The transcription factor PU.1 is critical for viability and function of human brain microglia
Abstract
Microglia are the predominant resident immune cells of the brain and can assume a range of phenotypes. They are critical for normal brain development and function but can also contribute to many disease processes. Although they are widely studied, the transcriptional control of microglial phenotype and activation requires further research. PU.1 is a key myeloid transcription factor expressed by peripheral macrophages and rodent microglia. In this article, we report the presence of PU.1 specifically in microglia of the adult human brain and we examine its functional role in primary human microglia. Using siRNA, we achieved substantial PU.1 protein knock-down in vitro. By assessing a range of characteristic microglial proteins we found decreased viability of adult human microglia with reduced PU.1 protein expression. This observation was confirmed with PU.1 antisense DNA oligonucleotides. An important function of microglia is to clear debris by phagocytosis. We assessed the impact of loss of PU.1 on microglial phagocytosis and show that PU.1 siRNA reduces the ability of adult human microglia to phagocytose amyloid-beta1-42 peptide. These results show that PU.1 controls human microglial viability and function and suggest PU.1 as a molecular target for manipulation of human microglial phenotype.
INTRODUCTION
As the main resident immune cells of the brain, microglia are involved in most, if not all, brain diseases. Microglia are surveyors of the brain micro-environment and their motile processes constantly search for areas of local injury and disturbances in homeostasis where they respond to ameliorate the damage (Hanisch and Kettenmann, 2007). Microglia are of myeloid origin (Ginhoux et al., 2010) and perform a variety of functions in the brain; cell–cell communication via secreted molecules, monitoring extracellular ion and neurotransmitter levels, surveying synapses and clearing extracellular debris by phagocytosis (Graeber, 2010; Hanisch and Kettenmann, 2007). In the healthy brain, microglial protrusions have been shown to directly contact astrocytes, neuronal cell bodies, and blood vessels (Nimmerjahn et al., 2005). It is well documented that microglia have a spectrum of activation states under different conditions with different stimuli (Graeber, 2010). However, we have a limited understanding of how they come to assume these different phenotypes and functions in the brain. Thus, the basic molecular mechanisms of microglial activation and their role in neurological disease require further research, as microglia are a potential target for treatments for many neurodegenerative diseases including Epilepsy, Alzheimer's disease, Motor Neuron disease and Parkinson's disease, as well as for acute insults such as stroke and traumatic brain injury (Cunningham, 2012; Klegeris et al., 2007; Saijo and Glass, 2011; Tai et al., 2007; Yang et al., 2010).
Transcription factors are regulators of gene expression and therefore of cell phenotype. To address the question of control of microglial phenotype, we investigated the microglial transcription factor PU.1. PU.1 is dynamically and heterogeneously expressed within hematopoietic lineages where high PU.1 expression activates genes of the myeloid lineage and PU.1 is expressed primarily in monocytic cells (Back et al., 2005). We have previously shown in rodents that high levels of PU.1 protein are expressed in resting microglia as well as in activated microglia following injury (Walton et al., 2000).
Transcription of the PU.1 gene is regulated by other hematopoietic transcription factors, predominantly C/EBPα, as well as by PU.1 itself (Cai et al., 2007; Kummalue and Friedman, 2003; Wang et al., 1999). In myeloid cells, C/EBPα facilitates PU.1 binding to its enhancer (Leddin et al., 2011; Yeamans et al., 2007). PU.1 can activate its own promoter elements in an autoregulatory loop (Chen et al., 1995) and PU.1 sense mRNA is further regulated by its own noncoding antisense transcript that controls translation of PU.1 sense mRNA (Ebralidze et al., 2008).
PU.1 transcription factor binds to a purine-rich sequence known as the PU-box found near the promoters of target genes, and regulates their expression in coordination with other transcription factors and cofactors. PU.1 controls a vast network of myeloid genes which are relevant to neuroimmunology and brain inflammation. PU.1 activates Erg2 transcription which in turn leads to upregulation of several macrophage genes (Weigelt et al., 2009). PU.1 is essential for myeloid cell differentiation (Feng et al., 2008; Forsberg et al., 2010) and has been found to regulate genes involved in a range of monocytic functions such as phagocytosis and chemotaxis (Lloberas et al., 1999). For example, PU.1 regulates expression of secreted molecules TNFα (Fukai et al., 2009), IL-18 (Koyama et al., 2004) and CXCL9 (Ellis et al., 2010); cell surface receptors CXCR1 (Wilkinson and Navarro, 1999) and CD11b (Pahl et al., 1993); and intracellular protein DAP12 (Henkel et al., 2002). Importantly, cells expressing PU.1 are responsive to macrophage colony-stimulating factor (M-CSF) (Henkel et al., 2002) due to PU.1 regulation of the M-CSF receptor gene c-fms (Zhang et al. 1994). Ghani et al. (2011) have provided evidence that PU.1 directly controls expression of at least four microRNAs (miR-146a, 342, 338, and 155), implicating PU.1 as a master coordinator of myeloid microRNA expression (Ghani et al., 2011).
Although most of the research on PU.1 and microglia has been carried out using rodent cells and models, it is becoming increasingly clear that there are important differences between rodent microglia and their human counterpart (Davis, 2012; Dragunow, 2008). Age has also been shown to have an impact on immune cell responses and activation (Lynch et al., 2010; Streit, 2006). Furthermore, the microenvironment of the brain may affect immune responses in ways that are different to those of the periphery. While the majority of research on PU.1 has been carried out on peripheral immune cells, PU.1 function has not been investigated in human microglia. Here we demonstrate PU.1 expression in the adult human brain and investigate the role of this transcription factor in adult human microglia. Using small interfering RNA (siRNA), we show that PU.1 is involved in adult human microglial viability and phagocytosis.
MATERIALS AND METHODS
Tissue
Autopsy adult human brain tissue was obtained from the Neurological Foundation of New Zealand Human Brain Bank under the University of Auckland Human Subjects Ethics Committee. Biopsy adult human brain tissue was obtained from patients undergoing surgery for intractable temporal lobe epilepsy. This research was approved by the Northern Regional Ethics Committee and informed consent was obtained from all tissue donors.
