Neuroinflammation in white matter tracts of Cnp1 mutant mice amplified by a minor brain injury
Abstract
Oligodendrocytes make myelin for rapid impulse propagation and contribute to the long-term survival of myelinated axons. The mechanisms by which oligodendroglial dysfunction(s) contribute to slowly progressive neurodegeneration are not well understood. Here, we demonstrate in Cnp1 mutant mice that secondary axonal degeneration in the subcortical white matter is associated with an age-dependent activation of both, innate and adaptive immune responses, including an expansion of infiltrating CD8+ T cells. While the detrimental role of lymphocytes in inherited myelin diseases is known, the role of activated microglia for the hypothetical cycle of inflammation/degeneration is unclear. We used a mild standardized cryolesion of the right parietal cortex to activate microglia at the vulnerable age of mouse puberty (postnatal day (P) 28). When applied to Cnp1 mutant mice, analyzed more than 3 months later, minor brain injury had acted as a “second hit” and significantly enhanced astrogliosis, microgliosis and axon degeneration, but not T cell infiltration. Interestingly, exacerbated neuropathological changes were also reflected by specific deterioration of working memory on top of an essentially normal basic behavior. We propose a model in which oligodendroglial dysfunctions can trigger a vicious cycle of neurodegeneration and low-grade inflammation that is amplified by nonspecific activators of the innate immune system. This interaction of genetic and environmental factors may be relevant for neuropsychiatric diseases associated with secondary neuroinflammation.
INTRODUCTION
In the higher nervous system, oligodendrocytes are best known for their role in synthesizing myelin, a multilayered membrane sheath that electrically insulates axons for rapid impulse propagation. Myelinated axons, bundled in “white matter” tracts, provide long range connections in the central nervous system and are critical for motor-sensory as well as for higher cognitive functions (Fields, 2008; Nave, 2010).
We have previously reported a novel role of oligodendrocytes in maintaining the functional integrity and long-term survival of myelinated axons. Importantly, this function of oligodendrocytes appears independent of the physiological role of myelin in speeding up impulse propagation. For example, mice lacking expression of the oligodendroglial protein 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNP) develop normally and are myelinated (Lappe-Siefke et al., 2003). However, despite the presence of myelin, these mice develop a progressive degeneration of myelinated axons that is associated with transport defects, axonal swellings (spheroids) and axonal degeneration, followed by premature death at around 1 year of age (Edgar et al., 2009; Lappe-Siefke et al., 2003; Rasband et al., 2005). We described a similar histological and ultrastructural phenotype in the myelinated Plp1 null mice (Edgar et al., 2004; Griffiths et al., 1998; Yin et al., 2006), in which neurodegeneration occurs later and is associated with premature death at about 2 years of age. In both mutants signs of degeneration can be detected in the first postnatal weeks and months, but restricted at this age to a subset of small caliber axons (Edgar et al., 2009). In contrast, a clearly dysmyelinated mutant, the MBP-deficient shiverer mouse, lacks these signs of secondary axon loss (Griffiths et al., 1998). Thus, the function of oligodendrocytes in myelin assembly can be genetically uncoupled from a second oligodendroglial function in maintaining axonal integrity.
Although oligodendrocytes may be essential for the survival of all myelinated axons, we hypothesized that this “neuroprotective” function is most important for axons that undergo phases of physiological stress, such as caused by low-grade inflammation in human myelin diseases. Since axonal degeneration in Cnp1 mutant mice is by itself associated with microglial activation (Lappe-Siefke et al., 2003), we hypothesized that the overall phenotype of this mouse model reflects a vicious cycle of low-grade inflammation and neurodegeneration, the extent of which could be modulated by non-genetic factors, including those that trigger the innate immune system of the brain. Here we show that a mild parietal cortical neurotrauma in juvenile mice “amplifies” the neuropathology of Cnp1 mutants and is associated with cognitive defects in working memory. We suggest a model in which minor brain trauma acts as a “second hit” in a neurodegenerative disease, which thereby determines the course of pathology in white matter tracts, most likely reflecting a vicious cycle of axonal degeneration and secondary inflammation.
