Regulation of AQP4 surface expression via vesicle mobility in astrocytes
Abstract
Aquaporin 4 (AQP4) is the predominant water channel in the brain, expressed mainly in astrocytes and involved in water transport in physiologic and pathologic conditions. Besides the classical isoforms M1 (a) and M23 (c), additional ones may be present at the plasma membrane, such as the recently described AQP4b, d, e, and f. Water permeability regulation by AQP4 isoforms may involve several processes, such as channel conformational changes, the extent and arrangement of channels at the plasma membrane, and the dynamics of channel trafficking to/from the plasma membrane. To test whether vesicular trafficking affects the abundance of AQP4 channel at the plasma membrane, we studied the subcellular localization of AQP4 in correlation with vesicle mobility of AQP4e, one of the newly discovered AQP4 isoforms. In cultured rat astrocytes, recombinant AQP4e acquired plasma membrane localization, which resembled that of the antibody labeled endogenous AQP4 localization. Under conditions mimicking reactivation of astrocytes (increase in cytosolic cAMP) and brain edema, an increase in the AQP4 plasma membrane localization was observed. The cytoskeleton remained unaffected with the exception of rearranged actin filaments in the model of reactive astrocytes and vimentin meshwork depolymerization in hypoosmotic conditions. AQP4e vesicle mobility correlated with changes in the plasma membrane localization of AQP4 in all stimulated conditions. Hypoosmotic stimulation triggered a transient reduction in AQP4e vesicle mobility mirrored by the transient changes in AQP4 plasma membrane localization. We suggest that regulation of AQP4 surface expression in pathologic conditions is associated with the mobility of AQP4-carrying vesicles.
INTRODUCTION
The aquaporin-4 (AQP4) water channel is one of the three AQPs identified in brain cells in vitro and in vivo (Amiry-Moghaddam and Ottersen, 2003; Badaut et al., 2002; Hasegawa et al., 1994; Jung et al., 1994; Nielsen et al., 1997). The strongest expression of AQP4 isoforms in rodent and nonhuman primate brain is in astrocytic end feet surrounding the blood–brain barrier (Arcienega et al., 2010; Nagelhus et al., 1998; Neely et al., 1999; Nielsen et al., 1997). In addition, AQP4 was identified in astrocytic processes in contact with synapses (Badaut et al., 2000,b; Nielsen et al., 1997). It was suggested that AQP4 is involved in water transport in several physiologic processes and that it plays an important role in astrocyte swelling and brain edema formation/resolution under various pathologic conditions, both in vitro (Arima et al., 2003; Yamamoto et al., 2001) and in vivo (Ke et al., 2001; Manley et al.2000; Papadopoulos et al., 2004). In the brain, astrocytes interact with neurons and blood vessels (Haydon, 2001; Nedergaard et al., 2003). Thus, the astrocyte pool of AQP4 is in a unique position to control water transport in brain edema, which is associated with many neurologic disorders (brain tumor, brain abscess, meningitis, stroke, head injury) and extracranial pathologies that have a secondary effect on the brain (Papadopoulos et al., 2004), including reactivation of astrocytes around the damaged area (Eddleston and Mucke, 1993; Jing et al., 2007; Pekny et al., 1999; Pekny and Pekna, 2004; Pekny and Nilsson, 2005). Water transport through the cell membrane may be regulated by the permeability properties of AQP4 (Gunnarson et al., 2008; Nicchia et al., 2011) and the heterogeneity of AQP4 crystalline-like orthogonal arrays of particles (Hirt et al., 2011). In addition, water transport may be regulated by the mobility of AQP4 vesicles to/from the plasma membrane, but this process has not been studied in detail. To study the latter possibility, we measured the mobility of AQP4 vesicles and AQP4 plasma membrane abundance. For this, we selected the newly described basic AQP4 isoform AQP4e. Water channel AQP4 exists in several isoforms that have been described over the last decade. In addition to M1 and M23, the first two described rat brain AQP4 isoforms (Jung et al., 1994), four new AQP4 isoforms were recently identified by screening the cDNA rat brain library (Moe et al., 2008). The new isoforms were named AQP4b, AQP4d, AQP4e, and AQP4f and their role and tissue localization remains to be unraveled and confirmed. The newly described AQP4 isoform AQP4e, also described as Mz isoform, is the largest known AQP4 isoform and, similar to M1 and M23, transports water across the plasma membrane (Moe et al., 2008; Rossi et al., 2011). In this study we aimed to describe properties of AQP4e vesicle mobility and to correlate this with the AQP4 plasma membrane localization in pathologic conditions.
In general, astrocytic vesicle trafficking in astrocytes can be altered in reactive astrocytes (Potokar et al., 2010, 2011), however, the characteristics of AQP4 vesicle traffic remain unknown, and it is unclear how AQP4 trafficking relates to the extent of AQP4 localized at the plasma membrane. To address these questions we simulated pathologic conditions: (1) an in vitro model of reactive astrocytosis induced by dibutyryl cAMP (dbcAMP) (Daginakatte et al., 2008; Nicchia et al., 2008); (2) high levels of intracellular free calcium [Ca2+]i; and (3) hypoosmotic cell swelling (Pangršič et al., 2006), which occurs in brain edema (Nase et al., 2008; Papadopoulos et al., 2004; Risher et al., 2009).
