Resting state basal ganglia network in idiopathic generalized epilepsy†
The study was performed according to the standards set by the Declaration of Helsinki. The study was approved by the Ethics Committee of West China Hospital.
Abstract
The basal ganglia, a brain structure related to motor control, is implicated in the modulation of epileptic discharges generalization in patients with idiopathic generalized epilepsy (IGE). Using group independent component analysis (ICA) on resting-state fMRI data, this study identified a resting state functional network that predominantly consisted of the basal ganglia in both healthy controls and patients with IGE. In order to gain a better understanding of the basal ganglia network(BGN) in IGE patients, we compared the BGN functional connectivity of controls with that of epilepsy patients, either with interictal epileptic discharges (with-discharge period, WDP) or without epileptic discharge (nondischarge period, NDP) while scanning. Compared with controls, functional connectivity of BGN in IGE patients demonstrated significantly more integration within BGN except cerebellum and supplementary motor area (SMA) during both periods. Compared with the NDP group, the increased functional connectivity was found in bilateral caudate nucleus and the putamen, and decreases were observed in the bilateral cerebellum and SMA in WDP group. In accord with the proposal that the basal ganglia modulates epileptic discharge activity, the results showed that the modulation enhanced the integration in BGN of patients, and modulation during WDP was stronger than that during NDP. Furthermore, reduction of functional connectivity in cerebellum and SMA, the abnormality might be further aggravated during WDP, was consistent with the behavioral manifestations with disturbed motor function in IGE. These resting-state fMRI findings in the current study provided evidence confirming the role of the BGN as an important modulator in IGE. Hum Brain Mapp, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
The basal ganglia consist of four main nuclei: the striatum, the globus pallidus, the subthalamic nucleus, and the substantia nigra (SN). Anatomical studies have shown connections between the basal ganglia and functionally disparate regions of cerebral cortex. These connections are characterized by parallel, predominantly segregated, closed-loop projections (reviewed in [McHaffie et al., 2005]). Alexander et al. proposed the existence of five segregated parallel functional loops, in which information from the motor, oculomotor, dorsolateral prefrontal, lateral orbitofrontal, and anterior cingulate cortices was received by the striatum, then sent via connections to specific basal ganglia nuclei, and subsequently projected back to same cortex via the thalamus [Alexander et al., 1986]. Other studies have proposed a tripartite model of basal ganglia connections. In tripartite models the striatum is separated into associative, sensorimotor, and limbic regions [Parent and Hazrati, 1995]; Selemon and Goldman-Rakic, 1985]. Anatomical evidence has confirmed that network connectivity exists between the nuclei of the basal ganglia and the cerebral cortex. The basal ganglia have been implicated in a variety of motor-related function, including motor selection, preparation, and execution [Gerardin et al., 2004]. In addition, basal ganglia dysfunction has been associated with disturbances in movement. Some evidence suggests that the nuclei of the basal ganglia are involved in the pathophysiology of movement disorders such as Parkinson's disease, Huntington's chorea, and Tourette's syndrome.
Recently, functional brain imaging has provided an appropriate method for studying functional neuroanatomy in vivo. Resting-state fMRI has been widely used to investigate functional connectivity in healthy controls. So far, more than 10 resting state networks (RSNs) have been discovered. However, RSN representing basal ganglia was discussioned in few studies. Damoiseaux et al. first demonstrated the involvement of changes in the connectivity between the basal ganglia and some cortical areas in aging [Damoiseaux et al., 2008]. Other studies have used higher-order probabilistic independent component analysis (PICA) to study basal ganglia connectivity in youth [Abou-Elseoud et al., 2010; Kiviniemi et al., 2009]. In addition, a study by Robison et al. suggested a RSN involving the basal ganglia and SMA, corresponding to the motor control circuit [Robinson et al., 2009]. However, the functional role of the BGN in various neural disorders is not fully understood.
According to the international classification of epileptic seizure, generalized seizures resulted from disturbances involving the entire brain [Commission on Classification and Terminology of the International League Against Epilepsy 1981]. Patients with IGE were found to have various abnormal brain functions, such as abnormity in cognition, behavior, executive function, and controlled motor [Caplan et al., 2008; Hommet et al., 2006]. It has been suggested that abnormally increased activity in subcortical structures including the basal ganglia may be crucial for motor manifestations [Blumenfeld et al., 2009]. Furthermore, IGE is characterized by bilateral synchronous epileptic discharge bursts with normal background activity (including spike and wave discharge; SWD), which reflects abnormal oscillations in corticothalamic networks. The basal ganglia has been suggested to modulate the occurrence of SWD through feedback circuits to the thalamus and cerebral cortex. Some evidence indicates that an intact SN may be necessary for the propagation of seizure activity (see review in [Norden and Blumenfeld, 2002]). Besides, some studies have examined regional cerebral blood flow (CBF) changes in the basal ganglia using single photon emission computed tomography (SPECT), during ictal and postictal periods with dystonic posturing in temporal lobe epilepsy [Newton et al., 1992] and secondary generalized tonic-clonic seizures[Blumenfeld et al., 2009; Li et al., 2009]. Seeck et al. found the reduced volume in putamen and caudate nucleus in IGE [Seeck et al., 2005]. We previously conducted a simultaneous EEG and fMRI study, revealing decreased blood oxygenation level-dependent (BOLD) signals associated with interictal SWD in the basal ganglia. These evidences indicate that the two main features of IGE, generalized epileptic discharge and behavioral abnormality, may be related to basal ganglia function, thus the functional connectivity related to basal ganglia may be altered in IGE. However, at present the importance and modulation role of the basal ganglia in IGE has not been investigated in detail using functional imaging.
Resting-state fMRI has recently been used to examine diverse disorders such as epilepsy [Luo et al., 2011; Nioche et al., 2009; Zhang et al., 2009a, b], schizophrenia [Calhoun et al., 2009], and Alzheimer's disease [Dickerson and Sperling, 2009; Wang et al., 2007]. Evidence has emerged indicating the disturbance of RSNs in epilepsy, including the default mode network (DMN) [Luo et al., 2011] and an attention network [Zhang et al., 2009a]. As such, measuring the resting-state BGN may provide crucial information about abnormal motor function and regulation sensitivity of epileptic discharge in IGE compared with healthy controls. Using resting-state fMRI, we examined the BGN in healthy controls and patients suffering from epilepsy with generalized seizure in the present study. In addition to fMRI measurements, we conducted simultaneous EEG recordings for patients. As measurements of functional RSNs may be influenced by epileptic discharges, the patient data set was divided into two groups; one group(WDP): interictal SWD were recorded during the fMRI data acquisition, where epileptic discharges is considered a paroxysmal event for baseline brain activity, and the other data group (NDP): where SWD activity was absent. This study aims at identifying differences in BGN functional connectivity between control group and epilepsy patient groups. In addition, we sought to uncover the role of the basal ganglia played in IGE by comparative investigation among the three groups.
