Elevated and atmospheric-level methane consumption by soil methanotrophs of three grasslands in China
Abstract
Background
Methane (CH4) oxidation driven by soil aerobic methanotrophs demonstrates the capacity of grassland as a CH4 sink.
Methods
In this study, we compared the oxidation characteristics of atmospheric-level and elevated concentration (10%) CH4 in a typical grassland (steppe) on the Loess Plateau, an alpine meadow (meadow) on the Qinghai-Tibet Plateau, and an inland arid-area artificial grassland (pasture) in northwest China and investigated the communities of active methanotrophs and their contribution to CH4 oxidation using DNA-based stable-isotope probing and Illumina Miseq sequencing.
Results
The results showed that the oxidation of atmospheric CH4 only occurred in steppe and meadow soils where the USCγ group of methanotrophs was numerically dominant in the methanotroph community. Pasture soils, with their very low relative abundance of USCγ, did not show atmospheric CH4 oxidation. However, a DNA-stable isotope probing experiment with 10% CH4 indicated that conventional CH4 oxidizers (Methylocaldum and Methylocystis) rather than USCγ communities assimilated significant amounts of 13CH4 for growth.
Conclusions
The CH4 oxidation mechanisms in the three experimental grassland soils varied significantly. The USCγ group may be obligate oligotrophic microorganisms or their growth requires specific unknown conditions.
INTRODUCTION
Methane (CH4) is an important greenhouse gas that induces global warming by absorbing Earth's longwave radiation (Khalil & Saberi, 1987). Its contribution to contemporary global warming is second to that of carbon dioxide. The global average concentration of CH4 in the atmosphere has reached 1.920 μL L−1 (Lan et al., 2023), which is 267% greater than the preindustrial revolution level of 0.720 μL L−1 (Dlugokencky et al., 2011). In upland soils, consumption of CH4 by CH4-oxidizing bacteria (methanotrophs) can reach ~ 30 Tg year−1 (Wuebbles & Hayhoe, 2002). With an uptake rate of 4.3 Tg CH4 year−1, grassland soils under well-aerated conditions are recognized as one of the major sinks of global soil CH4 (Dutaur & Verchot, 2007). In China, grassland is the second largest sink of CH4, consuming 0.65 Tg CH4 year−1, after forest, which consumes 0.68 Tg CH4 year−1 (Y. Wang et al., 2014). Therefore, grassland CH4 absorption is of great significance for balance of the atmospheric CH4 concentration.
As early as the beginning of the 20th century, the first strain of methane-oxidizing bacteria (methanotrophs) was isolated and cultured in the laboratory (Hutton & Zobell, 1949), but it was not until the early 1970s that methane-oxidizing microorganisms began to be scientifically named and analyzed (Whittenbury et al., 1970). According to their morphology, metabolic pathway, membrane structure, and major phosphatidic acid components, aerobic methanotrophs are classed as type I (γ-Proteobacteria) and type II (α-Proteobacteria) (Hanson & Hanson, 1996). A review by McDonald et al. (2008) reported that Methylobacter, Methylomicrobium, Methylomonas, Methylocaldum, Methylosphaera, Methylothermus, Methylosarcina, Methylohalobius, Methylosoma, and Methylococcus genera belong to type I and Methylocystis, Methylosinus, Methylocella, and Methylocapa belong to type II. Type I methanotrophs metabolize CH4 via the ribulose phosphate metabolic pathway and via the lower level of the serine cycle metabolic pathway, while type II methanotrophs metabolize CH4 via the serine cycle only (Trotsenko & Murrell, 2008). In recent years, continuing advances in molecular sequencing technology have enabled methanotroph research of a wider range of habitats, to the point of the discovery of Verrucomicrobia methanotrophs in extreme thermophilic and acidophilic environments (Dunfield et al., 2007; Islam et al., 2008; Pol et al., 2007). It has been found that all culturable aerobic methanotrophs have a low affinity for methane, so they usually populate habitats with elevated methane concentrations (up to 10 000 μL L−1), such as lakes and paddy fields (Costello & Lidstrom, 1999; Keller et al., 2013).
The phenomenon of oxidation of atmospheric CH4 by methanotrophs has been found since 1982 (Harriss et al., 1982), but until 2003, they were named the upland soil cluster (USC), with subgroups USCα and USCγ (Knief et al., 2003). Subsequent studies have found that these novel methanotrophs exist in different types of well-aerated soils (Deng et al., 2019; Henckel et al., 2000; Kou et al., 2020). Methanotrophs solely dependent on atmospheric CH4, however, have resisted culture until very recently. Tveit et al. (2019) reported the isolation and characterization of a methanotroph that can grow in the atmosphere and utilize CH4 at its atmospheric concentration as a carbon and energy source. This methanotroph was found to be closely related to USCα and named Methylocapsa gorgona MG08. Tveit et al. (2019) reported that M. gorgona MG08 has typical facultative physiological and metabolic characteristics and is capable of oxidizing not only atmospheric CH4 but also elevated CH4. Other studies have shown that some specific conventional methanotrophs in paddy soils have the potential to oxidize low-concentration CH4 by oxidizing elevated CH4 initially (Cai et al., 2016; Hao et al., 2019). Similar studies have not been reported in grassland soils.
