Stability of clinical condition in mild cognitive impairment is related to cortical sources of alpha rhythms: An electroencephalographic study
Abstract
Previous evidence has shown that resting eyes-closed cortical alpha rhythms are higher in amplitude in mild cognitive impairment (MCI) than Alzheimer's disease (AD) subjects (Babiloni et al. [2006a]: Human Brain Mapp 27:162–172; [2006b]: Clin Neurophysiol 117:252–268; [2006c]: Neuroimage 29:948–964; [2006d]: Ann Neurol 59:323–334; [2006e]: Clin Neurophysiol 117:1113–1129; [2006f]: Neuroimage 31:1650–1665). This study tested the hypothesis that, in amnesic MCI subjects, high amplitude of baseline cortical alpha rhythms is related to long-term stability of global cognition on clinical follow-up. Resting electroencephalographic (EEG) data were recorded in 100 amnesic MCI subjects during eyes-closed condition. EEG rhythms of interest were delta (2–4 Hz), theta (4–8 Hz), alpha1 (8–10.5 Hz), alpha2 (10.5–13 Hz), beta1 (13–20 Hz), and beta2 (20–30 Hz). Cortical EEG sources were estimated by low-resolution brain electromagnetic tomography (LORETA). Global cognition was indexed by mini mental state evaluation (MMSE) score at the time of EEG recordings (baseline) and about after 1 year. Based on the MMSE percentage difference between baseline and 1-year follow-up (MMSEvar), the MCI subjects were retrospectively divided into three arbitrary groups: DECREASED (MMSEvar ≤ −4%; N = 43), STABLE (MMSEvar ≈ 0; N = 27), and INCREASED (MMSEvar ≥ +4%; N = 30). Subjects' age, education, individual alpha frequency, gender, and MMSE scores were used as covariates for statistical analysis. Baseline posterior cortical sources of alpha 1 rhythms were higher in amplitude in the STABLE than in the DECREASED and INCREASED groups. These results suggest that preserved resting cortical neural synchronization at alpha frequency is related to a long-term (1 year) stable cognitive function in MCI subjects. Future studies should use serial MMSE measurements to confirm and refine the present results. Hum Brain Mapp, 2011. © 2010 Wiley-Liss, Inc.
INTRODUCTION
Elderly subjects with mild cognitive impairment (MCI) are characterized by an objective impairment of memory and/or other cognitive functions, not yet fulfilling the clinical picture of dementia [Flicker et al.,1991; Petersen et al.,1995,2001]. At group level, the MCI condition is considered as a risk factor of Alzheimers' disease (AD) [Arnaiz and Almkvist,2003; Galluzzi et al.,2001; Scheltens et al.,2002]. Indeed, recent studies have shown that 6–25% of MCI subjects progress to AD within a year [Bachman et al.,1993; Gao et al.,1998; Petersen et al.,2001], while only about 4% of normal elderly subjects (Nold) convert to AD within a year (Nold; [Frisoni et al.2004; Petersen et al.,2001]. However, not all MCI patients deteriorate over time [Bennett et al.,2002; Larrieu et al.,2002], because AD cumulative incidence rate ranges from 40 to 60% [Bennett et al.,2002; Fisk et al.,2003; Larrieu et al.,2002]; therefore, indexes of global cognition such as mini-mental state evaluation (MMSE) or ADAScog can remain stable or even improve over time, possibly due to vitamin deficit correction, suspension of therapies having side effects on cognition, cognitive training, and general changes in life style. In some cases, this improvement is followed by a subsequent worsening of the MMSE score as an effect of the AD or other causes of dementia. To our knowledge, the neural substrate of this phenomenon is unknown, despite its obvious importance for the understanding of MCI condition and for clinical application.
Electroencephalogram (EEG) seems to be an ideal candidate to address the above issue, because it probes general mechanisms of cortical neural synchronization allowing the temporal summation of postsynaptic potentials at the basis of scalp EEG rhythms. In the condition of resting state eyes closed, these rhythms showed changes in power along the pathological aging. When compared with Nold subjects, AD patients have been characterized by high power of delta (0–4 Hz) and theta (4–7 Hz) rhythms and low power of posterior alpha (8–12 Hz) and/or beta (13–30 Hz) rhythms [Babiloni et al.,2004;; Dierks et al.,1993,2000; Huang et al.,2000; Jeong,2004; Ponomareva et al.,2003; Prichep et al.,2005]. These EEG abnormalities have been associated with altered regional cerebral blood flow/metabolism and with impaired global cognitive function as evaluated by MMSE [Jeong,2004; Rodriguez et al.,1998,1999a,b; Sloan et al.,1995]. Furthermore, posterior alpha rhythms have shown a power decrement in MCI compared to Nold subjects [Babiloni et al.,2006b; Elmstahl and Rosen,1997; Huang et al.,2000; Jelic et al.,2000; Koenig et al.,2005; Zappoli et al.,1995]. Moreover, it has been shown that EEG theta power (3.5–7.5 Hz) is high in MCI subjects who will convert to AD [Prichep et al.,2006] and that EEG coherence alone or in combination with power density automatically distinguish Nold and AD subjects [Adler et al.,2003] and predict the progression from MCI to AD [Jelic et al.2000; Rossini et al.,2006].
This study tested the hypothesis that, in MCI subjects, high amplitude of baseline resting cortical alpha rhythms is related to long-term stability of global cognition, as a reflection of a preserved cortical neural synchronization generating “default” EEG rhythms.
METHODS
Subjects
In this study, 100 amnesic MCI subjects were enrolled. Furthermore, 45 cognitively normal elderly (Nold) subjects and 45 Alzheimers' disease patients were recruited as control groups to preliminarily confirm that the enrolled MCI subjects were characterized by the typical changes of the EEG rhythms observed in previous studies [Babiloni et al.,2006a; Rossini et al.,2007].