PARTICIPANTS AND METHODS
Participants
A total of 29 patients with IGE were recruited from epilepsy clinics for the EEG-fMRI study at the Neurology Department in the West China Hospital, Sichuan University. All patients underwent clinical brain structural MRI and 24-hour video EEG. No patient exhibited any radiological abnormalities. Diagnosis was established according to the diagnostic scheme published by the International League Against Epilepsy in 2001 [Engel, 2001]. The clinical epilepsy syndromes were established with generalized tonic-clonic seizures (GTCS) only in 14 patients, childhood absence epilepsy in 8 patients, juvenile absence epilepsy in 2 patients, epilepsy with myoclonic absence in 4 patients, and juvenile myoclonic epilepsy in 1 patient. The clinical patient details are shown in Table I. Thirteen patients were receiving antiepileptic medication, but the effect was poor. For the other 16 patients, nine of them [eight patients with absence seizure (patients No. 2, 4, 15, 17, 21, 24, 25, 26) and one with GTCS (patient No. 27)] were newly diagnosed epilepsy, four (No. 1, 14, 18, 19) have terminated medical treatment more than 3 years, and three (No. 3, 6, 7) never received normal medical treatment. A total of 25 right-handed, age-, and sex-matched healthy participants were selected for the control group. The study was approved by the Ethics committee of the West China Hospital, and was performed according to the standards set by the Declaration of Helsinki. Informed consent was obtained from each participant or parents (for children).
| No. | Sex | Age | Age at seizure onset | Seizure type frequency | Antiepileptic drugs | History/family history | Frequency of SWD (Hz) | No. volumes with SWD |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 12 | 2 | GTCS 1–2/m | None | — | 2.5–4 | 11 |
| 2 | F | 9 | 7 | AS several/d | None | — | 3–4 | 24 |
| 3 | M | 16 | 11 | GTCS 2–4/m | None | — | 2–4 | 12 |
| 4 | F | 8 | 6 | AS 20–30/d | None | — | 3 | 22 |
| 5 | M | 6 | 5 | GTCS 1/m | VPA | — | 3 | 5 |
| 6 | F | 14 | 11 | GTCS 1–1.2/m | None | — | 2–4 | 19 |
| 7 | M | 12 | 8 | GTCS 3/m | None | — | 3–5 | 4 |
| 8 | F | 11 | 9 | AS 2–5/d MS 1–2/w | VPA | — | 3.5 | 7 |
| 9 | F | 14 | 9 | AS 2–5/w MS 1–2/m | TCM, PHT, VPA | — | 2–3 | 40 |
| 10 | M | 13 | 8 | AS 5–8/d MS 2–3/w | PB VPA | Brother with As | 3–6 | 8 |
| 11 | M | 13 | 8 | AS 5–8/d MS 2–3/w | PB VPA | Brother with As | 3–6 | 35 |
| 12 | M | 24 | 10 | AS several/d | VPA | — | 3 | 12 |
| 13 | M | 8 | 7 | GTCS 3/m | VPA CZP LTG | — | 3 | 9 |
| 14 | M | 18 | 9 | GTCS 1/m | None | — | 2.5–3.5 | 21 |
| 15 | M | 5 | 4 | AS 40/d | None | — | 3 | 6 |
| 16 | F | 9 | 7 | AS several/d | None | — | 3–4 | 9 |
| 17 | F | 9 | 4 | AS 40/d | VPA, CZP, LTG | — | 3 | 12 |
| 18 | M | 19 | 9 | MS 2–5/w | None | — | 2.5–3 | 5 |
| 19 | M | 12 | 2 | GTCS 0.5–1/m | None | — | 2.5–4 | 8 |
| 20 | F | 20 | 10 | GTCS 1–3/m | VPA | — | 2–4 | 27 |
| 21 | F | 5 | 4 | AS 10/d | None | Hypoxia history at birth | 3 | — |
| 22 | M | 17 | 4 | GTCS 0.5/y | VPA | — | 3–3.5 | — |
| 23 | M | 21 | 13 | GTCS 1–2/m | VPA | — | 2–4 | — |
| 24 | M | 14 | 9 | AS 7–8/d | None | — | 3 | — |
| 25 | M | 18 | 5 | AS 2–3/d | None | — | 3–4 | — |
| 26 | M | 10 | 5 | AS 2–3/d | None | — | 2.5–3.5 | — |
| 27 | F | 12 | 11 | GTCS 1/m | None | — | 3–4 | — |
| 28 | M | 16 | 13 | GTCS 2/m | VPA | Mother with GTCS | 2–4 | — |
| 29 | M | 19 | 16 | GTCS 0.5/y | VPA | — | 2.5–3 | — |
- There are other data sets without discharge in patients from 12nd to 20th. Together with patients from 21st to 29th, they were separated into nondischarge group.
- AS: absence seizure, GTCS: generalized tonic clonic seizure, MS: myoclonic seizure, d: day, m: month, w: week, y: year, VPA: valproic acid, LTG: lamotrigine, CZP: clonazepam, TCM: traditional Chinese medicine, PB: phenobarbitone.
Data Acquisition
BOLD-sensitive MRI data were acquired using gradient-echo echo-planar imaging (EPI) sequences in a 3T MRI scanner (EXCITE, GE Milwaukee) with an eight-channel-phased array head coil. The imaging parameters were as follows: thickness: 5 mm (no gap), TR = 2,000 ms, TE = 30 ms, FOV = 24 cm ×24 cm, flip angle = 90°, matrix = 64 × 64. Two hundred and five volumes (30 slices per volume) were acquired during 410 seconds of a fMRI session. To ensure steady-state longitudinal magnetization, the first five volumes were discarded. We performed between two and five fMRI sessions, depending on patient endurance. During data acquisition, participants were required to relax with eyes closed, without falling asleep. Anatomical T1-weighted images were acquired using a three-dimensional (3D)-spoiled gradient recalled (SPGR) sequence, generating 156 axial slices [thickness: 1 mm (no gap), TR = 8.5 ms, TE = 3.4 ms, FOV = 24 cm ×24 cm, flip angle = 12°, matrix = 512 × 512].