All of the specimens studied in this article were from confirmed temporal lobe epilepsy cases with varying degrees of mild, moderate-marked mesial temporal sclerosis (most were graded 3–4 by neuropathological examination). Patients had most recently been taking a range of medications alone or in combination, including phenytoin, tegretol, topiramate, lamotrigine, and sodium valproate. There were no obvious associations between the results of any of the reported microglial experiments and drug use or degree of mesial temporal sclerosis.
Human Glial Cell Isolation and Culture
Glial cells were isolated from adult human brain tissue using methods previously described by Gibbons et al. (2007, 2011). Approximately 2 g of white and grey matter from the middle temporal gyrus was washed twice in Hanks balanced salt solution (HBSS; Ca2+ and Mg2+ free, Gibco BRL). The meninges and visible blood vessels were removed, then the tissue was diced into small (∼1 mm3) cubes and placed in 10 mL g−1 tissue warm enzyme dissociation mix containing 2.5 U mL−1 papain (Worthington) and 10 U mL−1 DNase (Invitrogen) in HBSS and incubated for 30 min at 37°C with agitation. An equal volume of DMEM/F12 supplemented with 10% fetal bovine serum (FBS), 1% Penicillin-Streptomycin-Glutamine (Gibco BRL) (final concentrations: penicillin (100 U mL−1), streptomycin (100 μg mL−1) and L-glutamine (0.29 mg mL−1)) was added and the tissue gently triturated. The cell suspension was passed through a 100 μm nylon cell strainer (Becton Dickinson), centrifuged for 10 min at 160 g, the pellet resuspended in 20 mL medium and plated into 75 cm2 tissue culture flask (Nunc) which was incubated at 37°C in 95% air/5% CO2. After 24 h, the debris and unattached cells were removed, centrifuged for 10 min at 160 g and replated onto the adhered cells for a further 24 h. Finally, the debris was removed and the cells carefully washed with medium. Cells were cultured for 1–2 weeks, detached by trypsinization with 0.25% Trypsin/1 mM EDTA, and plated at 50,000 cells mL−1 on 96-well plates for experiments. The yield of cells per tissue sample varies between cases, with an average of 2.2 ± 0.4 × 106 cells per gram tissue (mean ± SEM, n = 5 cases). The proportion of different cell types is also variable between cultures, with an average of 13.2% ± 1.3% PU.1-positive microglia, and 1.1% ± 0.02% GFAP-positive astrocytes (mean ± SEM, n = 3 cases). Routine validation of cell phenotypes was conducted as per Gibbons et al. (2007).
siRNA Transfection
PU.1 (SASI_Hs02_00315880) and control (Universal Negative Control #1) siRNA were purchased from Sigma–Aldrich. Two to three days after plating, primary human mixed cell cultures were transfected with 50 nM siRNA delivered by Lipofectamine RNAiMax (Invitrogen) (5 μL per 1 μM siRNA) in DMEM/F12 supplemented with FBS and L-glutamine. The transfection mix was left at room temperature (RT) for 15 min prior to adding to cells. No antibiotics were used in the transfection media and were only reintroduced 2 days after transfection. Cells were cultured for up to 7 days post-transfection.
DNA Oligonucleotide Transfection
PU.1 antisense (5'aaacccttccattttgcA), and control sense (5'tgcaaaatggaagggttT), DNA oligonucleotides were purchased from Sigma–Aldrich. Two to three days after plating, primary human mixed cell cultures were transfected with 150 nM oligonucleotides delivered by Fugene HD (Roche) (2.5 μL per 1 μM oligonucleotide) as for siRNA above.
Immunochemistry
To label cells cultured in vitro, cells were fixed in 4% paraformaldehyde for 15 min at RT. Cells were washed for 10 min with phosphate-buffered saline containing 0.2% Triton X-100 (PBS-T). All antibodies were diluted in immuno-buffer (PBS-T containing 1% goat serum and 0.04% merthiolate). Cells were incubated with primary antibody (see Table 1) overnight at 4°C with gentle rocking. Alexa Fluor-conjugated and biotinylated secondary antibodies (Table 1) were applied for 3 h at RT with gentle rocking. For colorimetric protein detection, ExtrAvidin-HRP was applied for 2 h at RT followed by 3,3'-diaminobenzidine tetrahydrochloride (DAB) reaction. PBS-T was used for all washes.
