The human dorsal premotor cortex facilitates the excitability of ipsilateral primary motor cortex via a short latency cortico-cortical route
Abstract
In non-human primates, invasive tracing and electrostimulation studies have identified strong ipsilateral cortico-cortical connections between dorsal premotor- (PMd) and the primary motor cortex (M1HAND). Here, we applied dual-site transcranial magnetic stimulation (dsTMS) to left PMd and M1HAND through specifically designed minicoils to selectively probe ipsilateral PMd-to-M1HAND connectivity in humans. A suprathreshold test stimulus (TS) was applied to M1HAND producing a motor evoked potential (MEP) of about 0.5 mV in the relaxed right first dorsal interosseus muscle (FDI). A subthreshold conditioning stimulus (CS) was given to PMd 2.0–5.2 ms after the TS at intensities of 50-, 70-, or 90% of TS. The CS to PMd facilitated the MEP evoked by TS over M1HAND at interstimulus intervals (ISI) of 2.4 or 2.8 ms. There was a second facilitatory peak at ISI of 4.4 ms. PMd-to-M1HAND facilitation did not change as a function of CS intensity. Even at higher intensities, the CS alone failed to elicit a MEP or a cortical silent period in the pre-activated FDI, excluding a direct spread of excitation from PMd to M1HAND. No MEP facilitation was present while CS was applied rostrally over lateral prefrontal cortex. Together our results indicate that our dsTMS paradigm probes a short-latency facilitatory PMd-to-M1HAND pathway. The temporal pattern of MEP facilitation suggests a PMd-to-M1HAND route that targets intracortical M1HAND circuits involved in the generation of indirect corticospinal volleys. This paradigm opens up new possibilities to study context-dependent intrahemispheric PMd-to-M1HAND interactions in the intact human brain. Hum Brain Mapp, 2012. © 2010 Wiley-Liss, Inc.
INTRODUCTION
The human primary motor hand area (M1HAND) is an important executive area for manual motor control. M1HAND is located in the anterior bank of the central sulcus having a characteristic knob-like structure with an omega shaped surface in the axial plane and a hook-like surface in the sagittal plane [Yousry et al.,1997]. The anatomical connectivity pattern of M1HAND has been thoroughly studied in non-human primates and underscores the pivotal role of this region in manual motor control [Lemon,2008]. The M1HAND is a major source of fast-conducting monosynaptic projections to the cervical motor neurons [He et al.,1993; Martino and Strick,1987], which are thought to be critically involved in the generation and control of highly skilled hand movements [Lemon,2008]. In addition, the M1HAND receives direct cortico-cortical inputs from a set of premotor areas, including the dorsal and ventral premotor cortex and supplementary motor area [Dum and Strick,1991; He et al.,1995; Kermadi et al.,1998; Luppino et al.,1993]. Although these premotor areas send descending projections to the cervical spinal cord, they exert substantial influence on descending motor control indirectly via their cortico-cortical inputs into M1HAND [Schmidlin et al.,2008].
In humans, transcranial magnetic stimulation (TMS) is a well established means of studying the excitability of fast monosynaptic corticospinal projections originating in M1HAND by recording the motor evoked potentials (MEPs) in contralateral hand muscles [Barker et al.,1985; Hallett,2000]. Epidural invasive recordings at the level of the cervical spinal cord have consistently shown that a single suprathreshold TMS pulse gives rise to a series of descending volleys in the corticospinal pathway [Amassian et al.,1987; Di Lazzaro et al.,2010]. When the pulse induces a posterior-to-anterior current in M1HAND, TMS primarily elicits so-called indirect waves (I-waves) [Di Lazzaro et al.,1999a; Patton and Amassian,1954]. I-waves are generated trans-synaptically by indirect excitation of the corticospinal neurons via intracortical circuits [Di Lazzaro et al.,1999a; Patton and Amassian,1954]. The interactions among circuits within motor cortex involved in the production of I-waves can be studied non-invasively using paired-pulse TMS [Tokimura et al.,1996; Ziemann and Rothwell,2000]. Facilitatory I-wave interaction can be demonstrated in the human primary motor cortex by applying pairs of magnetic stimuli to M1HAND through the same coil. The motor response to paired-pulse TMS is consistently facilitated, when the interval between the stimuli is around 1.0–1.5 ms, 2.5–3.0 ms, or 4.5 ms [Di Lazzaro et al.,1999b; Tokimura et al.,1996; Ziemann et al.,1998].
Invasive recordings in animals have shown that cortical electrical stimulation of the premotor or somatosensory cortex can also elicit multiple I-waves in the pyramidal tract, but only if the primary motor cortex is intact [Amassian et al.,1987]. This finding indicates that premotor-to-motor and sensory-to-motor cortico-cortical inputs influence intracortical circuits of M1HAND that generate motor commands in fast-conducting corticospinal output neurons. The activation of cortico-cortical inputs to M1 HAND are thought to underlie the modulatory effects of a conditioning stimulus (CS) of a connected cortical area on corticospinal excitability in M1HAND [Mochizuki et al.,2004]. Accordingly, microstimulation studies in monkeys showed short-latency facilitation of intracortically evoked test responses in M1HAND that was present less than 3 ms after the excitation of premotor-to-motor projections [Cerri et al.,2003; Prabhu et al.,2009; Shimazu et al.,2004; Tokuno and Nambu,2000].
Using a conditioning-test approach with a conditioning coil placed over a non-primary motor area and a test coil over M1HAND, dual-site TMS (dsTMS) has been recently established as a valuable tool to probe the excitability of distinct cortico-cortical inputs from ipsilateral and contralateral frontal and parietal areas to the M1HAND [Baumer et al.,2006; Civardi et al.,2001; Ferbert et al.,1992; Koch et al.,2007a; Mochizuki et al.,2004]. These studies found inhibitory or facilitatory interactions or both depending on the dsTMS protocol [Davare et al.,2008; Koch et al.,2007b]. In general, the conditioning effects on corticospinal excitability in M1HAND critically depended on the timing of the CS relative to the test stimulus (TS) given to M1HAND. Usually, the CS had to be given several milliseconds (4–12 ms) before the TS to impact on corticospinal excitability.