During fMRI acquisition, EEG data was continuously recorded using a 10/20 system with 32 Ag/AgCl electrodes attached to the scalp with conductive cream. Two ECG channels were simultaneously recorded. The amplifier was a Mizar 40 (EBNeuro, Florence, Italy), with 32 channels applied for MR. Data was sampled at 4,096 Hz. The EEG dynamic range was ±65.5 mV to prevent MRI artifact waveforms that could saturate the EEG/ECG. Since the electrodes would result in the pressure or uncomfortableness, once the patient complained the next session would be terminated. The MR artifact was filtered online [Garreffa et al., 2003] using the software BE-MRI Toolbox (Galileo New Technology, Florence, Italy). To ensure patient safety and judge whether the absence seizure occurs during the scanning, a scheme same as our previous study was adopted [Li et al., 2009]. The parents of the patients stayed in the MR scanner suite during the fMRI examination. When SWD occurred in the online filtered EEG, the parents were asked to monitor the patient for any clinical signs of seizure. If the GTCS was present, the fMRI scanning stop, and first aid was given such as diazepam injected. If the brief absence seizure was present, the fMRI scanning continued. However, the data with absence seizure was excluded in the current study. The absence seizure inside the scanner were defined strictly according to the factors mentioned in our previous study [Li et al., 2009]: (1) the SWDs were similar to those recorded outside the scanner during typical absence seizures presented with a longer duration (>4 seconds) [Van Luijtelaar et al., 1991], and (2) the parents of the patients observed the clinical absence when the SWD burst occurred. Interictal SWD timing and duration were marked independently by two skilled electroencephalographers and disagreements about the markers were resolved by discussion. Based on whether SWD was detected during a particular session or not, the fMRI data was divided into the WDP group and the NDP group. Simultaneous EEG was not recorded in healthy participants.
Data Preprocess Analysis
Preprocessing of fMRI data was conducted using the SPM2 software package [statistical parametric mapping available at: http://www.fil.ion.ucl.ac.uk/spm]. The slice time correction, three-dimensional motion detection and correction, spatial normalization to the Montreal Neurological Institute (MNI) template supplied by SPM, and spatial smoothing using an isotropic Gaussian kernel (8 mm full width at half maximum) were included. Only participants with head motion of less than 1 mm and 1° during EEG-fMRI acquisition were included.
ICA Decomposition and Basal Ganglia Network Identification
Group spatial ICA was applied to the fMRI data of all three groups of participants using GIFT software (available at: http://icatb.sourceforge.net/, version 2.0a) [Calhoun et al., 2001]. Dimension estimation was performed on all participants to determine the number of independent components (ICs) using the minimum description length (MDL) criterion [Li et al., 2007]. For computational feasibility, principal component analysis (PCA) was used to reduce data dimensions through three data reduction stages: individual subject, concatenated through participants in subgroup, concatenated through subgroups. Subsequently, the FastICA algorithm [Hyvarinen, 1999] was used in IC estimation. IC time-courses and spatial maps for each participant were back-reconstructed, using the aggregated components and the results from the data reduction step [Calhoun et al., 2001; Jafri et al., 2008]. To assess the voxels that contributed to a particular IC, the intensity values in each spatial map were converted to z-values [Calhoun et al., 2001; Mantini et al., 2007]. The z-maps of each component were then gathered in each group for a random-effect analysis using a one-sample t-test in SPM2. The threshold was set at P < 0.01 with a false discovery rate (FDR) [Genovese et al., 2002] correction. Subsequently, further selection of the BGN was performed using anatomical information according to previous studies [Abou-Elseoud et al., 2010; Damoiseaux et al., 2008; Kiviniemi et al., 2009; Robinson et al., 2009]. In this intrinsic functional network, we expected the pallidum, putamen, subthalamic nucleus, SN, and thalamus to be comprised. Since slow spontaneous BOLD fluctuations (<0.1 Hz) contributed to resting-state connectivity, Fourier spectrum power analysis was performed for time-courses of the each selected component.
Statistical Comparisons of the Basal Ganglia Network
We conducted two-sample t-tests in SPM2 to examine the differences between groups. First, the BGN mask was defined based on a union set of the activation maps from patient and control groups. Statistical comparisons were then carried out using this mask between groups. The significance level was set at P < 0.01 (FDR-corrected). In this study, some patients were included in two groups for two periods (with and without discharge) As such, when examining the difference between the two periods (with and without discharge), we checked the difference between groups with two-sample t-tests, and separately examined the participants simultaneously included in both groups by paired sample t-tests to produce the difference within-subjects in a smaller sample size.
Correlation Analysis Between Imaging and Clinical Features
To detect the underlying relationship between alteration of BGN in patients and clinic features, correlation analysis was performed. The voxels showing significant differences between groups were extracted as a mask consisting of several regions of interest (ROI). The mean z-values in these ROIs were correlated to the epilepsy duration (two patient groups) and discharge number (here, the number of volumes with SWD was adopted, only for the WDP group). The Pearson's correlation coefficients were acquired with threshold P < 0.05 (uncorrected).
RESULTS
Through reviewing EEG data by experts, 18 sessions from 18 patients (patient No.12–29 in Table I) were selected as the NDP group. No epileptic discharge was found and the head motion criteria were fulfilled (translation <1 mm, or rotation <1°) in these sessions. In parallel, 20 sessions from 20 patients (patient No.1–20 in Table I) were classified as data for the WDP group. The number of volumes with SWD was exactly counted to represent discharge number and displayed in Table I. There were nine patients (patient No.12–20 in Table I) included in both groups, because periods with and without discharge were acquired. The mean age in the NDP group was 14.2 years (SD = 5.69) and 12.6 years (SD = 4.90) in the WDP group. There was no significant difference (P = 0.351) in age between the two groups. Two of 25 controls were excluded due to excessive head motion. There were 23 participants in the final control group. The mean age was 13.65 years (range: 6–23, SD = 5.02) in control group.
Basal Ganglia Network Identification Within Group
Using group ICA, 45 components were estimated for the control group. We found an independent component (IC45) including the putamen, pallidum, subthalamus nucleus, SN, amygdala, parahippocampus, uncus, thalamus, SMA, and cerebellum (Fig. 1, and Table II). The spatial form of this component included the main nuclei of basal ganglia, which is consistent with previous results [Robinson et al., 2009].