Grassland ecosystems are the most important methane sinks besides forests. Previous studies on CH4 oxidation fluxes in grassland soils have mostly focused on environmental or agronomic factors, such as soil moisture (Dijkstra et al., 2011; Liu et al., 2021; Qi et al., 2022), temperature (Gu et al., 2019; Odriozola et al., 2014; van den Pol-van Dasselaar et al., 1998), nitrogen fertilizer application (Blankinship et al., 2010; Dai et al., 2013; Nanba & King, 2000), and grazing intensity (Abell et al., 2009; van den Pol-van Dasselaar et al., 1999; X. Zhou et al., 2010). Although methanotrophs play a major role in the oxidation process, studies on them have been limited to reporting the presence of USCγ and conventional type I and type II methanotrophs in the soil of typical grasslands in Inner Mongolia (Kou et al., 2020; Ma et al., 2016), alpine meadows on the Tibetan Plateau (Deng et al., 2019; Kou et al., 2020), and typical grasslands on the Loess Plateau (Y. Wang et al., 2022). Little is known about active methanotrophs responsible for the oxidation of CH4 in grassland soils. Therefore, the aim of this study was to explore the mechanism of CH4 oxidation in grassland soils.
In this study, incubation experiments in the laboratory were used to study the response of three grassland soils to atmospheric and elevated CH4. At the same time, soil DNA-stable-isotope probing (SIP) microenvironments and high-throughput sequencing were used to study the community of methanotrophs responsible for atmospheric CH4 oxidation. We hypothesized that the three different grasslands would have varied response mechanisms to atmospheric and elevated concentrations of CH4.
MATERIALS AND METHODS
Soil sampling
The sample areas were all located in Gansu Province, China, including two natural grasslands (alpine meadow and typical steppe) and one artificially cultivated grassland (typical pasture). The natural grasslands were located in Huanxian County of Qingyang City and Maqu County of Gannan Tibetan Autonomous Prefecture, respectively. Huanxian County belongs to the hill and gully area of the Loess Plateau, which has a temperate continental monsoon climate. More than 90% of the whole territory is covered by loess, and the thickness of the soil layer is between 60 and 240 m. Maqu County is at the eastern end of the Qinghai-Tibet Plateau, at the junction of Gansu, Qinghai, and Sichuan provinces. The plateau is a continental alpine humid area with low temperatures and strong winds and rain or snow, and without four seasons (only a cold and warm season). The artificial grassland was located in Linze County of Zhangye City, which has a continental desert steppe climate. The climate is dry, rainfall is low, evaporation is high, it is windy, and the climate is characterized by four distinct seasons. The three grassland soils at Huanxian, Maqu, and Linze are hereafter referred to as steppe, meadow, and pasture, respectively. In each grassland, soil samples were collected from three areas (50 m × 50 m). Ten soil cores to a depth of 10 cm were taken from each area using a soil corer (10 cm diameter) and bulked. Plant residues, stones, and other inert materials in the soil samples were removed and passed through a 2 mm sieve. A subsample was stored at −20°C for DNA extraction, and the rest of the sample was stored at 4°C for 1 week for chemical analysis and soil incubation. The physical and chemical properties of soil were determined as described by Y. Wang et al. (2022). The geographical, climatic, and soil characteristics of the three grasslands are shown in Table 1.