MATERIALS AND METHODS
Animals
All experiments had been approved by the local Animal Care and Use Committee. Cnp1 null mutant mice of mixed 129Sv-C57Bl/6 background were generated previously by homologous recombination in embryonic stem cells (Lappe-Siefke et al., 2003). Genotyping was performed with primers Cnp-E3s, 5′-GCC TTC AAA CTG TCC ATC TC-3′; Cnp-E3as, 5′-CCC AGC CCT TTT ATT ACC AC-3′ and puro3, 5′-CAT AGC CTG AAG AAC GAG A-3′. Genomic DNA for PCR analysis was isolated from tail biopsies using Invisorb Spin Tissue Mini Kit (Invitek) according to the manufacturer's directions. Behavioral experiments were performed after 10 backcrosses to the C57BL/6NCrl (Charles River Laboratories, Sulzfeld, Germany) mouse strain and included age-matched male wild-type mice (Cnp1+/+, n = 43), Cnp1 heterozygotes (Cnp1+/−, n = 22) and Cnp1 null mutants (Cnp1−/−, n = 47). Mice were housed in groups (5 per cage) in standard plastic cages and maintained in a temperature-controlled environment (21°C) on a 12 h light/dark cycle with food and water ad libitum. Behavioral tests were performed in a blind fashion during the light phase of the day between 10 am and 5 pm. The order of testing was as follows: Elevated plus maze, open field, hole board, rota-rod, social interaction, 8-arm maze, and fear conditioning. Age of mice at the beginning of testing was 3.5 months, i.e., 10 weeks after surgery (see below). 1 week after behavioural testing, i.e., at the age of 4.5 months, a subset of the mice (Cnp1−/−, lesioned: n = 7; Cnp1−/−, sham operated: n = 7; Cnp1+/−, lesioned: n = 4; Cnp1+/−, sham operated: n = 4; Cnp1+/+, lesioned: n = 6; Cnp1+/+, sham operated: n = 6) was examined in a blinded fashion by histology and immunohistochemistry (see below).
Surgery
At the age of 28 days (mouse puberty) mice were anaesthetized with an intraperitoneal (i.p.) injection of 0.25% tribromoethanol (Avertin) (0.125 mg/g). The parietal skull was exposed through a scalp incision and a cryolesion was placed on the right parietal cortex (coordinates from bregma: 1.5 mm posterior, 1.5 mm lateral). For that, a cone-shaped copper cylinder with tip diameter of 1 mm was cooled with liquid nitrogen (−183°C). Its tip was stereotactically placed in direct contact with the exposed parietal/occipital skull and kept in place for 60 s. Sham-operated animals went through the same procedure without cooling the metal cone. Numbers of mice in the four experimental groups were as follows: wildtype/sham (n = 22), wildtype/lesion (n = 21), null mutant/sham (n = 23) and null mutant/lesion (n = 24), heterozygous/sham (n = 11), heterozygous/lesion (n = 11).
Behavioral Testing
Elevated plus maze
In this test of anxiety mice were placed in the central platform, facing an open arm of the plus-maze. Behavior was recorded by an overhead video camera and a PC equipped with “Viewer” software (Biobserve, Bonn, Germany) to calculate the time each animal spends in open or closed arms. The proportion of time spent in open arms was used for estimation of open arm aversion (fear equivalent).
Open field
Spontaneous activity in the open field was tested in a gray Perspex arena (120 cm in diameter, 25 cm high) divided into three virtual zones: central, intermediate and peripheral. The mouse was placed in the center of the arena and the test started as soon as the mouse reached the wall. Over a 7-min period, the mouse was allowed to freely explore the open field. The behavior was recorded by a PC-linked overhead video camera. “Viewer” software was used to calculate velocity, distance traveled. and time spent in each of the zones. Additionally, the initial latency to reach the wall from the center was recorded.