Several studies have successfully applied the dsTMS approach to study the inputs from contralateral dorsal premotor cortex (PMd) to M1HAND [Baumer et al.,2006; Koch et al.,2006; Mochizuki et al.,2004; O'Shea et al.,2007]. The reason for this is that commercially available coils are relative large precluding the concurrent stimulation of ipsilateral PMd and M1HAND. According to a meta-analysis of functional imaging studies the rostral and caudal part of the PMd are located on average 8 and 23 mm anterior to M1HAND [Picard and Strick,2001]. In this study, we used specifically designed minicoils to perform dsTMS of left PMd and M1HAND. We hypothesized that ipsilateral PMd-to-M1HAND inputs might show a facilitatory interaction with intracortical circuits in M1HAND generating late I-waves. Given that the late I-waves leave the M1HAND several milliseconds after a magnetic stimulus is applied to M1HAND, we hypothesized that an ipsilateral CS should still be able to modulate the motor response evoked in M1HAND via a short latency pathway when the premotor CS is given after the TS.
METHODS
Subjects
Eighteen male healthy subjects aged between 21 and 40 years (mean 27.4 years ± 5.2) took part in the study. All participants were consistently right-handed according to the Edinburgh handedness inventory. Informed consent was obtained from all subjects prior to their participation in the study. The experiments were approved by the Ethics Committee of University of Kiel, Germany. During the experiment, participants were seated in a reclining chair with a head rest. Participants were asked to keep the eyes open and relax.
Set-Up for Dual-Site Transcranial Magnetic Stimulation
TMS of left M1HAND and PMd was performed with two custom-made figure-of eight minicoils attached to a P-Stim 160 stimulator (Mag&More, Munich, Germany). The geometry of the minicoils used a modified figure-of-eight design. The in-plane dimensions of the coil were 56 mm (x-axis) and 104 mm (y-axis). The handle of the coil was mounted on the coil perpendicularly to the plane of the coil (z-axis). The modified figure-of-eight coil contained two layers of wires with identical geometry which were serially connected. Each layer consisted of two wings with eight windings per wing. The windings of each wing were arranged eccentrically having a drop-like geometry with an angular component. This geometry shifted maximal stimulation in the x-axis away from the geometric center of the coil towards one edge of the coil (Fig. 1).
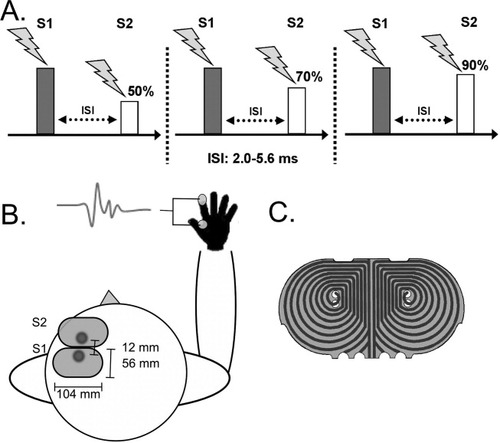
Experimental setup. A: Stimulation paradigm. Conditional stimulus (S2) was applied after the test stimulus (S1) over M1HAND. ISI (interstimulus interval) was randomly varied in one session. Three sessions with different S2 (50-, 70-, 90%) intensities were performed. B: View of the two mini-coils aligned to each other. The stimulation current was switched in one coil and achieve same current direction in both coils. C: Schematic drawing of the decentral coil windings.
The first minicoil was centered over the M1HAND. The M1HAND was functionally localized, placing the coil over the site where a single suprathreshold pulse elicited a maximal MEP in the right first dorsal interosseous muscle (FDI). The coil was placed tangentially on the scalp with the x-axis being approximately in parallel to the central sulcus (45–60 degree relative to the mid-sagittal line). The coil was positioned over the M1HAND such that the site of M1HAND stimulation was located in the anterior part of the coil.
The second coil targeting the adjacent left PMd was placed immediately anterior to and with the x-axis oriented in parallel to the coil targeting left M1HAND. To minimize the distance between the primary motor and dorsal premotor sites of stimulation, the anterior coil was placed over the PMd in a mirrored fashion with the site of maximal stimulation being located in the posterior part of the coil. The coils were aligned to each other so that the two hot spots were as close as possible. This allowed us to reduce the distance between the sites of effective stimulation to ∼3–4 cm. A detailed drawing of the coil is presented in Figure 1C.
Conditioning-Test Design
We used dsTMS to probe cortico-cortical connectivity between left PMd and M1HAND. Using a conditioning-test approach, the CS was given to left PMd and TS to left M1HAND (Fig. 1). Single pulses with biphasic pulse configuration were applied with the second phase of the pulse inducing a posterior–anterior (PA) current in the cortex. The pulse direction was changed in the PMd coil to stimulate in the same current direction.
After functional localization of the M1HAND stimulation site, we determined the active motor threshold (AMT) and resting motor threshold (RMT). The RMT was defined as the stimulus intensity at which a single biphasic pulse elicited an MEP of at least 50 μV peak-to-peak amplitude in five out of ten trials [Rossini et al.,1994]. The TS was used to adjust the intensity of the premotor TMS pulse. Since we had no specific predictions regarding the optimal intensity of the premotor CS, we used three different intensities for premotor TMS (50-, 70-, and 90% of TS-Intensity). The TS was given at an intensity which evoked MEPs with peak-to-peak amplitudes of about 0.5 mV.
In contrast to previous studies, the premotor CS was given after the TS to M1HAND. The inversion in temporal order with the TS given over M1HAND preceding the CS over left PMd was motivated by the following considerations: a suprathreshold TMS pulse over the M1HAND causes multiple descending volleys in the corticospinal tract with the latest volleys (i.e., late I-waves) leaving the cortex several milliseconds after the TMS pulse is applied. Assuming that the premotor-to-motor pathway is monosynaptic, we reasoned that ipsilateral premotor-to-motor conduction time should be less than 2 ms. If so, the premotor CS should still be able to facilitate late I-wave generation in ipsilateral M1HAND when given shortly after the TS over M1HAND.
