Nutritive value of forages consumed by ruminants during the dry season in the Western Highlands of Cameroon
Abstract
Background
In the Western Highlands of Cameroon (WHC), information on the nutritional value of fodder species consumed by ruminants is very limited.
Methods
Through interviews with farmers and monitoring of animals on the range, information was obtained on the types of fodder resources consumed by the ruminants. Samples of each forage species were collected in 15 districts, mixed, chopped, and dried in a ventilated oven at 60°C, and then ground for chemical composition analysis.
Results
Twenty-two forage species were identified. Among these species, Vernonia amygdalina (29.43% ± 0.45% dry matter [DM]) and Pennisetum clandestinum (87.21% ± 1.33% DM) were, respectively, the highest in protein and neutral detergent fiber contents. Manihot esculenta was one of the most energy-rich forages in terms of forage unit for lactation and forage unit for meat production. Hierarchical ascending classification revealed three main groups of forages, respectively, rich in crude fiber (Group 1), protein (Group 2), and energy (Group 3).
Conclusions
The present study identified 22 forage species browsed by ruminants in WHC. Further studies should be carried out to determine the antinutritional factors and to evaluate their nutrient value using in vitro or in situ digestibility techniques.
INTRODUCTION
In tropical areas, the nutritional value of fodder resources is a recurrent problem for livestock farmers (Awono et al., 2012). The production performance of livestock is strongly linked to the nutritional value of forages in the pastures (Klein et al., 2014). Lack of data on the inventory and nutritional value of forage species present in a locality is a hindrance to the planning and formulation of rangeland management plans (Suheel et al., 2015). In Cameroon, Lucha and Chuyong (2016) and Pahimi et al. (2020) carried out studies on the inventory of forage species in two agroecological zones. They identified 151 forage species in the Northwest region and 41 species in the North region. However, these studies were limited only to the inventory of fodder resources, without any attention to their nutritional value.
In recent decades, the number of livestock farmers increased in the Western Highlands of Cameroon (WHC), leading to an increase in forage demand, especially during the dry season, where forage shortage is crucial (Ministère de l'Elevage, des Pêches et des Industries Animales [MINEPIA], 2011). Improvement of the nutritional value of forage species in this area could be an asset to enhance meat and milk production in Cameroon. The general objective of the present study is to contribute to a better understanding of ruminant feeding in Cameroon through the design of a database on available forage resources with their nutritive values. More specifically, the aim was to identify and determine the nutritional value of forage species consumed by ruminants in the WHC.
MATERIALS AND METHODS
Description of the study area
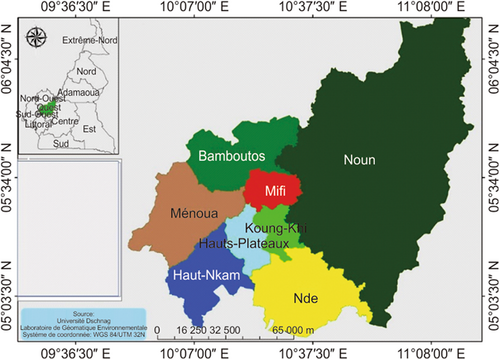
The WHC (Figure 1) is characterized by a rainfall of 1500–2000 mm with a 9-month rainy season and a 3-month dry season. The average annual temperature ranges from 22°C to 25°C. The soils are ferralitic, sandy-clay, generally not deep, and rejuvenated by erosion. Rich in humus, they are suitable for food and perennial crops.
Sampling
Five divisions in which ruminant breeders had a large herd were chosen, namely, Menoua, Bamboutos, Ndé, Mifi, and Noun. On the same basis, three districts were chosen per division. In these three districts, the snowball sampling or chain sampling method consisting of identifying new breeders from other breeders to form part of the sample was used. The latter were asked to provide information on the fodder species consumed by their livestock. Subsequently, with their permission, direct observation of the animals on the field was carried out to identify the forage species. Samples were collected from 15 districts at the flowering stage. Fresh samples were chopped, mixed and sun-dried for 6 days on the field. The dried samples were finely crushed and preserved in plastic bags to prevent the absorption of moisture for the evaluation of the chemical composition.