| Item | Steppe | Meadow | Pasture |
|---|---|---|---|
| Ecoregion | Semi-arid Loess Plateau | Alpine meadow Qinghai-Tibet Plateau | Northwest inland arid region |
| Sampling location | Huanxian County | Maqu County | Linze County |
| 37°07′ N, 106°49′ E | 34°58′ N, 101°53′ E | 39°11′ N, 100°06′ E | |
| Soil type | Yellow soil | Subalpine meadow soil | Salinized meadow soil |
| Average annual rainfall (mm) | 359.3 | 620 | 112.9 |
| Altitude (m) | 1650 | 3650 | 1400 |
| Annual average temperature (°C) | 7.1 | 1.2 | 7.6 |
| Dominant plant species | Stipa bungeana; Artemisia capillaris; Lespedeza davurica | Carex kansuensis; Festuca ovina; Poa poophagorum; Elymus nutans | Medicago sativa; Festuca elata |
| Soil moisture (%) | 5.41 ± 0.4 | 17.41 ± 0.9 | 11.71 ± 0.8 |
| pH | 8.59 ± 0.1 | 6.33 ± 0.2 | 7.84 ± 0.1 |
| Total nitrogen (g kg−1) | 0.65 ± 0.1 | 5.66 ± 0.04 | 1.44 ± 0.1 |
| Total carbon (g kg−1) | 15.10 ± 0.4 | 65.93 ± 2.3 | 28.23 ± 1.5 |
| NO3−-N (mg kg−1) | 1.36 ± 0.01 | 0.62 ± 0.01 | 0.79 ± 0.01 |
| NH4+-N (mg kg−1) | 0.23 ± 0.03 | 12.87 ± 0.3 | 0.18 ± 0.01 |
Soil incubation with atmospheric and elevated CH4
Atmospheric and elevated CH4 oxidation capacities of three grassland soils were determined by a soil incubation study. Fresh soil of each grassland (7 g, with 60% water-holding capacity) was placed in three replicate 120 mL glass serum vials and sealed with black butyl stoppers. There were two treatments: ambient air with 2.4 μL L−1 CH4 and elevated CH4 (10% 12CH4 [99.9% 12C] or 13CH4 [99 atom% 13C; Sigma-Aldrich Co.]) with 20% O2 and 70% Ar. Sealed bottles were incubated at 28°C in the dark for 50 days. The aim of the 50 day incubation was to provide sufficient time for oxidation to occur. The CH4 concentrations at the beginning (D0) and end (D50) were detected by gas chromatography (Agilent 7890A). The soil 13C-atom isotopic percentages were measured using an isotope ratio mass spectrometer (Thermo Fisher Scientific Inc.) after vacuum freeze-drying at −50°C and grinding to pass through a 0.15 mm sieve.
Genomic DNA extraction, DNA-SIP, and quantitative polymerase chain reaction (qPCR)
The total genomic DNA of all the soil samples (D0 and D50) was extracted from 0.5 g of soil using a FastDNA® SPIN kit for soil (MP Biomedicals) according to the manufacturer's instructions. The extracted DNA was dissolved in 100 µL of deionized sterile water, and its concentration and quality were determined using a UV spectrophotometer (NanoDrop Technologies). The concentration of the DNA ranged from 30 to 120 ng µL−1 with A260/A280 ranging from 1.70 to 1.90. The DNA extracted from the soils incubated for 50 days was subjected to ultrahigh-speed density gradient centrifugation as described by Xia et al. (2011). Nucleic acids were separated from the CsCl solution by precipitation using 700 μL of polyethylene glycol 6000, purified by washing with 70% ethanol, and then dissolved in 30 µL of TE buffer, stored at −20°C for subsequent 13C-DNA identification through qPCR of the pmoA gene. A qPCR assay was performed targeting the pmoA gene using the primer pairs A189F and mb661r (Costello & Lidstrom, 1999) using the CFX96 Optical Real-Time Detection System (Bio-Rad Laboratories Inc.). The qPCR reaction mixture (total volume, 20 μL) contained 10 μL of SYBR® Premix Ex Taq (TaKaRa Biotech), 0.5 μL of each primer (10 μM), 1.0 μL of DNA template (DNA concentration: 10–20 ng μL−1), and 8 μL of DNase/RNase-free water. The qPCR thermal profile consisted of an initial denaturation step at 95°C for 2 min, followed by 39 cycles of 95°C for 10 s, 55°C for 30 s, and 72°C for 30 s, followed by plate readings at 80°C. The real-time qPCR standards were obtained using a 102–108 gene copies µL−1 dilution series of plasmids containing pmoA gene fragments. Plasmid DNA, used for calibration, was prepared using the pEASYT1 Cloning Kit (Beijing TransGen Biotech Co., Ltd.) and the TaKaRa MiniBEST Plasmid Purification Kit Ver. 4.0 (TaKaRa Bio Inc.) containing a cloned target gene according to the manufacturer's instructions. The amplification efficiencies for the primer pairs were between 83.6% and 98.2%, with R2 values of 0.990–0.999.