| Antibody | Company | Catalogue no. | Dilution |
|---|---|---|---|
| Rabbit anti-PU.1 | Cell Signaling | 2258 | 1:500 |
| Mouse anti-CD45 | Abcam | ab8216 | 1:500 |
| Mouse anti-HLA-DP, DQ, DR | Dako | M0775 | 1:500 (cells); 1:1,000 (tissue) |
| Mouse anti-GFAP | Sigma-Aldrich | G3893 | 1:5,000 |
| Rabbit anti-GFAP | Dako | Z0334 | 1:5,000 |
| Mouse anti-Prolyl 4-hydroxylase | Dako | M0877 | 1:1,000 |
| Rabbit anti-DAP12 | Santa Cruz | Sc-20783 | 1:500 |
| Rabbit anti-CX3CR1 | Abcam | Ab8021 | 1:100 |
| Goat anti-Iba-1 | Abcam | Ab5076 | 1:1,000 |
| Mouse anti-CD68 | Abcam | Ab955 | 1:500 |
| Rabbit anti-CREB | Cell Signaling | 9197 | 1:500 |
| Rabbit anti-ATF2 | Santa Cruz | Sc-187 | 1:500 |
| Mouse anti-NeuN | Chemicon | MAB377 | 1:500 |
| Mouse anti-MAP2 | Sigma-Aldrich | M4403 | 1:500 |
| Goat anti-rabbit IgG Alexa Fluor® 594 | Invitrogen | A11012 | 1:500 |
| Goat anti-mouse IgG Alexa Fluor® 488 | Invitrogen | A11001 | 1:500 |
| Goat anti-mouse IgG Alexa Fluor® 594 | Invitrogen | A11005 | 1:500 |
| Goat anti-rabbit IgG Alexa Fluor® 488 | Invitrogen | A11008 | 1:500 |
| Goat anti-rabbit biotinylated | Sigma-Aldrich | B7389 | 1:500 |
| Goat anti-mouse biotinylated | Sigma-Aldrich | B7264 | 1:500 |
| ExtrAvidin-HRP | Sigma-Aldrich | E2886 | 1:500 (cells); 1:1,000 (tissue) |
| Mouse anti-β-actin | Abcam | AB6276 | 1:5,000 |
| Anti-rabbit horseradish peroxidase | GE Healthcare | NA934V | 1:2,000 |
| Antimouse horseradish peroxidase | GE Healthcare | NA934V | 1:2,000 |
Tissue from two biopsy cases and six autopsy cases was used for immunohistochemistry. Fixed-frozen adult human brain autopsy and biopsy tissue was cut on a freezing microtome (Microm HM450) into 50 μm sections and processed for light and confocal microscopy according to procedures outlined by Waldvogel et al. (2007). Briefly, free-floating sections were incubated for 20 min in 50% methanol and 1% H2O2, washed with PBS-T, and incubated in primary antibody for 3 days at 4°C with gentle rocking. Sections were then washed and incubated overnight at RT in biotinylated secondary antibody before washing and incubating for 4 h at RT in ExtrAvidin-HRP. Sections were then washed and exposed to DAB and 0.01% H2O2 for 15–20 min to produce a brown reaction product. After washing, the sections were mounted onto glass slides using a gelatin and chromic potassium sulfate solution and left to dry overnight. The slides were dehydrated through a graded alcohol series to xylene and coverslipped with DPX mounting medium (Merck).
For acid and base antigen retrieval, sections from three autopsy cases were washed in H2O and then incubated in either 2 or 4 M hydrochloric acid (HCl) or 1 mM ethylenediaminetetraacetic acid (EDTA) for 2 h at RT. Sections were then washed and processed as normal. No immunohistochemical labeling was seen when primary antibody was omitted.
Fluorescent labeling of tissue sections was carried out as above except that sections were mounted using PBS and coverslipped directly with Prolong Gold (Invitrogen). A Leica SP2 confocal laser-scanning microscope was used to obtain confocal images.
To label nuclei, cells and tissue were stained with Hoechst 33258 (Sigma–Aldrich) for 30 min at RT protected from light. Cells were washed in TNE buffer (pH 7.4) containing 10 mM Tris, 200 mM NaCl, and 1 mM EDTA prior to, and following, incubation in 20 μM Hoechst 33258 diluted in TNE buffer. Tissue sections were incubated in 1 μM Hoechst 33258 diluted in PBS-T buffer and PBS-T was used for washes.
Alamar Blue Cell Viability Assay
Following treatment of cells with PU.1 siRNA, 10 μL of Alamar Blue solution (AbD Serotec) was added to each well and cells were incubated at 37°C for 1 h. Fluorescence was measured on a FLUOStar Optima plate reader (BMG Labtech) with an excitation of A544 and an emission of A590.
Western Blot
Cells intended for Western Blot analysis were cultured in six-well plates. Wells were washed with PBS and 120 μL lysis buffer (2% sodium dodecyl sulfate, 4% glycerol and 62.5 mM Tris, pH 6.8; supplemented with protease inhibitor (Roche Diagnostics)) was added per well and cells were collected using a cell scrapper (Nunc). Samples were heated to 95°C for 10 min, cooled on ice and stored at −20°C. Protein concentrations were determined using the Biorad DC assay. All gel electrophoresis was performed using Invitrogen systems and buffers according to manufacturer's instructions. Samples with equal amounts of protein (50 μg) were loaded into precast Bis–Tris gels and resolved by gel electrophoresis using Xcell SureLock Mini Cell. Proteins were then electrotransferred onto polyvinylidene difluoride membrane (PVDF, Hybond-P; Amersham) using the Xcell Blot Module. After protein transfer, PVDF membranes were blocked for 1 h with 0.1% Tween-20 (Sigma) Tris Buffered Saline solution (TBST) supplemented with 5% skim milk on a rocker, and incubated with primary antibody (see Table 1) diluted in 5% bovine serum albumin (Gibco)-TBST overnight at 4°C with gentle shaking. PVDF membranes were subsequently washed 3 × 10 min with TBST and probed with species-specific secondary antibody (see Table 1) linked to horseradish peroxidase diluted in 5% skim milk TBST for 2 h at RT with gentle shaking. Membranes were washed again and proteins visualized using the Amersham ECL Plus kit (GE Healthcare) and LAS-3000 imager (Fujifilm).
Reverse Transcription and Quantitative Real Time Polymerase Chain Reaction
Quantitative real time reverse transcription-polymerase chain reaction (qRT-PCR) was carried out as described by Monzo et al. (2011). Briefly, total RNA was extracted from cells and tissue using Trizol (Invitrogen) and cDNA was formed using SuperScript III (Invitrogen). PU.1 RNA was quantified using SYBR Green (Invitrogen) and Applied Biosystems 7900HT Fast Real-Time PCR System. Primers for PU.1 were obtained from Invitrogen (Forward: 5'CGACCATTACTGGGACTTCCA; Reverse: 5'GGAGCTCCGTGAAGTTGTTCTC). PU.1 RNA amount was made relative to β-ACTIN internal control.
Phagocytosis Assays
To evaluate the effect of PU.1 siRNA on primary adult human microglial phagocytosis of amyloid-β1–42 amino acid peptide (Aβ42; Bachem), phagocytosis assays were performed as previously described (Gibbons et al., 2011; Smith et al., 2010). Four days after siRNA transfection, 5 μM Aβ42 was added to the cells for 24 h. Cells were washed twice with PBS to remove excess Aβ42 and fixed with 4% PFA for 15 min at RT. Thioflavin S was used to visualize phagocytosed Aβ42 (Smith et al., 2010).