Forage species identification
Forage species were identified using the botanical guides “Graminée du Cameroun” (Van Der Zon, 1992) and “Guide des ligneux” (Geerling, 1982). The identification of fodder plants was based on their morphological characteristics, according to these guides.
Analysis of forage chemical composition
Analysis of forage chemical composition consisted of determining the moisture content by drying the different forages in an oven at 103°C for 12 h. Ash content was determined by incinerating samples in a muffle furnace at 500°C for 6 h. Crude protein (CP), ether extracts (EEs), and crude fiber (CF, 100% dry matter [DM]) were determined using the Kjeldahl, Soxlhet, and Weende methods, respectively (Association of Official Analytical Chemists [AOAC], 2000). Organic matter (OM) was determined from the difference between the percentages of DM and ash. Carbohydrate was calculated as the difference between the percentages of OM, CP, and EEs. The neutral detergent fiber (NDF) and acid detergent fiber (ADF) were determined using the method of Van Soest et al. (1991).
Nutritional value of forages
The nutritional value of each forage species identified (energy and protein value) was estimated from the chemical composition. This research used the French system for assessing forage nutritive value initially developed in the 1970s by the “Institut National de la Reserche Agronomique” (commonly known as INRA, and since 2020 renamed INRAE—National Research Institute for Agriculture, Food, and the Environment). The system has been refined over several decades and has been described by Colin-Schoellen et al. (2000). Effectively, the system rates the nutritive value of a feed compared to the feed value for ruminants of 1-kg barley, with separate calculations, depending on whether the feed is consumed by ruminants for milk or meat production, and considering a range of feed quality criteria including estimated dietary protein utilization.
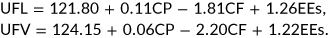 ()
()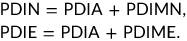 ()
() ()
()Statistical analysis
The identified forage species were subjected to hierarchical ascending classification (HAC). This method allows classification of individuals into groups with common characteristics in a dendrogram. Data on the chemical composition and nutritional value were subjected to a one-way analysis of variance (ANOVA) to compare forage species and their groups. Duncan's test at a 5% significance level was used for mean separation. The software SPSS 20.0 was used to perform ANOVA (SPSS, 2020), and HAC was performed using the XLSTAT 25.1 (XLSTAT, 2021) software package.
RESULTS
Chemical composition and nutritional value of forage species
In the WHC, 22 forage species belonging to 11 botanical families were identified: Poaceae (8), Fabaceae (5), Convolvulaceae (1), Myrtaceae (1), Malvaceae (1), Meliaceae (1), Euphorbiaceae (1), Asparagaceae (1), Asteraceae (1) Musaceae (1), and Lauraceae (1) (Table 1). Tables 2 and 3 summarize the chemical composition and nutritive values of the forages, respectively. The highest protein (29.43% ± 0.45% DM) and PDIN (18.48 ± 1.45 g kg−1 DM) contents were recorded in Vernonia amygdalina, while the highest NDF (87.21% ± 1.33% DM) and PDIE (109.03 ± 3.27 g kg−1 DM) contents were recorded in Pennisetum clandestinum and Manihot esculenta, respectively. DM, OM, and protein contents ranged from 84.61% to 92.23%, 71.77% DM to 86.77% DM, and 12.31% DM to 23.49% DM, respectively. Apart from Imperata cylindrica, Cynodon dactylon, Digitaria ciliaris, and Entandrophragma cylindricum, all the studied forages had UFL and UFV values greater than 0.70 UF kg−1 DM.
