The role of the unaffected hemisphere in motor recovery after stroke
Abstract
The contribution of the ipsilateral (nonaffected) hemisphere to recovery of motor function after stroke is controversial. Under the assumption that functionally relevant areas within the ipsilateral motor system should be tightly coupled to the demand we used fMRI and acoustically paced movements of the right index finger at six different frequencies to define the role of these regions for recovery after stroke. Eight well-recovered patients with a chronic striatocapsular infarction of the left hemisphere were compared with eight age-matched participants. As expected the hemodynamic response increased linearly with the frequency of the finger movements at the level of the left supplementary motor cortex (SMA) and the left primary sensorimotor cortex (SMC) in both groups. In contrast, a linear increase of the hemodynamic response with higher tapping frequencies in the right premotor cortex (PMC) and the right SMC was only seen in the patient group. These results support the model of an enhanced bihemispheric recruitment of preexisting motor representations in patients after subcortical stroke. Since all patients had excellent motor recovery contralesional SMC activation appears to be efficient and resembles the widespread, bilateral activation observed in healthy participants performing complex movements, instead of reflecting maladaptive plasticity. Hum Brain Mapp, 2010. © 2010 Wiley-Liss, Inc.
INTRODUCTION
Evidence is accumulating that the human brain is able to compensate for lost motor function after stroke. Despite a huge body of literature on brain plasticity in animal models, however, the mechanisms and biological basis for this recovery process in humans remains incompletely understood. With the advent of functional magnetic resonance imaging (fMRI) it has become possible to monitor the neural correlates of recovery after stroke noninvasively. During the performance of various motor tasks different brain activation patterns between stroke patients and healthy participants, including enlarged areas of activation within the damaged hemisphere and an additional recruitment of brain regions in the healthy hemisphere, have been observed in the past [Calautti et al.,2007; Johansen-Berg et al.,2002; Nair et al.,2007; Ward et al.,2003a; Ward et al.,2003b; Ward and Cohen,2004]. Although it is generally agreed upon that each of these additionally activated brain areas reflect some aspect of reorganization, it apparently is a major challenge to disentangle the role of each component of this network in recovery from stroke. Especially findings on the functional significance of the contralesional (ipsilateral) motor cortical areas for outcome after subcortical motor stroke have been contradictory. Recent fMRI studies evaluating different motor tasks have reported an enhanced activation in premotor and sensorimotor areas of the undamaged hemisphere during the early stages of spontaneous recovery after subcortical stroke [Calautti et al.,2007; Johansen-Berg et al.,2002; Loubinoux et al.,2003; Ward et al.,2003b]. These studies demonstrate a continuous decline of these activation patterns during later stages in well-recovered patients and persistent involvement of ipsilateral sensorimotor structures only in patients with poor recovery. Besides pure motor tasks similar findings also could be observed in patients with brain reorganization during language recovery after infarction within the territory of the left middle cerebral artery [Saur et al.,2006]. In contrast, other studies have indicated that activation of ipsilateral motor structures might also be beneficial for performance in the chronic stage [Gerloff et al.,2006; Puh et al.,2007; Lotze et al.,2006].
Because the majority of the studies outlined above used rather simple motor paradigms, which could also be performed by handicapped patients, it is well conceivable that tasks of increasing complexity might shed further light on the functional contribution of the undamaged hemisphere to recovery, especially in the chronic stage. Various PET and fMRI studies demonstrate a linear increase of the BOLD response magnitude in parallel with the presentation rate at the level of the sensorimotor cortex [e.g. Sadato et al.,1996; Blinkenberg et al.,1996; Jäncke et al.,1998a,b]. This positive linear rate effect reflects higher neural activity associated with increasing requirements of motor control during faster finger movements [Jäncke et al.,1998a,b]. Under the assumption that incremental movement rates are associated with an increased functional demand on the motor system [Blinkenberg et al.,1996; Kim, et al.,2005; Sabatini et al.,1993] we used fMRI and acoustically paced movements of the right index finger at six different frequencies (2.0, 2.5, 3.0, 4.0, 5.0, and 6.0 Hz) to investigate the behavioral significance of motor regions recruited ipsilaterally in a group of patients with chronic striatocapsular infarction of the left hemisphere and with initial severe paresis of their hands and near complete recovery compared with a group of healthy, age-matched participants. We hypothesized that functionally relevant areas within the ipsilateral motor system should show a close coupling between the movement rate and the hemodynamic response in patients.