Diagnostic Criteria
The present inclusion and exclusion criteria for amnesic MCI subjects were based on previous seminal reports [Albert et al.,1991; Devanand et al.,1997; Flicker et al.,1991; Petersen,2004; Petersen et al.,1995,1997,2001; Portet et al.,2006; Rubin et al.,1989; Zaudig,1992]. Summarizing, the inclusion criteria were as follows: (i) objective memory impairment on neuropsychological evaluation, as defined by performances ≥1.5 standard deviation below the mean value of age- and education-matched controls for a battery of neuropsychological tests to assess cognitive performance in the domains of memory (see in the following paragraph), language, executive function/attention, and visuo-construction; (ii) normal activities of daily living as documented by the history and evidence of independent living; and (iii) Clinical Dementia Rating (CDR) Score of 0.5. The exclusion criteria included (i) mild AD, as diagnosed by standard protocols including NINCDS–ADRDA [McKhann et al.,1984] and DSM-IV; (ii) evidence [including magnetic resonance imaging (MRI) procedures] of concomitant cerebral impairment such as frontotemporal degeneration, cerebrovascular disease, and reversible cognitive impairment (including pseudo-depressive dementia); (iii) marked fluctuations in cognitive performance compatible with Lewy body dementia and/or features of mixed cognitive impairment including cerebrovascular disease (particular attention was devoted to this point given the working hypothesis focused on cognitive stability in MCI subjects); (iv) evidence of concomitant extra-pyramidal symptoms; (v) clinical and indirect evidence of depression as revealed by the Geriatric Depression Scale (GDS; [Yesavage et al.,1982–1983] scores lower than 14 (no depression); (vi) other psychiatric diseases, epilepsy, drug addiction, alcohol dependence (as revealed by a psychiatric interview), and use of psychoactive drugs including acetylcholinesterase inhibitors or other drugs enhancing brain cognitive functions; and (vii) current or previous uncontrolled or complicated systemic diseases (including diabetes mellitus) or traumatic brain injuries. Of note, the routine clinical protocol did not include the assessment of possible vitamin/mineral deficiencies in the recruited MCI subjects. All subjects explained the importance of a wealth life style and sleep-awake cycle. They were also informed about the importance of vitamins and minerals in the diet and invited to ensure a regular and abundant daily intake of mixed vegetables and fresh fruits.
A battery of neuropsychological tests was performed to assess cognitive performance in the domains of memory, language, executive function/attention, and visuo-construction abilities. The tests to assess memory were the immediate and delayed recall measure of the Rey Auditory Verbal Learning Test [Carlesimo,1996; Rey,1958], the delayed recall of Rey figure [Rey,1968], the delayed recall of a three-word list [Chandler et al.,2004], the delayed recall of a story [Spinnler and Tognoni,1987], and/or Busckhe–Fuld. The tests to assess language were the 1-min verbal fluency for letters [Novelli,1986], the 1-min verbal fluency for fruits, animals or car trades [Novelli,1986], and the Token test [De Renzi and Vignolo,1962; Spinnler and Tognoni,1987]. The tests to assess executive function and attention were the Trail Making Test part A and B [Reitan,1958], the attentive matrices [Spinnler and Tognoni,1987], and the Digit forward and the Digit backward [Orsini et al.,1987]. Finally, the tests to assess visuo-construction were the copy of Rey figures [Rey,1968], the Raven of Progressive matrices [Raven,1965], and/or the Clock Drawing test [Shulman et al.,1993].
In the MCI subjects, MMSE score was obtained at the date of the EEG recordings (baseline I MMSE) and approximately after 1 year (follow-up II MMSE). Based on the percentage difference of the MMSE score between baseline and 1-year follow-up (MMSEvar), the MCI subjects were divided into three subgroups of 19 patients: the DECREASED group with MMSEvar ≤ about −4% (i.e., subjects loosing equal or more than 4% of the MMSEvar; N = 43), the INCREASED group with MMSEvar ≥ about +4% (i.e., subjects gaining equal or more than 4% of the MMSEvar; N = 30), and the STABLE group with MMSEvar between −4% and +4% (N = 27). We used the MMSE score rather than the memory score for the subjects' classification, because the former is typically used for the screening of global cognition in follow-up studies on MCI and AD subjects. At this early stage of the research, the MMSE threshold for the classification was arbitrarily chosen to divide the present MCI population into three groups of MCI with comparable age, gender, and education. We are aware that the three groups might not include amnesic MCI subjects intrinsically different from each other at the border of the categories and that these limits did not correspond to any neurophysiological or neuropathological entity. They have just a heuristic value at this early stage of the research. With the MMSEvar limits at ±4%, MCI subjects who “substantially” change follow-up MMSE score are supposed to be much more represented in the DECREASED and INCREASED groups than in the STABLE group. Noteworthy, it has been reported that the test–retest reliability of the MMSE score is supposed to range from about 0.80–0.95 as test–retest correlation [Tombaugh and McIntyre, 1992]. Furthermore, it has been suggested that the use of a short-term follow-up of few days would help the estimation of the MMSE reliability ([Becker,2001; Becker and Markwell2000] for details on the MMSE reliability problem). Unfortunately, the general design of this study did not allow it. The data collection closely followed the routine clinical practice in each centre, because we received research funds just to support for coordination and pooling data of clinical practice.
Probable AD was diagnosed according to NINCDS–ADRDA [McKhann et al.,1984] and DSM IV criteria. The recruited AD patients underwent general medical, neurological, and psychiatric assessments. Patients were also rated with a number of standardized diagnostic and severity instruments that included mini-mental state evaluation (MMSE; [Folstein et al.,1975], CDR Scale ([Hughes et al.,1982], GDS [Yesavage et al.,1982], Hachinski Ischemic Score (HIS; [Rosen et al.,1980], and Instrumental Activities of Daily Living scale (IADL; [Lawton and Brodie,1969]). Neuroimaging diagnostic procedures (MRI) and complete laboratory analyses were carried out to exclude other causes of progressive or reversible dementias to have a clinically homogenous mild AD group. Exclusion criteria included any evidence of (i) frontotemporal dementia, diagnosed according to current criteria [Knopman et al.,2005], (ii) vascular dementia, diagnosed according to NINDS–AIREN criteria [Roman et al.,1993], (iii) extra-pyramidal syndromes, (iv) reversible dementias (including depressive pseudodementia), and (v) Lewy body dementia, according to the criteria by McKeith [2005].
