reduction in correlation strength on excluding time windows of task-related activity from both the original and residual time courses. These time windows were taken to encompass 25 s before each visual cue until 40 s after the final cued (or uncued) finger taps performed by the subjects. For subject S1 (Fig. 1A), the final 34 s of the time courses were also excluded. Similar results were obtained for the VN, DMN, and DAN with and without removing the task related periods, whereas the reduction in correlation strength was significantly smaller (P = 0.002) for the MN when the task-related periods were excluded from the analysis.
To assess the contribution of the spontaneous events to the power spectra of the cortical networks, we computed the ratio between the spectral power of the original time courses and the GLM residuals for the frequency range 0.005–0.1 Hz. The spectral power was computed on a voxelwise basis for the correlated voxels in each network and then averaged over the same voxels, for the original and residual data separately (Fig. 4). Regressing out the events resulted in a reduction in spectral power by a factor of 2.0–3.2 (MN), 1.6–2.6 (VN), 1.5–2.1 (DMN), and 1.5–2.3 (DAN), across subjects.
DISCUSSION
This study probes the origin of the slow fluctuations observed in fMRI data acquired from a subject who is apparently at rest. Our results demonstrate that individual spontaneous BOLD activation events occur in fMRI data acquired during apparent rest. These spontaneous events encompassed one or more cortical regions; cortical regions consistently observed within and between subjects corresponded to the MN, VN, DMN, and DAN [Raichle et al., 2001; Fox et al., 2005, 2006; Beckmann et al., 2005; Smith et al., 2009]. We also found that the correlation of fluctuations within these networks varied considerably over time and peaked at the time of spontaneous events. Regressing these spontaneous events out of the fMRI signal reduced both the correlation strength and the power in spectral frequencies below 0.1 Hz in these networks, suggesting that the spontaneous events contribute significantly to correlations that are frequently observed over long-time windows. Our findings of spontaneous events supports the nonstationary nature of cortical network activity recently reported [Chang and Glover, 2010]. Here, we show that part of the nonstationary nature of resting state activity is due to individual spontaneous BOLD responses that occur in addition to slow fluctuations typically observed at rest. The origin of the fMRI signal fluctuations at rest is not completely understood, but it has been related to slow changes in rhythmic neuronal activity [e.g., Leopold et al., 2003, He et al., 2008, Nir et al., 2008, Liu et al., 2010] that potentially do not manifest as a BOLD response. The reduction in both correlation and spectral power after regressing out the detected spontaneous events suggests that a more dynamic form of activity, perhaps individual mental tasks, also contributes.
Some of the spontaneous events involving the motor system were related to small physical movements of the subject, such as hand movement detected by concurrent EMG measurements (Fig. 1A). Some activity was detected in the primary motor cortex that was not detected in the EMG and may be related to movements of other parts of the body (Supporting Information Fig. 2; S5/A), but none of this activity was related to head movement as detected by the motion parameters. For some spontaneous events, hand movement detected by the EMG coincided with activity in the other networks that were active both with the motor system but also at times when no activity in the motor system was detected. However, some peaks in the EMG signal above the noise floor (4 × standard deviation of the EMG signal, excluding task-related tapping) had a relatively low amplitude and short duration (<20 ms), which is considered to be not physiologically meaningful [Dumitru and King, 1999]. Therefore, it is possible that any muscle activity associated with these peaks did not yield signal changes in the motor system that were detectable with our analysis. Several events were detected, however, that were not related to apparent physical movement and may be indicative of individual mental tasks or states [Masson et al., 2007; Christoff et al., 2009]. It is likely that the presence of a queued hand movement will have modified the baseline neuronal activity, and this will be an interesting topic for further work.
Spontaneous events encompassed cortical networks associated with more than one function (Table I) and the spatial extent of activity varied between different events that were assigned to the same function. This may be due to “trial-to-trial” variations in the spatial pattern of cortical activations as detected with sparse PFM and GLM [Windischberger et al., 2002]. Alternatively, regions of a network may exhibit different patterns of activity depending on the particular function or mental task, as has been shown for example for the posterior parietal cortex engagement in memory retrieval [Shannon and Buckner, 2004] and attention modulation [Corbetta and Shulman, 2002]. Additionally the time-varying connectivity between regions in a given network or with other networks (de Pasquale et al., 2010; Britz et al., 2010; Musso et al., 2010; Chang and Glover, 2010] will also affect the spatial extent of spontaneous BOLD activation events.