Hole board
The hole board test measures exploratory activity. The apparatus consisted of a 21 × 21 × 36 cm3 transparent Perspex chamber with a non-transparent floor raised 5 cm above the bottom of the chamber with 12 equally spaced holes, 2 cm in diameter. Mice were allowed to explore the chamber for 3 min and the number of explored holes (head dips) was scored by a trained experimenter.
Rota-rod
Rota-rod is a test for motor function, balance and coordination and comprises a rotating drum (Ugo Basile, Comerio, Varese, Italy), which is accelerated from 4 to 40 revolutions per minute over the course of 5 min. Each mouse was placed individually on a drum and the latency of falling from the drum was recorded using a stop-watch. To assess motor learning, the rota-rod test was repeated 24 h later.
Sociability and social memory test
Sociability and social memory test was performed in a rectangular Perspex box that was divided with two transparent walls, forming three chambers (each: 20 × 40 × 22 cm3). Dividing walls had rectangular openings (5 cm wide) allowing access into each chamber. First, during habituation phase, the openings were closed and the experimental mouse was placed in the middle chamber and allowed to explore it for 5 minutes. After the habituation period, the sociability test was performed. For that, an unfamiliar C57BL/6J male mouse (stranger 1) was placed in one of the side chambers. This mouse was enclosed in a small (7.5 x 11.5 x 7.5 cm) wire cage, which allowed nose contact between two mice but prevented fighting. The identical empty wire cage was placed in the opposite chamber. Both openings to the side chambers were then opened and the experimental mouse was allowed to freely explore the entire box for 10 min. The amount of time spent in each chamber and the number of entries into each chamber were recorded by the video-tracking system “Viewer.” At the end, the social memory test was performed. A second, unfamiliar mouse (stranger 2) was placed into the previously empty wire cage and the experimental mouse had again free access to all chambers for 10 min and could choose between the first, already-investigated mouse (stranger 1), and the novel unfamiliar mouse (stranger 2). All mice used as strangers had been previously habituated to the placement in the small wire cage and had no prior contact with the experimental mice.
8-arm radial maze
Working memory was assessed in the 8-arm maze, which consisted of eight transparent Plexiglas arms (30 × 6 × 6 cm3) radiating from the octagonal starting chamber. Each arm was separated from the central chamber by vertical aluminum doors. A magazine cup with a photocell detector of head entries was located at the end of each arm, in which strawberry milk reward was delivered. The maze was placed in a room, which contained extra-maze visual cues that were always kept in the same position when testing. First, before starting the habituation phase, mice were given the strawberry milk for 24 h in their home cages. After this period, the bottles with milk and water were removed and mice were water-deprived. Water deprivation was applied during the whole experimental period. Immediately after finishing the daily test sessions, mice were given water in their home cages for 60 min. The habituation phase consisted of 4 trials, 2 per day, in which mice were closed in one of the arms for 15 min. During this period the milk reward was delivered every 2 min in the magazine cup and mice were allowed to collect the reward after making a head entry into the magazine. After two days of habituation the training phase has started and consisted of 10 daily trials. During each trial, mice were put in the central chamber and after 20 s all doors were raised simultaneously, allowing the mice a choice of any arm. Because the milk reward was given in each arm only once (after making a head entry into the magazine), mice had to remember which arm had been already visited. The training trial was terminated immediately after all eight portions of milk were consumed or after 15 min had elapsed. Following parameters were measured: time required to finish the trial, number of errors (defined as head entry to the magazine in a revisited arm) and number of correct responses made before the occurrence of an error.
Cued and contextual fear conditioning
The fear conditioning test was performed as described in (Radyushkin et al., 2005). In brief, mice were trained within the same session for both contextual and cued fear conditioning. After a 2-min period, in which baseline freezing was assessed, mice received two paired presentations of a 10 s, 5 kHz, 85 dB tone (conditioned stimulus, CS) and a 2 s, 0.4 mA foot shock (unconditioned stimulus, US). The contextual memory was assessed 72 h after the training. Mice were monitored over 2 min for freezing in the same conditioning chamber. Four hours later cued memory test was performed. For that mice were placed in a new chamber with novel visual cues and baseline freezing (pre-cue) was measured for 2 min. After that, the 85 dB tone (conditioned stimulus, CS) was presented for 2 min and freezing upon tone was quantified. Duration of freezing behavior, defined as the absolute lack of movement (excluding respiratory movements), was recorded by a video camera and a PC equipped with “Video Freeze” software (MED Associates, St. Albans, Vermont, USA).