The Nold subjects were recruited mostly among nonconsanguineous patients' relatives. All Nold subjects underwent physical and neurological examinations as well as cognitive screening (including MMSE and GDS). Subjects affected by chronic systemic illnesses, those receiving psychoactive drugs, or with a history of neurological or psychiatric disease were excluded. All Nold subjects have a GDS score lower than 14 (no depression).
Table I summarizes the relevant demographic and clinical data of the Nold, MCI, and AD subjects. Table II summarizes the data of the MCI subjects belonging to the DECREASED, STABLE, and INCREASED groups.
| Nold | MCI | AD | |
|---|---|---|---|
| N | 45 | 100 | 45 |
| Gender (M/F) | 17/28 | 34/66 | 16/29 |
| Age (years) | 67.8 (±1.3 SE) | 70.5 (±0.7 SE) | 69.7 (±1.7 SE) |
| Education | 7.7 (±0.5 SE) | 7.9 (±0.4 SE) | 8.0 (±0.6 SE) |
| MMSE | 28.1 (±0.2 SE) | 26.5 (±0.2 SE) | 19.1 (±0.7 SE) |
| MCI_Decreased | MCI_Stable | MCI_Increased | |
|---|---|---|---|
| N | 43 | 27 | 30 |
| Gender (M/F) | 18/25 | 5/22 | 19/11 |
| Age (years) | 70.7 (±1.0 SE) | 68.5 (±1.5 SE) | 72.0 (±1.3 SE) |
| Education | 8.5 (±0.7 SE) | 7.4 (±0.8 SE) | 7.6 (±0.7 SE) |
| MMSE | 26.8 (±0.2 SE) | 27.8 (±0.3 SE) | 24.9 (±0.4 SE) |
| MMSE (follow up) | 24.2 (±0.3 SE) | 27.8 (±0.3 SE) | 27.1 (±0.3 SE) |
| MMSEvar | −9.7 (±1.1 SE) | 0.04 (±0.3 SE) | 9.1 (±1.0 SE) |
- The MCI subjects were retrospectively divided into three groups of 19 patients: the DECREASED (MMSEvar ≤ −4%), STABLE (MMSEvar ≈ 0), and INCREASED (MMSEvar ≥ +4%) groups.
EEG Recordings
EEG data were recorded in resting subjects (eyes-closed). These data were acquired (0.3–70 Hz bandpass) from 19 electrodes positioned according to the international 10–20 system (i.e., Fp1, Fp2, F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1, and O2). To monitor eye movements, the horizontal and vertical electrooculogram (0.3–70 Hz bandpass) was simultaneously recorded. All data were digitized in continuous recording mode (5 min of EEG; 128–256 Hz sampling rate, the sampling rate being fixed in each recording research unit of this multicentric study). In all subjects, EEG recordings were performed in the late morning. According to standard good EEG practice, the subjects' general conditions were preliminary checked with a brief interview on the quality of the sleep in the night preceding the experiment and on the use of psychoactive agents. In the case of conditions incompatible with EEG recordings of good quality, the experiment was postponed. To keep constant the level of vigilance, an operator controlled online the subject and the EEG traces, verbally alerting the subject any time there were signs of behavioral and/or EEG drowsiness (this occurred in a large minority of subjects) Benzodiazepines, antidepressant and/or antihypertensive drugs (when present) were withdrawn for about 24 h before the EEG recordings to pair the period from the last assumption of the drugs and EEG recording across the MCI and AD subjects. Of note, this protocol minimized drowsiness and slowing of EEG rhythms that are some times observable after a disturbed night, in early morning, and during postlunch digestion periods. Furthermore, it made the results of this study comparable with those of previous relevant EEG studies on dementia [Babiloni et al.,2006b,c,e,f,2007,2009,2010].
Preliminary Analysis of the EEG Data
The recorded EEG data were analyzed and fragmented off-line in consecutive 2-s epochs. We rejected the EEG epochs associated with operator's markers indicating drowsiness, verbal warnings, eyes opening, arm/hand movements, or other events disturbing the EEG recordings. Furthermore, the EEG epochs with ocular, muscular, and other types of artifact were preliminary identified by a computerized automatic procedure. EEG epochs with sporadic blinking artifacts (less than 15% of the total) were then corrected by an autoregressive method [Moretti et al.,2003]. Finally, two independent experimenters—blind to the diagnosis at the time of the EEG analysis—manually confirmed the EEG segments accepted for further analysis.
Spectral Analysis of the EEG Data
The digital FFT-based power spectrum analysis (Welch technique, Hanning windowing function, no phase shift) was evaluated to calculate the individual alpha frequency (IAF) peak, defined as the frequency associated to the strongest EEG power at the extended alpha range [Klimesch,1999]. Mean IAF peak was 9.4 Hz (±0.15 standard error, SE) in the Nold subjects, 9.3 Hz (±0.12 SE) in the MCI subjects (whole group), and 8.3 Hz (±0.20 SE) in the AD subjects. A statistically significant ANOVA difference was found among the groups [F(2.187) = 13.67; P < 0.00001], indicating that the IAF peak was lower in frequency in the AD than Nold and MCI groups. In the MCI subjects, mean IAF peak was 9.5 Hz (±0.21 SE) in the STABLE group, 9.1 Hz (±0.21 SE) in the DECREASED group, and 9.4 Hz (±0.21 SE) in the INCREASED group. No statistically significant ANOVA difference was found among these three groups (P > 0.05). Nevertheless, the IAF peak was used as a covariate (together with age, education, gender, and resting eyes-closed alpha rhythms) in the statistics on EEG cortical sources. Indeed, the IAF is a frequency of special importance, because it is associated with maximum power of resting eyes-closed EEG rhythms [Klimesch,1999]. The above procedure minimized the possibility that small differences in the IAF peak could confound the comparisons of cortical alpha sources among the Nold, MCI, and AD groups.
The frequency bands of interest were delta (2–4 Hz), theta (4–8 Hz), alpha 1 (8–10.5 Hz), alpha 2 (10.5–13 Hz), beta 1 (13–20 Hz), and beta 2 (20–30 Hz; [Babiloni et al.,2004,2006a,b,c,d,e]. The choice of the fixed EEG bands did not account for the IAF peak. However, this should not affect the results, because more than 90% of the subjects had the IAF peak within the alpha 1 band (8–10.5 Hz).