High-throughput sequencing of 16S ribosomal RNA and pmoA genes
The 16S ribosomal RNA (rRNA) and pmoA genes were amplified from the DNA extracts using the universal bacterial primer pair 515F/907R (Stubner, 2002) and A189f/mb661r (Costello & Lidstrom, 1999), respectively, by Illumina MiSeq sequencing. The PCR components for V4–V5 regions of 16S rRNA genes with a 12 base pair (bp) sample specific adaptor sequence were attached to the 5′ end of the 515F primer, and pmoA genes with a 6 base pair (bp) sample specific adaptor sequence were attached to the 5′ end of the A189f primer. The PCR of 16S rRNA and the pmoA gene was performed in a 50 μL mixture containing 25 μL of TaKaRa Premix Ex Taq (TaKaRa Biotech), 1 μL of each primer (10 μM), 2.0 μL of DNA template, and 21 μL of DNase/RNase-free water. The PCR thermal profile for amplifying the 16S rRNA gene consisted of an initial denaturation step at 95°C for 3 min, followed by 30 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 45 s. The PCR thermal profile for amplifying the pmoA gene consisted of an initial denaturation step at 95°C for 3 min, followed by 39 cycles of 95°C for 3 min, 55°C for 30 s, and 72°C for 30 s. In both thermal profiles, the final elongation step was at 72°C for 5 min. The 16S rRNA and pmoA gene amplicons were verified on 1.2 g 100 mL−1 agarose gels stained with Goldview to confirm amplicon size and specificity (single band), purified using a Mini BEST DNA Fragment Purification Kit, version 3.0 (TaKaRa Biotech), quantified using a UV spectrophotometer (NanoDrop Technologies), and mixed at an equimolar ratio. The amplicon library was constructed using TruSeq Nano DNA LT Sample Prep Kit Set A (24 samples) and then sequenced using the Illumina MiSeq system (Illumina) with the Reagent Kit v2 2 × 250 bp for the 16S rRNA gene and the Reagent Kit v2 2 × 300 bp for the pmoA gene.
Raw FASTQ data of 16S rRNA and pmoA genes were processed using mothur software (version 1.41.0) (Schloss et al., 2009) to merge the pair-end reads, sample splitting, and preliminary quality control. Reads with 370–380 bp length were selected for the 16S rRNA gene and 470–471 bp for the pmoA gene. The command “chimera.vsearch” was used to check and remove the chimeras in the sequences. Then, the “classify.seqs” command was used for the taxonomic classification of the high-quality sequences (total 1 195 626 for the 16S rRNA gene and 293 595 for the pmoA gene) with a cutoff of 70% using the parameter settings of the “Wang” method (Q. Wang et al., 2007). Detailed sequencing processing is described in Cai et al. (2020). A phylogenetic tree was constructed with MEGA7.0 software (Molecular Evolutionary Genetics Analysis, version 7.0) using the neighbor-joining method (Kumar et al., 2016).
Statistical analysis
The physical and chemical properties of soil were presented as the mean ± standard error of triplicate values of each grassland. Excel 365 was used for data sorting, and Origin 2021 was used for drawing. The statistical analysis of the abundance of the pmoA gene and the relative abundance of 16S rRNA methanotroph-like sequences and pmoA gene was conducted using one way analysis of variance using IBM SPSS Statistics 20.0 (SPSS Inc.). Mean comparisons were carried out using Duncan's post hoc test. A p < 0.05 was considered to be statistically significant.
RESULTS
Atmospheric and elevated concentration CH4 oxidation potential and methanotroph abundance
There were significant differences in the potential for oxidation of atmospheric CH4 in steppe, meadow, and pasture soils, with oxidation in the steppe and meadow soils but not in the pasture soil. At the start of the soil incubation, the concentration of CH4 in the headspace of all bottles was approximately 2.4 ± 0.01 μL L−1 (Figure 1a). After the incubation of soil for 50 days, the concentration in the headspace of bottles with meadow and steppe soils oxidation reduced values to 0.2 μL L−1, while the concentration of that with pasture soil remained the same as the initial CH4 concentration, at about 2.54 ± 0.01 μL L−1.
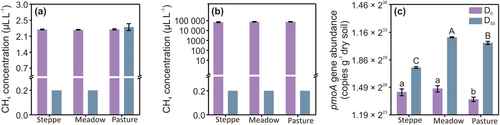
All three soils were able to oxidize the elevated concentration of CH4. At the start of the soil incubation, the CH4 concentrations in the soils from steppe, meadow, and pasture were 69 949, 76 679, and 76 816 μL L−1, respectively. After 50 days, the CH4 concentration decreased to 0.2 μL L−1 (Figure 1b).
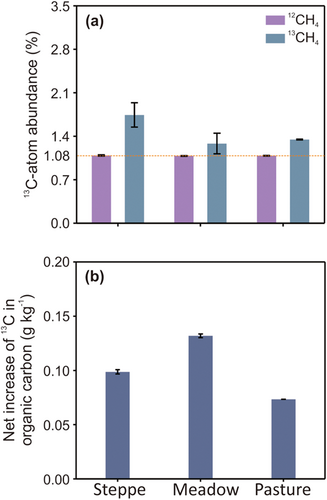
The 13C abundance of organic carbon for all three soils at D0 was 1.08% ± 0.006%. Oxidation of 13CH4 resulted in values of the 13C abundance of organic carbon in the steppe, meadow, and pasture soils increasing to 1.7% ± 0.2%, 1.3% ± 0.2%, and 1.4% ± 0.01%, respectively (Figure 2a). The net increase in the organic 13C content in three grassland soils also changed (Figure 2b). In agreement with this, qPCR analysis indicated that the abundance of the pmoA gene increased significantly by D50, reaching 5.3 × 108, 6.6 × 109, and 4.15 × 109 copies g−1 dry soil, and showing increases of 7.72, 72.47, and 112.14 times the values at D0 of 7.0 × 107, 9.0 × 107, and 4.0 × 107 copies g−1 dry soil in the steppe, meadow, and pasture soils, respectively (Figure 1c).