MATERIALS AND METHODS
Participants
The demographic and clinical data of the patients are summarized in Table I. The mean age in the patient group was 58.9 ± 7.8 years (n = 8, 4 females) and in the control group 58.4 ± 6.6 years (n = 8, 4 females), respectively. The mean NIHSS at the time of the event was 5.38 ± 1.85 and all patients had experienced substantial clinical recovery (mean NIHSS: 1.75 ± 0.46; P < 0.001; t-test). Mean time since stroke was 3.5 ± 0.5 years.
| Patient | Age | Duration | NIHSS 1 | MRC 1 | NIHSS 2 | MRC 2 | CVRF | Medication | Gender | Lesion |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 = A.S. | 69 | 35 | 8 | 2 (1) | 2 | 4 (3) | Hypertension | Enalapril, aspirin, metoprolol | m | Left putamen/ pallidum CVA |
| 2 = I.W. | 63 | 37 | 5 | 3 (1) | 2 | 3 | Hypertension, hypercholestremia | Enalapril, aspirin, atorvastatin | f | Left putamen/ pallidum CVA |
| 3 = I.Z. | 56 | 35 | 3 | 3 | 1 | 4 | Hypercholestremia, patent foramen ovale | Atorvastatin, marcumar | f | Left internal capsule CVA |
| 4 = B.M. | 49 | 49 | 8 | 2 | 2 | 4 | Nicotine abuse | Aspirin | m | Left internal capsule CVA |
| 5 = J.G. | 59 | 39 | 4 | 3 | 2 | 4 | Hypertension | Enalapril, aspirin | m | Left putamen/ pallidum and internal capsule CVA |
| 6 = S.S. | 47 | 32 | 5 | 3(2) | 1 | 4 | Patent foramen ovale | Marcumar | f | Left putamen/ pallidum and internal capsule CVA |
| 7 = A.E. | 62 | 42 | 4 | 3 | 2 | 4 | Nicotine abuse, hypertension | Enalapril, aspirin, metoprolol | m | Left putamen/ pallidum and internal capsule CVA |
| 8 = K.S. | 66 | 44 | 6 | 2 (1) | 2 | 4 (3) | Hypercholestremia, patent foramen ovale | Atorvastatin, marcumar | f | Left putamen/ pallidum and internal capsule CVA |
- CVA, cerebral vascular accident; Duration, time between CVA onset and fMRI; NIHSS 1, NIH stroke scale at the onset of CVA; NIHSS 2, NIH stroke scale when fMRI was perfomed; MRC 1, Medical Research Council (MRC) scale at the onset of CVA for wrist extension (and finger extension in brackets if different); MRC 2 = Medical Research Council (MRC) scale for wrist extension (and finger extension in brackets if different) when fMRI was perfomed; CVRF, cardiovascular risk factors.
Patients were recruited from the out- and inpatient services. Inclusion criteria were (1) first-ever striatocapsular ischemic stroke within the left hemisphere (≥24 months after the event; Fig. 1), (2) near complete recovery of motor deficit (Medical Research Council scale >3/5) (3) ability to perform acoustically paced finger tapping up to 6 Hz without mirror movements (5) right-handedness according to the Edinburgh handedness inventory, and (6) adequate language and cognitive function to understand consenting and study-related instructions. The neurological disorders were assessed at the time of the event and at the time of the fMRI study using the MRC scale and the NIH Stroke Scale (NIHSS). Eight healthy volunteers, recruited from the community, served as participants. They were carefully selected and each underwent a detailed interview, as well as a general physical and neurological examination. None of them reported a history of any neurological, psychiatric or cardiac disease (cardiovascular risk factors such as hypertension, diabetes mellitus, hyperlipidemia or hypercholesterolemia and smoking) or medication of centrally active drugs. All controls were also right-handed according to the Edinburgh handedness inventory. Informed consent had been obtained in accordance with the Declaration of Helsinki and the local Ethics Committee. All participants were asked to refrain from caffeine at least six hours before the fMRI study.
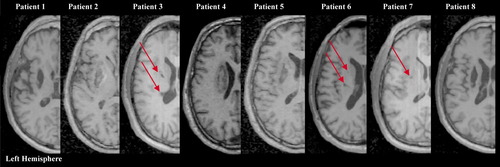
Axial T1-weighted MRI scans at the level of maximum infarct volume for each patient performed at the time of fMRI. Patient numbers correspond with those in the Table I. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Motor Paradigm
Functional MRI was conducted while patients and controls performed acoustically paced motor tasks with their right index finger (see Fig. 2 for a schematic display of the experimental setting) [Riecker et al.,2003; Riecker et al.,2006]. Isochronous series of clicks with six frequencies (2.0; 2.5; 3.0; 4.0; 5.0, and 6.0 Hz) served as acoustical pacing signals (duration of each series of clicks = 6 s) and were presented 15 times each in a pseudorandomized order with onset-to-onset intervals ranging from 12 to 24 s. The clicks were generated by manual editing (Cool Edit Pro 2002, Version 2.0, Syntrillium Software Corporation, Phoenix, USA) of a recorded “natural” sound (stroke of a pen against the desk). Altogether, the experiment encompassed 3 × 90 series of clicks (6 rates × 15 trials). During this task, participants were asked to press a button designed for fMRI experiments in response to the periodic acoustical trigger stimulus. Prior to the fMRI experiment, participants practiced the motor task several times together with measures of electromyography (left ipsilateral extensor digitorum communis muscle) to detect mirror movements. Participants' responses were recorded in digitized form, so that each single signal from the response button could be verified and frequency, reaction time and error rate of the finger movements could be determined. Error rates were defined as the deviating number of taps compared to the number of required auditory cues. A repeated measures analysis of variance (ANOVA; group × frequency) was performed to evaluate differences across the two groups on task accuracy. A camera system allowed for the monitoring of associated finger or mouth movements inside the scanner.