| Families | Species |
|---|---|
| Asparagaceae | Draceana fragrans |
| Asteraceae | Vernonia amygdalina |
| Convolvulaceae | Ipomoea batatas |
| Euphorbiaceae | Manihot esculenta |
| Fabaceae | Desmodium uncinatum |
| Fabaceae | Desmodium intortum |
| Fabaceae | Arachis glabrata |
| Fabaceae | Calliandra calothyrsus |
| Fabaceae | Centrosema pubescens |
| Lauraceae | Persea americana |
| Malvaceae | Sida acuta |
| Meliaceae | Entandrophragma cylindricum |
| Musaceae | Musa paradisiaca |
| Myrtaceae | Psidium guajava |
| Poaceae | Bracharia ruziziensis |
| Poaceae | Cynodon dactylon |
| Poaceae | Panicum maximum |
| Poaceae | Digitaria ciliaris |
| Poaceae | Pennisetum purpureum |
| Poaceae | Pennisetum clandestinum |
| Poaceae | Trypsacum laxum |
| Poaceae | Imperata cylindrica |
| Species | DM (%) | Chemical composition (% DM) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ash | OM | CH | EE | CP | CF | NDF | ADF | ||
| Brachiaria ruziziensis | 91.29 ± 1.21a | 11.48 ± 0.86b | 79.71 ± 1.75c | 63.46 ± 1.24b | 1.20 ± 0.07e | 15.07 ± 0.59e | 28.35 ± 0.41b | 63.02 ± 1.05c | 35.38 ± 0.75d |
| Panicum maximum | 90.94 ± 1.90a | 12.26 ± 1.72b | 78.85 ± 2.46c | 62.27 ± 2.34b | 2.90 ± 0.27d | 13.86 ± 0.67e | 24.90 ± 0.98c | 54.40 ± 0.12d | 29.06 ± 0.23f |
| Pennisetum purpureum | 91.59 ± 0.39a | 11.97 ± 1.37b | 79.72 ± 1.56c | 58.71 ± 1.41c | 7.35 ± 0.32a | 13.46 ± 0.62e | 29.90 ± 1.01b | 80.39 ± 1.23b | 44.22 ± 1.06b |
| Pennisetum clandestinum | 89.97 ± 0.54b | 10.07 ± 0.67c | 79.86 ± 1.16c | 60.82 ± 1.37b | 3.80 ± 0.25c | 15.24 ± 0.34e | 30.42 ± 0.60b | 87.21 ± 1.33a | 49.05 ± 2.11b |
| Trypsacum laxum | 90.80 ± 0.72b | 9.95 ± 1.43c | 81.04 ± 1.69b | 64.54 ± 0.64b | 4.01 ± 0.15bc | 12.31 ± 1.46f | 31.17 ± 1.19a | 71.25 ± 0.32b | 40.11 ± 0.06c |
| Cynodon dactylon | 89.80 ± 0.22b | 11.8 ± 0.59b | 78.04 ± 1.09c | 51.15 ± 0.40d | 2.70 ± 0.09d | 24.15 ± 1.15b | 25.87 ± 0.89c | 51.20 ± 0.62d | 37.15 ± 0.36d |
| Digitaria ciliaris | 89.26 ± 0.32b | 17.49 ± 0.74a | 71.77 ± 1.17d | 47.75 ± 0.45e | 1.8 ± 0.29e | 22.22 ± 0.95c | 27.07 ± 0.85b | 55.19 ± 0.22d | 31.19 ± 0.77e |
| Imperata cylindrica | 84.61 ± 1.12d | 7.94 ± 0.47d | 76.73 ± 0.17c | 56.16 ± 0.35d | 1.20 ± 0.20e | 19.36 ± 0.81d | 33.41 ± 0.25a | 53.20 ± 0.12d | 31.12 ± 0.46e |