The reversed timing adopted in the present study was inspired by a conditioning-test paradigm where two stimuli are given through the same coil over the M1HAND to test facilitatory intracortical interactions in the M1HAND at I-wave latency [Tokimura et al.,1996; Ziemann et al.,1998]. In this paradigm, a slightly subthreshold CS follows a suprathreshold TS and results in MEP facilitation at interstimulus intervals (ISIs) that correspond to the latency of late I-waves, peaking at 2.4–2.8 and 4.4 ms. Therefore, we varied the ISIs between the first motor TS and the second premotor CS between 2.0 ms and 5.2 ms in steps of 0.4 ms.
The main experiment consisted of three blocks of measurements in which the intensity of the premotor CS was varied. CS intensities were set at 50-, 70-, and 90% of TS. The order of blocks was counterbalanced across subjects to avoid order effects. Apart from the CS intensity, experimental procedures were identical across blocks. In each block, 10 conditioned MEPs per ISI were collected as well as 20 unconditioned MEPs (TS over M1HAND alone). There were also 10 trials in which the CS was given alone. The experimental conditions were applied in a pseudorandom order. A customized Signal script and a CED [Cambridge Electronic Design (Signal, CED), Cambridge, UK] device were used to trigger the TMS devices and control the order of experimental conditions.
Recordings
In all participants, MEPs were recorded through a pair of surface electrodes placed over the right FDI muscle, using a belly–tendon montage. Raw signals were amplified and bandpass filtered (3 Hz–1 kHz). Signals were digitized using a CED 1401 laboratory interface and stored at a sample rate of 5 kHz. Auditory (speakers) feedback of EMG activity was given to the subjects to ensure complete relaxation. Peak-to-peak amplitudes of MEPs were analyzed offline on a personal computer using NuCursor software (J. Rothwell, Institute of Neurology, University College of London, UK).
Control Experiment 1
Ten subjects (five males, age range: 20–29 years) participated in this experiment of whom seven had taken part in the main experiment. This experiment was designed to detect a possible spread of excitation from PMd to ipsilateral M1HAND. Participants performed a tonic contraction of the right FDI muscle at ∼20% of their maximal force level. Continuous audio-visual feedback of the EMG activity was given to the subjects to assist them in maintaining a constant level of contraction. During contraction, we applied single TMS pulses over the PMd under the same stimulation conditions as in the main experiment. Like in the main experiment, a second coil was placed over the motor hot spot of the M1HAND to mimic as closely as possible the experimental set-up of the main experiment. Only the coil over the PMd was discharged. Stimulus intensity was progressively increased using stimulus intensities of 70-, 90-, 110-, and 130% of individual RMT. Fifteen trials were recorded per stimulus intensity. For each intensity, the rectified EMG traces were visually inspected, averaged, and analyzed for the presence of a MEP or silent period.
Control Experiment 2
To demonstrate whether the conditioning effects of the premotor CS in the main experiment were topographically specific, we re-examined 10 male subjects who had taken part in the main experiment (age range: 23–31 years). In two separate blocks of measurements, the conditioning stimulus was either applied to left PMd or 3 cm rostrally to the PMd site over the dorsolateral prefrontal cortex (dlPFC). The order of blocks was counterbalanced across subjects. In addition, we slightly modified the conditioning-test paradigm in this control experiment. Instead of adjusting the intensity of the conditioning stimulus to the intensity of the test stimulus, the conditioning intensity was defined in relation to individual RMT of the M1HAND. The conditioning stimulus applied over left PMd or left dlPFC had the same stimulus intensity which was 70- or 90% of RMT, respectively. If a participant had shown maximal MEP facilitation at 50- or 70% TS intensity in the main experiment, the PMd stimulus was adjusted to 70% RMT in the control experiment. Those participants in whom the conditioning PMd stimulus had induced the strongest MEP facilitation at 90% TS intensity, the conditioning stimulus intensity was set at 90% of RMT. By adjusting the intensity of the CS to individual RMT rather than TS intensity, it was possible to directly relate the results of control Experiments 1 and 2. This slight modification in the conditioning-test paradigm did not affect the PMd-to-M1HAND interaction.
Control Experiment 3
This control experiment was designed to probe the temporal specificity of the observed facilitatory effects induced by the PMd stimulus in the main experiment. In addition, we wished to test whether direct depolarization of the proximal corticospinal axon at the axonal bend in the subcortical while matter might have played a role. If so, we reasoned that the facilitatory effect of the PMd stimulus on MEP amplitude would be particularly pronounced when the depolarizing effects of the PMd and M1HAND had the possibility to sum up in the proximal segment of the corticospinal axon. Therefore, we modified the dsTMS protocol using a very short ISI of 0.5 ms. Ten subjects (four men, age range: 20–31 years) were examined using the same experimental setup described in the main experiment. The conditioning stimulus was given to left PMd already 0.5 ms after the TS over M1HAND. Fifteen conditioned and 15 unconditioned stimuli (M1HAND alone) were applied in pseudorandom order.
Control Experiment 4
This control experiment was designed to directly compare the facilitatory effects of dsTMS of left PMd and M1HAND and the standard paired-pulse TMS protocol that has been introduced to probe facilitatory I-wave interaction in the M1HAND [Tokimura et al.,1996; Ziemann and Rothwell,2000].