The spatial and temporal correspondence between spontaneous events and slowly fluctuating spontaneous activity was assessed using seed-correlation analysis based on seed regions in the precuneus (DMN), extrastriate visual cortex (VN), and IPS (DAN). These regions were all found to be spontaneously active at some point for all subjects (Fig. 2 and Supporting Information Fig. 2), and to exhibit slowly fluctuating patterns of activity at rest [Beckmann et al., 2005; De Luca et al., 2006; Damoiseaux et al., 2006; Fox et al., 2005, 2006; Smith et al., 2009; Greicius et al., 2003; Nir et al., 2006]. These three networks were found to be active independent of the finger-tapping task, as they exhibited correlations over the entire time course on short-time scales (Figs. 2F and 3 and Supporting Information Fig. 3). The correlated areas found for these seeds were similar between subjects (Fig. 3 and Supporting Information Fig. 3), and generally larger than activated areas detected in the same regions of the brain for individual spontaneous events (Fig. 2D vs. Fig. 3D).
However, the correlation strength for each network fluctuated considerably over time (Fig. 4) for all subjects, in support of recent evidence on the non-stationary nature of spontaneous fluctuations at rest [de Pasquale et al., 2010; Britz et al., 2010; Musso et al., 2010; Chang and Glover, 2010]. We have shown here that part of this nonstationary nature of activity is due to spontaneous events. Both task-related and spontaneous BOLD events coincided with instances of increased correlation for all networks (Fig. 4B–D). For the motor/sensory network, the finger-tapping task was associated with considerably increased correlation in the relevant motor regions, as expected, and exclusion of these events by regressing them out of the signal time course substantially reduced the correlation across these regions at the time of the event also as expected (Fig. 4A). Exclusion of the spontaneous events for the other networks also reduced the correlation in the particular time window but correlation remained (Fig. 4B–D). It should be noted that similar results were obtained for these networks when task-related periods were excluded from this analysis, indicating that task-related periods did not make a significant contribution to the measured correlation for these networks. The reduction in correlation, however, will depend on the effectiveness of the GLM in regressing out the spontaneous events, which will depend, for instance, on variations in the shape of the hemodynamic response function. Another possibility is that the spontaneous events removed had a small spatial extent compared to the corresponding network and so may have small effect on the network correlation. After exclusion of the events, spectral power was reduced particularly in frequencies <0.1 Hz (Fig. 4) which was expected since the duration of the events was on the order of a few seconds and thus evidently in the low-frequency range. To investigate the relationship between correlation and spectral power, we further compared the average correlation over time to the total spectral power for the entire range sampled (<0.25 Hz) for each network. We found that the decrease in network correlation on removal of events was linearly related to the decrease in spectral power (R2 = 0.50 for spontaneous events and R2 = 0.97 for task-related activity) indicating that individual spontaneous events explain part of the spontaneous slow fluctuations observed in these networks with fMRI. The remaining part may be due to either spontaneous events that were not detected or slow fluctuations in neuronal activity as has been suggested in previous electrophysiological studies [Leopold et al., 2003; He et al., 2008; Nir et al., 2008; Liu et al., 2010]. These studies have shown that slow fluctuations in rhythmic neuronal activity particularly in the gamma power range (∼>25 Hz) and in low frequencies (<4 Hz) are evident as signal power changes in frequencies below 0.1 Hz and are likely correlates of the slow fluctuations in the BOLD signal. These neuronal fluctuations are associated with increases or decreases in cortical excitability or neuronal synchronization [see Raichle, 2010, Schroeder and Lakatos, 2009 for Reviews]. In this context, the increased network correlation that coincided with spontaneous events, suggests that they may reflect information transfer across brain regions, though the extent to which the spontaneous events cause or result from measured changes in network correlation in fMRI remains to be proved.
While further investigation is required to elucidate the neurophysiologic or behavioral nature of spontaneous BOLD events that are not related to apparent movement, our findings highlight that there is an interplay between individual spontaneous BOLD activation events and slow fluctuations at rest, and open up the possibility of examining functional connectivity at a more dynamic level.