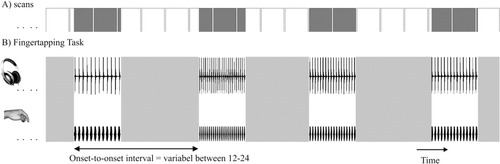
Schematic representation of the experimental set-up. (A) Time course of scan acquisition: Each box represents the measurements across one complete brain volume comprising 28 slices. Note the jittering effect between activation periods (gray-shaded areas) and scanning intervals (white boxes). (B) Finger tapping task: Subjects performed acoustically paced movements of the right index finger at six different frequencies (2.0, 2.5, 3.0, 4.0, 5.0, and 6.0 Hz) 15 times each in a pseudo-randomized order with onset-to-onset intervals ranging from 12–24 s.
fMRI Data Acquisition
Participants lay supine in a 1.5 T whole-body scanner (Siemens Sonata, Erlangen, Germany), their heads being secured by foam rubber to minimize movement artifacts. Twenty-eight parallel axial slices (thickness = 4 mm, gap = 1 mm) were obtained across the entire brain volume using an echo-planar imaging sequence (64 × 64 matrix, field of view = 192 × 192 mm2, TE = 39 ms, TR = 3 s, flip angle = 90°). The experiment encompassed three scanning sessions comprising 555 (=3 × 185) image volumes altogether. Five initial dummy scans were used for the equilibration of T1 saturation effects within each session. For anatomical localization of activated areas, the fMRI maps were superimposed on a T1-weighted 3D sequence (MPRAGE; 128 sagittal slices, thickness = 1.5 mm, 256 × 256 matrix, field of view = 256 × 256 mm2, TE = 4 ms, TR = 9.7 ms) averaged across all participants.
fMRI Data Analysis
Overview
After data preprocessing for each individual, (1) single subject analysis during the activation phases for each production rate separately and also as a parametric analysis including the calculation of the main effects and the rate/response functions were performed in order to determine the influence of stimulus frequency on the magnitude of the BOLD signal. (2) These data were used for the subsequent group analyses and (3) for subtraction analysis between controls and patients. Moreover, all cortical brain structures identified as significant activated areas with the single subject analysis served as the volumes of interest for (4) the subsequent computation of correlation coefficients across the entire time series of the BOLD effect (functional connectivity).
Data Preprocessing
fMRI data were transformed to an ANALYZE-compatible format and analyzed using the SPM5 software package (Wellcome Department of Imaging Neuroscience, London, UK). Each anatomical T1-weighted image was realigned to the standard T1 template provided by the SPM5 software package. Coregistration of the functional images then relied on the same transformation matrix. Subsequently, spatial normalization and correction of MRI images into a standard space as defined by an ideal template was performed. Finally, the normalized data sets were smoothed with an isotropic Gaussian kernel (10 mm full-width at half maximum).
Analysis of Main Effects and Rate/Response Functions
The resulting contrast images during finger tapping from each participant provided the data base for all subsequent steps of statistical analysis. On the basis of a boxcar reference function (“trains of events”) a parametric approach using polynomial basis algorithms up to the second order allowed for the determination of the relationship between movement rates of the right index finger and regional BOLD signal. This procedure models three different rate/response functions: (a) categorical on-off responses to movement rates irrespective of presentation rate (main effect), (b) linear change in BOLD signal along with increasing movement rates (first order term), and (c) nonlinear (quadratic) relationships between stimulus frequency and hemodynamic activation (second order term). After single subject analysis second level group analysis was performed using an Analysis of Variance. Also a subtraction analysis between controls and patients (and vice versa) was performed to detect group differences. For anatomical localization of activated areas, the fMRI maps were superimposed on transverse sections of the structural MR images averaged across all participants. The height threshold of the present study at voxel level was set at P < 0.05 (corrected for multiple comparisons).
Analysis of Categorical Effects
The resulting contrast images during finger tapping from each participant provided the data base for all subsequent steps of this analysis. On the basis of a boxcar reference function (“trains of events”) the data recorded during the various activation phases were analysed separately for each stimulus frequency and participant. Group means and standard deviation of hemodynamic activation at each rate were calculated. Subsequently, at the respective activation maximum within each volume of interest group means and standard deviation of hemodynamic brain activity was determined and displayed as a bar graph (see Fig. 5). Beyond the parametric statistics, this analysis allows for an additional characterization of the various linear and nonlinear response functions.