| Desmodium uncinatum | 91.93 ± 0.92a | 7.55 ± 0.28d | 84.38 ± 1.20ab | 58.9 ± 1.37c | 3.32 ± 0.05c | 22.13 ± 0.36c | 16.47 ± 0.99d | 57.56 ± 2.01d | 32.04 ± 1.64e |
| Desmodium intortum | 91.82 ± 1.93a | 9.56 ± 1.20c | 82.61 ± 1.82b | 56.20 ± 1.57c | 4.99 ± 0.21b | 21.42 ± 0.27c | 22.56 ± 0.98c | 65.33 ± 0.12c | 39.26 ± 1.08c |
| Calliandra calothyrsus | 92.23 ± 0.60a | 5.63 ± 0.98e | 86.77 ± 1.03a | 63.34 ± 1.24b | 1.30 ± 0.11e | 22.10 ± 0.29c | 21.09 ± 0.68c | 23.37 ± 1.31f | 18.34 ± 0.05f |
| Centrosema pubescens | 88.27 ± 0.13c | 9.34 ± 1.25c | 78.93 ± 1.02c | 42.61 ± 1.27e | 8.5 ± 0.18a | 27.82 ± 0.31b | 18.36 ± 0.51d | 51.33 ± 0.22d | 40.06 ± 1.08c |
| Arachis glabrata | 90.4 ± 0.96b | 9.6 ± 1.57c | 80.8 ± 1.22b | 52.78 ± 1.67d | 3.00 ± 0.08d | 25.02 ± 0.28b | 23.31 ± 0.18c | 45.33 ± 0.52e | 29.23 ± 1.18e |
| Ipomoea batatas | 88.76 ± 1.35c | 12.19 ± 1.80b | 76.69 ± 0.96c | 49.41 ± 1.01de | 5.67 ± 0.34b | 21.80 ± 0.74c | 12.76 ± 0.94e | 80.08 ± 1.09b | 60.87 ± 2.01a |
| Sida acuta | 90.20 ± 1.13b | 9.78 ± 0.11c | 80.45 ± 1.18b | 52.14 ± 0.87d | 5.74 ± 0.10b | 22.55 ± 0.35c | 15.75 ± 0.21d | 30.12 ± 0.61f | 17.46 ± 0.30f |
| Manihot esculenta | 91.97 ± 1.64a | 9.16 ± 1.03c | 82.51 ± 1.79b | 54.69 ± 2.47d | 8.11 ± 0.06a | 20.15 ± 1.89d | 12.50 ± 0.47e | 83.33 ± 1.01a | 53.14 ± 1.19b |
| Entandrophragma cylindricum | 89.11 ± 0.62b | 7.97 ± 1.13d | 81.14 ± 1.01ab | 66.47 ± 1.97a | 1.20 ± 0.11e | 13.47 ± 1.21f | 29.10 ± 0.37b | 59.31 ± 0.11c | 38.99 ± 1.31d |
| Psidium guajava | 90.78 ± 0.25b | 5.72 ± 0.03e | 85.05 ± 1.79a | 67.46 ± 1.37a | 1.10 ± 0.10e | 16.49 ± 1.11e | 19.49 ± 0.54d | 68.31 ± 1.01b | 45.14 ± 0.19c |
| Draceana fragrans | 88.45 ± 0.15c | 11.48 ± 0.33b | 76.97 ± 1.53c | 45.71 ± 1.27e | 5.86 ± 0.18b | 25.4 ± 0.18b | 26.44 ± 0.64bc | 69.01 ± 0.01b | 35.18 ± 0.09d |
| Musa paradisiaca | 90.35 ± 0.16b | 10.97 ± 0.84b | 79.38 ± 1.11c | 54.58 ± 1.07d | 1.40 ± 0.42e | 23.39 ± 0.28c | 24.83 ± 0.16c | 68.19 ± 0.12b | 49.15 ± 0.25b |
| Vernonia amygdalina | 89.26 ± 0.15b | 12.06 ± 0.14b | 77.21 ± 1.23c | 42.23 ± 1.72e | 5.54 ± 0.23b | 29.43 ± 0.45a | 16.38 ± 0.36d | 58.19 ± 0.25c | 38.51 ± 0.29d |
| Persea americana | 91.26 ± 1.05a | 7.36 ± 0.61d | 83.87 ± 1.89ab | 67.06 ± 1.02a | 1.54 ± 0.77e | 15.31 ± 0.11e | 22.99 ± 0.86c | 63.10 ± 0.39b | 35.75 ± 0.62d |
- Note: Means followed by different letters within a column are significantly different (p < 0.05).