Quantitative Image Analysis of Cell Number and Phagocytosis
Quantification of PU.1-immunopositive, CD45-immunopositive and total Hoechst-positive cell number, and phagocytosis of Aβ42 was achieved using a Discovery-1 automated fluorescence microscope (Molecular Devices) and Metamorph (6.2.6 software, Molecular Devices) image analysis system as previously described (Smith et al., 2010). Results were logged automatically to Microsoft Excel spreadsheets.
Statistical Analysis
Representative data are displayed as mean ± standard error of the mean (SEM). Experiments were performed in at least triplicate. Statistical analysis was carried out using unpaired t tests and One-way ANOVA followed by Tukey's multiple comparison test (GraphPad Prism 4.02). P values of <0.05 were considered statistically significant differences.
RESULTS
PU.1 is a Marker of Cultured Adult Human Primary Microglia
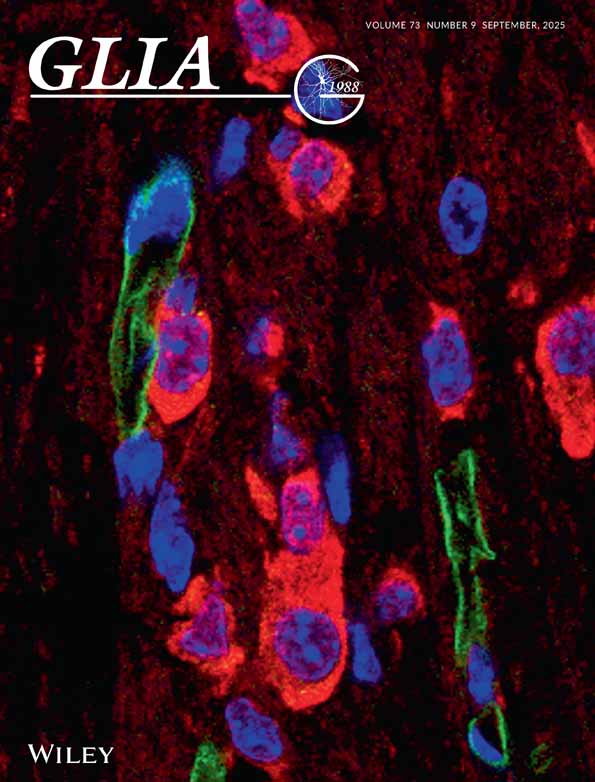
Isolation of cells from human autopsy and biopsy cortical brain tissue gives rise to a mixed population of cells including microglia, astrocytes and fibroblast-like leptomeningeal cells (Gibbons et al., 2007, 2011). With specific cell markers we can identify and quantify microglia-specific effects within this mixed cell population (Gibbons et al., 2011). Primary microglia isolated from both biopsy and postmortem adult human brain tissue, selectively express the transcription factor PU.1 and we found that microglia always coexpress PU.1 and leukocyte common antigen (the protein tyrosine phosphatase CD45), a pan microglia marker (Fig. 1A–C). Levels of PU.1 expression in microglia are highly consistent between different cultures (representative images are shown in Fig. 1). HLA-DP,-DQ,-DR is a highly inducible marker of adult human microglia and was expressed by some, but not all, PU.1-immunopositive microglia, suggesting that it may label a subset of “activated” microglia in vitro (Fig. 1D). However, all microglia expressing HLA-DP,-DQ,-DR also expressed PU.1 (Fig. 1D) and we did not find a significant difference in PU.1 staining intensity between HLA-DP,-DQ,-DR positive or negative microglia. We used a range of other commonly used microglial markers to confirm PU.1 as a microglial-specific protein and found PU.1 to be coexpressed with all CD68, DAP12 (an adaptor protein expressed by cells of the monocytic lineage), CX3CR1 (fractalkine receptor), and Iba-1 (allograft inflammatory factor 1) immunoreactive microglia (Supp. Info. Fig. 1). PU.1 is specific for microglia in mixed cell populations isolated from human brain tissue as it was not found in glial fibrillary acidic protein (GFAP)-positive astrocytes (Fig. 1E; Supp. Info. Fig. 2) or in fibroblast-like leptomeningeal cells that express the collagen-synthesising enzyme prolyl 4-hydroxylase, a known marker of fibroblasts (Fig. 1F).

Primary human microglia express PU.1 in vitro. (A) PU.1 (red) expression in microglial nuclei in mixed cell cultures isolated from adult human brain tissue. (B) All microglia in mixed cell cultures coexpress PU.1 (red) in their nuclei and CD45 (green) on their cell surface. (C) Figure 1B overlaid with Hoechst to label all nuclei (blue). PU.1 is specifically expressed by CD45-positive microglia in mixed cell cultures. (D) HLA (green) labels a subset of PU.1-positive microglia. (E) GFAP-positive astrocytes (green) do not express PU.1 (pink). (F) Prolyl 4-hydroxylase-positive fibroblast-like leptomeningeal cells (green) also do not express PU.1 (pink). Scale bar = 50 μm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
PU.1 is a Marker of Microglia in the Adult Human Brain
To confirm that PU.1 is expressed by microglia in the adult human brain, as well as in primary human microglia in vitro, immunohistochemistry for PU.1 was performed on fixed-frozen sections of human brain tissue. PU.1 was found to be ubiquitously expressed in biopsy human brain tissue in both the grey and white matter (Fig. 2A). Double-labeling immunofluorescence studies were carried out with a range of glial and neuronal markers to confirm PU.1 as a microglia-specific marker. In adult human brain tissue, PU.1 was coexpressed by cells labeled with the other widely used microglia markers HLA-DP,-DQ,-DR, and CD45 (Fig. 2B,C). However, as in vitro, HLA-DP,-DQ,-DR was only coexpressed by a subset of PU.1-immunopositive microglia. We did not find colabeling of PU.1 with astrocytic (GFAP) or neuronal (Neuronal Nuclei (NeuN) and Microtubule-Associated Protein 2 (MAP2)) markers.