Cortical Source Analysis of EEG Rhythms by LORETA
LORETA software as provided at http://www. unizh.ch/keyinst/NewLORETA/LORETA01.htm was used for the estimation of cortical sources of EEG rhythms [Pascual-Marqui and Michel,1994; Pascual-Marqui et al.,1999,2002]. LORETA is a functional imaging technique belonging to a family of linear inverse solution procedures [Valdes et al.,1998] modeling 3D distributions of EEG sources [Pascual-Marqui et al.,2002], which has been successfully used in recent EEG studies on brain aging [Babiloni et al.,2004,2006a,b,c,d,e; Dierks et al.,2000].
LORETA computes 3D linear solutions (LORETA solutions) for the EEG inverse problem within a three-shell spherical head model including scalp, skull, and brain compartments. The brain compartment is restricted to the cortical gray matter/hippocampus of a head model co-registered to the Talairach probability brain atlas and digitized at the Brain Imaging Center of the Montreal Neurological Institute [Talairach and Tournoux,1988]. This compartment includes 2,394 voxels (7-mm resolution), each voxel containing an equivalent current dipole. Of note, the EEG electrode positions were not co-registered to individual brain source models; unfortunately, the official LORETA package did not include software to do so, and we could not obtain the digitalization of the electrode position from our clinical units.
LORETA solutions consisted of voxel current density values able to predict EEG spectral power density at scalp electrodes, independently of the electrode reference used. These solutions were normalized by dividing the LORETA current density values at each voxel for the power density value obtained averaging the LORETA current density values across all frequencies (0.5–45 Hz) and all 2,394 voxels of the brain volume. After the normalization, the solutions lost the original physical dimension and were represented by an arbitrary unit scale (for sake of brevity and clarity, we refereed to this scale as LORETA current density). This procedure reduced intersubjects variability and fitted the LORETA solutions in a Gaussian distribution [Leuchter et al.,1993; Nuwer,1988].
Solutions of the EEG inverse problem are underdetermined and ill conditioned when the number of spatial samples (electrodes) is lower than that of the unknown variables (current density at each voxel). To properly address this problem, the cortical LORETA solutions predicting scalp EEG spectral power density were regularized to estimate distributed rather than punctual EEG source patterns [Pascual-Marqui and Michel,1994, Pascual-Marqui et al.,1999,2002]. In line with the low-spatial resolution of the adopted technique, we used MATLAB software to collapse the voxels of LORETA solutions at frontal, central, parietal, occipital, temporal, and limbic regions of the brain model coded into Talairach space. The Brodmann areas listed in Table III formed each of these regions of interest (ROI). A main advantage of the regional analysis of LORETA solutions—using an explicit source model coregistered into Talairach space—was that the model could disentangle rhythms of contiguous cortical areas. In other words, EEG rhythms from the occipital source were disentangled with respect to those of the contiguous parietal and temporal sources.
| Loreta Brodmann areas into the regions of interest | |
|---|---|
| Frontal | 8, 9, 10, 11, 44, 45, 46, 47 |
| Central | 1, 2, 3, 4, 6 |
| Parietal | 5, 7, 30, 39, 40, 43 |
| Temporal | 20, 21, 22, 37, 38, 41, 42 |
| Occipital | 17, 18, 19 |
| Limbic | 31, 32, 33, 34, 35, 36 |
- LORETA solutions were collapsed in frontal, central, parietal, occipital, temporal and limbic ROIs.
Statistical Analysis of the LORETA Solutions
A first statistical session aimed at evaluating the control hypothesis that regional EEG sources as revealed by the LORETA solutions had amplitude sensitive to the cognitive status of the amnesic MCI subjects when compared with the control groups formed by the Nold and mild AD subjects. The confirmation of this hypothesis would validate the procedures relative to subjects' recruitment and EEG data analysis. To this aim, the LORETA solutions from the Nold, amnesic MCI, and mild AD subjects were used as an input for an ANOVA. Subjects' age, education, gender, IAF peak, and MMSE were used as covariates. Mauchly's test evaluated the sphericity assumption. Correction of the degrees of freedom was made with the Greenhouse–Geisser procedure. Duncan test was used for post hoc comparisons (P < 0.05). The ANOVA analysis used the factors Group (Nold, MCI, mild AD; independent variable), Band (delta, theta, alpha 1, alpha 2, beta 1, and beta 2), and ROI (central, frontal, parietal, occipital, temporal, and limbic). The control hypothesis would be confirmed by the following two statistical results: (i) a statistical ANOVA effect including the factor Group (P < 0.05); (ii) a post hoc test indicating statistically significant differences of the LORETA solutions with the patterns Nold < MCI < mild AD or Nold > MCI > mild AD (Duncan test, P < 0.05).
A second statistical session aimed at evaluating the working hypothesis that cortical sources of alpha rhythms were higher in amplitude in the STABLE than DECREASED group; this statistical analysis also aimed at evaluating if these cortical sources differed in the MCI groups when compared with the Nold and the AD groups. The ANOVA used the factors Group (STABLE, DECREASED, INCREASED, Nold, and AD; independent variable), Band (delta, theta, alpha 1, alpha 2, beta 1, and beta 2), and ROI (central, frontal, parietal, occipital, temporal, and limbic). Subjects' age, education, gender, IAF peak, and I MMSE were used as covariates. In addition, we took into account the individual MMSEvar and the “regression” of the follow-up MMSE score toward the MMSE group mean. For a given subject, such “regression” was defined by two variables: (i) the group mean of the baseline MMSE score minus the individual value of the baseline MMSE score; and (ii) the group mean of the baseline MMSE score minus the individual value of the follow-up MMSE score. Practically, we included the individual MMSEvar and the two “regression” values as three additional covariates into the main ANOVA. Of note, a previous reference study [Tombaugh,2005] has shown a regression to the group mean of the MMSE score across test repetitions in Nold subjects, Namely, higher MMSE values at the first examination tend to be lower at the second examination, whereas the lower values at the first examination tend to be higher at the second one.