We report that AQP4 plasma membrane localization increased after treatment with dbcAMP and after hypoosmotic stimulation. The extent of colocalization of AQP4 and plasma membrane correlated with changes in AQP4e vesicle mobility, while the effect on the cytoskeleton was stimulation-specific. We propose that, in astrocytes, vesicle dynamics importantly contributes to surface expression of AQP4 water channel.
MATERIALS AND METHODS
Cell Cultures
Cortical astrocytes were isolated from 3-day-old Wistar rats (Schwartz and Wilson, 1992) and cultured in growth medium (high-glucose Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (FBS), 1 mM pyruvate, 2 mM glutamine and 25 μg mL−1 penicillin/streptomycin at 37°C, 95% air/5% CO2) (Pangršič et al., 2006). Astrocytes were plated onto 22-mm diameter glass coverslips coated with poly-L-lysine (PLL) or BD Matrigel Matrix (MTG; BD Biosciences) according to the manufacturers' instructions and were used within 3 days. All chemicals for maintaining the cell cultures and for the experiments were purchased from Sigma, unless stated otherwise.
Cell and Vesicle Labeling
Subconfluent astrocytes were transfected with pAQP4e-GFP (AQP4e) and pAQP4d-GFP(AQP4d) using Lipofectamine transfecting reagent (Invitrogen) according to the manufacturer's instructions and supplied by UltroserG serum (3%, Life Technologies), or labeled with LysoTracker dye (200 nM, 5 min, 37°C, Invitrogen). Plasma membrane was labeled with fluorescent lipophilic Vybrant® DiD labeling solution (DiD, 5 μM, 2 min, 37 C, Invitrogen).
For immunocytochemistry, cells were rinsed in phosphate buffered saline (PBS), fixed in 4% formaldehyde (15 min) and permeabilized with 0.1% Triton X-100 (10 min) at room temperature. DiD-labeled cells were fixed in 2% formaldehyde. The nonspecific background staining was prevented by blocking solution (3% bovine serum albumin (BSA) and 10% goat serum in PBS, 37 C, 1 h). Antibodies were diluted in 3% BSA in PBS and applied to cells (37 C, 2 h or 4 C overnight for primary antibodies and 37 C for 45 min for secondary antibodies). When two primary antibodies (raised in different species) were used, they were applied sequentially. Cells were mounted onto glass slides using Slowfade Gold antifade reagent (Invitrogen). Primary antibodies used were: rabbit polyclonal to AQP4 (1:400, Alomone Labs), LAMP1 (1:300, Abcam), β-actin (1:200, Abcam), and vimentin (1:200, Abcam); mouse monoclonal to cis-Golgi marker GM130 (1:700, BD Biosciences), α-tubulin (1:100, Sigma), GFAP (1:100, Sigma), and EEA1 (1:300, BD Biosciences). Secondary antibodies used were: goat anti-rabbit or goat anti-mouse IgG conjugated to fluorescent dyes (Alexa Fluor 488 or 546, 1:600, Invitrogen).
Imaging
Imaging was performed with an inverted confocal microscope (Zeiss LSM 510 META, Zeiss LSM 780) using an oil-immersion objective (63×/NA 1.4). EGFP and the Alexa Fluor 488 were excited by the 488-nm line of the argon laser and the emission light was collected through the bandpass filter (505–530 nm). Alexa Fluor 546 was excited with the He/Ne laser (543 nm) and the emission light was filtered with the longpass filter, with the cutoff below 560 nm. To excite DiD dye, the He/Ne laser was used (633 nm) and the emission light was filtered with the longpass filter with the cutoff below 650 nm. To eliminate possible bleed through, the green and red emission fluorescence was acquired sequentially. In live cells, time series images were recorded in 2-s intervals. Recordings of unstimulated vesicle mobility were performed in 300 mOsm (isoosmolar) extracellular solution (control) consisting of (in mM): 130 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 D-glucose, 10 HEPES (pH 7.2). Cells were stimulated with: (A) dbcAMP (1 mM, 3 h and 24 h, 37 C); dbcAMP treatment induces stellate morphology in astrocytes, high levels of GFAP expression, and modified expression of a high number of genes, which are all features of reactive gliosis (Daginakatte et al., 2008); (B) calcium ionophore ionomycin (4 μM, 1 min, room temperature), which triggers an increase in [Ca2+]i (Potokar et al., 2008); and (C) with 200 mOsm hypoosmolar solution (HYPO), which evokes cell swelling (Pangršič et al., 2006).
Analysis of Colocalization and Vesicle Mobility
The extent of the colocalization between the fluorescent probes was determined using the ColocANA software tool (Celica, Slovenia) (Kreft et al., 2004; Potokar et al., 2006). Briefly, the software summed the area above the 20% threshold level for each channel and for costained regions of the image and counted the number of green, red, and costained pixels (Kreft et al., 2004). The degree of colocalization is expressed as the extent (%) of costained pixels in the cell in comparison with pixels representing aquaporin or the plasma membrane.
Vesicle mobility was analyzed using ParticleTR software (Celica, Slovenia). The parameters of vesicle mobility, such as the current time (time from the beginning of a single vesicle tracking), step length (displacement of a vesicle in a 2-s time interval), TL (the total length of the vesicle pathway analyzed), velocity, MD, and directionality were estimated as described (Potokar et al., 2005, 2007) for periods of 30 s from two independent cultures. Directionality and subgrouping into directional vesicles (DV) and nondirectional vesicles (NDV) was described previously (Potokar et al., 2005). Statistical analysis was performed with the Student t test and ANOVA. Values are expressed as mean ± SEM. Regression lines were compared by Stattools (www.stattools.net).