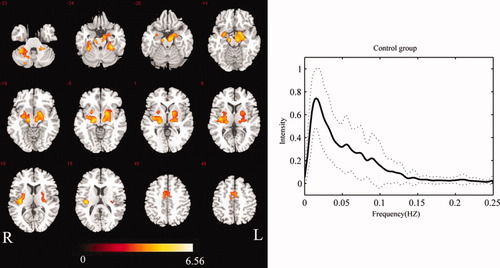
Group-level BGN map for controls (P < 0.01 FDR-corrected) (left), and mean frequency content of controls' BGN time-course (right). The solid line denotes the mean over participants. The range of the standard deviation is shown by the two dashed lines. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
| Region | Talairach coordinate | T value | ||
|---|---|---|---|---|
| X | Y | Z | ||
| Substantia Nigra (B) | −12 | −15 | −14 | 6.36 |
| 13 | −17 | −13 | 6.16 | |
| Pallidus (B) | −22 | 4 | 1 | 4.53 |
| 18 | 3 | −3 | 5.70 | |
| Putamen (B) | −21 | 6 | −7 | 4.53 |
| 30 | −13 | 12 | 4.01 | |
| Claustrum (B) | −33 | −19 | 12 | 5.99 |
| 33 | −18 | 10 | 4.38 | |
| Subthalamic nucleus (R) | 12 | −18 | −5 | 5.20 |
| Thalamus (B) | −18 | −25 | 2 | 5.48 |
| 18 | −22 | 3 | 5.89 | |
| Amygdala (B) | −24 | 1 | −18 | 5.34 |
| 24 | −5 | −18 | 5.36 | |
| Parahippocampal (B) | −29 | −15 | −24 | 6.56 |
| 12 | −15 | −22 | 6.37 | |
| Uncus (L) | −30 | −14 | −37 | 6.56 |
| Insula | −33 | −20 | 12 | 4.22 |
| 33 | −25 | 12 | 6.12 | |
| Cerebellar Tonsil (B) | −30 | −42 | −35 | 6.16 |
| 30 | −39 | −34 | 6.22 | |
| SMA (B) | −9 | −12 | 52 | 4.55 |
| 6 | −6 | 52 | 4.35 | |
In the NDP group, IC40 was identified as BGN activity from 42 components. Similarly, 42 components were decomposed for the WDP group, and IC41 was identified as BGN activity. The spatial forms were similar in these two groups, with both showing components in the putamen, pallidum, caudate nucleus, subthalamus, SN, amygdala, hippocampus, parahippocampus, insula, and thalamus. Compared with the control group, the SMA and cerebellum in the two patient groups did not show significant changes in connectivity. However, the caudate nucleus and insula were involved. The maps of the BGN in the two patient groups are shown in Figures 2 and 3.
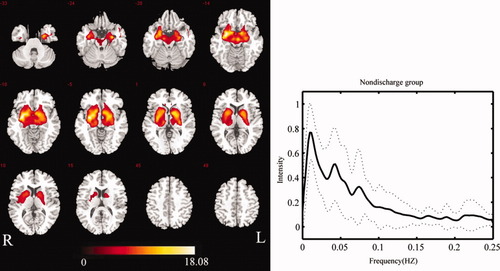
Group-level BGN map for patients in NDP group (P < 0.01 FDR-corrected) (left), and mean frequency content of BGN time-course of patients in NDP group (right). The solid line denotes the mean over participants. The range of the standard deviation is shown by the two dashed lines. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
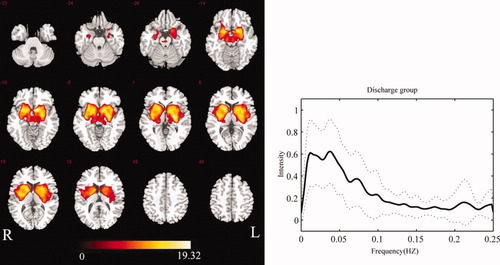
Group-level BGN map for patients in WDP group (P < 0.01 FDR-corrected) (left), and the mean frequency content of BGN time-course of patients in WDP group (right). The solid line denotes the mean over all participants. The range of the standard deviation is shown by the two dashed lines. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The power spectra of the time-courses of independent components were also utilized to identify RSNs. For each group, the power spectra of the selected component showed that the frequency content was mainly concentrated below 0.1 Hz (Figs. 1-3). The peak frequency was 0.015 Hz in the control group. Two peak frequencies were revealed in the patient groups, 0.011 Hz and 0.043 Hz in the NDP group, 0.011 Hz and 0.039 Hz in the WDP group. We performed comparisons of power spectral density in low-frequency bin (0.01–0.1Hz) among three groups. No significantly statistical difference was found.
Between-Group Analysis (Control-Patients) of Basal Ganglia Network
Between-group differences in functional connectivity within the BGN were examined using two-sample t-tests in SPM2 (significance level was set at P < 0.01 corrected with FDR). Compared with the control group, increased functional connections among the main nuclei in the basal ganglia, amygdala, parahippocampus, and hippocampus were found in two patient groups (Fig. 4; details shown in Tables III and IV). The results illustrated that the functional integration among the main nuclei in basal ganglia increased significantly in measurement periods both with and without discharge in epilepsy patients.