Analysis of fMRI Time Series in Terms of “Functional Connectivity”
Brain connectivity represents an elusive concept varying across different domains of research [Horwitz,2003]. On the basis of a decision by consensus at a recent workshop [Lee et al.,2003] functional connectivity was defined as the “temporal correlation between spatially remote neurophysiological events” [Friston et al.,1993]. To obtain a measure of functional connectivity, region-specific time series were compared with each other [Riecker et al.,2005]). After data preprocessing, the hemodynamic responses obtained during the finger tapping task were calculated separately in each participant. These individual data provided the basis for the analysis of functional connectivity. All the cortical brain areas which had been characterized by a significant hemodynamic activation effect as determined by the preceding parametric analysis served as volumes of interest (VOIs). The region-specific time series of each volume of interest from each participant included the first eigenvariate of all voxels within a 5-mm radius sphere centered on the respective local maximum. Computation of the combined time series within SPM5 relied on the “VOI times-series-extraction-tool”. Afterwards, all regions of interest for each participant were compared with each other. Thus, the within-subject correlation matrices across all region-specific time series could be calculated. Subsequently, group means and standard deviations were determined across all participants. Using SPSS for windows (SPSS Version 15), the computed correlation coefficients were assigned to the categories “very high”, “high”, and “intermediate”. The respective correlation coefficients within the various brain regions were then transformed into gray values to get a more straightforward visualization of the functional connectivity between the different brain structures (see Fig. 6). Additionally we used the Mann-Whitney U-test as a nonparametric method to analyze whether the correlation matrices of each region demonstrate significant differences between controls and patients. This test is defined as a nonparametric test (distribution-free) used to compare two independent groups of sampled data. This test could be described as an alternative to the independent group t-test, when the assumption of normality or equality of variance is not met.
Head Movement
Head displacements during fMRI measurements influence spin excitation history and, thus, modify the BOLD signals of the respective scans. In order to account for these artifacts, the SPM5 software estimates the movement parameters during the realignment of functional images by comparing each slice to its reference, usually the first scan of a measurement series. As a second step of analysis, SPM5 performs a mathematical adjustment based on a moving average-autoregression model of spin excitation history effects to remove persisting movement-related components [Frackowiak et al.,1997]. It could be demonstrated that this procedure removes as much as 90% of the variance due to movement-related effects in an fMRI time series. The present experiment measured continuously the complete brain volume using a TR of 3 s. We checked the head movement parameters in all (x-, y-, and z-) directions after the realignment step of signal analysis. On the basis of our experiences from former studies, we decided, prior to the present experiment, to exclude those data sets with more than 2-mm motion in any direction. As described above participants performed several test runs outside the scanner as well as prior to the experiment inside the scanner in order to get acquainted with the test material and to learn how to avoid strong/sudden head and associated jaw, lip, tongue, and shoulder/arm movements. Thus, because of this extremely careful preparation of the participating participants none of them had to be excluded from the study. The mean head movement parameters were: x-direction 1.27 mm (±0.34), y- direction 1.43 (±0.29), and z-direction 1.38 (±0.28).
RESULTS
Behavioral Data
For each participant, the average tapping frequency, the tapping interval, the reaction time at the beginning of each block, and the error rates (defined as a deviating number of taps compared with the number of auditory cues given during the scanning procedure) were determined. The behavioral results of healthy control participants and patients are summarized in Fig. 3. A repeated measures analysis of variance (ANOVA; group × frequency) indicated that the actual finger tapping frequency (F(1,14) = 291.679, P ≤ 0.001), the tapping interval (F(1,14) = 286.453, P ≤ 0.001), and the error rates (F(1,14) = 297.561, P ≤ 0.001) differed significantly between both groups. In contrast, the reaction time (F(1,14) = 0.054, P ≤ 0.853) did not show a significant difference. Overall, there was no significant main effect for frequency × group interaction. Even though our participants (especially our patients) did not report that the higher stimulus rates had required much more effort on task accuracy, this analysis revealed an increasing difference in patients compared to healthy controls during the higher frequency conditions (4.0, 5.0, and 6.0 Hz). According to visual control inside and electromyography outside the scanner, (mirror) movements of the left hand did not take place in any of the patients.