RESULTS
Cortical Sources of Resting EEG Rhythms in Nold, MCI, and AD Subjects
For illustrative and control purposes, Figure 1 maps the grand average of the LORETA solutions (i.e., relative power current density at cortical voxels) modeling the distributed EEG cortical sources at delta, theta, alpha 1, alpha 2, beta 1, and beta 2 bands in the Nold, MCI (STABLE, DECREASED, and INCREASED as a whole group), and AD subjects. The Nold group presented alpha 1 sources with the maximal values of amplitude distributed in parieto-occipital regions. Delta, theta, and alpha 2 sources had moderate amplitude values when compared with alpha 1 sources. Finally, beta 1 and beta 2 sources were characterized by lowest amplitude values. Compared to the Nold group, the MCI group showed a decrease in amplitude of parietal, occipital, and temporal alpha 1 sources. With respect to the Nold and MCI groups, the AD group showed an amplitude increase of widespread delta and theta sources, along with a strong amplitude reduction of parietal, occipital, and temporal alpha 1 sources. These differences were statistically significant as shown by an ANOVA interaction [F(50, 3,600) = 4.19; P < 0.00001] among the factors Group (AD, MCI, and Nold), Band (delta, theta, alpha 1, alpha 2, beta 1, and beta 2), and ROI (central, frontal, parietal, occipital, temporal, and limbic).
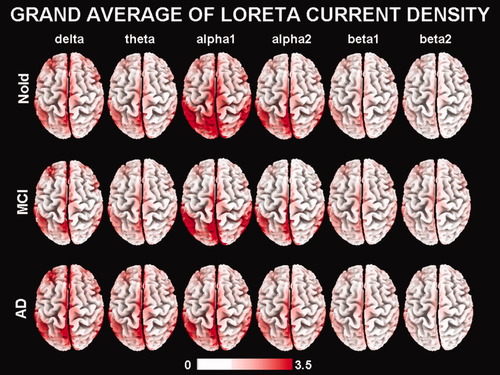
Grand average of LORETA solutions (i.e., normalized relative current density at the cortical voxels) modeling the distributed EEG sources for delta, theta, alpha 1, alpha 2, beta 1, and beta 2 bands in Nold, MCI, and AD groups. The left side of the maps (top view) corresponds to the left hemisphere. Legend: LORETA, low-resolution brain electromagnetic tomography. Color scale: all power estimates were scaled based on the averaged maximum value (i.e., alpha 1 power value of occipital region in Nold). Note that the LORETA solutions consisted of voxel current density values able to predict EEG spectral power density at scalp electrodes. These solutions were normalized by dividing the LORETA current density values at each voxel for the power density value obtained averaging the LORETA current density values across all frequencies (0.5–45 Hz) and all 2,394 voxels of the brain volume. After the normalization, the solutions lost the original physical dimension and were represented by an arbitrary unit scale. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Cortical Sources of Resting EEG Rhythms in Amnesic MCI Subjects Belonging to the STABLE, DECREASED, and INCREASED Groups
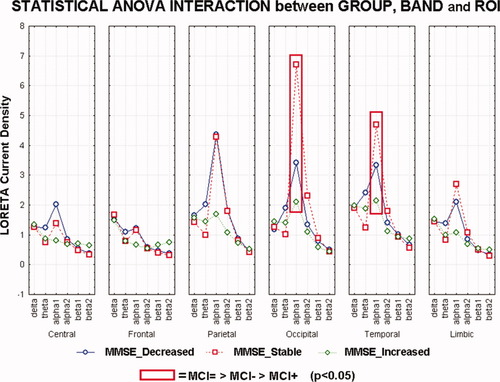
Figure 2 maps the grand average of the LORETA solutions (i.e., relative power current density at cortical voxels) modeling the distributed EEG cortical sources for delta, theta, alpha 1, alpha 2, beta 1, and beta 2 bands in the STABLE, DECREASED, and INCREASED groups of the amnesic MCI subjects. As mentioned earlier (see Methods section), the differences among the groups were evaluated by an ANOVA including the factors Group (STABLE, DECREASED, INCREASED, and Nold; AD), Band (delta, theta, alpha 1, alpha 2, beta 1, and beta 2), and ROI (central, frontal, parietal, occipital, temporal, and limbic). Figure 3 shows mean regional normalized LORETA solutions (distributed EEG sources) relative to an ANOVA interaction [F(50, 2,425) = 1.98; P < 0.0001] among all the factors. In the figure, the LORETA solutions had the shape of EEG relative power spectra. Notably, profile and magnitude of these spectra in the groups differed across various cortical macro-regions. For illustrative purposes, we also included the distributed EEG cortical sources of the Nold and AD groups.
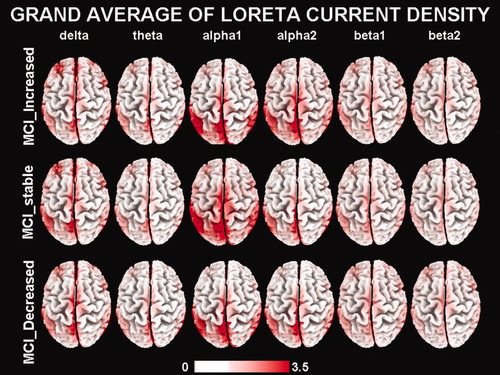
Grand average of LORETA solutions (i.e., normalized relative current density at the cortical voxels) modeling the distributed EEG sources for delta, theta, alpha 1, alpha 2, beta 1, and beta 2 bands in the DECREASED (MMSEvar ≤ −4%), STABLE (MMSEvar between −4 and 4%), and INCREASED (MMSEvar ≥ +4%) groups of amnesic MCI subjects. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
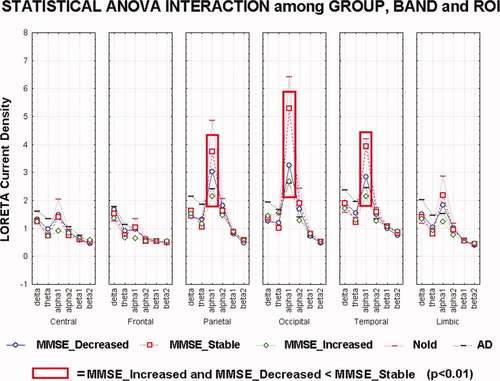
Results of the main ANOVA for the evaluation of the hypothesis concerning cortical sources from the investigated EEG rhythms: statistical interaction among the factors Group (DECREASED, STABLE, and INCREASED), Band (delta, theta, alpha 1, alpha 2, beta 1, and beta 2), and ROI (central, frontal, parietal, occipital, temporal, and limbic) in the three groups of amnesic MCI subjects. Noteworhty, these subjects were classified into the INCREASED and DECREASED groups on the basis of the MMSEvar limits at ±4% and into the STABLE group on the basis of the MMSEvar values between −4% and +4%. Only for illustrative purposes, the distributed EEG cortical sources of the Nold and AD groups were included in the figure. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The planned post hoc testing also showed that parietal, occipital, and temporal alpha 1 sources were higher in amplitude in the STABLE group than in the DECREASED and in DECREASED than in INCREASED groups (P < 0.01).