RESULTS
Recombinant AQP4e Resides Predominantly at the Plasma Membrane
Several AQP4 isoforms are expressed in rat brain: three basic isoforms M1/a, M23/c, Mz/e and their splicing variants b, d, f (Jung et al., 1994; Moe et al., 2008; Neely et al., 1999; Rossi et al., 2011). All three basic isoforms transport water across the plasma membrane, including the newly described AQP4e. Water transport across the plasma membrane could be determined by the plasma membrane extent of AQP4e, which could be governed by the mobility properties of AQP4e vesicles.
First we quantitatively tested the plasma membrane localization of endogenously expressed AQP4 labeled by the anti-AQP4 antibody and both transfected AQP4 isoforms (plasma membrane isoform e and d, predicted to be mainly intracellular). The plasma membrane was labeled with DiD (Fig. 1A). The extent of AQP4e at the plasma membrane was similar to endogenous AQP4 (P = 0.21) and significantly higher than AQP4d (Fig. 1). We observed that the composition of the cell adherence substrates affects the plasma membrane localization of AQP4. Endogenous AQP4 was significantly more colocalized to the plasma membrane in astrocytes attached to the BD Matrigel basement membrane matrix (MTG) than in astrocytes attached to poly-L-lysine (PLL) (Fig. 1D).
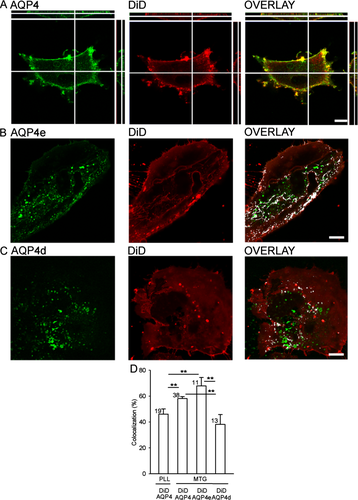
AQP4e is the predominant plasma membrane AQP4 isoform in rat astrocytes. (A) Colabeling of astrocyte with AQP4 antibody and the lipophilic membrane dye DiD. DiD and AQP4 show similar plasma membrane distribution. The cross-sections right and above panels A show the absence of staining in the cell interior. (B) The plasma membrane of AQP4e-GFP (AQP4e) transfected astrocytes was labeled with DiD. The overlay shows overlapping fluorescence (white colocalization mask) between AQP4e and DiD (66% in the image shown). (C) AQP4d-GFP (AQP4d) transfected astrocytes with DiD-labeled plasma membrane. The overlay shows overlapping fluorescence (white colocalization mask) between AQP4d-GFP and DiD (33% in the cell shown). (D) The extent of colocalization between fluorescently labeled plasma membrane (DiD) and fluorescently labeled endogenous AQP4 or transfected AQP4 isoforms (e and d). The colocalization between DiD and endogenous AQP4 was 46% ± 4% in the PLL attached cells, significantly lower than in the MTG attached cells: endogenous AQP4 (58% ± 2%) and transfected AQP4e (67% ± 7%). AQP4d had the lowest colocalization with DiD (38% ± 8%). **P < 0.01. Numbers in bars indicate the number of cells analyzed. Scale bars: 10 μm.
AQP4d also localized to the plasma membrane, which is in contrast to the report by Moe et al. (2008) for the HeLa cell line. Given the observed discrepancy in the plasma membrane localization of AQP4d, we further tested the subcellular localization of both transfected isoforms (Fig. 2). In the Golgi apparatus (GA), the localization of AQP4d was significantly higher than AQP4e and endogenous AQP4 (P < 0.001) (Fig. 2A,C), yet the latter two were similarly localized to the GA (P = 0.39, Fig. 2C). On the other hand, comparison of endogenous AQP4, AQP4e, and AQP4d showed significantly higher colocalization with late degradation compartments identified by anti-LAMP1 labeling: endogenous AQP4 was significantly less colocalized (P < 0.001) than AQP4d and AQP4e that were comparable (P = 0.10; Fig. 2B,C). Endogenous AQP4 was also found in early endosomes, labeled by anti-EEA1 (8.1% ± 1%, Fig. 2C). In addition, AQP4e was to a minor extent (2.5% ± 1%, Fig. 2C) colocalized with LysoTracker, which labels acidic compartments, such as endosomes and lysosomes (Blanchette et al., 2009) and was optimized for labeling of endosomes/lysosomes in astrocytes (Potokar et al., 2010). The localization of endogenous AQP4 was compared with the localization of the predominant plasma membrane isoform, AQP4e. The measured colocalization between both AQP4s was high (86% ± 1%, Fig. 2C).