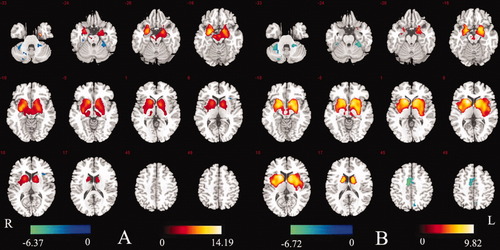
(A) Map of difference between NDP group and control group (P < 0.01 FDR-corrected). Increased connection in NDP group is shown by red–yellow color. Decreased connection in NDP group was colored by blue. (B) Map of difference between WDP group and control group (P < 0.01 FDR-corrected). Increased connection in WDP group is shown by red–yellow color. Decreased connection in WDP group is shown in blue. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
| Contrast | Region | Talairach coordinate | T value | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| NDP > Control | |||||
| Substantia Nigra (B) | −12 | −18 | −5 | 5.45 | |
| 15 | −24 | −9 | 5.30 | ||
| Pallidus (B) | −16 | 5 | 1 | 8.55 | |
| 15 | 5 | 5 | 7.03 | ||
| Putamen (B) | −18 | 6 | 10 | 7.64 | |
| 15 | 6 | 0 | 8.70 | ||
| Subthalamic nucleus (B) | −11 | −19 | −3 | 4.21 | |
| 12 | −17 | −5 | 5.16 | ||
| Caudate (B) | −12 | 19 | −6 | 9.78 | |
| 15 | 22 | −6 | 7.97 | ||
| Thalamus (B) | −11 | −20 | −1 | 3.63 | |
| 12 | −19 | 0 | 5.04 | ||
| Amygdala (B) | −24 | 1 | −18 | 14.19 | |
| 24 | −8 | −18 | 8.90 | ||
| Parahippocampal (B) | −18 | −5 | −20 | 7.15 | |
| 15 | 4 | −18 | 10.26 | ||
| Hippocampal (B) | −27 | −12 | −14 | 8.56 | |
| 28 | −11 | −13 | 9.96 | ||
| NDP < Control | |||||
| Cerebellar Tonsil (B) | −33 | −42 | −42 | 6.37 | |
| 30 | −39 | −34 | 5.48 | ||
| Contrast | Region | Talairach coordinate | T value | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| WDP > Control | |||||
| Substantia Nigra (B) | −9 | −21 | −8 | 3.83 | |
| 9 | −21 | −8 | 4.28 | ||
| Pallidus (B) | −16 | 5 | 1 | 8.91 | |
| 15 | 5 | 5 | 8.44 | ||
| Putamen (B) | −33 | −15 | −1 | 7.64 | |
| 15 | 10 | −7 | 9.64 | ||
| Caudate(B) | −15 | 10 | 14 | 9.38 | |
| 15 | 15 | 4 | 9.82 | ||
| Subthalamic nucleus (L) | −11 | −19 | −3 | 3.63 | |
| Thalamus (L) | −15 | −28 | 2 | 3.57 | |
| Amygdala (B) | −24 | 1 | −18 | 6.56 | |
| 27 | −2 | −14 | 9.26 | ||
| Parahippocampal (B) | −24 | 2 | −16 | 7.97 | |
| 21 | −34 | −9 | 4.00 | ||
| Hippocampal (B) | −27 | −12 | −14 | 5.74 | |
| 28 | −11 | −13 | 5.27 | ||
| Insula (B) | −38 | −2 | 9 | 7.27 | |
| 36 | 3 | 8 | 8.79 | ||
| WDP < Control | |||||
| Cerebellar Tonsil (B) | −33 | −42 | −42 | 6.72 | |
| 30 | −39 | −34 | 5.78 | ||
| SMA (B) | −9 | −12 | 48 | 6.32 | |
| 12 | −9 | 52 | 5.51 | ||
Between-Group Analysis (WDP Compared With NDP) of Basal Ganglia Network
We examined the differences in brain activity between periods with and without discharge in epilepsy patients. Although the spatial pattern of the BGN appeared similar in these two groups from visual inspection, a two-sample t-test in SPM2 revealed a significant group difference in connectivity (significance level was set at P < 0.01 corrected with FDR). Compared with the NDP group, decreased functional connectivity was found in the cerebellum, SMA, and bilateral uncus. In contrast, functional connectivity in the bilateral caudate nucleus, putamen, and insula increased in the WDP group (Fig. 5A, details shown in Table V).
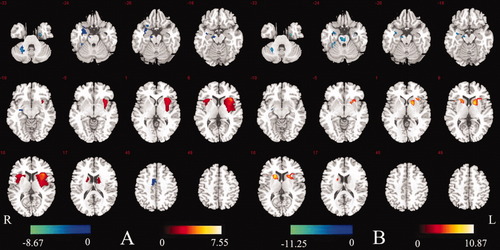
Map of difference between NDP group and WDP group (P < 0.01 FDR-corrected). (A) Between-groups test (18 vs. 20 participants); (B) within-subjects test (over nine participants). Comparison of WDP group with NDP group, showing increased connection in WDP group in red–yellow color, and decreased connection in WDP group in blue. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
| Contrast | Region | Talairach coordinate | T value | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| NDP > WDP | |||||
| SMA (R) | 6 | 9 | 45 | 4.21 | |
| Cerebellar Tonsil (R) | 26 | −54 | −33 | 8.67 | |
| Uncus (B) | −25 | −8 | −28 | 6.91 | |
| 23 | 1 | 23 | 4.11 | ||
| NDP < WDP | |||||
| Caudate (B) | −18 | 9 | 15 | 4.05 | |
| 15 | 11 | 15 | 3.94 | ||
| Putamen (B) | −29 | 3 | 7 | 5.47 | |
| 23 | 11 | 10 | 4.08 | ||
| Insula (B) | −40 | 18 | 5 | 7.55 | |
| 32 | 15 | 9 | 5.69 | ||
In addition, we conducted a paired sample t-test on the results from nine patients who were included simultaneously in both the with- and without-discharge groups. The regions of increased connectivity in the with-discharge period compared to without-discharge period were consistent with that of between the WDP group and the NDP group (Fig. 5A,B). In parallel, the decreased connectivity regions mentioned above were acquired except for SMA (Fig. 5B). The similarity between Figure 5A (pseudo-between-subjects) and Figure 5B (within-subjects) indicates that the difference between the two periods is reliable.
Based on three groups of data in Figures 1 to 3 above, Figure 6 shows the means and SDs of z-vlaues in the main structures involved in the BGN. z-Values were extracted by averaging the z-value for each peak voxel, which was localized with coordinates in Table V, and the nearest eight neighbors for each region for each participant. Here, six ROIs were selected, the SMA and cerebellum for motor control function, and bilateral caudate nucleus and putamen for modulation of SWD. It is clear that the functional connectivity in SMA and cerebellum that were responded to motor control function were the strongest in the control groups, followed by the NDP group and the WDP group. The functional connectivity in the bilateral caudate nucleus, putamen, and insula that were related to the modulation of SWD showed the opposite pattern, with the strongest connections in the WDP group, followed by the NDP and control groups (as shown in Fig. 6).