PU.1 protein is present in biopsy adult human brain tissue and is coexpressed with other microglial markers. (A) PU.1 (DAB, brown) is abundantly expressed in both the grey and white matter of biopsy adult human brain tissue. (B) PU.1 (darker DAB-Ni nucleus indicated by arrow) and HLA-DP,-DQ,-DR (lighter DAB processes) label the same microglial cell. (C) Confocal image of PU.1 (red) and CD45 (green) immunoreactive microglia. Hoechst (blue) stains all nuclei. (D) Postmortem adult human brain tissue does not show any PU.1 immunochemical staining. Scale bar = 20 μm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In contrast to the strong PU.1 protein expression in microglia from adult human biopsy brain tissue, the same immunochemical method could not detect PU.1 protein in postmortem brain tissue from a number of neurologically normal and diseased patients (Fig. 2D). To determine whether this might be due to fixation-related effects, acidic (2M and 4M HCl) and basic (1mM EDTA, pH 8) antigen retrieval methods were tested but did not result in specific PU.1 labeling (Supp. Info. Fig. 3).
PU.1 mRNA is Expressed in the Adult Human Brain
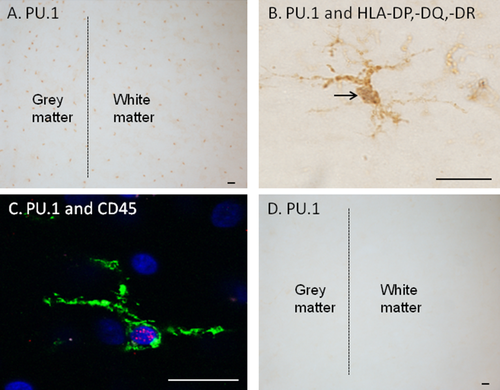
qRT-PCR was performed to determine whether, despite PU.1 protein being elusive in autopsy human brain tissue, PU.1 mRNA is present in the adult human brain. Levels of PU.1 mRNA expression were compared to those in a PU.1-positive human monocytic cell line (THP-1 cells), and to pure cultures of PU.1-negative fibroblast-like human leptomeningeal cells (Fig. 1F). The cultures of fibroblast-like human leptomeningeal cells only were obtained at late passage once the negligibly dividing microglia and astrocytes were no longer present (Gibbons et al., 2007). PU.1 mRNA is indeed found at a considerable level in autopsy adult human brain tissue (Fig. 3). Thus it is likely that PU.1 protein is degraded relatively quickly, prior to fixation of autopsy human brain tissue, compared to biopsy tissue.
PU.1 mRNA is present in adult human brain tissue. qRT-PCR analysis reveals that PU.1 mRNA is expressed in autopsy adult human brain tissue. The THP-1 human monocytic cell line was used as a positive control for PU.1 mRNA expression. As a negative control, pure cultures of human brain fibroblast-like leptomeningeal cells were obtained after three to four passages of mixed cell cultures and did not contain microglia or astrocytes.
Reduction of In Vitro PU.1 Protein Expression by siRNA
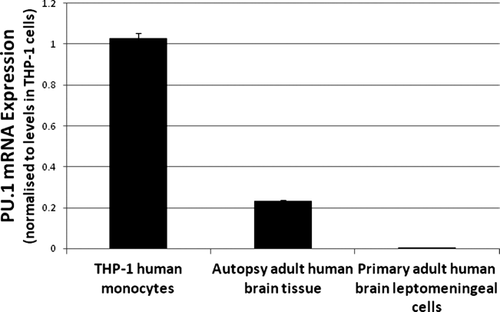
To assess the function of PU.1 protein in adult human microglia we used siRNA to reduce PU.1 protein expression. We achieved high transfection efficiencies in these primary adult cells and found that PU.1 siRNA produced 70.6 ± 2.4% (mean ± SEM; n = 10) PU.1 protein knock-down 4–7 days post-transfection (Fig. 4A–C). Quantification of PU.1 expression shows a significant decrease in the number of PU.1-positive cells (Fig. 4D). This method of PU.1 protein reduction has produced consistent results in microglia from more than 10 biopsy and postmortem tissue samples, demonstrating the efficacy of this method on microglia from a range of human cases, both diseased (Epilepsy, Huntington's and Parkinson's disease) and normal. Transfection of adult human microglia with siRNA was confirmed using a fluorescent-tagged siRNA to verify the uptake of siRNA by these cells (Supp. Info. Fig. 4).
Adult human microglia can be transfected with PU.1 siRNA to reduce PU.1 protein expression. (A) Microglia isolated from adult human brain tissue express high constitutive levels of PU.1 in their nuclei. (B) Microglial PU.1 expression is greatly reduced at 7 days following transfection with PU.1 siRNA. (C) Western blot showing reduced PU.1 expression after specific siRNA-targeted knock-down. β-actin was used as a loading control. (D) Quantification of immunocytochemical PU.1 expression shows that PU.1 siRNA significantly decreases the number of PU.1-expressing cells after transfection. Scale bar = 50 μm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Specificity of the loss of PU.1 protein expression was tested immunocytochemically using markers of other transcription factors expressed by adult human microglial cells; cAMP response element-binding protein (CREB) and activating transcription factor 2 (ATF2). The levels of CREB and ATF2 transcription factor expression were not affected by PU.1 siRNA (Supp. Info. Fig. 5).
PU.1 siRNA Reduces Microglial Viability
To determine the functional effects of loss of PU.1 expression with PU.1 siRNA, we measured the long-term viability of mixed cell cultures 7 days following transfection. At this time point PU.1 expression was greatly reduced (Fig. 5A,B) while total glial cell number was not notably altered (Fig. 5C,D). Using Alamar Blue solution to assess functional mitochondria in viable cells, we found that PU.1 siRNA does not significantly affect overall primary glial/leptomeningeal cell viability (Fig. 5G). Total cell counts of all Hoechst-labeled nuclei for control and PU.1 siRNA-treated cultures were also not significantly different (Fig. 5H). Astrocytes and fibroblast-like cells are not affected by PU.1 siRNA (Supp. Info. Fig. 6).