- Abbreviations: ADF, acid detergent fiber; CF, crude fiber; CH, carbohydrates; CP, crude protein; DM, dry matter; EE, ether extract; NDF, neutral detergent fiber; OM, organic matter.
| Species | Energy (UF kg−1 DM) | Estimated protein (g kg−1 DM) | ||
|---|---|---|---|---|
| UFL | UFV | PDIN | PDIE | |
| Arachis glabrata | 0.78 ± 0.61d | 0.86 ± 0.04c | 15.71 ± 1.31b | 51.94 ± 1.02f |
| Brachiaria ruziziensis | 0.74 ± 0.01d | 0.64 ± 0.01d | 9.46 ± 0.37d | 47.60 ± 2.04f |
| Calliandra calothyrsus | 0.88 ± 0.01c | 0.81 ± 0.01c | 13.88 ± 0.18c | 71.23 ± 3.76d |
| Centrosema pubescens | 0.95 ± 0.44b | 1.02 ± 0.16c | 17.41 ± 0.52a | 66.55 ± 1.33e |
| Cynodon dactylon | 0.46 ± 0.21f | 0.54 ± 0.19f | 12.96 ± 1.29c | 33.62 ± 1.45g |
| Desmodium intortum | 0.90 ± 0.02b | 0.82 ± 0.02c | 13.45 ± 0.17c | 62.91 ± 4.39e |
| Desmodium uncinatum | 0.99 ± 0.02b | 0.93 ± 0.02b | 13.89 ± 0.23c | 89.64 ± 5.07c |
| Digitaria ciliaris | 0.68 ± 0.17e | 0.77 ± 0.64d | 13.95 ± 1.17c | 36.95 ± 0.55g |
| Draceana fragrans | 0.74 ± 0.15d | 0.84 ± 0.13c | 15.95 ± 1.03b | 36.39 ± 0.81g |
| Entandrophragma cylindricum | 0.62 ± 0.28e | 0.72 ± 0.14c | 8.46 ± 1.45d | 48.30 ± 1.82f |
| Imperata cylindrica | 0.53 ± 0.24f | 0.64 ± 0.37d | 12.16 ± 0.35c | 18.38 ± 0.61h |
| Ipomoea batatas | 1.08 ± 0.02a | 1.04 ± 0.02a | 13.69 ± 0.46c | 97.58 ± 3.74b |
| Manihot esculenta | 1.12 ± 0.01a | 1.08 ± 0.01a | 12.65 ± 1.19c | 109.03 ± 3.27a |
| Musa paradisiaca | 0.72 ± 0.23d | 0.81 ± 0.05c | 14.69 ± 1.22b | 47.65 ± 0.84f |
| Panicum maximum | 0.82 ± 0.02b | 0.74 ± 0.02d | 8.70 ± 0.42d | 63.54 ± 5.12e |
| Pennisetum clandestinum | 0.73 ± 0.01d | 0.63 ± 0.01d | 9.57 ± 0.22d | 39.17 ± 2.61g |
| Pennisetum purpureum | 0.78 ± 0.02d | 0.68 ± 0.02d | 8.45 ± 0.39d | 44.03 ± 4.53f |
| Persea americana | 0.76 ± 0.25d | 0.83 ± 0.67c | 9.61 ± 1.69d | 73.38 ± 1.84bf |
| Psidium guajava | 0.83 ± 0.15b | 0.89 ± 0.23c | 10.36 ± 0.89d | 87.81 ± 1.17c |
| Sida acuta | 1.03 ± 0.10a | 0.98 ± 0.56b | 14.16 ± 0.22b | 88.39 ± 1.19c |
| Trypsacum laxum | 0.72 ± 0.02d | 0.61 ± 0.03d | 7.73 ± 0.92e | 41.12 ± 4.31f |
| Vernonia amygdalina | 0.96 ± 0.29b | 1.02 ± 0.35a | 18.48 ± 1.45a | 70.53 ± 1.88d |
- Note: Means followed by different letters within a column are significantly different (p < 0.05).