Ten subjects participated in this experiment (five men, age range: 24–33 years, five subjects had participated in the main experiment). In the first part of the experiment, short-latency intracortical facilitation was examined using a figure-of eight coil (MC-B70) charged by a MagPro stimulator (MagVenture, Farum, Denmark). Three stimulus conditions were applied in pseudorandom order (10 repetitions per condition). A biphasic TS was either given alone or followed by a conditioning pulse at ISIs of 2.4 or 2.8 ms. The intensity of the CS was set at 90% of the RMT, while the TS was adjusted to elicit a mean MEP amplitude of 0.5 mV in the relaxed right FDI muscle when given alone. In the second part of the experiment, we used the identical dual-site TMS setup as in the main experiment. A TS was either given alone to left M1HAND or together with a CS over left PMd at ISIs of 2.4- or 2.8 ms. PMd intensity was set here at 90% of the RMT. The order of the two experiments was counterbalanced across subjects.
Data Analysis
Data processing and analysis were performed with SPSS software (Release 16.0, Copyright SPSS). We hypothesized that TMS over left PMd activates facilitatory PMd-to-M1HAND monosynaptic connections that project on intracortical circuits in M1HAND involved in the generation of I-waves. Therefore, we expected that the premotor CS would produce its facilitatory effect at an ISI of 2.4 or 2.8 ms corresponding to the latency of the second I-wave [Ziemann et al.,1998].
We expected the optimal intensity at which the premotor CS produces premotor-to-motor facilitation to be uncoupled from the threshold to evoke MEPs over the M1HAND. Therefore, we examined in each subject at which CS intensity (i.e., 50-, 70-, and 90% of TS) the premotor CS produced maximal premotor-to-motor facilitation at an ISI of 2.4 or 2.8 ms. The individual paired-pulse excitability curve showing maximal paired-pulse facilitation was selected for group analysis and paired-pulse MEPs were normalized to the mean MEP amplitude elicited by the TS over left M1HAND.
We further predicted that the ISI producing maximal premotor-to-motor facilitation would slightly vary in time from subject to subject with some subjects showing peak facilitation at an ISI of 2.4 ms and others at 2.8 ms. Therefore, temporal realignment was performed to improve comparability across subjects and to better reveal I-wave periodicity. At the group level, individual excitability curves were aligned to the peak of MEP facilitation at the ISI of interest (2.4 or 2.8 ms). Hence, the peak of maximal paired-pulse facilitation was identical for all subjects after realignment. The normalized and realigned paired-pulse excitability curves were analyzed using repeated-measures analysis of variance (ANOVA) with the within-subject factor ISI (10 levels: 2.0, 2.4, 2.8, 3.2, 3.6, 4.0, 4.4, 4.8, 5.2, and 5.6 ms). Conditional on a significant F value, we performed Fisher's LSD post hoc pair-wise comparisons (P < 0.05; two-tailed; no adjustment for multiple comparisons) comparing mean MEP amplitudes of consecutive ISIs. The same statistical approach was applied for control Experiment 2.
In control Experiment 3, the one-way ANOVA included the main factor stimulation (M1HAND-PMd, unconditioned) to asses the excitability changes. The data acquired in control Experiment 4 we analyzed using a two-way ANOVA with the within-subject factors stimulation (2 levels: F8-Coil, PMd-M1) and states (3 levels: Test, ISI of 2.4, ISI of 2.8 ms). We performed simple linear regression analysis to test whether the relative magnitude of MEP facilitation achieved with standard paired-pulse TMS of M1HAND predicts the relative MEP facilitation induced by dsTMS of M1HAND and PMd.
RESULTS
Main Experiment
Mean AMT and RMT were 53.15% ± 10.89% and 65.07% ± 9.78% of maximum stimulator output, respectively. The amplitude of unconditioned MEP was matched among the three sessions in which premotor CS was given at 50% (0.58 ± 0.32 mV), 70% (0.49 ± 0.22 mV), or 90% TS (0.51 ± 0.26 mV). The premotor CS never elicited MEPs when given alone.
The premotor CS facilitated the MEP evoked by the TS over M1HAND at the ISIs of interest (Fig. 2). However, the facilitatory effect was evenly distributed among the three intensities of stimulation: The premotor CS produced a relative MEP facilitation of at least 25% in 11 subjects at a stimulus intensity of 50% TS, 12 subjects at a stimulus intensity of 70% TS, 12 subjects at a stimulus intensity of 90% TS. Paired-pulse facilitation peaked at an ISI of 2.8 ms in approximately two third of the cases, while peak facilitation occurred at an ISI of 2.4 ms in the remaining third (Fig. 2).
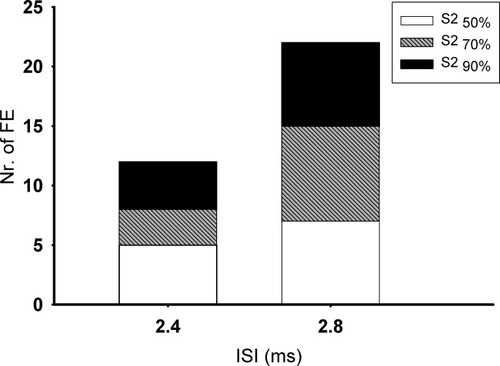
Number of facilitation effects (FE) at two relevant interstimulus intervals of 2.8- and 2.4-ms at three different S2 intensities.
In each subject, we selected the paired-pulse excitability curve showing maximal premotor-to-motor facilitation at ISI of 2.4 or 2.8 ms and normalized the paired-pulse MEPs to the mean MEP amplitude evoked by the TS over the M1HAND alone. We also performed a temporal realignment. In those subjects with paired-pulse facilitation peaking at an ISI of 2.4 ms, the paired-pulse excitability curve was shifted 0.4 ms to the right to align peak facilitation with the peak at an ISI of 2.8 ms. The mean paired-pulse excitability curve after temporal realignment is given in Figure 3. Using the normalized and temporally realigned MEP amplitudes as dependent variable, ANOVA revealed an effect for ISI [F (9, 108) = 2.2091, P = 0.027], indicating a significant difference in normalized MEP amplitudes across the ISIs. Post hoc comparisons revealed a difference in amplitudes for MEPs evoked at the aligned facilitation peak (ISI of 2.8 ms) relative to an ISI of 2 ms (P < 0.05), 2.4 ms (P < 0.005), 3.2 (P < 0.001), and 3.6 ms (P < 0.005). A second peak of MEP facilitation was present at an ISI of 4 ms with a significant MEP facilitation relative to MEPs evoked at an ISI of 3.2 ms (P < 0.01). Mean MEP amplitudes at an ISI of 4.0 ms also showed a trend towards a significant difference relative to paired-pulse TMS at ISIs of 3.6 ms (P = 0.06).