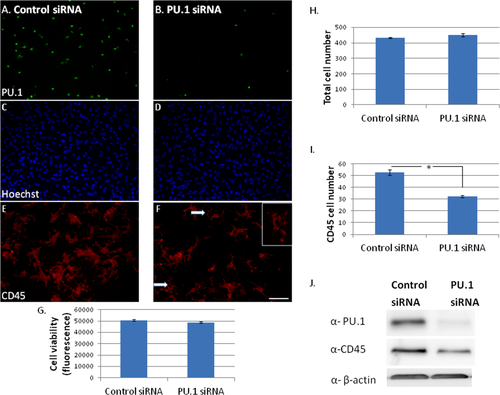
Reduced PU.1 protein expression decreases adult human microglial viability. (A and B) PU.1 siRNA reduces PU.1 protein expression (green) 7 days following transfection. (C and D) Total glial cell number (all nuclei labeled with Hoechst) is not affected by PU.1 siRNA. (E and F) CD45 microglial cell surface protein expression (red) is reduced 7 days following transfection with PU.1 siRNA. Arrows and inset indicate disintegrating microglia. (G) Total glial cell viability as measured by Alamar Blue assay was not changed by PU.1 siRNA. (H) Quantification of total Hoechst nuclei does not show a significant difference between control and PU.1 siRNA. (I) Quantification of CD45-positive microglial cell number shows a significant reduction in microglia number after PU.1, but not control, siRNA transfection. (J) Western blot showing reduced CD45 expression with siRNA-induced PU.1 protein knock-down. β-actin was used as a loading control. Scale bar = 50 μm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Despite no significant loss in total cell viability, we still found a reduced number of CD45-immunoreactive microglia amongst cells with PU.1 knock-down (Fig. 5E,F). The number of CD45-positive microglia was significantly decreased by PU.1 siRNA (Fig. 5I), as was total CD45 protein expression measured by Western blot (Fig. 5J).
Immunocytochemistry for other microglial proteins showed the same result as for CD45. We found that the number of DAP12 and CX3CR1 immunoreactive microglia was decreased with PU.1 siRNA compared to control siRNA (Fig. 6). There did not appear to be a specific decrease in staining intensity for DAP12 or CX3CR1 (Fig. 6A–D, respectively), only a decreased number of microglia expressing these proteins 7 days following transfection (Fig. 6E,F, respectively). In general, the PU.1-depleted microglia were less spread-out (insets in Fig. 6C,D) and some showed signs of disintegration (Fig. 5F, arrows and inset). These observations suggest that even though the microglia are still present with PU.1 siRNA, reduced levels of PU.1 expression are affecting their viability and phenotype.

Microglial DAP12 and CX3CR1 expression are reduced by PU.1 siRNA. Adult human microglia in a mixed cell culture express DAP12 adaptor protein (A) and CX3CR1 fractalkine receptor (C). 7 days following transfection with PU.1 siRNA there is reduced expression of DAP12 (B) and CX3CR1 (D). Insets in C and D show representative morphology of cells. Quantification of immunocytochemical images shows a reduction in DAP12-immunoreactive (E) and CX3CR1-immunoreactive (F) microglia following PU.1 siRNA transfection compared to control siRNA transfection. Scale bar = 50 μm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The finding that PU.1 siRNA reduces microglial viability was validated using DNA oligonucleotides against PU.1 as an alternative method of reducing PU.1 protein expression. As with siRNA, the antisense DNA oligonucleotides targeting PU.1 (150 nM) successfully reduced PU.1 protein expression compared to control sense oligonucleotides (Fig. 7A,B). This was again followed by a reduction in the number of CD45-immunoreactive microglia (Fig. 7B). Whilst the number of PU.1-expressing microglia was significantly reduced from 3 days post-transfection, the reduction in CD45-positive microglia was only evident 6 days post-transfection (Fig. 7). The number of microglia is decreased at 6 days compared to 3 days with both sense and antisense oligonucleotides, but the specific reductions in PU.1 (at 3 and 6 days) and CD45 (at 6 days) expression are significantly greater for antisense oligonucleotides.
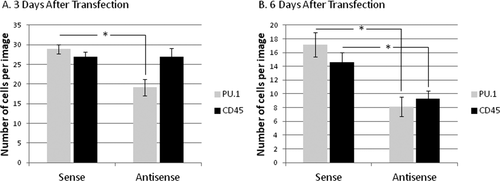
Transfection of adult human brain mixed cell cultures with PU.1 antisense DNA oligonucleotides results in reduced PU.1 and CD45 immunoreactive microglia. (A) 3 days after transfection, PU.1 expression in adult human mixed cell cultures is significantly reduced by PU.1 antisense DNA oligonucleotides, compared to sense oligonucleotides. However, CD45 expression is unaffected. (B) At 6 days after transfection, both PU.1 and CD45 expression is significantly reduced by PU.1 antisense DNA oligonucleotides.
To determine the optimal siRNA concentration for subsequent functional studies and to investigate the effects of high siRNA concentration on overall cell viability, concentration-response experiments were carried out using 25–100 nM siRNA. Concentrations of PU.1 siRNA above 50 nM caused a general decrease in cell number, probably through non-specific actions. For this reason 50 nM siRNA was chosen for later experiments as it provided more than 50% PU.1 protein reduction (Supp. Info. Fig. 7A) without a significant decrease in total cell number (Supp. Info. Fig. 7B).
PU.1 siRNA Reduces Microglial Phagocytosis of Aβ42 Peptide
Phagocytosis assays were performed with adult human microglia to investigate the role of PU.1 in this process. Cells were incubated with Aβ42 peptide following PU.1 siRNA transfection. With control siRNA transfection, phagocytosed Aβ42 was seen surrounding the microglial nuclei and filled up almost the entire cytoplasm (Fig. 8A,C). With PU.1 siRNA treatment, there was a dramatic reduction in the amount of Aβ42 that had been phagocytosed by the microglia, concomitant with a reduction in PU.1 expression (Fig. 8B,D). PU.1 knock-down significantly reduced the percentage of microglia that undertook phagocytosis of Aβ42 (Fig. 8E).