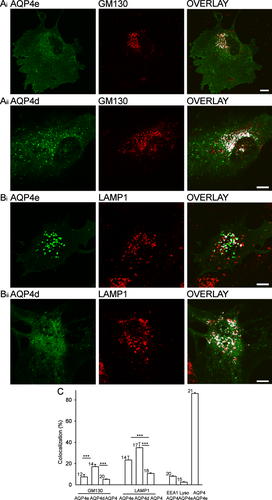
AQP4d but not AQP4e is strongly confined to LAMP1-positive vesicles. (Ai,ii) The fluorescence of AQP4e-GFP and AQP4d-GFP in single rat astrocytes (left panels), fluorescently labeled Golgi apparatus (GA, middle panels, anti-GM130 antibody), and superimposed fluorescence signals with colocalized pixels (white, right panels). The extent of colocalized fluorescence in the cells shown was 6% (Ai) and 16% (Aii). (Bi,ii) The fluorescence of AQP4e-GFP and AQP4d-GFP in single rat astrocytes (left panels), immunolabeled late endosomal compartments (middle panels, anti-LAMP1), and superimposed fluorescence signals with white colocalization masks (right panels). The extent of colocalized fluorescence in the cells shown was 24% (Bi) and 22% (Bii). (C) The average colocalization of GM130 with AQP4d (17% ± 2.1%), was significantly higher than with AQP4e (7.4% ± 2.4%) or endogenous AQP4 (5.2% ± 0.3%) (white bars). Both transfected AQP4 isoforms were colocalized significantly more with degradation compartments (AQP4e, 23.4% ± 3.8%; AQP4d, 34.9% ± 5.0%) than endogenous AQP4 (10.8% ± 0.6%) (gray bars), ***P < 0.001. The colocalization between EEA1 and AQP4 was 8.1% ± 0.5%, between LysoTracker and AQP4e 2.5% ± 0.5%, and between AQP4 and recombinant AQP4e isoform 86.0% ± 1.0%. Numbers in bars indicate the number of cells analyzed. Scale bars: 10 μm.
The data show that endogenous AQP4 in astrocytes resides predominantly at the plasma membrane and to a small extent in secretory and degradation compartments. The predominant plasma membrane isoform AQP4e follows this subcellular distribution and was used to monitor whether the extent of AQP4 in the plasma membrane correlates with vesicle mobility under various experimental conditions.
dbcAMP-Mediated Increase in the Localization of AQP4 at the Plasma Membrane and Decrease in the Mobility of AQP4e Vesicles
Quantitative analysis of superimposed fluorescence signals showed that dbcAMP treatment triggered an increase in AQP4 plasma membrane localization to: 53% ± 3% (15 min), 59% ± 2% (30 min), 63% ± 2% (3 h), 54% ± 2% (24 h) compared with control cells (41% ± 3%, P < 0.05, Fig. 3A). These results are consistent with the study by Nicchia et al. (2008), who qualitatively, but not quantitatively, reported that dbcAMP induced increased staining of AQP4 at the plasma membrane of rat astrocytes.
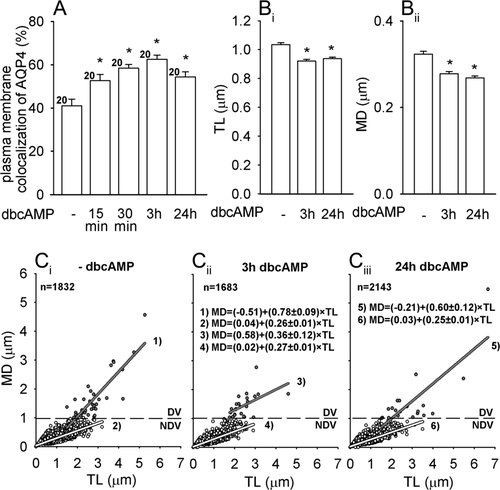
cAMP triggers a time-dependent increase in the plasma membrane localization of AQP4 and significantly decreases the mobility of AQP4e vesicles. (A) Plasma membrane colocalization of AQP4 (%) represents the extent of colocalization between the immunolabeled AQP4 and the fluorescently labeled plasma membrane (DiD). dbcAMP triggered a time-dependent increase in AQP4 plasma membrane localization reaching the peak after 3 h treatment, *P < 0.05. (Bi,ii) dbcAMP induced a significant decrease in TL and MD, *P < 0.05. The mobility was tested at the AQP4 plasma membrane localization peak after 3 h and after 24 h incubation with dbcAMP. (Ci–iii) The directionality of the vesicles (MD/TL). The linear equation (MD = y0 + a × TL) was fitted to the data. The slopes (a) of the line were significantly different (P < 0.05) between DV in nonstimulated vs. dbcAMP-stimulated cells (3 h) and between NDV in non-stimulated vs. dbcAMP-stimulated cells (24 h). Gray circles, lines—DV (directional vesicles), white circles, lines—NDV (nondirectional vesicles). n(n) denoted in C refers also to B. Figures 3-5: dashed lines: 1 μm, n(n) = number of cells (number of vesicle periods examined).