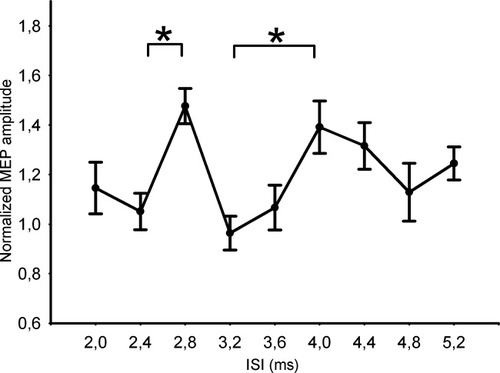
Results of the main experiment after realignment. The mean peak to peak amplitude of the conditioned MEP was expressed as a quotient of the mean peak-to-peak amplitude size of the unconditioned test pulse. *Significant results are marked at P < 0.05 (corrected).
Control Experiment 1
This experiment tested whether a premotor CS has any influence on voluntary EMG activity in the tonically contracting FDI muscle with increasing stimulus intensity. Four intensities were tested (i.e., 70-, 90-, 110-, and 130% of individual RMT). Although the amplitude of the recorded artefact gradually increased with the intensity of premotor TMS, the premotor CS failed to produce a MEP or silent period in the tonically contracting target muscle (Fig. 4).
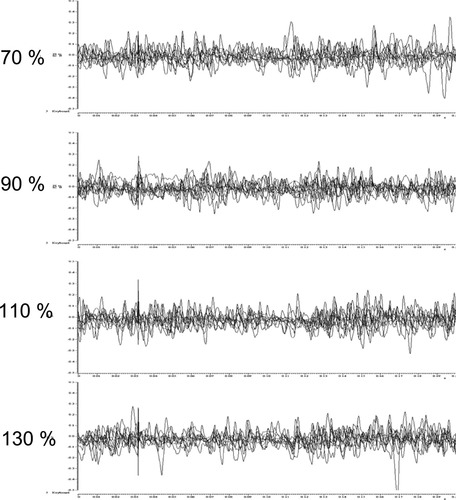
Control Experiment 1. No silent period activity or MEPs were monitored at increased stimulation intensities at PMd site in the periphery tonically activated muscles.
Control Experiment 2
Motor thresholds of the 10 subjects participating in this experiment did not differ from those in the main experiment. AMT und RMT were 55.78 ± 13.12 and 68.46 ± 11.20%, respectively. In the 10 subjects who were re-examined, we were able to replicate the facilitation found in the main experiment when using the CS intensity that had produced the most consistent facilitation but adapted to the percentage of the RMT. All subjects showed MEP facilitation either at 2.4 or 2.8 ms. One-way ANOVA of the temporally realigned group data showed a significant main effect of ISI [F (8, 56) = 3.3053, P = 0.004]. Post hoc tests revealed significant differences in mean MEP amplitude between the realigned ISI of 2.8 and MEPs elicited with ISIs at 2 ms (P < 0.005), 2.4 ms (P < 0.005), 3.2 ms (P < 0.001), and 3.6 (P < 0.001). Again, a second facilitatory peak emerged at 4 ms with a significant MEP facilitation relative to MEPs evoked at the ISI of 3.6 ms (P < 0.05; Fig. 5). When the S2 coil was positioned 3 cm rostrally to the PMd site targeting the dorsolateral prefrontal cortex, the paired-pulse facilitation was no longer present. Accordingly, the one-way ANOVA showed no effect of ISI [F (8, 40) = 0.9, P = 0.5].
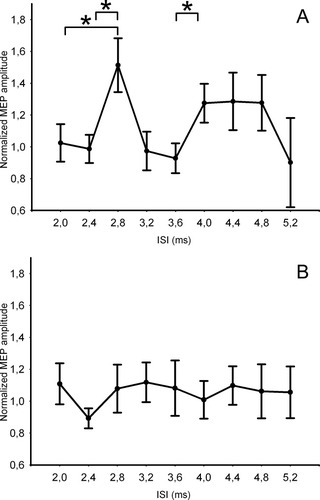
Results of the control Experiment 2, after realignment. A: Same coil setup as in the main experiment, CS Intensity adapted to RMT. B: Conditioning coil placed over the dorsolateral prefrontal cortex (3 cm rostrally to the PMd stimulation site). The mean peak to peak amplitude of the conditioned MEP was expressed as a quotient of the mean peak-to-peak amplitude size of the unconditioned test pulse.*Significant results are marked at P < 0.05 (corrected).
Control Experiment 3
In 10 individuals, the conditioning stimulus was applied to PMd already 0.5 ms after the test stimulus to M1HAND. No consistent MEP facilitation was observed at this ISI. Accordingly, the one-way ANOVA showed no main effect for stimulation [F (1, 9) = 0.01, P > 0.1]. Mean MEP amplitudes did not differ significantly between the stimulation conditions (M1HAND-PMd 0.65 ± 0.24 mV; M1HAND alone 0.65 ± 0.31).