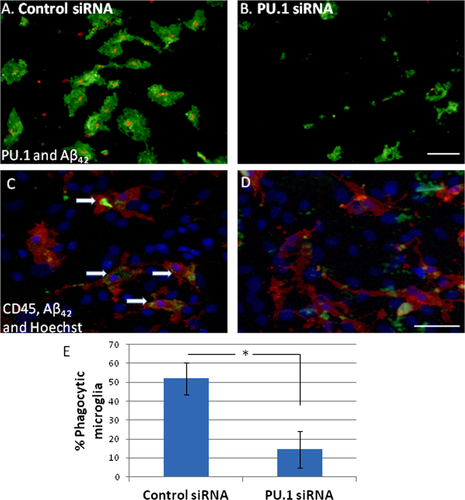
PU.1 siRNA reduces phagocytosis of amyloid-β1-42 (Aβ42) by human microglia. (A) Microglial nuclei labeled with PU.1 (red) are surrounded by fluorescently labeled Aβ42 peptide (green) which has been phagocytosed by the microglia. (B) PU.1 siRNA reduces PU.1 expression and dramatically reduces the amount of Aβ42 that has been phagocytosed by the microglia. (C and D) Microglial cells are labeled with cell surface marker CD45 (red). Hoechst (blue) labels all nuclei in the mixed cell culture. Aβ42 (green) can be seen inside control microglial cells (arrows, C) but not in those treated with PU.1 siRNA (D). (E) PU.1 siRNA significantly reduces the percentage of microglia that undergo phagocytosis. Scale bar = 50 μm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
This study establishes the presence of PU.1 and investigates its function in microglia in the adult human brain. Microglia are known to play a significant role in central nervous system homeostasis and disease. Thus, learning more about the factors, such as PU.1, that control the flexibility of microglial phenotype could provide strategies for manipulating microglia actions in the brain.
Most research on PU.1 in microglia has been conducted on rodent models despite increasing evidence of important distinctions between rodent microglia and their human counterparts (Davis, 2012; Dragunow, 2008; Gibbons et al., 2011; Smith et al., 2010). It may not always be appropriate to directly translate findings across different species or age groups. Thus, we have attempted to extend the current knowledge of PU.1 into the human context by investigating the role of PU.1 in microglia from the adult human brain. In doing so, our results may shed more light onto the role of PU.1 and microglia in the context of human neurodegenerative disease.
The importance of studying PU.1 in the context of the brain is highlighted in a paper by Ponomarev et al. (2010) which identifies a brain-specific microglia/macrophage deactivating C/EBPα-PU.1 pathway (Ponomarev et al., 2010). Furthermore, Durafourt et al. (2012) have found several differences between human adult microglia and blood-derived macrophages regarding cell surface protein expression, cytokine production and phagocytosis (Durafourt et al., 2012). Nevertheless, an increasing number of studies are investigating connections between the central nervous system and the peripheral immune system (Chen et al., 2008; Perry, 2010) and PU.1 may also influence the brain through its expression in peripheral immune cells (Weigelt et al., 2011).
PU.1 in the Adult Human Brain
We have previously shown that PU.1 is constantly expressed in rodent microglia (Walton et al., 2000). Here we further demonstrate (1) PU.1 protein expression in primary adult human microglia cultured in vitro from biopsy and postmortem brain tissue; (2) in situ expression of PU.1 protein in adult human biopsy brain tissue; (3) PU.1 mRNA expression in post-mortem adult human brain tissue (Figs. 1).
We can likely rule out the possibility that PU.1 expression in biopsy tissue is due to the underlying epileptic condition from our in vitro cell culture studies, in which we see PU.1 expression in microglia from all cases regardless of disease state. We have not noticed a difference in levels of PU.1 in microglia from neurologically normal and neurologically diseased individuals, however, it would be interesting to investigate whether alterations in PU.1 expression are seen in affected areas of the brain for particular diseases. The lack of PU.1 immunoreactivity in autopsy human brain tissue (Fig. 2D), despite PU.1 mRNA expression (Fig. 3), is possibly due to fast degradation of the protein following death. A previous report of immunohistochemical staining of human post-mortem brain tissue did not find nuclear localization of several transcription factors despite nuclear staining in biopsy tissue (MacGibbon et al., 1997). As expected if the PU.1 protein degrades during postmortem delay, antigen retrieval methods for immunohistochemistry in autopsy adult human brain tissue did not result in positive PU.1 labeling (Supp. Info. Fig. 2). The average post-mortem delay of tissue in the Neurological Foundation of New Zealand Human Brain Bank is >10 h, but with shorter postmortem delays, it may be possible to achieve PU.1 labeling in autopsy human brain tissue. Of course, it is also possible that the nonepileptic brain does not express PU.1 protein and that the observed expression of PU.1 protein in a range of nonepileptic and epileptic cell culture cases observed in this study is due to infiltration of peripheral monocytes that are known to express this protein. Although this is a possibility, we believe that it is unlikely as monocyte infiltration (and hence PU.1 protein) would also be expected in postmortem tissue but we did not observe any PU.1 protein in this tissue. Furthermore, the pattern of expression of PU.1 in the epileptic brain is very widespread in regions that are unlikely to be part of the epileptic focus. While more inducible markers such as HLA-DP,-DQ,-DR may provide a measure of particular subsets of microglia, PU.1 identifies a larger microglial population and we propose that PU.1 can serve as a marker for microglia cultured from the adult human brain.
Reduction of In Vitro PU.1 Protein Expression by siRNA
The tool that we used to investigate PU.1 function in adult human microglia was siRNA, which specifically targets and degrades PU.1 mRNA, reducing PU.1 protein levels. We achieved high transfection efficiencies in these primary adult cells with high levels of PU.1 protein knock-down 4–7 days post-transfection (Fig. 4). Direct targeting of PU.1 protein found only in microglia brain cells allows analysis of microglia-specific functions within a mixed primary cell culture. While a pure microglial culture would remove the possibility of stand-by effects from other cells, a mixed cell culture better models the mix of cells present in vivo (Gibbons and Dragunow, 2010). The outstanding efficiency with which these primary adult human cells are transfected demonstrates their usefulness as a model system for investigating basic cell biology as well as for drug discovery (Dragunow, 2008). If the ability of siRNA to knockdown PU.1 expression in human microglia in vitro translates to human microglia in vivo, then this approach may also serve as a possible therapeutic strategy to modulate neuroinflammation in neurodegenerative disorders.