The finding that plasma membrane enrichment of AQP4 occurs relatively quickly led to the question whether this may be related to altered AQP4 vesicle mobility. To test this presumption, AQP4e transfected astrocytes were treated with dbcAMP and the mobility parameters of the AQP4e vesicles were determined. The average track length (TL), maximal displacement (MD), and vesicle speed were affected significantly (Fig. 3B). TL (in μm) was significantly higher (1.03 ± 0.01, P < 0.05) in controls (no added dbcAMP), than in dbcAMP-treated cells [0.92 ± 0.01 (3 h) or 0.94 ± 0.01 μm (24 h)]. Similarly, dbcAMP significantly affected MD (in μm): (0.28 ± 0.00) (3 h) and 0.27 ± 0.00 (24 h) vs. controls (0.32 ± 0.01, P < 0.05, Fig. 3B). The effects on vesicle speed were small but significant (in μm s−1): 0.031 ± 0.001 (dbcAMP 3 h and 24 h) vs. 0.035 ± 0.001 (controls); P < 0.05. Vesicle directionality (MD/TL ratio) was also affected (Fig. 3C). The slopes (a) describing directionality were significantly lower in directional vesicles (DV) after 3 h stimulation vs. control (-dbcAMP): 0.79 ± 0.09 (-dbcAMP), 0.36 ± 0.12 (dbcAMP, 3 h), 0.60 ± 0.12 (dbcAMP, 24 h). NDV retained similar directionality, albeit it was significantly different between dbcAMP 3 and 24 h (Fig. 3). Also, the extent of DV was lower after dbcAMP treatment [2.1% (control), 1.4% (dbcAMP, 3 h), 0.9% (dbcAMP, 24 h); Fig. 3C].
dbcAMP may also trigger an increase in [Ca2+]i (Slamon et al., 2005). To test the effect of increased [Ca2+]i separately, we applied ionomycin (Potokar et al., 2005), which triggered significant reductions in MD. TL remained similar as in non-stimulated controls (Fig. 4A), but the directionality of AQP4e vesicles was severely affected: DV were absent (Fig. 4B). The slopes of the line fitted to the data were significantly higher (P < 0.05) in controls vs. stimulated cells.
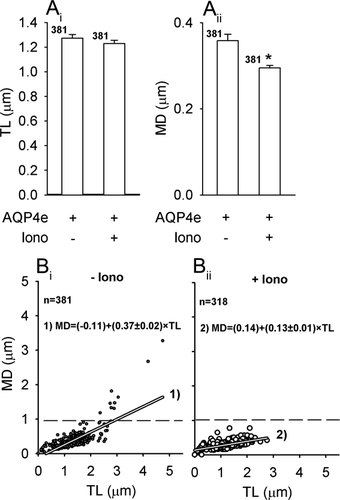
Ionomycin triggers a decrease in the mobility of AQP4e vesicles. (A) Ionomycin stimulation (Iono) induced a small decrease in the average TL and MD of AQP4e vesicles. TL before Iono, 1.27 ± 0.03 μm (Iono −); TL after Iono (Iono +), 1.23 ± 0.03 μm; MD (Iono −), 0.36 ± 0.03 μm; MD (Iono +), 0.29 ± 0.01 μm; **P < 0.01. (B) The directionality of vesicles represented by the ratio MD/TL and described by the line with the equation (MD = y0 + a × TL). The slopes (a) of the line were significantly different (P < 0.05) between DV and NDV at Iono− vs. Iono+.
We tested whether the changes in vesicle mobility are correlated with substantial rearrangements of the cytoskeleton. For this, we stimulated cells either with ionomyin or dbcAMP and immunolabeled the following cytoskeleton filaments: microtubules, actin filaments, GFAP and vimentin filaments. The results show that none of the labeled cytoskeleton filaments were depolymerized after stimulation with either ionomycin or dbcAMP. The only major rearrangement observed was in actin cytoskeleton, which acquired near plasma membrane distribution following dbcAMP application (Fig. 6), as described previously (Nicchia et al., 2008).
Altogether, these results show that dbcAMP increases the plasma membrane density of AQP4 and that effect of [cAMP]i and of [Ca2+]i on the mobility of AQPe-carrying vesicles is similar. Interestingly, the increase in the plasma membrane localization of AQP4 is accompanied by decreased mobility of directional vesicles transporting AQP4e isoform.
Hypoosmolarity Induced Transient Changes in AQP4e Vesicle Mobility Likely Also via Disintegration of the Vimentin Cytoskeleton
Hypoosmotic conditions also affected plasma membrane localization of AQP4 in rat astrocytes. In comparison with unstimulated cells, hypoosmotic stimulation triggered a transient increase in the AQP4 plasma membrane localization (Fig. 5A). Then we tested whether these changes were related to changes in AQP4e vesicle trafficking. Hypoosmotic stimulation first slightly but not significantly reduced the TL of vesicles: 1.93 ± 0.06 μm (−HYPO) to 1.82 ± 0.05 μm (HYPO 2 min). Subsequently TL significantly increased to 2.09 ± 0.07 μm (HYPO 10 min, P < 0.05 vs. HYPO 2 min; Fig. 5Bi). MD followed similar pattern: 0.53 ± 0.05 μm (-HYPO), 0.49 ± 0.03 μm (HYPO 2 min), 0.65 ± 0.05 μm (HYPO 10 min, P < 0.05 vs. HYPO 2 min; Fig. 5Bii). The average vesicle speed remained similar: 0.06 ± 0.002 μm s−1 (control), 0.06 ± 0.001 μm s−1 (HYPO 2 min) and was significantly higher after 10 min 0.07 ± 0.002 μm s−1 (HYPO 10 min, P < 0.05 vs. HYPO 2 min). The directionality of the vesicle pathways transiently decreased at HYPO 2 min, exclusively on the account of DV; the slopes at control (P = 0.002) and HYPO 10 min (P = 0.001) were significantly higher than HYPO 2 min (Fig. 5C). Taken together, an increase in the AQP4 plasma membrane localization overlaps with decreased mobility of AQP4e vesicles at HYPO 2 min and subsequent decrease in the AQP4 plasma membrane localization at HYPO 10 min overlaps with increased AQP4e vesicle mobility. Again, changes in mobility are predominantly on the account of DV.