Control Experiment 4
Figure 6 illustrates the results of control Experiment 4 which includes the data of 10 subjects. A two-factorial repeated-measures ANOVA revealed a statistical trend for the factor stimulation paradigm [F (1, 9) = 4.67, P < 0.1], and a significant main effect for the factor stimulus condition [F (2, 18) = 8.72, P < 0.005]. The interaction between the two factors was not significant [F (1, 9) = 4.67, P > 0.05]. Accordingly, there were no differences in the unconditioned MEPs evoked by single-pulse TMS over M1HAND in both stimulation paradigms (M1HAND-PMd dsTMS: 0.76 ± 0.33 mV; Paired-pulse TMS of M1HAND: 0.78 ± 0.38 mV). Overall, the conditioning pulses given 2.4 or 2.8 ms after the TS induced MEP facilitation in both paradigms, but paired-pulse TMS of M1HAND induced a more pronounced MEP facilitation compared to dsTMS of M1HAND and PMd (Fig. 6). For both ISIs, simple regression analysis showed that the relative magnitude of MEP facilitation achieved with paired-pulse TMS of M1HAND did not predict the relative MEP facilitation induced by dsTMS of M1HAND and PMd (for the ISI of 2.4 ms: r = 0.40, P > 0.1; for ISI of 2.8 ms: r = 0.43, P > 0.1).
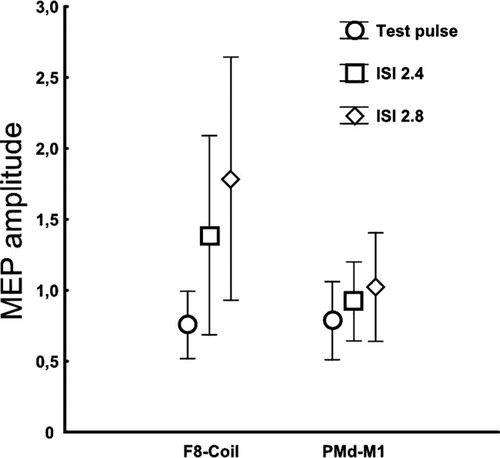
Results of the control Experiment 4. Test and conditioning pulses were applied by a figure of eight coil (F8-Coil) or as in the main experiment by ds-TMS of PMd-M1 at ISI of 2.4- and 2.8 ms. The absolute amplitude of the conditioned and unconditioned MEP (test pulse) are plotted.
DISCUSSION
Here, we used a novel dsTMS approach with specifically designed minicoils to probe non-invasively the ipsilateral cortico-cortical connectivity between the left PMd and M1HAND in healthy human subjects. A subthreshold pulse given to left PMd 2.4 or 2.8 ms after a suprathreshold stimulus over the left M1HAND facilitated the MEP amplitude in contralateral FDI relative to the MEP evoked by suprathreshold M1HAND stimulation alone. The precise timing of the facilitatory peak as well as the optimal CS intensity to elicit premotor-to-motor facilitation differed across participants. Most subjects showed facilitation at an ISI of 2.8 ms, but in a third of the subjects the optimal facilitatory interval was shorter occurring at 2.4 ms. Moreover, the optimal intensity to produce such facilitation was quite variable and showed no fixed relation to the excitability of the ipsilateral M1HAND as indexed by the motor threshold. The control experiments proved that the observed premotor-to-motor paired-pulse facilitation was topographically specific and not caused by a direct spread of stimulation to M1HAND. The observed PMd-to-M1HAND facilitation was also not caused by current induction in the coil overlying M1HAND during the discharge of the PMd coil. According to the manufacturer, the peak off-state current of the stimulating device is 0.1 A. Therefore, the maximal current that may be induced in the non-discharging M1HAND coil by the neighboring PMd coil is negligible, being more than 1,000 times below the current intensity needed to excite cortical neurons.
Facilitation of M1HAND Through PMd Inputs
Microstimulation and histological tracer studies have shown strong cortico-cortical connections between PMd and M1HAND in primates [Dum and Strick,2002; Matelli et al.,1984]. These PMd-to-M1HAND cortical inputs are thought to play an important functional role for manual motor control. Single-pulse electrical microstimulation in PMd elicited an early excitatory response peaking at ∼2.4 ms in the pyramidal cells of M1HAND followed by a late inhibitory phase [Tokuno and Nambu,2000]. This observation is of relevance to the present results because it shows that ipsilateral PMd stimulation can elicit a short-latency facilitatory response in ipsilateral primary motor cortex.
Little is known about the functional connections of the premotor motor areas in humans. Double pulse TMS paradigms have probed interactions between different cortical areas [Baumer et al.,2006,2009; Civardi et al.,2001; Koch et al.,2006,2007a]. Using two small figure-of-eight coils, a transcranial stimulus given 5 cm anterior to the M1HAND hot-spot had an inhibitory effect on corticospinal excitability of ipsilateral M1HAND when a subthreshold conditioning stimulus was given 6 ms before a transcranial test stimulus [Civardi et al.,2001]. This inhibitory conditioning effect gradually turned from inhibition into facilitation when the intensity of the conditioning stimulus was gradually increased from 90- to 120% of active motor threshold [Civardi et al.,2001]. Interestingly, the facilitatory effect of a suprathreshold conditioning stimulus on ipsilateral corticospinal excitability was suppressed by an additional subthreshold conditioning stimulus given 5 ms before the conditioning stimulus [Koch et al.,2007b].
So far, the large size of standard TMS coils has limited bifocal TMS of the ipsilateral PMd and M1HAND. Although the studies by Civardi et al. [2001] and Koch et al. [2007b] used dsTMS to probe intrahemispheric connectivity between rostral frontal areas and M1HAND, the conditioning pulse was applied rostrally from the PMd in the dorsolateral prefrontal cortex [Baumer et al.,2009]. This may explain why the temporal pattern of cortico-cortical interaction in the studies by Civardi et al. [2001] and Koch et al. [2007b] clearly differed from the pattern revealed by cortical microstimulation in primates [Tokuno and Nambu,2000]. Since the dorsolateral prefrontal cortex has no direct cortico-cortical connections with M1HAND [Dum and Strick,2005], we hypothesize that the inhibitory M1HAND response 6 ms after prefrontal conditioning represents “late inhibition” produced by polysynaptic (e.g., prefrontal-premotor-motor) projections [Civardi et al.,2001]. In summary, a direct comparison between the present study and the study by Civardi et al. [2001] is difficult because of the substantial differences with respect to the temporal sequence of the applied pulses over PMd and M1HAND and the positioning of the conditioning coil over PMd in the present study and prefrontal cortex in the study by Civardi et al.