- Abbreviations: DM, dry matter; PDIE, protein digestible in the small intestine supplied by rumen-undegraded dietary protein + protein supplied by microbial protein from rumen-fermented organic matter; PDIN, protein digestible in the small intestine supplied by rumen-undegraded dietary protein + protein supplied by microbial protein from rumen-degraded dietary protein; UFL, forage unit for lactation; UFV, forage unit for meat production.
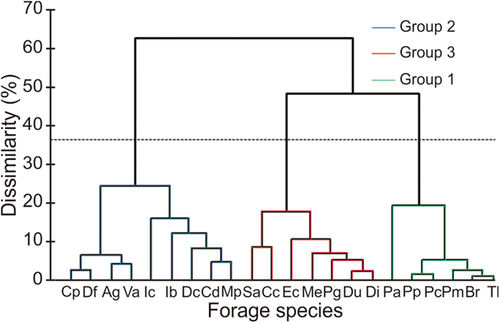
Classification of forage species
The dendrogram (Figure 2) grouped forage species so as to maximize dissimilarity between groups. On the horizontal axis, represented by a dotted line on the dendrogram, there are three groups: group 1 (G1), group 2 (G2), and group 3 (G3). Table 4 presents the description of the different groups of forages according to their number, species, and botanical family.
| Groups | Number | Species | Botanical family |
|---|---|---|---|
| G1 | 6 | Persea americana, Pennisetum purpureum, Pennisetum clandestinum, Panicum maximum, Bracharia ruziziensis, Trypsacum laxum | Lauraceae, Poaceae, Poaceae, Poaceae, Poaceae, Poaceae |
| G2 | 9 | Centrosema pubescens, Draceana fragrans, Arachis glabrata, Vernonia amygdalina, Imperata cylindrica, Ipomoea batatas, Digiatria ciliaris, Cynodon dactylon, Musa paradisiaca | Fabaceae, Asparagaceae, Fabaceae, Asteraceae, Poaceae, Convolvulaceae, Poaceae, Poaceae, Musaceae |
| G3 | 7 | Sida acuta, Calliandra calothyrsus, Entandrophragma cylindricum, Manihot esculenta, Psidium guajava, Desmodium uncinatum, Desmodium intortum | Malvaceae, Fabaceae, Meliaceae, Euphorbiaceae, Myrtaceae, Fabaceae, Fabaceae |
Results of hierarchical classification of forage species were obtained, identifying three groups based on a criterion of maximum dissimilarity between groups. Chemical composition and nutritional value data for the three groups are presented in Table 5.
| Item | Group 1 | Group 2 | Group 3 | p Value |
|---|---|---|---|---|
| Chemical composition | ||||
| DM | 90.97 ± 0.56b | 88.79 ± 1.74c | 94.14 ± 1.16a | 0.040 |
| Ash | 10.51 ± 1.82a | 11.43 ± 2.69a | 7.91 ± 2.72b | 0.016 |
| OM | 80.50 ± 1.78a | 77.59 ± 2.62b | 83.27 ± 2.24a | 0.001 |
| CH | 62.81 ± 2.71a | 49.15 ± 5.00c | 59.88 ± 5.97b | 0.001 |
| CP | 11.91 ± 5.19c | 24.28 ± 3.08a | 19.75 ± 3.46b | 0.001 |
| EE | 6.15 ± 3.58 | 3.96 ± 2.52 | 4.39 ± 3.47 | 0.445 |
| CF | 27.95 ± 3.29a | 23.15 ± 6.30b | 19.56 ± 5.42c | 0.037 |
| NDF | 69.89 ± 12.20 | 59.08 ± 11.09 | 55.33 ± 21.33 | 0.236 |
| ADF | 38.92 ± 7.09 | 39.16 ± 10.11 | 34.91 ± 13.29 | 0.696 |
| Nutritional value | ||||
| UFL | 0.75 ± 0.03b | 0.71 ± 0.16c | 0.97 ± 0.10a | 0.001 |
| UFV | 0.68 ± 0.08c | 0.80 ± 0.15b | 0.93 ± 0.10a | 0.008 |
| PDIN | 8.92 ± 0.75c | 14.41 ± 3.01a | 13.15 ± 1.32b | 0.001 |
| PDIE | 51.47 ± 13.81b | 45.59 ± 18.81c | 86.65 ± 15.46a | 0.001 |
- Note: Means followed by a different letter within a row are significantly different (p < 0.05).