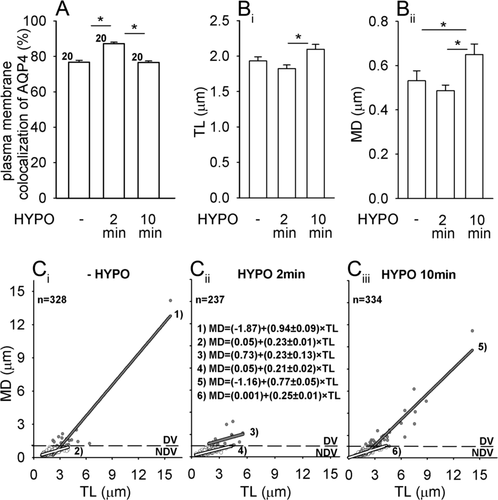
Hypoosmotic stimulation induces a transient increase in the plasma membrane localization of AQP4 and alterations in AQP4e vesicle mobility. (A) Plasma membrane colocalization of AQP4 (%) denotes the extent of colocalization between the immunolabeled AQP4 and the fluorescently labeled plasma membrane (DiD). Compared with unstimulated cells (79.7% ± 1.02 %, - HYPO), hypoosmotic stimulation first triggered an increase in the AQP4 plasma membrane colocalization (87.1% ± 0.86%, HYPO 2 min), followed by a decrease (76.6% ± 0.86%, HYPO 10 min), *P < 0.05. n, number of cells. (B) Average TL and MD transiently decreased and subsequently increased in hypoosmotic conditions, *P < 0.05. (C) Directionality of vesicles (MD/TL) described by the line (MD = y0 + a × TL). The slope (a) of DV was significantly lower at HYPO 2 min and of NDV significantly higher at HYPO 10 min vs. 2 min.
We also explored whether hypoosmotic stimulation affects the integrity of the cytoskeleton meshwork. To achieve this, several types of astrocyte cytoskeleton components were immunolabeled under control conditions and after hypoosmotic stimulation. The hypoosmolar solution triggered a substantial fragmentation of vimentin filaments after 2 min, but microtubules, actin filaments, and glial fibrillary acidic protein (GFAP) filaments appeared unaffected (Fig. 6).
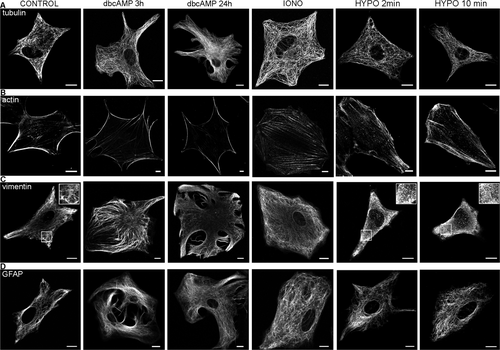
Different changes in the cytoskeleton are evident in reactive astrocytes and after hypoosmotic stimulation. Several types of cytoskeleton in rat astrocytes were immunolabeled under isoosmotic conditions (CONTROL), at high [Ca2+]i (IONO), and after stimulation with dbcAMP (3 and 24 h) or hypoosmotic solution (HYPO; 2 and 10 min): (A) microtubules with anti-α tubulin; (B) actin filaments with anti-β actin; (C) vimentin network with anti-vimentin; and (D) glial fibrillary acidic protein with anti-GFAP antibody. Note that dbcAMP induced rearrangement of actin filaments and hypoosmotic conditions triggered depolymerization of vimentin filaments, selected areas (white squares) are 2× magnified in insets (C). Bars: 10 μm.
DISCUSSION
In this study, we show that AQP4 is primarily located at the plasma membrane of cultured cortical astrocytes (Figs. 1 and 2) and that the directional mobility of AQP4 carrying vesicles importantly contributes to the plasma membrane localization of AQP4 channel in conditions mimicking reactive astrocytosis and brain edema (Figs. 3-5). Alterations observed in the vesicle mobility correlate well with alterations in the density of AQP4 at the plasma membrane.
AQP4e Is the Essential Plasma Membrane Isoform in Cultured Rat Astrocytes
AQP4 immunofluorescence in cultured cortical astrocytes was reported to display a punctuate pattern, which appears to be distributed across astrocytes (Nicchia et al., 2005, 2008). We have shown that such a pattern is particularly prominent at the plasma membrane and that AQP4 plasma membrane localization can be affected by the nature of the cell attachment substrate. A higher AQP4 plasma membrane signal was measured in cells attached to the substrate, where a major component is laminin, one of the key functional components of all basement membranes, including the one covering astroglial end feet (Sixt et al., 2001; Wolburg et al., 2009). These results show that it is important to consider the type of cell attachment substrate when interpreting the extent of AQP4 in the plasma membrane in in vitro experiments.