The increase of the conditioning pulse intensity changed the inhibition in a slight facilitation, by possibly involving adjacent cortical areas. And indeed another recent work presented a similar facilitation of the M1 after a suprathreshold PMd pulse at 6 ms [Koch et al.,2007b]. Different stimulation current directions in the conditioning- and test coil and a CS-intensity might account for this different pattern of PMd-M1 interaction. Furthermore, due to space considerations the exact stimulation site remains not completely defined.
The results of our study support the long held view that direct fast connections from PMd to the ipsilateral M1HAND are involved in the generation of I-waves [Ziemann and Rothwell,2000]. Indeed, surface stimulation of the dorsal premotor area in primates elicited repetitive descending I-waves that were abolished after ablation of the ipsilateral primary motor cortex [Patton and Amassian,1954]. When setting out to probe this premotor-to-motor route, we modified the paired-pulse TMS paradigm originally developed for probing facilitatory I-wave interactions in the premotor motor cortex at short ISI [Tokimura et al.,1996; Ziemann et al.,1998]. In that paradigm, two transcranial stimuli are applied through the same coil centered over the motor hot spot to target circuits in the M1HAND that generate this short-latency intracortical facilitation. While analyzing the same range of ISIs, we used two magnetic coils rather than a single coil in the present study and applied the first suprathreshold pulse to M1HAND and the subsequent low-intensity pulse over PMd. Using this dsTMS paradigm, the low-intensity premotor stimulus conditioned the corticomotor response evoked by the suprathreshold stimulus in ipsilateral M1HAND. Premotor-to-motor facilitation occurred when the conditioning stimulus given to the PMd 2.4 or 2.8 ms after TMS of the M1HAND. This implies that premotor-to-motor facilitation occurred via a short latency pathway that reached ipsilateral M1HAND quickly enough to interact with the intracortical excitation evoked by direct TMS of the M1HAND. Our results suggest an interaction between the PMd input to M1HAND and some intracortical circuits in the M1HAND involved in I-wave generation. Yet we can not make any inferences about the true latency of the premotor-to-motor interaction or comment on which I-wave circuits were specifically facilitated by the premotor input. Here invasive recordings of the induced corticospinal descending volleys at the level of the spinal cord might help to tackle this question [Di Lazzaro et al.,2010].
Temporal realignment of the paired-pulse excitability curves across subjects revealed a distinct ISI-specific peak of MEP facilitation with no consistent facilitation at the ISI before or after the facilitatory peak. However, a second facilitatory peak emerged at an ISI of 4.0 ms suggesting an undulatory pattern of premotor-to-motor facilitation. On the basis of the timing and undulatory character of the observed premotor-to-motor MEP facilitation, it is conceivable that the conditioning premotor stimulus activated a short latencyPMd-to-M1HAND cortico-cortical pathway which interacted with intracortical circuits in the M1HAND implicated in the generation of corticospinal descending I-waves.
Site of Facilitatory Interaction
In our first control experiment, the PMd stimulus alone failed to induce a MEP or silent period in the pre-activated contralateral FDI muscle, even when stimulus intensity was increased to 130% of RMT of the left M1HAND. In other words, a premotor stimulus intensity of 130% RMT was still subthreshold for producing significant descending corticospinal excitation even though the corticospinal system was more excitable due to voluntary preactivation of the target muscle. The failure of TMS over PMd alone to induce any motor response in the activated target muscle even at high stimulus intensities excludes the possibility that the MEP facilitation induced by dual-site TMS was caused by direct spread of magnetic stimulation from the PMd coil to M1HAND.
The failure to elicit a motor response with high-intensity TMS over PMd also speaks against a spinal mechanism. In the second control experiment, the PMd was stimulated only with 70% or 90% of individual RMT, yet the I-wave like PMd-to-M1HAND facilitation of the MEPs elicited over ipsilateral M1HAND was clearly evident. Of note, I-wave like PMd-to-M1HAND facilitation was present at conditioning intensities that were substantially lower than the maximal stimulus intensity used in the first control experiment (i.e., 130% of RMT). This implies that the stimulus intensities that were used in the second control experiment for PMd stimulation were considerably below the threshold for activating descending motor projections from PMd to the spinal cord. Therefore, we argue that the I-wave interaction like MEP facilitation observed in both, the main experiment and the second control experiment, was caused by a cortico-cortical rather than a subcortical (spinal) mechanism.
In the main experiment, conditioning TMS was given to left PMd at 50-, 70-, and 90% of TS. At each of these intensities, premotor TMS induced MEP facilitation, but only in a subgroup of subjects. There was no clear relationship between the intensity of PMd stimulation and the resulting facilitation of corticospinal excitability in M1HAND. The lack of a relationship between stimulus intensity of the PMd stimulus and the resulting MEP facilitation also speaks against a direct descending effect of PMd stimulation on the excitability of spinal circuits. Experiments using transcranial electrical stimulation of M1HAND or brain stem stimulation at the level of the foramen magnum might help to further exclude a spinal mechanism. However, these stimulation methods are usually quite painful and require preactivation of the target muscle which limits the comparability with the present results in which PMD-M1HAND facilitation was recorded at rest and without major discomfort.
The results of the first control experiment provided further evidence that the facilitatory effect of premotor TMS was not caused by spread of excitation to M1HAND or descending corticospinal volleys directly originating from PMd. In this experiment, we gradually increased the intensity of PMd stimulation to 130% of individual RMT (estimated over M1HAND), while recording the electromyographic activity in the tonically contracting right FDI muscle. In both cases, spread of excitation to M1HAND or direct descending excitation of the spinal neurons by the PMd input, one would have expected increases of facilitation as a function of the intensity of the PMd stimulation. This was not the case. Even at 130% RMT, premotor TMS failed to induce a silent period or a MEP in the contralateral target muscle.