Methanotroph composition changes during the incubation with elevated concentration of CH4
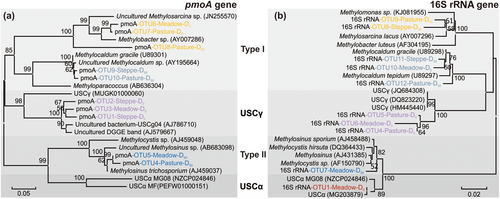
The operational taxonomic units identified from the pmoA gene sequences (Figure 3a) and methanotroph-like 16S rRNA sequences (Figure 3b) resulted in a similar phylogenetic tree topology. It was found that the soil mainly included methanotrophs closely related to type I (Methylocaldum, Methylosarcina, and Methylobacter) and type II (Methylocystis and Methylosinus), as well as USCα and USCγ.
The results of the pmoA gene sequences showed that, in steppe soil (D0), the relative abundance of USCγ (USCγ + JR3) was 95.9% ± 0.4%. That of Methylocaldum (type I) was 2.2% ± 0.2%. After 50 days, the relative abundance of Methylocaldum increased significantly (p < 0.01) to 92.4% ± 1.9%. In contrast, at D50, the relative abundance of USCγ decreased (p < 0.01) to 1.4% ± 0.5%. There were no statistically significant differences in the relative abundances of other methanotrophs. In meadow soil, USCγ decreased from 44.7% ± 6.1% (D0) to 0.5% ± 0.01% (D50), while Methylocystis increased significantly from 30.6% ± 3.1% (D0) to 97.4% ± 0.2% (D50). In pasture soil, USCγ and Methylocystis decreased from 4.7% ± 1.2% and 24.9% ± 0.9% at D0 to 0.4% ± 0.06% and 8.4% ± 4.0% at D50, respectively, while Methylobacter and Methylosarcina increased sharply from 0.8% ± 0.1% and 1.7% ± 0.2% at D0 to 5.9% ± 0.2% and 16.8% ± 5.9% at D50, respectively. Methylocaldum (type I) also increased (D50) but this was not statistically significant (p < 0.01) (Figure 4a,c).
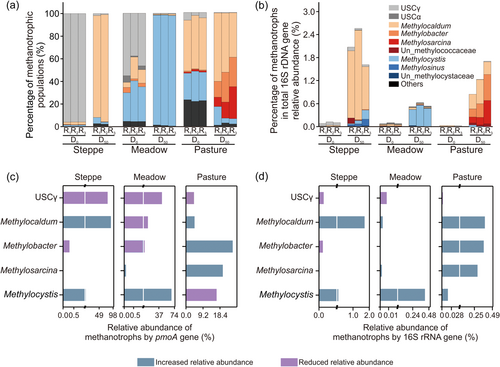
Sequencing of the 16S rRNA gene yielded essentially the same results as that of pmoA. In the steppe, Methylocaldum increased significantly from 0.022% ± 0.001% at D0 to 0.7% ± 0.3% at D50 (p < 0.01); the proportion of USCγ at D0 was 0.076% ± 0.001% and at D50, it was 0.063% ± 0.017%, and so there was no change. In the meadow, Methylocystis increased significantly from 0.0135% ± 0.0001% to 0.44% ± 0.03% (D50). In pasture, Methylocaldum (0.0162% ± 0.0038%), Methylosarcina (0.0018% ± 0.0018%), and Methylobacter (0.0036% ± 0.0036%) increased significantly to 0.41% ± 0.08%, 0.29% ± 0.13%, and 0.38% ± 0.13%, respectively (p < 0.05) at D50 (Figure 4b,d).
Detection of active methanotrophs during the incubation with elevated concentration of CH4
The 12CH4- and 13CH4-labeled methanotroph communities examined by the qPCR of pmoA genes and the methanotroph-like 16S rRNA genes in the MiSeq sequencing analysis of DNA-SIP buoyant density gradient fractions are shown in Figure 5a–c for the steppe, meadow, and pasture soils, respectively. The qPCR data for pmoA genes show that 13C-DNA and 12C-DNA had been separated, and the maximum copy number of the 13C-DNA-labeled pmoA gene appeared at a buoyant density gradient of 1.730–1.745 g mL−1, and the pmoA gene abundance was 7.67 × 107, 6.23 × 108, and 4.19 × 108 copies g−1 dry soil for steppe, meadow, and pasture soils, respectively. The maximum copy number of the 12C-DNA-labeled pmoA gene appeared at 1.715–1.725 g mL−1 of the buoyant density gradient, and the corresponding pmoA gene abundance was 5.01 × 109, 2.84 × 107, and 8.23 × 108 copies g−1 dry soil for steppe, meadow, and pasture soils, respectively.