The AQP4 channel exists in several isoforms with AQP4e being the largest channel that transports water in Xenopus oocytes (Moe et al., 2008). According to qualitative confocal analysis of recombinant AQP4e in an astrocyte cell line, this isoform is present at the plasma membrane and in the cytoplasm (Moe et al., 2008). We quantitatively confirmed the distribution of transfected AQP4e in the plasma membrane in primary cortical rat astrocytes, its localization was not significantly different from endogenous AQP4 (P = 0.21; Fig. 1). In addition to the plasma membrane, AQP4e was also localized to the GA, similar to endogenous AQP4, and the degradation compartments (Fig. 2), higher than endogenous AQP4, likely due to the overexpression of AQP4e. In comparison with the AQP4d isoform, AQP4e was less confined to the GA or LAMP1-positive compartments (Fig. 2). In contrast to findings on HeLa cell line (Moe et al., 2008), where AQP4d was found only in cytoplasm, we found it also in the plasma membrane. We can speculate that, in astrocytes, AQP4d may also be implicated in the regulation of water transport through the plasma membrane, however, this remains to be tested. The findings of AQP4 in LAMP1-positive compartments (Fig. 2) are consistent with data by Madrid et al. (2001). Once endocytosed, AQP4 may recycle back to the cell surface or enter the late endocytic pathway for degradation (Madrid et al., 2001).
The plasma membrane localization of AQP4 is probably governed by demands for water transport regulation. We propose that vesicle trafficking may be one of the key regulating steps. Altered water regulation occurs in trauma (Papadopoulos et al., 2004), where astrocytes also become reactive (Pekny and Nilsson, 2005), therefore we explored the dynamic properties of the predominant plasma membrane isoform, AQP4e (Moe et al., 2008) in the model of reactive gliosis and in hypoosmotic conditions.
AQP4e Vesicle Mobility Is Diminished in a Model of Reactive Gliosis
Nicchia et al. (2008) demonstrated that dbcAMP positively modulates the level of AQP4 protein expression after long-term treatment (24 h) and speculated that increase in AQP4 in plasma membrane is related to the acquisition of the reactive phenotype. Further tests showed that dbcAMP treatment increased the water permeability, measured after 24 h (Nicchia et al., 2008). We quantified the increase in AQP4 in plasma membrane after dbcAMP treatment and found that it can be observed as early as after 15 min and its increased localization remains high for 24 h (Fig. 3). Prompt changes implicate additional regulation in a much faster time domain than on the level of mRNA expression (Nicchia et al., 2008). It appears that, in reactive astrocytes, the alterations in water permeability might also be linked to the fast regulation of delivery/retraction of directional AQP4s vesicles to/from the plasma membrane.
This possibility was tested by measuring the mobility parameters of vesicles carrying AQP4e. In unstimulated conditions, the mobility of AQP4 vesicles resembled the mobility of slow recycling and endosomal vesicles (Potokar et al., 2008; Potokar et al., 2010; Stenovec et al., 2007). dbcAMP treatment to induce reactive astrocytosis diminished AQP4e vesicles TL by 10% and MD by 15% (Fig. 3B). This might be an important regulatory mechanism to alter the delivery/retraction ratio of AQP4 vesicles to/from the plasma membrane in reactive astrocytes. Decreased mobility with significantly lower directionality might contribute to restraining the AQP4 vesicles near the plasma membrane and may also be linked to dbcAMP-induced rearrangements of the F-actin cytoskeleton mesh already speculated to be one of the major factors responsible for increased AQP4 plasma membrane localization (Nicchia et al., 2008). After treatment with dbcAMP Nicchia et al. (2008) observed rearrangements of actin filaments along the plasma membrane, whereas non-stimulated astrocytes showed F-actin mainly organized in stress fibers. The same rearrangements of actin cytoskeleton were also observed in our study and they correlated in time with increased AQP4 plasma membrane localization of AQP4 and diminished AQP4e mobility (Fig. 6). In line with this, actin cytoskeleton dynamics have an important role in the mobility of several other vesicle types in astrocytes [ANP vesicles in the secretory pathway (Potokar et al., 2007), VGLUT1 and ANP vesicles in recycling pathways (Potokar et al., 2008; Stenovec et al., 2007) and endosomal/lysosomal vesicles (Potokar et al., 2011)]. The possibility that dbcAMP in astrocytes regulates AQP4 plasma membrane localization through phosphorylation, was rejected; in fact no phosphorylated AQP4 was found (Nicchia et al., 2008, Yamamoto et al., 2002). However, different cell types obviously exhibit different strategies, because, in epithelial MDCK cells, phosphorylation appeared crucial for intracellular targeting (Madrid et al., 2001). When interpreting the effects of dbcAMP on vesicle mobility, we have to take into account several other effects of dbcAMP, such as intermediate filament changes (Yamamoto et al., 2002) and increases in [Ca2+]i (Slamon et al., 2005). Mild alteration in staining of the intermediate filament protein GFAP reported by Nicchia et al. (2008) was absent in our experiments.