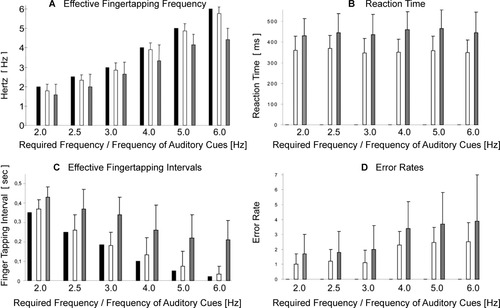
Behavioral measures of healthy control subjects and patients during finger tapping with six frequencies. Actual tapping frequency performed by healthy controls and patients versus auditory cues (A); reaction time in (ms) (B); actual tapping intervals (s) performed by healthy controls and patients versus auditory cues (C); and error rates defined as a deviating number of taps compared to the number of cues (D). Values are mean ± SD. Black bars = auditory cues, white bars = healthy control subjects, gray bars = patients.
fMRI Data
According to our hypothesis we defined the supplementary motor area (SMA) as well as the premotor cortex (PMC) and sensorimotor cortex (SMC) of both hemispheres as the regions of interest for the subsequent analyses.
Main Effects
During finger tapping at each single finger tapping frequency as well as across all frequencies main areas of activation included the left SMC, left basal ganglia (Putamen/Pallidum) and left thalamus (not displayed), right cerebellum (not displayed), and SMA (Figs. 4A,B and 5; Table II) in patients and controls. In addition to these regions both groups also demonstrated significant brain activation within the right SMC and right PMC across all frequencies. A subtraction analysis between healthy controls and patients (controls > patients) irrespective of presentation rate (main effect) demonstrated significant higher hemodynamic activation at the level of the left SMC and the left pre-SMA/left SMA proper (red colored brain areas in Fig. 4C, cf. also Table III). The reverse comparison of the respective main effects (patients > controls) did not reveal any areas of higher activation in the group of patients.
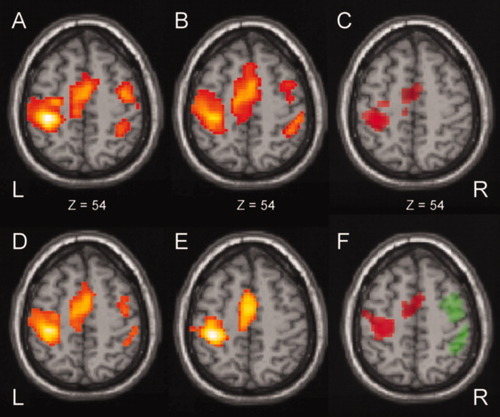
Statistical parametric maps of (1) the main effects for patients (A) and controls (B), (2) the positive linear BOLD signal changes along with increasing movement rates for patients (D) and controls (E) and (3) the results of the subtraction analysis of main effects (C) and positive linear BOLD signal changes (F) between the two groups. Red color indicates the significant activation patterns of the comparison controls > patients, green color indicates the significant activation patterns of the comparison patients > controls. L, left; R, right; z, distance to the intercommissural plane. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
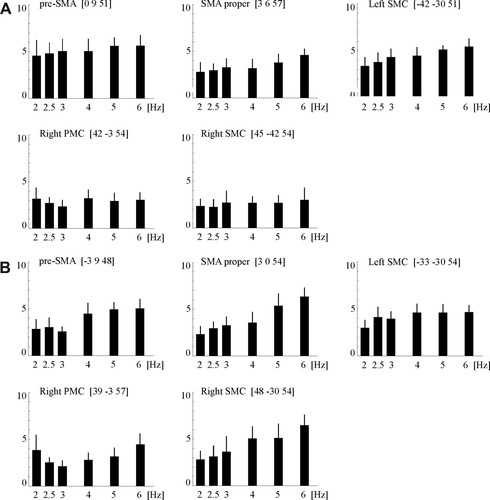
Parametric changes of BOLD signal intensity along with increasing movement rate of the right index finger in control subjects (A) and patients (B; size of effect and variance of BOLD signal intensity within the respective voxels calculated in arbitrary units by SPM5). SMA, supplementary motor area; PMC, premotor cortex; SMC, sensorimotor cortex; L, left; R, right; z, distance to the intercommisural plane;
| Patient group | Control group | ||||
|---|---|---|---|---|---|
| Main effect (all frequencies) | First order term (positive linear) | Main effect (all frequencies) | First order term (positive linear) | ||
| Pre-SMA | Left | 6.75/7.74 [−3 9 48] 285 voxels* | 5.73/8.57 [−3 15 48] 251 voxels* | 6.26/9.38 [0 9 51] 339 voxels* | 6.01/9.26 [−3 12 51] 248 voxels* |
| SMA proper | Mesial | 5.84/6.86 [3 0 54] 285 voxels* | 6.72/7.34 [−3 −3 63] 251 voxels* | 5.79/8.63 [3 6 57] 339 voxels* | 5.34/9.42 [3 0 57] 248 voxels* |
| Premotor cortex | Right | 4.97/4.65 [39 −3 57] 94 voxels | 4.76/8.80 [42 −6 57] 78 voxels | 6.02/5.09 [42 −3 54] 72 voxels | n.s. |
| SMC | Left | 6.43/8.59 [−33 −30 54] 351 voxels | 5.92/10.02 [−33 −33 57] 389 voxels | 7.33/13.47 [−42 −30 51] 401 voxels | 6.38/14.46 [−33 −30 57] 343 voxels |
| Right | 5.81/7.08 [48 −30 54] 157 voxels | 5.17/5.92 [24 −42 57] 122 voxels | 4.93/7.01 [45 −42 54] 104 voxels | n.s. | |
- Z scores (cursive) and T values represent activation maximum within each region. The extent of each activated area is given by the numbers of activated voxels. SPM-coordinates are printed in square brackets. Parametric analyses concerning the “First order term” (negative linear, e.g. decreasing activation in parallel with increasing frequency of movement) and positive as well as negative “Second order term” (quadratic effect) revealed no significant results in the patient or control group.