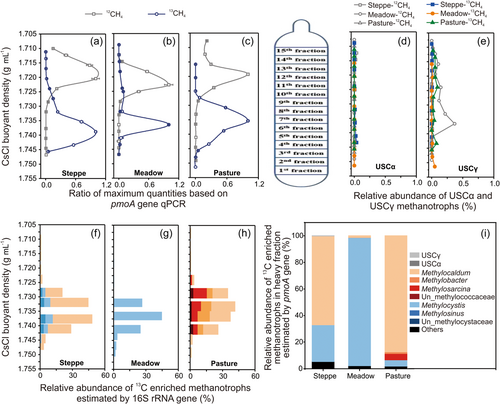
The high-throughput sequencing of the methanotroph-like 16S rRNA and pmoA genes in the total DNA of the steppe, meadow, and pasture soils after incubation (D50) and the different DNA-SIP buoyant density gradient fractions obtained from the steppe, meadow, and pasture soil DNA showed that 13CH4 was mainly assimilated by conventional type I and type II methanotrophs (Figure 5f–h). In the steppe, Methylocaldum of type I and Methylocystis of type II were labeled and accounted for 35.9% and 7.0% of the total 16S rRNA genes, respectively (Figure 5f). The rest of type I and type II methanotrophs accounted for only 0.6% and 4.8%, respectively (Figure 5f and Table 2). In the meadow, the main methanotroph was Methylocystis, which accounted for 44.1% of the total 16S rRNA genes (Figure 5g), with other methanotrophs accounting for only 1.3% (Figure 5g and Table 2). In the pasture, the proportion of labeled Methylocaldum, Methylosarcina, and Methylobacter accounted for 21.5%, 9.2%, and 8.6%, respectively, of the total 16S rRNA sequences in the heavy buoyant density gradient fraction (Figure 5h) and type I was the dominant methanotroph type, with type II accounting for only 2.1% (Figure 5h and Table 2). The sequencing of pmoA gene also confirmed the above result (Figure 5i and Table 3). It is worth noting that, in the three grasslands, USCγ was dominant in the steppe and meadows D0 soils (Figure 4a,b). However, the proportion of USC-like 16S rRNA sequences in the heavy buoyant density gradient fractions was very low (Table 2) in both soils (Figure 5d,e).
| Methanotrophs | Relative abundance in total 16S RNA gene reads | Relative abundance in total-MOB-like 16S rRNA gene reads | ||||
|---|---|---|---|---|---|---|
| Steppe (%) | Meadow (%) | Pasture (%) | Steppe (%) | Meadow (%) | Pasture (%) | |
| USCα | 0 | 0 | 0 | 0 | 0 | 0 |
| USCγ | 0.041 | 0.015 | 0 | 0.000 86 | 0.000 33 | 0 |
| Methylocaldum | 35.90 | 0.54 | 21.47 | 74.55 | 1.19 | 51.01 |
| Methylosarcina | 0.065 | 0.03 | 9.61 | 0.14 | 0.066 | 22.84 |
| Methylobacter | 27.25 | 0.045 | 8.56 | 0.57 | 0.098 | 20.34 |
| Methylocystis | 6.92 | 44.11 | 0.59 | 14.37 | 97.36 | 1.41 |
- Abbreviation: rRNA, ribosomal RNA.
| Methanotrophs | Relative abundance | ||
|---|---|---|---|
| Steppe (%) | Meadow (%) | Pasture (%) | |
| USCγ | 0.027 | 0 | 0.047 |
| Methylocaldum | 68.14 | 1.54 | 88.84 |
| Methylobacter | 0.055 | 0.074 | 1.34 |
| Methylosarcina | 0.069 | 0.037 | 4.52 |
| Methylocystis | 26.79 | 97.23 | 4.85 |
DISCUSSION
The results of this study are consistent with those of previous studies reporting the CH4 uptake capacity of grassland soils (Kato et al., 2013; Täumer et al., 2021; Wei et al., 2012). In our study, we found that the CH4 oxidation mechanisms in the three experimental grassland soils varied significantly. The steppe and meadow soils were able to oxidize atmospheric CH4, but the pasture soil was not. In the steppe and meadow soils, USCγ constituted 92.6% and 45% of the methanotrophs, respectively, while in the pasture soil, only 5.9% of methanotrophs consisted of USCγ (Figure 4a). Therefore, the proportion of USCγ in experimental soils may explain the varied consumption of atmospheric CH4 among the soils used in our study.