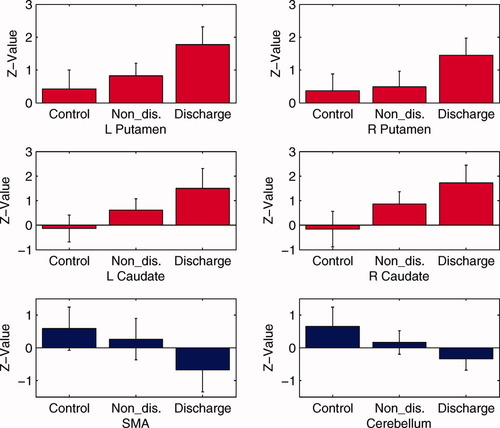
z-Values in the bilateral caudate nucleus, bilateral putamen, cerebellum, and SMA among three groups. z-Values were extracted by averaging the z-value for each peak voxel, which was localized with coordinates in Table V, and nearest eight neighbors for each region for each participant. The histogram denotes the z-value mean and one standard error of the mean in each group. The red histograms revealed increasing z-value in these clusters from control group, NDP group to WDP group. The blue histogram revealed the lowest z-values in cerebellum and SMA in the WDP group, followed by the NDP and control group, subsequently. The statistical differences are from the above Figures 4 and 5 with P < 0.01 (FDR-corrected). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Relationship Between BGN Imaging and Clinic Features
The z-value in each of the six ROIs was correlated with the epilepsy duration (two patient groups), and the discharge number (WDP group), respectively. For the WDP group, no significant correlation between the epilepsy duration and the z-value in any of six ROIs was found, however, the z-value in SMA (r = −0.555, P = 0.010) and cerebellum (r = −0.456, P = 0.042) were significantly negative correlated with the discharge number (see Fig. 7). The result, decreased functional connectivity in SMA and cerebellum along with the increased discharge number, might indicate that the frequent discharges might progressively aggravate the motor control function. Meanwhile, positive correlations were observed in bilateral caudate (L: r = 0.512, P = 0.020; R: r = 0.482, P = 0.031) (see Fig. 7). These results might reflect the positive modulation in BGN for discharges. For the NDP group, The significantly positive correlations between the epilepsy duration and z-value in bilateral putamen (L: r = 0.493, P = 0.037; R: r = 0.551, P = 0.017) were found (see Fig. 8). This positive correlationship might reflect the stable and continuous modulation function which increased with epilepsy duration in epilepsy.
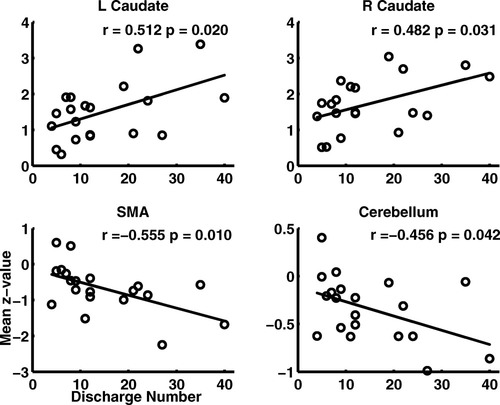
Correlation between the discharge number and the mean z-value in cerebellum, SMA, and bilateral caudate nuclei in WDP group. The discharge number represented the number of the volume with SWD.
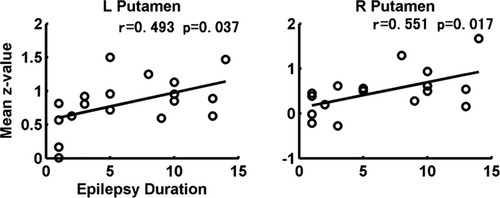
Correlation between the epilepsy duration and the bilateral putamen in NDP group.
DISCUSSION
This study shows that during the resting state, the BGN can be robustly identified using ICA in different participant groups (epilepsy patients and healthy controls). The RSN we found in controls includes not only the pallidum, putamen, SN, subthalamic nucleus, but also the SMA, insula, cerebellum, and limbic cortex. To the best of our knowledge, this study is the first demonstration of significant differences in BGN functional connectivity between controls and patients suffering from IGE, as well as of changes in BGN functional connectivity caused by SWD in IGE. The main findings from the current results are as follows: (1) the functional connections among the main nuclei in basal ganglia, amygdale, and hippocampus were strengthened in IGE patients compared to controls; (2) functional connectivity in the bilateral caudate nuclei, putamen, and insula increased in the WDP group relative to the NDP group; and functional connectivity in bilateral caudate nuclei significantly positive correlated to the discharge number in the WDP group; meanwhile, the relationship between the epilepsy duration and the functional connectivity in bilateral putamen was significantly positive in the NDP group; (3) compared with control group, functional connectivity decreased in the bilateral cerebellum and SMA in epilepsy patients, and the decrease was more pronounced during periods with discharge, compared with periods without, as well as significantly negative correlated to the discharge number in the WDP group.
BGN is a Resting State Functional Network
Using ICA to investigate RSN signal sources, the involvement of a component in the striatum has been reported in previous studies [Abou-Elseoud et al., 2010; Damoiseaux et al., 2008; Kiviniemi et al., 2009; Robinson et al., 2009]. Meanwhile, the BGN proposed in this work was similar to the RSN described by Robison et al., which included the basal ganglia and SMA and corresponded to the motor control circuit [Robinson et al., 2009]. Furthermore, the BGN found in the current study was similar to a result in previous studies that was obtained using functional connectivity analysis on resting-state fMRI data seeded at dorsal rostral/caudal putamen [Di Martino et al., 2008], at the amygdala (Robinson et al., 2009; Roy et al., 2009], and the SN [Nioche et al., 2009]. In addition, we found that the spatial features of the BGN were similar in healthy controls and epilepsy patients with and without discharge during the resting state. In the frequency domain, the peak frequency and spectral characteristics we found conformed to the RSN features reported in previous studies [Biswal et al., 1995; Fox and Raichle, 2007]. Taken together in the context of previous findings, resulted from two main methods studied RSN (ICA and functional connectivity analysis based on seed), our data indicate that the BGN proposed in the current work is a component of RSNs.