Concerning the rate of conversion/progression to AD, 26 of 100 amnesic MCI subjects converted to AD at 1-year follow-up (26% of conversion). Specifically, there was a higher amount of amnesic MCI subjects converted to AD in the STABLE (N = 4; mean MMSE score from 26.7 ± 0.4 SE at baseline to 26.9 ± 0.6 SE at 1-year follow-up) and INCREASED (N = 5; mean MMSE score from 22.9 ± 1 SE at baseline to 25.2 ± 0.6 SE at 1-year follow-up) groups than in the DECREASED group (N = 17; mean MMSE score from 26.5 ± 0.3 SE at baseline to 23.7 ± 0.6 SE at 1-year follow-up). In the INCREASED group, the amnesic MCI subjects who did not convert to AD at the 1-year follow-up (N = 25) showed a mean MMSE score from 25.3 ± 0.3 SE at baseline to 27.3 ± 0.3 SE at 1-year follow-up. In the DECREASED group, the amnesic MCI subjects who did not convert to AD at the 1-year follow-up (N = 26) showed a mean MMSE score from 27.1 ± 0.3 SE at baseline to 24.6 ± 0.4 SE at 1-year follow-up. In the STABLE group, the amnesic MCI subjects who did not convert to AD at the 1-year follow-up (N = 23) showed a mean MMSE score from 28.0 ± 0.3 SE at baseline to 27.9 ± 0.3 SE at 1-year follow-up.
On the whole, these results indicate that, in the MCI subjects, the amplitude of alpha sources was related to the stability of the global cognition along a period of 12 months.
Control Analyses
It should be remarked that the present results might be affected by the MMSE test–retest reliability. Indeed, recent findings in more than 2,000 subjects have shown that the MMSE test–retest reliability within 3 months was characterized by an error of about +1.8% (MMSEvar), mainly due to the effects of learning [Tombaugh,2005]. When this effect was removed by measuring the MMSE test–retest reliability after 5 years (negligible learning effect), the error (MMSEvar) was about of 0.9% [Tombaugh,2005]. Therefore, a good estimation of the mean MMSE test–retest reliability is of about ±1.5% (MMSEvar). On the other hand, an alternative solution for taking the MMSE test–retest reliability issue into account would be the use of corrected indices, for example, RCI-diff or RCI-Reg as suggested in a previous seminal study [Tombaugh,2005, Table IV]. These indices are much higher than ±1.5 of MMSE score. For instance, RCI-Reg (for 90% of confidence interval) varied from ±2.84 for the short test–retest intervals to ±3.75 for the long test–retest intervals in that seminal study, what corresponds to ±9.47% and ±12% of MMSE score, respectively. In other words, the reliable threshold for the group splitting might be about ±10–12% of MMSE score. To account for this issue, we performed two control analyses with additional MMSEvar limits. For the first control ANOVA, we classified the amnesic MCI subjects into the following groups: DECREASED = MMSEvar lower than −4% (N = 43), INCREASED = MMSEvar higher than +4% (N = 30), and STABLE = MMSEvar between −1.5% and +1.5% (N = 19) (Fig. 4 and Table IV). The ANOVA design and covariates were those of the main ANOVA analysis. The ANOVA factors were Group (DECREASED, STABLE, and INCREASED; independent variable), Band (delta, theta, alpha 1, alpha 2, beta 1, and beta 2), and ROI (central, frontal, parietal, occipital, temporal, limbic). The results showed a statistically significant interaction among all the factors [F(50,2225) = 3.43; P < 0.00001]. The post hoc testing indicated that the parietal, occipital, temporal, and limbic alpha 1 sources were higher in amplitude in the STABLE group than in the DECREASED and in DECREASED than in INCREASED groups (P < 0.01).
| MCI_Decreased | MCI_Stable | MCI_Increased | |
|---|---|---|---|
| N | 43 | 19 | 30 |
| Gender (M/F) | 18/25 | 5/14 | 19/11 |
| Age (years) | 70.7 (±1.0 SE) | 66.9 (±1.8 SE) | 72.0 (±1.3 SE) |
| Education | 8.5 (±0.7 SE) | 8.2 (±1.1 SE) | 7.6 (±0.7 SE) |
| MMSE | 26.8 (±0.2 SE) | 27.7 (±0.4 SE) | 24.9 (±0.4 SE) |
| MMSE (follow up) | 24.2 (±0.3 SE) | 27.7 (±0.4 SE) | 27.1 (±0.3 SE) |
| MMSEvar (%) | −9.7 (±1.1 SE) | 0.13 (±0.1 SE) | 9.1 (±1.0 SE) |
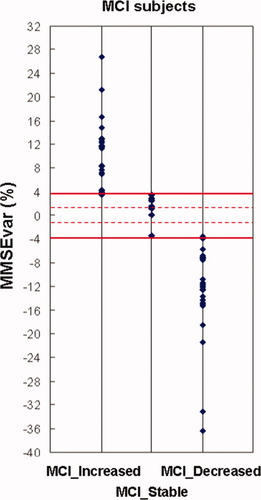
The three groups of amnesic MCI subjects (INCREASED, STABLE, and DECREASED) on the basis of the MMSEvar limits at ±4% (horizontal thick lines). The dot lines indicate the values of MMSEvar at ±1.5%. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
For the second control ANOVA, we classified the amnesic MCI subjects into the following groups: DECREASED = MMSEvar lower than −12% (N = 8), INCREASED = MMSEvar higher than +12% (N = 8), and STABLE = MMSEvar = 0% (N = 16) (Fig. 5 and Table V). The ANOVA design and covariates were those of the main ANOVA analysis. The ANOVA factors were Group (DECREASED, STABLE, and INCREASED; independent variable), Band (delta, theta, alpha 1, alpha 2, beta 1, and beta 2), and ROI (central, frontal, parietal, occipital, temporal, and limbic). Again, the results showed a statistically significant interaction among all the factors [F(50,725) = 1.40; P < 0.05]. The post hoc testing (Fig. 6) indicated that the occipital and temporal alpha 1 sources were higher in amplitude in the STABLE group than in the DECREASED and in DECREASED than in INCREASED groups (P < 0.05). In general, the results of these two control ANOVAs confirmed the findings of the main statistical analysis.