When the three soils were incubated with elevated concentration 13CH4, the pmoA gene copy numbers in the three soils increased significantly (Figure 1c), indicating an increase in the methanotroph population in the soils. These values are within the range of previously reported abundances of methanotrophs in grassland soil (Abell et al., 2009; Ma et al., 2016; Zheng et al., 2012). In parallel to the methanotroph population increase, it was found that, during the oxidation of elevated concentration 13CH4, the abundance of 13C in the organic carbon in the steppe, meadow, and pasture soils increased relative to the background value of 1.08% (Figure 2a). It is likely that during the incubation, the oxidation of elevated 13CH4 methane could have resulted in significant amounts of the 13C-labeled metabolites (methanol, formaldehyde, and formic acid) (Krause et al., 2017), indicating the cross-feeding of nonmethanotrophs. This was evident from detection of nonmethanotrophs in heavy DNA-SIP gradient fractions (Table 4).
| Bacterial classification | Relative abundance | ||
|---|---|---|---|
| Meadow (%) | Steppe (%) | Pasture (%) | |
| Methanotrophs | 45.30 | 48.16 | 37.77 |
| Taxa other than methanotrophs (phylum level) | |||
| Acidobacteria | 5.36 | 4.50 | 3.73 |
| Actinobacteria | 20.33 | 8.49 | 6.08 |
| Proteobacteria | 16.82 | 17.47 | 23.89 |
| Firmicutes | 0.72 | 0.65 | 0.72 |
| Chloroflexi | 0.55 | 1.08 | 1.31 |
| Armatimonadetes | 0.22 | 1.02 | 0.28 |
| Bacteria_unclassified | 7.41 | 9.73 | 11.48 |
| Bacteroidetes | 0.51 | 0.57 | 1.27 |
| Candidate_division_WPS-1 | 0.34 | 0.63 | 0.35 |
| Candidatus_Saccharibacteria | 0.01 | 0 | 0 |
| Gemmatimonadetes | 1.02 | 1.58 | 0.50 |
| Nitrospirae | 0.06 | 0.13 | 0.08 |
| Planctomycetes | 0.88 | 5.47 | 11.78 |
| Thaumarchaeota | 0.04 | 0.21 | 0.12 |
| Verrucomicrobia | 0.30 | 0.10 | 0.14 |
The increased methanotroph population was mainly represented by conventional type I and type II methanotrophs (Figure 4a) owing to their ability to assimilate carbon from elevated CH4 (Figure 5). We found that, in the steppe, meadow, and pasture soils studied here, USCγ did not assimilate carbon atoms from elevated concentrations of labeled CH4 (13CH4). However, a recent study reported that newly isolated methanotrophs related to USCα were able to assimilate carbon from elevated CH4 (Chiri et al., 2020; Tveit et al., 2019). Clearly, if the USCγ methanotrophs had this function in our study, they would have been labeled, but we did not detect USCγ in the heavy fraction of DNA-SIP. This may have been due to the conventional methanotrophs having an absolute advantage in the utilization of elevated CH4 and consuming almost all the 13CH4. Although USCγ possibly used 13CH4 slowly for growth, compared with a large number of enriched conventional methanotrophs, we did not detect USCγ in the 16S rRNA and pmoA gene sequencing. However, we cannot ignore the defects of 16S rRNA and pmoA gene sequencing for accurate quantification (Alteio et al., 2021). The differences in carbon assimilation from elevated CH4 by USCα and USCγ methanotrophs clearly warrant further investigation in future work.
We found that the compositions of methanotrophs in the steppe, meadow, and pasture soils were significantly different (Figure 4a, D0), which was probably affected by the soil heterogeneity, geographical conditions, and climate (Knief, 2015; Nazaries et al., 2018). The steppe grassland methanotroph community was dominated by USCγ (JR3), accounting for more than 90%, which is consistent with the greater USCγ methanotroph community previously reported from Inner Mongolia steppe grasslands (Ma et al., 2016; X. Q. Zhou et al., 2008). Soil pH has been identified as a key factor controlling methanotroph communities, with USCγ more likely to be detected in soils with pH > 6, so the high soil pH values in our experimental soils (Table 1) may have contributed to the dominance of USCγ compared to meadow and pasture soils (Deng et al., 2019). The methanotroph community in the meadow soil was different, consisting of USCs, type I, type II, and unclassified (others). A similar methanotroph composition from meadow soils was previously reported (Wei et al., 2012; Zheng et al., 2012). The diversity of methanotrophs in alpine soil may also be affected by the rich plant diversity of alpine grassland. Vegetation types will directly or indirectly affect the uptake of CH4 by methanotrophs (Y. Yang et al., 2013; M. Yang et al., 2016). In our study, pasture, a cultivated grassland, had the most unidentified methanotrophs (23%). Compared with natural grassland, the range of disturbance to the soil environment caused by human activities, such as irrigation, fertilization, land preparation, and vegetation changes, may have caused significant changes in the methanotrophic community of pasture (Anna et al., 2018). The difference in the proportion of type I and type II methanotrophs in pasture may be due to the stronger salt tolerance of type I methanotrophs compared with type II methanotrophs (Hao et al., 2019).