Increases in [Ca2+]i Decrease the Mobility of AQP4e Vesicles
An increase in [Ca2+]i differentially affects vesicle mobility in rat astrocytes and appears to be vesicle type specific (Potokar et al., 2007, 2008, 2010; Stenovec et al., 2007). The speed of AQP4e vesicles was unaffected by increases in [Ca2+]i, as observed in vesicles on their way to the plasma membrane (Potokar et al., 2005, 2007). AQP4e vesicles were slow, according to TL and MD, resembling vesicles in degradation pathways (Potokar et al., 2008, 2010) and decreased only mildly after ionomycin treatment compared with the two- to threefold decrease observed in ANP recycling vesicles (Potokar et al., 2008). Their directionality, however, was greatly affected by ionomycin addition (Fig. 4) similar to ATP vesicles (Pangršič et al., 2007) and ANP recycling vesicles (Potokar et al., 2008, 2010). The mechanisms of Ca2+-dependent regulation of vesicle traffic are largely unclear (Potokar et al., 2010), but changes in [Ca2+]i may well induce alterations in cytoskeleton functions and in vesicle attachment to the cytoskeleton. These data may give us an insight on whether the astrocyte cytoskeleton is engaged in pre- and postfusion vesicle mobility; specific engagement of the cytoskeleton in the mobility of AQP4 vesicles is yet to be unraveled. By looking closely at the alterations in mobility pattern, we may assume that the initial small decrease in vesicle mobility in hypoosmotic conditions is associated with the transient increase in [Ca2+]i (Benfenati et al., 2001; Pangršič et al., 2006; Thrane et al., 2011) followed by delayed rearrangements of the cytoskeleton that may favor increased vesicle mobility. Defragmentation of vimentin cytoskeleton (Fig. 6) may thus affect vesicle mobility by decreasing steric hindrance in the dense network of filaments. In addition to changes in vesicle mobility, the observed increase of the plasma membrane localized AQP4e in reactive astrocytosis could reflect increased rate of exocytosis of the primed AQP4e-carriying vesicles.
Hypoosmotic Stimulation Affects AQP4 Plasma Membrane Localization, AQP4e Vesicle Mobility, and Cytoskeleton Dynamics
Despite high clinical importance, the molecular mechanisms of brain edema formation, development, and alleviation remain poorly understood. The role of the main water channel AQP4 in brain edema formation has been described (Manley et al., 2000); however, the exact mechanisms of brain edema remodeling remain to be resolved. APQ4 responses to water imbalance are most probably regulated at different levels. Several studies have reported response at the level of expression; however, these data are somehow conflicting. On one hand, an upregulated expression of AQP4 was detected in humans and rodents in pathophysiologic conditions (Aoki et al., 2003; Saadoun et al., 2002, 2003; Sun et al., 2003; Taniguchi et al., 2000) and downregulation was detected adjacent to the site of injury (Sun et al., 2003). But Ke et al. (2001) detected a decrease in AQP4 expression in vasogenic edema induced by traumatic lesions at day 1 post injury, while in others AQP4 expression in edematous regions remained unaltered. In addition to various responses measured in different regions, also different AQP4 isoforms may have different functions or regulatory mechanisms (Neely et al., 1999). The tissue distribution of different isoforms is not conclusive and it is best described for M1 and M23, which were confirmed in perivascular astrocytic end feet (Neely et al., 1999). AQP4e is probably localized in glia end feet together with M1 and M23 (S⊘rb⊘ et al., 2008; 2012).
Regardless of different isoforms, during the early stages of brain edema formation astrocyte swell (Nase et al., 2008; Papadopoulos et al., 2004; Risher et al., 2009). A 30% reduction in osmolarity almost instantaneously triggered ∼20% increase in soma volume, measured in tissue (Thrane et al., 2011), and 5–60% increase in the cross-sectional area of cultured rat astrocytes (Pangršič et al., 2006; Takano et al., 2005). The increase in cell volume may be accompanied by the increased rate of membrane insertion of exocytic vesicles (Pasantes-Morales et al., 2002), although it was suggested that in astrocytes hypotonicity-induced cell swelling is mainly due to the plasma membrane unfolding (Pangršič et al.,2006). The prompt changes in cell volume must require fast adaptations in AQP4 permeability or availability (plasma membrane insertion or retraction), which depends on trafficking of vesicles carrying AQP4. None of the previous studies investigated AQP4 trafficking at the single vesicle level and we demonstrated a correlation between changes in AQP4 plasma membrane localization and changes in the mobility of AQP4e-carrying vesicles in hypoosmotic conditions. Although, an increase in cellular volume per se could affect vesicle mobility, we believe that altered mobility of AQP4e vesicles cannot be regarded simply as a consequence of the cell volume changes, especially because the directionality of vesicles was affected the most and we also observed alterations in cytoskeleton arrangement. In the conditions mimicking brain edema, the mobility of AQPe-carrying vesicles may be the rate limiting step for regulating the plasma membrane amount of AQP4 channel.
In summary, the data show that the plasma membrane localization of AQP4 and vesicle mobility of one of the major AQP4 isoforms is altered in reactive astrocytes and after hypoosmotic stimulation. Increased plasma membrane localization is correlated with diminished mobility of vesicles, which is probably correlated to the rearrangements of the cytoskeleton in reactive astrocytes and in hypoosmotic conditions. As Nicchia et al. (2008) have pointed out, an increase in the cytoplasmic concentration of cAMP triggers rearrangements in actin cytoskeleton, which may play an important part in retaining AQP4e vesicles near the plasma membrane. In hypoosmotic conditions, increased plasma membrane localization was again correlated with diminished mobility and vice versa. However, prominent changes in actin cytoskeleton were absent, but vimentin filaments were affected. We assume that different mechanisms are responsible for surface expression of AQP4 channels in different pathological conditions, but both significantly affect vesicle mobility, especially their directional mobility, which appears to be determined by cytoskeletal elements (Potokar et al., 2007).
Acknowledgments
The authors thank Dr Mateja Prebil for assistance with cell culture preparations.