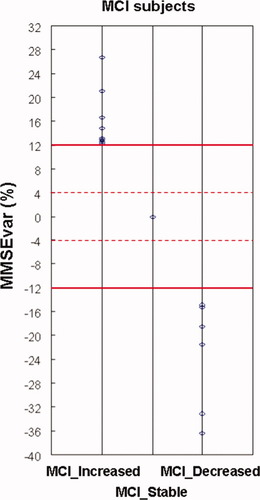
The three groups of amnesic MCI subjects (INCREASED, STABLE, and DECREASED) on the basis of the MMSEvar limits at ±12% (horizontal thick lines). The dot lines indicate the values of MMSEvar at ±4%. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
| MCI_Decreased | MCI_Stable | MCI_Increased | |
|---|---|---|---|
| N | 8 | 16 | 8 |
| Gender (M/F) | 3/5 | 3/13 | 3/5 |
| Age (years) | 70.8 (±2.3 SE) | 67.4 (±2.0 SE) | 70.1 (±1.3 SE) |
| Education | 9.1 (±1.7 SE) | 8.9 (±1.2 SE) | 7.5 (±1.3 SE) |
| MMSE | 27.0 (±0.2 SE) | 27.8 (±0.5 SE) | 22.7 (±0.5 SE) |
| MMSE (follow up) | 21.3 (±0.8 SE) | 27.8 (±0.5 SE) | 26.4 (±0.6 SE) |
| MMSEvar (%) | −21.2 (±3.1 SE) | 0.0 (±0.0 SE) | 16.3 (±1.8 SE) |
Results of the control ANOVA for the evaluation of the hypothesis concerning sources from the investigated brain rhythms: statistical interaction among the factors Group (DECREASED, STABLE, INCREASED, Nold, and AD), Band (delta, theta, alpha 1, alpha 2, beta 1, and beta 2), and ROI (central, frontal, parietal, occipital, temporal, and limbic) in the three groups of amnesic MCI subjects. Noteworthy, these subjects were classified into the INCREASED and DECREASED groups on the basis of the MMSEvar limits at ±12% and into the STABLE group on the basis of the MMSEvar values at ±0%. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
This study tested the hypothesis that, in amnesic MCI subjects, high amplitude of resting cortical alpha rhythms is statistically correlated with stability of global cognitive function at 1-year follow-up, possibly as a reflection of preserved cortical neural synchronization at the basis of “default” EEG rhythms.
We observed that posterior cortical sources of alpha rhythms (8–10.5 Hz) were lower in power in the amnesic MCI and AD subjects than in the normal elderly subjects. The opposite was true for occipital cortical sources of delta rhythms. These control results were in line with previous EEG evidence of our Consortium showing progressive differences of resting eyes-closed cortical alpha rhythms along Nold, MCI, and AD subjects [Babiloni et al.,2006b,c,d,2007,2010; Rossini et al.,2006).
Based on the MMSE percentage difference between baseline and 1-year follow-up (MMSEvar), the amnesic MCI subjects were retrospectively divided into the DECREASED (MMSEvar ≤ −4%), STABLE (MMSEvar ≈ 0), and INCREASED (MMSEvar ≥ +4%) groups. Among others, three covariates were of special interest, namely the individual MMSEvar and two variables for the individual regression of the follow-up MMSE score to the MMSE group mean. Furthermore, we performed two control ANOVAs using even more stringent MMSEvar limits (i.e., ±1.5% and 0% for the STABLE group; ±12% for the INCREASED and DECREASED groups), in line with a reference study [Tombaugh,2005]. As an original finding, all statistical analyses showed that baseline posterior cortical sources of alpha rhythms (8–10.5 Hz) were higher in amplitude in the STABLE than in the DECREASED and INCREASED groups. Furthermore, there were less amnesic MCI subjects converted to AD (1-year follow-up) in the STABLE (N = 4) and INCREASED (N = 5) groups than in the DECREASED (N = 17), with a global annual conversion rate to AD of about 26% in line with previous evidence showing an annual conversion rate comprised between 6 and 40% [Petersen et al.,2001; Jack et al.,2005]. Of note, the five amnesic MCI subjects of the INCREASED group who converted to AD had a quite low-mean MMSE score at baseline (22.9 ± 1 SE) and remained close to a MMSE score of 25 (25.2 ± 0.6 SE) at 1-year follow-up. In the whole, the present findings indicate that, at group level, the amnesic MCI subjects who remained cognitively stable at 1-year follow-up were characterized by cortical sources of resting alpha rhythms with relatively high amplitude.
The discussion of the present findings should take into account at least the following two issues. What is the meaning of the instability/stability over time of cognition in amnesic MCI subjects? And which should be the link between the resting cortical alpha rhythms of amnesic MCI subjects and their cognitive stability/instability? At the present early stage of research, we can just speculate about these issues. Previous evidence has shown that about 40–50% of MCI subjects do not deteriorate over a long period of several years, and standard indexes of global cognition can temporarily improve and then worsen over time [Bennett et al.,2002; Larrieu et al.,2002]. The improvement of the global cognition might be due to vitamin deficit correction, suspension of therapies having side effects on cognition, cognitive training, general changes in life style, brain adaptation, or cholinergic intervention, whereas its decrement might depend on depression and/or several kinds of dementias such as cerebrovascular, reversible (including pseudo-depressive dementia), Lewy body, or mixed ones. For this reason, we devoted a particular attention to the exclusion of all subjects suspected falling in these categories. However, hidden preclinical processes may nonlinearly interact to affect the evolution of cognitive functions in the present amnesic MCI subjects, and this interaction may be reflected at least in part by changes in “default” cortical neural synchronization producing resting (EEG) alpha rhythms.