- * Due to an overlap between pre-SMA and SMA proper the number of activated voxels are given for both regions together.
| Main effect (all frequencies) | First order term (positive linear) | ||||
|---|---|---|---|---|---|
| Controls > Patients | Patients > Controls | Controls > Patients | Patients > Controls | ||
| Pre-SMA | Left | 3.81/4.49 [0 9 51] 128 voxels* | n.s. | 4.02/3.98 [0 6 54] 115 voxels* | n.s. |
| SMA proper | Mesial | 5.74/6.41 [3 3 54] 128 voxels* | n.s. | 4.73/5.17 [3 0 54] 115 voxels* | n.s. |
| Premotor cortex | Right | n.s. | n.s. | n.s. | 4.18/5.03 [39 −3 54] 87 voxels |
| SMC | Left | 4.32/5.99 [−39 −33 54] 185 voxels | n.s. | 4.67/5.21 [−42 −30 54] 192 voxels | n.s. |
| Right | n.s. | n.s. | n.s. | 3.99/4.28 [48 −33 51] 94 voxels | |
- Z scores (cursive) and T values represent activation maximum within each region. SPM-coordinates are printed in square brackets. The extent of each activated area is given by the numbers of activated voxels. Parametric analyses concerning the “First order term” (negative linear, e.g. decreasing activation in parallel with increasing frequency of movement) and positive as well as negative “Second order term” (quadratic effect) revealed no significant results in the patient or control group.
- * Due to an overlap between pre-SMA and SMA proper the number of activated voxels are given for both regions together.
Linear and Nonlinear Rate/Response Functions
At the level of the left pre-SMA, the left SMA proper and the left SMC the hemodynamic response increased linearly with the frequency of the finger movements in both groups (Figs. 4D,E and 5; Table II). In contrast, an additional linear increase of the BOLD response with higher tapping frequencies at the level of the right PMC and SMC was only seen in the group of patients (Figs. 4D and 5; Table II). A subtraction analysis of linear increasing hemodynamic signal changes along with increasing movement rates (controls > patients) demonstrated a stronger increase of the hemodynamic BOLD signal changes along with increasing frequency within pre-SMA, SMA, proper and SMC of the left hemisphere in the control group (red colored brain areas in Figs. 4F and 5; cf. Table II). In contrast, the reverse comparison (patients > controls) demonstrated a higher increase of the signal changes within PMC and SMC of the right hemisphere (green colored brain areas in Figs. 4F and 5; cf. also Table II). The remaining parametric analyses concerning the “First order term” (negative linear, e.g. decreasing activation in parallel with increasing frequency of movement) and positive as well as negative “Second order term” (quadratic effect) revealed no significant results in the patient or control group.
During finger tapping at each single finger tapping frequency as well as across all frequencies a typical pattern of activation was observed in both groups, which included the pre-SMA, the SMA proper and the contralateral sensorimotor cortex (SMC; Fig. 5). The categorical analysis demonstrated an increasing hemodynamic brain activity along with increasing index finger movements in these brain areas. In addition to these regions, right-sided PMC and SMC demonstrated different BOLD signal changes along with increasing index finger movements in both groups. Analysis at each single finger tapping frequency exhibited an increasing hemodynamic brain activation within these areas in patients, whereas the analysis in the control group demonstrated whether in- nor decreasing effects along with rising index finger movements (see Fig. 5). These findings are corroborated by the parametric analysis of linear and nonlinear rate/response functions (see above). Therefore, these findings suggest that near complete brain reorganization following striatocapsular stroke within the left hemisphere could be achieved by an upregulation with recruitment of compensatory right PMC and SMC.
fMRI Time Series in Terms of “Functional Connectivity”
The results of the functional connectivity analyses between the activated cortical regions of interest for both groups are summarized in Figure 6. As expected, very high correlations between the left pre-SMA, left SMA proper, and left SMC were found in both groups. In addition, there was a very high correlation between the left SMA proper and the right SMC, as well as between the right SMC and the right PMC and between right SMC and the left SMC in the patients. Nonparametric Mann-Whitney-U-Tests were used to evaluate whether groups differ in the strength of correlation between the respective brain areas. In healthy controls, the correlation coefficients between SMA proper and the left motor cortex were significantly stronger compared to patients (P < 0.01). In contrast, the patient group demonstrated significantly higher correlation coefficients between SMA proper and right SMC, between SMC of both sides and between the right SMC and right PMC compared to controls (P < 0.01).