Main Anatomical Components Involved in the BGN
Previous anatomical research has reported that the basal ganglia projects widely to a range of cerebral cortical regions through corticostriatal connections, and on the basis of the tripartite model the cortical-basal ganglia-cortical connections have been suggested to act as parallel, closed-loop projections [McHaffie et al., 2005; Selemon and Goldman-Rakic, 1985]. These cortical areas connected to the basal ganglia are considered to be divided into associative cortex, sensorimotor cortex, and limbic portions. Our data from healthy controls revealed that the SMA and limbic cortex were included in the basal ganglia network. This is consistent with the prediction of the sensorimotor and limbic loops [Parent and Hazrati, 1995; Selemon and Goldman-Rakic, 1985]. The absence of associative cortex in the BGN does not necessarily imply the lack of connection between these areas and basal ganglia. A possible reason is that some areas of associative cortex might be divided into other RSNs, such as attention and memory networks. On the other hand, our network also included the posterior insula and anterior cerebellum. A previous meta-analysis of basal ganglia functional connectivity [Postuma and Dagher, 2006] reported that the insula and cerebellum were strongly coactivated with the putamen. The anatomical connections projecting from the dorsal posterior insula to the dorsal putamen have previously been described in the primate [Chikama et al., 1997]. Moreover, the posterior insula and anterior cerebellum have been implicated to be involved in motor function. The BGN confirmed previous resting state fMRI findings reporting that the RSN included basal ganglia and corresponded to the motor control circuit [Robinson et al., 2009]. Moreover, our present results expanded this RSN circuit to include more brain regions related to motor function, corresponding to limbic loops.
The enhanced connectivity we found within the basal ganglia in the control group may reflect coactivity among the main nuclei of the basal ganglia. The striatum and subthalamic nucleus are two primary input sites that predominantly receive afferents from the cerebral cortex, limbic structures, and the thalamus. The input signal is then relayed, via direct and indirect routes, and transmitted to the principal output nuclei, the internal globus pallidus, and the SN pars reticulate (SNpr). The output nuclei project directly to the thalamus, midbrain, and medulla, and indirectly to target cortical and limbic regions through thalamus. Moreover, complex connections exist within the basal ganglia [Selemon and Goldman-Rakic, 1985; McHaffie, 2005]. For example, the SNpr receives glutamatergic, tonic excitatory input from the subthalamus, as well as inhibitory phasic GABAergic input from the striatum. In striato-nigral pathway, there are two projections, direct monosynaptic GABAergic projections, and indirect polysynaptic projection which included the pallidum and subthalamic nucleus between two sites. Efficient synaptic activity along these nuclei could facilitate network synchronization among neurons in these regions. Although, functional neuroimaging could not identify the pathways between neurons, the functional network connectivity we detected may be related to these underlying pathways. In addition, disturbed functional connectivity within the basal ganglia may reflect abnormal activity within the basal ganglia circuit. This type of basal ganglia abnormality may be involved in neurological conditions such as Parkinson's disease [Ceballos-Baumann, 2003].
Regulation of BGN in Epileptic Discharge
We observed that the pattern of BGN in patients was largely similar to controls. However, enhanced integration within the basal ganglia, amygdala, and parahippocampus was found in patients compared with controls. This may indicate that some circuits within the BGN are related with the generalization of epileptic discharges. There is evidence that bilaterally synchronous epileptic discharges reflect abnormal oscillations in corticothalamic networks in patients with IGE [Crunelli and Leresche, 2002; Danober et al., 1998; Timofeev and Steriade, 2004]. The basal ganglia are widely considered to play an important role in the regulation of discharge (see review in [Norden and Blumenfeld, 2002]). In addition, a number of studies have suggested that an intact SN is necessary for propagation of seizure activity [Deransart et al., 2001; McNamara et al., 1984], and many circuits related to SN have been shown to play an important role in epilepsy [Deransart et al., 2003; Paz et al., 2007]. Specifically, the pathway between the SNpr and the superior colliculus, while the later is considered to be a dorsal midbrain anticonvulsant zone [Garant and Gale, 1987; McNamara et al., 1984], participated in regulation of epileptic discharge. Additional evidence that came from experiments involving neuropharmacology [Danober et al., 1998; Deransart et al., 1998] and deep brain stimulation in epilepsy [Loddenkemper et al., 2001] also confirmed that basal ganglia interference was involved in modulating epileptic discharge and seizure activity. And the above comparisons among the control, patients with discharge and without discharge show that the modulation enhanced the integration in BGN of patients when compared with the controls, and the modulation during epileptic discharge was stronger than during the nondischarge period. In the WDP group, the association of the time-course of BGN and the onset of interictal SWD was analyzed. We found that the responses have a decreasing trend after discharges, and the maximum of the decrease approximately 7 seconds after the onset of SWD (Supporting Information). The findings in part suggested that the BGN in WDP group maybe related to the IED. Furthermore, other functional imaging studies have demonstrated that basal ganglia activity is related to interictal SWD, and to generalized seizure in patients with secondarily generalized epilepsy or IGE [Blumenfeld et al., 2009; Li et al., 2009].
Caudate Participation in Modulation of Generalized Epileptic Discharge
An interesting finding in the present study was that significant changes were detected in the activity of the caudate nucleus in patients, but not in controls. All participants were required to close their eyes during fMRI scans. The absence of the caudate nucleus involvement in controls was consistent with previous reports [Robinson et al., 2009]. In their work, the caudate component was apparent in a fixation condition. The authors presumed that caudate component was a task-related response related to the suppression of ocular saccades [Robinson et al., 2009]. However, we found caudate nucleus involvement in the patient groups in the present study. This finding may suggest that the caudate nucleus is involved in modulating epileptic discharge in conjunction with other nuclei of the basal ganglia. In fact, the caudate nucleus was associated with interictal SWD and generalized seizure activity found in an fMRI study we recently conducted [Li et al., 2009]. Furthermore, compared with that in the NDP, the bilateral caudate nucleus and putamen involvement we found in the WDP may indicate that multiple regions of the basal ganglia cooperated in the modulation of epileptic discharge, or were themselves affected by epileptic discharge. The other evidence came from correlation analysis between the mean z-value in bilateral caudate nuclei and the number of discharge, positive relationship, supported that the caudate nucleus played a crucial role for modulation of epileptic discharge.
BGN From Control to Nondischarge and Discharge
In the present study, we focused on the functional networks involving the basal ganglia rather than examining the whole-brain response to epileptic discharge. Using simultaneous EEG recording, the fMRI data could be divided into the WDP group and the NDP group. We found similarly strengthened functional connections among the main nuclei in basal ganglia in both groups when compared with the control. This result confirms that the BGN plays an important modulatory role in generalized epileptic discharge, and may reflect physiopathologic changes in the basal ganglia related to epilepsy. The results suggest that these changes may be related to the modulation of interictal SWD and generalized seizure over a long period. In particular, the positive relationship between the mean z-value in bilateral putamen and epilepsy duration in the NDP group may also implicate the stable strengthened modulation function which increases with epilepsy duration in epilepsy. The decreasing functional connectivity in the bilateral caudate nuclei and putamen in the WDP group relative to the NDP group might indicate that the strength of the disturbed modulation was more predominant during periods with discharges.