In our second control experiment, we replicated the premotor-to-motor paired-pulse facilitation in the same subjects using the optimal stimulus intensity settings as revealed in the main experiment. This facilitatory effect was no longer present, when the conditioning coil was shifted 3 cm rostrally to target the dorsolateral prefrontal cortex, indicating site-specific facilitation over the PMd site.
Using a short ISI of 0.5 ms, the third control experiment tested whether the stimuli given to PMd and M1HAND interacted at the axonal level by directly stimulating the corticospinal axon in the subcortical white matter. MEP measurements revealed no facilitatory effect at 0.5 ms, arguing against a direct summation of TMS effects at the proximal corticospinal axon.
Inter-Individual Variability
The MEP facilitation elicited by PMd conditioning varied among subjects. This was the case for the optimal ISI (2.4 or 2.8 ms) but also for the optimal intensity of the premotor pulse to elicit PMd-to-M1HAND facilitation. One possible explanation for this variability might be inter-individual differences in the anatomical connectivity strength of the direct connections linking these two adjacent areas. In a recent study it was shown that individual differences in white matter microstructure as expressed by the fractional anisotropy reflect variations in functional connectivity [Boorman et al.,2007]. In analogy, differences in anatomical connectivity between M1HAND and PMd might account for interindividual differences. Future structural combining diffusion-MRI and dsTMS measurements of ipsilateral PMd-to-M1HAND will clarify this issue. There might also be a considerable inter-individual variability in the functional expression of PMd-to-M1HAND facilitation at rest, when the motor system is idling. A more consistent PMd-to-M1HAND facilitation might be observed in the context of sensomotoric mapping. In addition, the relatively rigid procedure to place the coil over PMd resulted in inter-individual differences in PMd stimulation contributing to the variability of PMd-to-M1HAND facilitation. Neuronavigated TMS of the PMd site based on individual activation patterns as revealed by functional MRI might give more consistent results.
Physiological Considerations
Direct facilitatory connections between contralateral PMd and M1HAND have been demonstrated with dsTMS [Baumer et al.,2006; Civardi et al.,2001; Ferbert et al.,1992; Koch et al.,2007a; Mochizuki et al.,2004]. Because of the large size of conventional TMS coils it was not possible to apply dsTMS to the study of intrahemispheric PMd-M1HAND connections. The newly designed minicoils with eccentric wiring enabled us to apply temporally coordinated TMS to two adjacent, at a distance less then 2 cm close cortical areas. This inspired us to modulate the activity of the ipsilateral M1HAND and PMd and to analyze the short latency facilitatory interaction between these motor areas. The latencies of the observed facilitatory peaks at ISIs of 2.4 ms or 2.8 and 4.0 ms are in concordance with the timing of the descending I-waves in epidural recordings after TMS stimulation of the motor cortex [Burke et al.,1993]. Studies which involved electrical cortical stimulation and data from epidural recordings as well as animal studies confirmed the cortical origin of these descending volleys [Di Lazzaro et al.,1999a; Patton and Amassian,1954; Tokimura et al.,1996]. The excitability of the facilitatory circuits involved in I-wave generation have been studied in the intact human brain with paired-pulse TMS using one figure-of-eight coil [Tokimura et al.,1996; Ziemann et al.,1998]. The exact physiological substrate for this short-latency cortical facilitation at I-wave latencies is not fully clarified. Although the same coil is used to probe facilitatory I-wave interactions in M1HAND, this does not necessarily imply that both pulses excite the same interaction by stimulating two segregated but functionally interacting neural populations. It might be possible that I-waves are generated by excitation of different chains of interneurons in M1HAND that receive cortico-cortical inputs from connected premotor or sensory cortical areas [Sakai et al.,1997]. Our results are compatible with this view and suggest that premotor inputs into the M1HAND interact with the intracortical circuits in M1HAND that generate descending I-waves in the corticospinal tract.
At present, it is not possible to directly address the question whether the pathway mediating the PMd-to-M1HAND interactions projects on the same I-wave circuits that are tested with standard paired pulse protocols in M1HAND. The currently available TMS hardware used to probe PMD-to-M1 interactions and standard I-wave interactions in M1 differ substantially. Therefore, different neural populations are probed in the stimulated M1 with the two protocols. This notion is supported by the control experiment in which we compared the facilitatory effects induced by dsTMS of left PMd and M1HAND and the standard paired-pulse TMS protocol applied over the M1HAND [Tokimura et al.,1996; Ziemann and Rothwell,2000]. Paired-pulse TMS of M1HAND with a large figure-of-eight coil induced a more pronounced MEP facilitation relative to dsTMS of M1HAND and PMd with highly focal minicoils (Fig. 6). In addition, the inter-individual variations in I-wave facilitation induced by paired-pulse TMS of M1HAND did not predict the relative MEP facilitation induced by dsTMS of M1HAND and PMd. How much these differences reflect true neurobiological differences is hard to tell given the marked differences in the TMS hardware. We hypothesize that in both paradigms, the facilitation of corticospinal excitability relies on intracortical neural circuits in the M1HAND, but that the paradigms probe the excitability of different neural circuits in the M1HAND. Future studies have to tackle this question, once both paradigms can be performed with the same TMS hardware.
Relevance of the Study
Premotor cortical areas and especially PMd play an important role in the preparation and sensory guidance of hand movements [Picard and Strick,2001; Schluter et al.,1998]. Direct connections from premotor areas to M1HAND have been demonstrated in animal studies and especially in primates, yet little is know about these pathways in humans. Our study demonstrates, for the first time, with the aid of highly focal TMS a direct short latency facilitatory premotor to motor route. The observed temporal pattern of the interaction resembles classical I-wave interaction that can be demonstrated with paired-pulse stimulation over the M1HAND. This route was highly specific for the stimulated region of PMd and no interaction occurred if the coil was moved further rostrally. As PMd plays an important role for implementing sensory or visual information for movement preparation and fine motor tuning [Petrides,1985] it is likely that this short latency route might play a crucial role in context-dependent modulation of M1HAND excitability via the PMd during manual motor control.