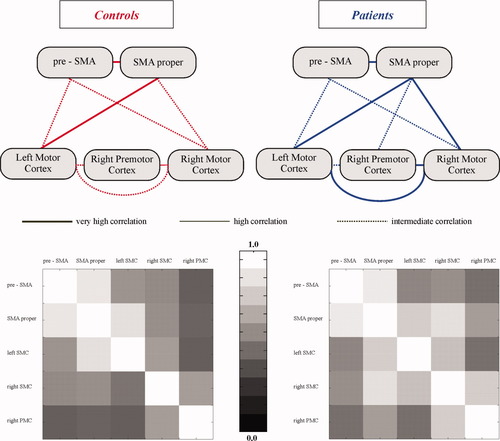
The upper panel displays the results of a quantitative functional connectivity analysis: computed correlation coefficients across the time series of the blood oxygen level-dependent (BOLD) signal within the volumes of interest. The diagram depicts very high (>0.9), high (>0.8), and intermediate correlations (>0.6). Low correlations are not displayed. The lower panel shows the correlation coefficients for both groups displayed as grey scale values. Note: The averaged gray scale values (lower panel) are in accordance with the results from the Mann-Whitney-U-Test demonstrating higher correlation coefficients between SMA proper and right SMC, between SMC of both sides and between the right PMC and SMC in patients compared to controls. Vice versa, higher correlation coefficients were seen in controls compared with patients between SMA proper and left-sided SMC. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
In this study we used fMRI and acoustically paced movements of the right index finger at six different frequencies to analyze the contribution of ipsilateral motor regions to motor behavior in a subset of patients who had experienced near complete motor recovery following a striatocapsular stroke within the left hemisphere. A close correlation between the increase of the BOLD-signal magnitude and the frequency of the finger movements at the level of the right PMC and SMC clearly indicates that the contralesional (ipsilateral) hemisphere contributes to motor performance and thus to recovery in these patients (Figs. 4 and 5). In addition, the fMRI time series analysis in terms of functional connectivity supports the notion that this enhanced ipsilateral activity may in part be the consequence of an increased drive from supplemental motor areas to this hemisphere (e.g., right-sided PMC and SMC; Fig. 6).
To date, the contribution of the ipsilateral, nonaffected hemisphere to recovery after stroke is controversial. In early fMRI studies an increased task-related activation within ipsilateral motor structures had been observed in acute stroke patients compared with healthy participants [Cao et al.,1998; Cramer et al.,1997]. These findings were generally interpreted in favor of a functional role of the ipsilateral hemisphere for recovery. However, in the past few years this interpretation has been challenged by subsequent studies, which have reported a persistence of these enhanced activation patterns in patients with poor recovery, whereas a decreased recruitment of ipsilateral motor structures was observed in patients with an improvement of motor function during follow-up [Calautti et al.,2007; Johansen-Berg et al.,2002; Loubinoux et al.,2003; Ward et al.,2003b; Saur et al.,2006]. These observations pointed to a negative association between functional outcome and the degree of task-related activity within the nonaffected hemisphere. Several TMS experiments have even indicated that contralesional brain activation may inhibit ipsilesional cortex and thus be detrimental for motor recovery [Duque et al.,2005; Murase et al.,2004]. In fact, reducing this enhanced excitability with repetitive transcranial magnetic stimulation (TMS) in stroke patients has been shown to improve motor performance of the paretic hand [Mansur et al.,2005]. Our finding of a close relationship between the magnitude of the BOLD signal changes within the ipsilateral SMC and the functional demand in extremely well recovered patients does not corroborate the hypothesis that the recruitment of these structures generally represents maladaptive plasticity or an epiphenomenon. Furthermore, a recent study used fMRI to investigate short-term reorganization in the right premotor cortex after transcranial magnetic stimulation disrupted the left premotor cortex in healthy participants. It could be demonstrated that there was a compensatory increase in activity in the right premotor cortex and in connected medial premotor areas demonstrating recovery of function after disruption by transcranial magnetic stimulation [O'Shea et al.,2007]. Similarly, using a multimodal approach encompassing EEG, PET and TMS Gerloff and colleagues could recently demonstrate that contralesional motor areas significantly contribute to motor performance in patients with subcortical stroke [Gerloff et al.,2006]. At least indirectly our results are supported by the clinical observation that patients suffering from a second stroke in the opposite hemisphere not only develop a new contralateral hemiparesis but also an impairment of the recovered limb [Ago et al.,2003; Fisher,1992; Song et al.,2005].