- Abbreviations: ADF, acid detergent fiber; CF, crude fiber; CH, carbohydrates; CP, crude protein; DM, dry matter; EE, ether extract; NDF, neutral detergent fiber; OM, organic matter; PDIE, protein digestible in the small intestine supplied by rumen-undegraded dietary protein + protein supplied by microbial protein from rumen-fermented organic matter; PDIN, protein digestible in the small intestine supplied by rumen-undegraded dietary protein + protein supplied by microbial protein from rumen-degraded dietary protein; UFL, forage unit for lactation; UFV, forage unit for meat production.
Chemical composition and nutritional value of forage species groups
Table 5 presents an overview of the chemical and nutritional value of the different groups of forages. Forages in G2 are richer in protein and PDIN, but poor in PDIE (p < 0.05) compared to forages in G1 and G3. Forages in G3 had significantly higher (p < 0.05) UFL, UFV, and PDIE compared to forages in G1 and G2. Forages in G1 are, respectively, richest and poorest in CF and CP as compared to G2 and G3.
DISCUSSION
A total of 22 forage species were identified by herd owners in the WHC. This number is very much lower than the 151 and 41 forage species recorded, respectively, by Lucha and Chuyong (2016) and Pahimi et al. (2020) in the Northwest and North regions of the same country. This may be because most of the land in WHC is used for agriculture and the space reserved for livestock is made up of marginal land with very low fertility, such as the sides of mountains and the edges of roads. The forages identified were classified into groups rich in CG (G1), in protein (G2), and with high UFL (G3). The highest DM content (91.93% ± 0.92%) was recorded in D. intortum, while the lowest content was recorded in I. cylindrica (84.61% ± 1.12%). In the Doucen region of Algeria, Djennane (2016) reported DM content ranging between a maximum value of 97.89% in Plantago notata and a minimum value of 18.82% in Maresia nana. According to Vélez-Terranova et al. (2022), the DM content of forages is higher in the dry season due to the increase in the rate of evapotranspiration in the plants, thus reducing the availability of water in the leaves. In the present study, the highest content of ash (17.49%) was recorded in D. ciliaris. This result is close to the 19.86% of ash recorded in Astragalus armatus by Zirmi-Zembri and Kadi (2016) in Algeria. Since the plant can take up minerals from the soil, its ash content depends strongly on the mineralogical composition of the soil (Vélez-Terranova et al., 2022). The highest protein content (29.43% ± 0.45% DM) was recorded in V. amygdalina. Oboh (2006) stated that the leaves of V. amygdalina are rich in CP (33.3%); however, these authors warned that tannin (0.6%) and cyanide (1.1 mg kg−1) contents of this species are likely to cause toxicity symptoms, including hemolysis of erythrocytes in mammals. The maximum and minimum CF contents were recorded, respectively, in I. cylindrica (33.41% ± 0.25% DM) and M. esculenta (12.50% ± 0.47% DM). In Algeria, Djennane (2016) recorded the highest content of fiber in Launanen residifolia (47.09% DM) and the lowest in Centaurea dimerphategia (7.27% DM). The NDF values ranged from a maximum of 87.21 ± 1.33% DM in P. clandestinum to a minimum of 23.37% ± 1.31% DM in C. calothyrsus. Elsewhere, Shah et al. (2019) recorded the maximum and minimum NDF contents, respectively, in Thysanolaena maxima (79.22% DM) and Ficus semicordata (53.21% DM) in India. In the present study, the fiber content in P. clandestinum was observed to be 87.21% ± 1.33% DM, compared with 60.86% reported by Camacho-Ospina et al. (2021) for the same species in the municipality of San Miguel de Sema, Columbia. This difference may be explained by the variation in the soil composition and the physiological stage of the plants sampled. HAC revealed three main groups of forages, essentially rich in CG (G1), protein (G2), and energy (G3). G1 consisted mainly of plants belonging to the Poaceae family that are known to be generally rich in fiber (Klein et al., 2014). The CP content of studied forages in the WHC varied from 12.31% to 23.49% DM. The present result differs from the findings of Zirmi-Zembri and Kadi (2016) and Badourou et al. (2021), who, respectively, recorded values ranging from 0.70% to 28.60% DM and 5.64% to 13.13% DM for the forage species in Algeria and Benin. The protein content varied from one species to another and this can depend on the nitrogen content of the soil. Indeed, nitrogen fertilization increases the forage protein content and the quantity of biomass produced by forage plants (Tendonkeng et al., 2011).