We have also established that antisense DNA oligonucleotides give the same result of reduced PU.1 protein expression as siRNA (Fig. 7). Both siRNA and antisense DNA oligonucleotides specific for PU.1 result in a reduced number of CD45-imunoreactive microglia (Fig. 5 and 7, respectively). However, siRNA was more effective at reducing PU.1 protein expression at a lower concentration (50 nM) compared to DNA oligonucleotides (150 nM).
PU.1 siRNA Reduces Microglial Viability
PU.1 has been shown to be an important factor in rodent myeloid cell differentiation, proliferation and survival (Anderson et al., 1998; Lloberas et al., 1999). Here we show that reduced PU.1 expression leads to reduced adult human microglial viability. We characterized the PU.1-depleted microglia with antibodies to CD45, DAP12, and CX3CR1, and found a reduced number of microglia expressing these proteins (Figs. 5 and 6). Anderson et al. (2001) have shown that CD45 is not expressed by PU.1-deficient myeloid cells and that PU.1 regulates murine CD45 gene expression (Anderson et al. 2001). The result of reduced DAP12 expression with reduced levels of PU.1 was similarly reported by Henkel et al. (2002) using mouse monocytic precursor cells and by Weigelt et al. (2007) using murine macrophage RAW264.7 cells (Henkel et al., 2002; Weigelt et al., 2007). While we observed a reduction in the number of CD45 and DAP12 immunopositive microglia with PU.1 siRNA, we did not observe reduced staining intensity of CD45 or DAP12 in individual microglia treated with PU.1 siRNA. Either our experimental paradigm does not allow us time to see specific reductions in CD45 and DAP12 expression, or compensatory mechanisms/low turnover rates result in stable CD45 and DAP12 protein expression in these adult human microglia.
Specific regulation of CX3CR1 gene transcription by PU.1 has not been reported, although several other transcription factor binding sites have been found for the human CX3CR1 gene promoter regions (Barlic et al., 2004; Garin et al., 2002). Nevertheless we found a reduction in the number of CX3CR1-expressing microglia when PU.1 expression was reduced by siRNA, suggesting that a general decrease in microglial cell number and reduced microglial viability result from loss of PU.1 expression. Furthermore, disintegration and rounding of some microglia after PU.1 siRNA transfection suggest a loss in membrane integrity (insets in Figs. 5F and 6C,D).
PU.1 siRNA Reduces Phagocytosis of Aβ42 Peptide
To assess the long-term effect of reduced PU.1 on human microglia function we assessed their phagocytosis of Aβ42. Phagocytosis is an important innate function of microglia during development and throughout adulthood. In Alzheimer's disease microglia may be helpful in clearing the brain of extracellular Aβ42 deposits (Bard et al., 2000; Boche et al., 2010; Rogers et al., 2002). However, microglial phagocytosis can also be detrimental in the case of “primary phagocytosis” which actively induces cell death (Neher et al., 2011), and it may also lead to autoimmunity via antigen presentation (Huizinga et al., 2012).
We found that PU.1 siRNA consistently decreased microglial phagocytosis of Aβ42 (Fig. 8). The PU.1-regulated genes which are involved in this process are yet to be confirmed but some potential candidates are cytoskeletal genes (Berclaz et al., 2002; Weigelt et al., 2009) and DAP12 adapter protein (Henkel et al., 2002). Berclaz et al. (2002) have previously shown that PU.1 rescues phagocytosis in mouse macrophages in a process involving cytoskeletal changes. Additionally, downregulation of the M-CSF receptor in vivo could reduce M-CSF-stimulated phagocytosis (Zhang et al. 1994). There are also likely to be previously unidentified PU.1-regulated proteins or microRNAs which can impact the phagocytic process.
We used a phagocytosis assay which has previously been validated by confocal microscopy and cytochalasin D inhibition of actin polymerization (Smith et al., 2010). However it is possible that some of the Aβ42 associated with microglia is adhered to the cell surface as opposed to phagocytosed.
It is possible that decreased phagocytic ability of microglia is a consequence of overall decreased microglial viability. However, previous reports demonstrate that decreased viability is not always associated with decreased phagocytosis. We have reported that whilst Valproic acid (VPA) treatment of BV2 microglia reduces their viability, it also increases their phagocytic capacity (Smith et al., 2010). Conversely, 1 mM VPA treatment of primary adult human microglia does not reduce their viability, but does reduce their phagocytic capacity alongside a decrease in PU.1 expression (Gibbons et al., 2011). Altogether this data suggests that there is a more specific mechanism(s) at play whereby reduced PU.1 leads to decreased phagocytosis in primary human microglia.
In conclusion, this article establishes the importance of the transcription factor PU.1 to microglia in adult human brains. We show that PU.1 has important roles in the function of human microglia. Ultimately we may be able to harness PU.1, or downstream targets of PU.1, to modulate microglial activity for treatment of brain disorders.
Acknowledgments
The authors are most grateful to the donors and their families for the gift of brain tissue for the ongoing research into brain disorders. The authors thank Lynair Roberts, specialist epilepsy nurse at Auckland City Hospital, for co-ordination of tissue collection; Claire Lill and Inna Semenyajenko (Center for Brain Research Biobank) for help with tissue processing; Dr. E. Scott Graham and Dr. Thomas Park for critical proof-reading of manuscript; Natacha Coppieters for help with immunohistochemistry pretreatments; Dr. Shu Chin Ma (Gus Fisher Postdoctoral Research Fellow) for early technical help with the siRNA studies; and the University of Auckland Biomedical Imaging Research Unit for assistance with confocal microscopy.