In our as well as previous studies it could be ruled out that mirror movements were responsible for the enhanced activation of ipsilateral SMC [Butefisch et al.,2005; Gerloff et al.,2006]. It is also noteworthy that activation of the ipsilateral SMC is not confined to stroke victims, but can also be found in healthy participants during performance of complex, as well as nondominant hand tasks [Catalan et al.,1999; Chen et al.,1997; Hummel et al.,2003; Sadato et al.,1996; Verstynen et al.,2005]. Therefore, it is well conceivable that the execution of the motor tasks required recruitment of this bilateral network in our recovered stroke patients, which is physiologically engaged with complex tasks. Although the patients did not experience the motor tasks more effortful than the control participants a quantitative analysis of their actual movements produced during scanning clearly revealed that the degree of effort must have increased at the highest stimulation frequencies to maintain the speed, accuracy and pacing. With respect to the concept of “plasticity” following a cerebral ischemic event it is thus more likely the observed enhanced activation within the ipsilateral SMC represents an upregulation of activity in a pre-existing motor network, rather than being the consequence of axonal sprouting with formation of new synapses [Chen et al.,2002].
The results of the functional connectivity analysis points towards a crucial role of supplemental motor areas in organizing movement of the impaired limb following stroke.
In support of this notion, activation within these motor structures has been associated with the initiation, programming, selection, learning and responsiveness to internally cued movements or to the selection of the appropriate neuronal populations necessary to execute a movement [Gerloff et al.,1997]. Although we did not perform additional TMS experiments to evaluate the potential recruitment of uncrossed pyramidal fibers, it is highly unlikely that this pathway can account for the enhanced activation within the ipsilateral hemisphere, based on the results of previous TMS and fMRI studies [Butefisch et al.,2005; Foltys et al.,2003; Gerloff et al.,2006; Nair et al.,2007].
Unexpectedly, we did not find a motor region, which showed a larger extent of activation in patients compared with the controls, irrespective of regarding each stimulation frequency separately or performing a group analysis across all frequencies. Only the analysis of positive linear BOLD signal changes along with increasing movement rates demonstrated a difference in terms of higher activation at the level of the right-sided PMC and SMC in patients compared to controls which together with the fMRI time series analysis can be interpreted as an increased drive from supplemental motor areas to this hemisphere. This finding contrasts with most previous fMRI studies dealing with this issue, which have consistently described an overactivation (with respect to the extent of activation) in several motor regions especially in acute but also in chronic stroke patients compared to age-matched control participants. Although it is rather unlikely that the patient characteristics were completely different between the current and previous studies alterations of the microvascular system (through diabetes, hypertension and hyplercholesterolemia for instance) have been shown to significantly influence BOLD signal changes [Pineiro et al.,2002], which could partially account for this finding. It is also important to emphasize that we only studied well-recovered chronic stroke patients who were able to perform finger tapping with a frequency of up to 6 Hz. Thus, our stimulation protocol clearly differs from those used in previous studies, which limits the comparability of the activation maps.
Strengths of our study include the homogeneous patient population as well as the meticulous assessments of the actual movement produced during scanning including speed, accuracy and pacing, whereas the lack of additional TMS experiments or longitudinal fMRI measurements pose a limitation to our results. In addition, a semi-quantitative score of the perceived task difficulty was not obtained in the participants. While we used a parametric variation of movement frequency to detect differences between patients and controls, a recent study has indicated that the functional demand on the primary motor system is more likely related to movement quantity rather than movement frequency [Kim et al.,2005]. On the other hand, an enhanced recruitment of the contralesional motor cortex in chronic stroke patients has also been reported by other groups using complex motor tasks [Gerloff et al.,2006; Schaechter et al.,2008], supporting our principal findings. Finally, it should be stressed that our results were obtained in highly selected, well recovered stroke patients, so that they cannot be generalized to all stroke victims.
In conclusion, our results support the model of an enhanced bihemispheric recruitment of preexisting motor representations in patients after subcortical stroke, sustaining the notion that these regions are functionally integrated into the reorganized network subserving recovered hand function. Since all patients had an excellent motor recovery contralesional PMC and SMC activation appears to be efficient and resembles the widespread, bilateral activation observed in healthy participants performing complex movements. Moreover, functional connectivity in terms of a time series analysis demonstrated that the SMA significantly influences this activity during voluntary movements in the chronic stage of recovery. Finally, our fMRI results in exceptionally well recovered patients and the fMRI time series analysis in terms of functional connectivity are not in line with the hypothesis that the recruitment of these brain areas represents maladaptive plasticity. From an anatomical point of view, it is more likely that the enhanced activation patterns within the right hemisphere represents an upregulation of activity in a pre-existing motor network, rather than being the consequence of axonal sprouting.
Acknowledgements
The authors thank Mario Prosiegel for helpful comments.