V. amygdalina and M. esculenta were found to be very rich in PDIN and PDIE. This finding could be explained by their high levels of protein and energy, respectively. According to Baumont et al. (2009), there is a positive correlation between protein content and PDIN, and energy content and PDIE. Zirmi-Zembri and Kadi (2016) identified Calycotum spinosa as forage providing the most digestible protein (PDIN and PDIE > 200 g kg−1 DM) to ruminants. M. esculenta was a forage with very high energy content as measured by UFV (1.12 ± 0.01 UF kg−1 DM) and UFL (1.08 ± 0.01 UF kg−1 DM) in the WHC, while Zirmi-Zembri and Kadi (2016) reported Haloxylon scopariumas the forage with the highest UFL (1.5 UF kg−1 DM) and UFV (1.6 UF kg−1 DM) in Algeria. This difference can be explained by the fact that the nutritive value of forage varies from one species to another and soil factors vary from one area to another (Klein et al., 2014). According to Bouazza et al. (2012), forage is of good quality if it has CP > 10% DM and NDF < 30% DM, and of poor quality if it contains CP < 10% and NDF > 50%. A forage is considered a good energy source if it has UFL ≥ 0.7 and UFV ≥ 0.6. C. calothyrsus (CP = 22.10% ± 0.29% DM; NDF = 23.37% ± 0.12% DM) could then be considered as good-quality forage because of its high protein and low fiber contents. Forages with a high NDF (NDF > 50%) content are less digestible by the animals. In the present study, the majority of forages had more than 50% NDF. Values for NDF in this study could be expected to be comparatively high because samples were collected at the flowering stage and the fiber content increases with the physiological progression of the plant into reproductive development (Klein et al., 2014).
To improve the production performance of ruminants, it is essential to provide forages rich in nutrients necessary for the proper functioning of the rumen microorganisms. Forages from G1, G2, and G3 can also be assessed according to their potential to provide rumen microorganisms with the nutrients that they need. The energy and protein values of forages from G2 and G3 indicated that they are suitable to stimulate the proper functioning of rumen microorganisms. G1 forages, which have high fiber content, could limit ruminal digestion. In similar research, Sidi et al. (2016) recorded three groups of woody plants rich in fiber, energy, and protein in the community rangelands of Northeast Benin.
CONCLUSIONS
Twenty-two forage species palatable to ruminants in the WHC were characterized through their chemical composition and nutritional value. They were classified into groups of forages rich in CF (G1), protein (G2), and energy (G3). Further studies should be carried out to determine the antinutritional factors present and the in vitro or in situ digestibility of these forages.
AUTHOR CONTRIBUTIONS
Leslie Tieubou Tsopgni: Formal analysis; investigation; methodology; writing—original draft. Jules Lemoufouet: Methodology; supervision. Felix Meutchieye: Supervision. Langston Wilfried Edie Nounamo: Formal analysis; investigation. Camile Nyembo Kondo: Investigation. Jean Raphaël Kana: Conceptualization; methodology; supervision; validation. Mama Mouchili: Formal analysis; supervision. Back Armel Feudjio: Investigation.
ACKNOWLEDGMENTS
The authors would like to acknowledge the staff of the Animal Production and Nutrition Research Unit from the University of Dschang for their assistance during the chemical composition analysis and the processing of data.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data generated or analyzed during this study are available from the corresponding author upon reasonable request.