The DNA-SIP results showed that some of the conventional methanotrophs in grassland soils became active under a high CH4 source (Figure 5i). This finding is important because pockets of elevated CH4 can occasionally occur in grasslands when favorable conditions arise for CH4 production, such as after a heavy rainfall event (Hu et al., 2005). Under such conditions, the low-affinity methanotrophs may become active and potentially consume substantial CH4, thus markedly reducing grassland emissions. However, events such as heavy rainfall can also inhibit methanotroph activity by limiting O2 availability and gas diffusion. In our study, when soils were exposed to elevated CH4 concentrations, the active methanotrophs in the three grassland soils were markedly different. There is limited literature covering the study of active microorganisms in grassland soils (Li et al., 2020; Y. Wang et al., 2022). As such, the specific reasons for the difference in active microorganisms in different grasslands need further research. In our study, type II Methylocystis was the main active methanotroph in meadow grassland soil, type I Methylocaldum was the main active methanotroph in pasture soil, and in the steppe soils, the active methanotrophs consisted of both Methylocaldum and Methylocystis. The differences in soil physiochemical properties might have caused the variation in physiologically distinct active methanotrophs in these three grassland soils (Lüke et al., 2014; Nazaries et al., 2018). Several previous studies have reported that type II methanotrophs dominate and survive mostly under nutrient-limited adverse environmental conditions (Hanson & Hanson, 1996). In contrast to this, in our study, Methylocystis was the active methanotroph in nutrient-rich alpine meadow grassland soil. Type II methanotrophs were also dominant in nutrient-rich paddy soils (Baani & Liesack, 2008; Gu et al., 2019), indicating that type II methanotrophs may have wider adaptability to nutrient-rich environments. In addition, Methylocystis has the ability to consume CH4 at elevated concentrations and atmospheric-level concentrations, which means that Methylocystis can play a key role in consuming atmospheric CH4 when growth is periodically supported by a high CH4 source. It has been found that Methylocystis are dominant in hydromorphic soil and active at low CH4 concentrations (Knief et al., 2006). High soil organic carbon levels, continuous addition of animal excreta, and periodic water saturation with heavy rainfall and snowmelt in alpine meadow grassland soils support the creation of methanogenic hotspots that can provide favorable growth conditions for Methylocystis species (Y. Yang et al., 2013). The dominance of Methylocaldum in the active methanotrophs of pasture soils can be attributed to its growth preference in high pH conditions and its ability to withstand high salinity compared with type II methanotrophs (Sherry et al., 2015). Although Methylocystis and Methylocaldum represent a very small proportion of the in situ methanotroph community in steppe soil, they become dominant and active when enough substrate is provided (Figure 4f–h).
CONCLUSIONS
The CH4 oxidation mechanisms in the three studied grassland soils varied significantly. The oxidation of atmospheric CH4 occurred in both steppe and meadow soils but not in pasture soils. The proportion of USCγ methanotrophs in experimental soils may explain the varied consumption of atmospheric CH4 among the soils. The DNA-SIP results showed that some of the conventional methanotrophs in grassland soils became active under a high CH4 source. Type II Methylocystis was the main active methanotroph in meadow grassland soil, type I Methylocaldum was the main active methanotroph in pasture soil, and in the steppe soils, the active methanotrophs consisted of both Methylocaldum and Methylocystis. However, the USCγ methanotrophs were not able to assimilate CH4 for growth at elevated concentrations of CH4, suggesting that the USCγ group may be obligate oligotrophic microorganisms or their growth requires specific unknown conditions.
AUTHOR CONTRIBUTIONS
Yufang Wang: Data curation; formal analysis; investigation; methodology; software; writing—original draft. Yuanfeng Cai: Methodology; project administration; software. Fujiang Hou: Formal analysis; investigation; resources. Saman Bowatte: Resources; supervision; writing—review and editing. Zhongjun Jia: Project administration; resources; supervision; writing—review and editing.
ACKNOWLEDGMENTS
We thank Prof. Shenghua Chang and Dr. Zechen Peng of Lanzhou University for invaluable assistance with data collection and Rong Huang, Xi Wang and Deling Sun at the Institute of Soil Science, Chinese Academy of Sciences for assistance with sample analysis.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
All the high-throughput sequences were submitted to the Sequence Read Archive (SRA) of the NCBI database under the accession number SRP233521 and SRP310780 for the 16S rRNA gene, and the accession numbers SRP226136 and SRP310781 for pmoA gene.