Concerning the resting alpha rhythms (8−10 Hz), there is consensus that they represent the dominant resting oscillations of human brain [Klimesch,1996; Klimesch et al.,1997,1998; Rossini et al.,1991; Steriade and Llinas,1988] and have been linked to intelligent quotient, memory, and cognition [Klimesch,1999]. An important neuroanatomical substrate of alpha rhythms is the cholinergic innervation from basal forebrain to cerebral cortex, including a gross branch connecting visual occipital regions. This innervation would be targeted by neurodegenerative processes in AD [Helkala et al.,1996; Holschneider et al.,1999; Mesulam et al.,2004; Ricceri et al.,2004; Teipel et al.,2005], especially in AD patients not responding to long-term cholinergic therapy [Tanaka et al.,2003]. Instead, brainstem cholinergic innervation of the thalamus would be relatively spared [Geula and Mesulam,1989,1996,1999; Mash et al.,1985; Mesulam et al.,2004]. To confirm the pivotal role of cholinergic systems in the generation of resting EEG rhythms, the following lines of evidence have been reported: (1) scopolamine—a cholinergic antagonist—reproduced in the healthy the typical abnormal pattern of alpha and theta rhythms observed in AD [Osipova et al.,2003]; (2) loss of cholinergic connections to cerebral cortex reduced the power of resting posterior alpha sources in amnesic MCI subjects [Babiloni et al.,2009]; (3) posterior sources of resting alpha rhythms were especially impaired in AD not responding to cholinergic therapy at 1-year follow-up [Babiloni et al.,2006f]; (4) posterior cortical alpha rhythms were more preserved in the MCI subjects in whom the global cognitive status is impaired more for the white-matter vascular lesions than for the neurodegenerative processes [Babiloni et al.,2009,2010]; in this sense, changes of posterior cortical alpha rhythms might be a marker of neurodegenerative processes rather than a marker of white-matter vascular lesions, in line with previous evidence showing that posterior cortical alpha rhythms are more affected in AD patients than in subjects with subcortical vascular dementia [Babiloni et al.,2004]. However, this does not mean that attentional processes just depend on cholinergic systems in MCI and AD subjects. Together with cholinergic systems, unbalance of monoaminergic [Dringenberg,2000] and glutamatergic [Di Lazzaro et al.,2004] might affect cortical excitability and EEG rhythms in MCI and AD.
Within this theoretical framework, it can be speculated that in the present amnesic MCI subjects, instability of cognitive function over time (1-year follow-up) is related to abnormal resting alpha rhythms mainly due to the impairment of cholinergic systems rather than to diffuse white-matter vascular lesion. However, any oversimplification and overlinearization would prevent the understanding of the underlying processes. Indeed, the relationships among alpha generation, neurodegeneration at cholinergic systems, and abnormalities of regional cerebral blood flow are more complex of the above tentative explanations. On one hand, total prevalence of cerebrovascular lesion has been found to be significantly higher in AD than in the healthy [Jellinger and Mitter-Ferstl,2003]. In AD patients, cognitive and clinical status has been affected by the severity of both neurodegenerative and cerebrovascular lesions in thalamus, hippocampus, basal ganglia, anterior cingulate, and parieto-temporal areas [de Jong et al.,2008; Etiene et al.,1998]. Furthermore, cerebrovascular lesions have been associated with greater overall severity of clinical dementia and poorer cognitive performance [Heyman et al.,1998], especially in the earliest stages of AD or in subjects older than 80 years [Esiri et al.,1999; Jellinger,2001; Lee et al.,2000; Mungas et al.,2001]. For similar severity of dementia symptoms, there have been fewer neurodegenerative lesions in AD patients with vascular lesions than in those without vascular lesions, as whether neurodegenerative and cerebrovascular lesions are additive/synergistic causes of AD [Nagy et al.,1997; Snowdon et al.,1997; Zekry et al.,2002]. On the other hand, the treatment of AD with cholinesterase inhibitors has been found to affect not only the mechanisms of EEG generation but also regional cerebral blood flow in areas related to attentional and memory functions [Claassen and Jansen,2006]. Clinical and cognitive status of AD patients has been in part explained by amyloid angiopathy of small vessels [Zekry et al.,2003]. Furthermore, AD patients carrying ApoE4 allele as a genetic risk of AD have presented an increment of vessel intima-media thickness values with respect to noncarriers and cerebrovascular dementia patients [Altamura et al.,2007]. In contrast, no relation was found between ApoE4 allele and the presence/grade of carotid plaques both in AD and cerebrovascular dementia patients [Altamura et al.,2007]. Finally, evolution of cognitive function in AD patients has been unfavorable as a function of impaired cerebral vasomotor reactivity [Silvestrini et al.,2006].
In conclusion, this study tested the hypothesis that, in amnesic MCI subjects, high amplitude of baseline cortical alpha rhythms correlates with global cognition at 1-year follow-up. Results showed that baseline posterior cortical sources of alpha 1 rhythms were higher in amplitude in the STABLE than in the DECREASED and INCREASED groups. These results suggest that preserved resting cortical neural synchronization at alpha frequency is related to a long-term (1 year) stable cognitive function in amnesic MCI subjects, although this study did not define procedures for the classification of amnesic MCI subjects into neurophatological or neurophysiological subgroups. Future studies should shed light on the underlying complex psychophysiological relationships among the time evolution of cognitive impairment, neurodegenerative, and cerebrovascular lesions in amnesic MCI subjects by serial follow ups including the assessment of MMSE score, neurostructural and biological markers of AD, and possible deficiencies of vitamins/minerals in the blood.
Acknowledgements
The research was granted by the Fatebenefratelli Association for Biomedical Research (AFaR), IRCCS Fatebenefratelli of Brescia, and Tosinvest Sanita' (Cassino, Pisana). Dr. Roberta Lizio has contributed to this study in the framework of her Ph.D. fellowship in Neurophysiology at the Department of Physiology and Pharmacology, University of Rome Sapienza, Rome, Italy. We thank Dr. Patrizio Pasqualetti for statistical advice.