Disturbed Motor Function in IGE
The basal ganglia have been implicated in a variety of motor-related functions. Our results, showing the absence of cerebellum and SMA involvement in the BGN in two groups of patients, may be related to motor function disturbances in IGE. Accordingly, recent studies have shown abnormalities in behavior and executive function in IGE [Caplan et al., 2008; Hommet et al., 2006; McDonald, 2008].The decreased connections that we found in the cerebellum and SMA in epilepsy patients were accompanied by greater decreases in the connections of patients in the WDP compared with that of patients in the NDP. The degressive tendency may reflect aggravated motor abnormality during SWD. Meanwhile, the z-value in SMA and cerebellum significantly negative correlated to the discharge number, which suggested that the motor abnormality might be more and more serious with the increase of epileptic activities. In line with this interpretation, previous studies have reported behavioral impairments during spike-wave activity [Goode et al., 1970].
Methodological Consideration and Suggestions for Future Research of the BGN
Several potential confounds may have influenced on our result that should be considered. First, many antiepileptic drugs (AEDs) have been reported to cause decreased cerebral metabolic rate and/or cerebral blood flow [Theodore, 1988; Matsuda et al., 1996]. The effects of AEDs may modulate the BOLD signal to affect the functional connectivity. Especially, the sedation drug such as midazolam might change the functional connectivity. Greicius et al. found that the functional connectivity in DMN significantly decreased during the conscious sedation with midazolam in healthy subjects [Greicius et al., 2008]. Another recent study reported that the sedation with midazolam induces low frequency fluctuations increase in resting-state functional networks [Hlinka et al., 2010]. In this work, though 16 of 29 patients did not receive the AEDs, the AEDs effects still should be considered. As a supplementary test, two-sample t-test was performed between patients with and without receiving AEDs in the NDP group and WDP group, respectively, and no significant difference was found in the BGN regions for each of the two groups. This fact might indicate that the AEDs effects for resting-state BGN were limited. However, it is unclear whether AED effect exists in other networks, and caution in interpretation is recommended for the future resting-state fMRI studies in epilepsy. Secondly, the behavior and executive function testing was not performed in this study. However, the evidences have been accumulated to demonstrate the existence of motor functional abnormality [Caplan et al., 2008; McDonald, 2008; Hommet et al., 2006]. We held the option that the altered BGN should be correlated to the disturbed motor function in IGE.
Furthermore, some methodological considerations should be mentioned. The EEG data was not acquired for the controls, and the EEG electrodes might affect the BOLD signal. Here, the EEG system works professionally in MRI environment, and the artifact depth was generally less than 8 mm [Krakow et al., 2000]. Meanwhile, ICA was used in this study, and the aimed independent component with crucial information located at subcortical brain structures, which is distant with the scalp electrodes. The influence of electrodes might be slight and negligible. For group ICA, the multiple data reduction steps were performed before IC estimation, primarily for computational reasons to reduce the amount of required memory (see review in [Calhoun et al., 2009]). In this work, according to the manual of GIFT, we adopt three reduction stages for the number of our subjects is about 20. To confirm the above result, the two-stage reduction was also tested in GICA process for the three groups (whereas, 20 health controls were included in control group for the computational reasons) and the results were similar with that obtained with the three-stage reduction and displayed in this text. The similar results indicate that the affects of the two or three reduction steps are slight for the comparison study in small samples. However, a further improved and stable algorithm for reduction needs to be developed in the future [Zhang et al., 2010]. Besides, there is no validated way to identify the number of ICs in ICA analysis. The number of ICs may affect the extraction of BGN. In a previous study, a striatum component was emerged at model order more than 40 [Abou-Elseoud et al., 2010]. In this work, the minimum description length (MDL) criterion [Li et al., 2007] implanted in GIFT was used to determine the number of ICs, and the determined number of ICs was more than 40 in all three groups. Besides, in current practice, there are two widely adopted approaches to obtain the connectivity, ICA and region of interest (ROI) based correlation analysis, and their mathematical basis is quite different, the results may be different for a common dataset, we need to take care of this fact when we compare the results of them from different literatures.
Because the anatomical connections from the basal ganglia project to widespread cortical regions, disturbances in the BGN may affect various functional domains. Motor abnormality in Parkinson's disease is associated with basal ganglia dysfunction [Dejean et al., 2008]. As such, the BGN might be a candidate marker for neuro- and psychopathologies involving the basal ganglia. Not only abnormality within the BGN but also the functional network connectivity (FNC) between BGN and other RSNs may contribute in investigating the physiopathological mechanisms of diseases related to the BGN. Jafri et al. [ 2008] first examined FNC to evaluate changes in the interrelationship between different RSNs in schizophrenia. Previous studies have revealed different functional connectivity abnormalities in various neural networks related to epilepsy using resting state fMRI, including the epileptogenic network [Bettus et al., 2008], the attention network [Zhang et al., 2009a], and the DMN [Luo et al., 2011]. As abnormalities in the BGN can reflect impaired motor function in epilepsy patients and the regulation of epileptic discharge, examining the FNC of the BGN may be beneficial for the classification of various types of epilepsy and to evaluating types of therapy. In addition, analyzing the connectivity of RSNs associated with the BGN may provide insight into diseases related to the basal ganglia including Parkinson disease, and other types of epilepsy.
CONCLUSION
In summary, this study revealed a resting state functional network, which we termed as the BGN. This network consists of the basal ganglia, SMA, insula, cerebellum, and limbic cortex. This network is in accord with the anatomical connections of the basal ganglia. BGN activity may reflect the intrinsic relations among the nuclei of the basal ganglia and the motor control circuit, which is functionally related to the basal ganglia. Moreover, we found that IGE was associated with significant differences in BGN activity. Compared with controls, patients with IGE illustrated significantly enhanced integration within the BGN during the two periods with and without discharge. Compared with the WDP group, increased functional connectivity was found in the bilateral cerebellum and SMA, but functional connectivity in the bilateral caudate nucleus and putamen decreased in the NDP group. These findings shed some lights on the underlying physiopathological changes in the BGN involved in IGE. The differences between patients and controls may be due to interictal SWD and generalized seizure over a long period. This study provides evidence that the BGN, as a modulator, plays an important role in IGE, and that behavioral abnormalities may be related to functional changes in the BGN.