FACS Analysis
For FACS analysis, blood was flushed out from mice by transcardial perfusion (PBS+EDTA). Spinal leukocytes were isolated with Percoll gradient from homogenized tissue and stained with fluorescent antibodies (anti-CD45 PerCP clone 30-F11, anti-CD8 APC clone 53.6.7, BD Pharmingen) after blocking the Fc receptor (anti-mouse CD16/32, Biolegend).
Histology and Immunostaining
Mice were anesthetized with Avertin and perfused through the left ventricle with 15 mL of Hank's balanced salt solution (Lonza, Switzerland), followed by 50 mL of 4% paraformaldehyde in PBS. Brains were harvested and postfixed in 4% paraformaldehyde overnight at 4°C and then embedded in paraplast. Totally, 5 μm microtome sections (Microm HM400, Walldorf, Germany) were prepared and stained by haematoxylin-eosin (H&E) or Nissl to study cytoarchitecture. For DAB based immunostaining of paraffin sections Dako-LSAB2 system or Vectastain Elite ABC kit (Vector laboratories) were used according to manufacturer's instructions. Primary antibodies were directed against APP (1:750, Chemicon), CD3 (1:150, Serotec), CNPase (1:150, Sigma), GFAP (1:200, Novocastra), Iba-1 (1:1000, Wako), and Mac3 (1:400, Pharmingen).
Morphometry
Light microscopic images from hippocampal gray matter (bregma −1.82 mm), hippocampal fimbria (bregma −1.82 mm) and anterior commissure (bregma 0.38 mm) were captured at 20x or 40x magnification and overlapping digitized images were fused using Photoshop CS and ImageJ software. Given brain areas in 2 fused images per animal from left and right hemispheres were blindly analyzed for GFAP positive area, number of APP positive axonal spheroids, and Iba-1 and Mac3-positive microglia. APP positive spheroids clearly not associated with a nucleus were quantified using the cellcounter plugin from ImageJ with a post magnification of 100%. For the quantification of Iba-1- or Mac3-positive microglia, nuclei surrounded by DAB positive deposits were quantified manually (for hippocampal gray matter and hippocampal fimbria) or by semi-automated analysis using an ImageJ software plug-in (anterior commissure) (available online at http://www1.em.mpg.de/acmac3). For semi-automated analysis of GFAP-positive areas a plug-in for the ImageJ software was implemented (available online at http://www1.em.mpg.de/gfap). CD3-positive T-cells were quantified in corpus callosum, hippocampal fimbria, and striatal white matter tracts (collectively referred to as white matter), and in hippocampal gray matter and cortex (collectively referred to as gray matter; bregma −1.82 mm).
Statistics
Statistical significance was evaluated using 3-way and 2-way repeated measurement ANOVA, and two-sided Student's t-test. Data are represented as mean ± SEM in figures and text. The data were analyzed using Prism 4 (GraphPad Software, San Diego, CA). P-values < 0.05 were considered significant.
RESULTS
Effect of a Mild Neurotrauma on Behavior in Cnp1−/− Mice
As previously reported, Cnp1 null mutant mice (Cnp1−/−) develop several clinical abnormalities, beginning gradually at about 4–5 months of age, including ataxia, muscle weakness, and hindlimb paralysis, all secondary to progressive axon loss (Lappe-Siefke et al., 2003). Thus, for a basic behavioral test battery, including higher cognitive function tests, we analyzed age-matched male mutants and wildtype controls (Cnp1+/+) beginning at 3.5 months of age (i.e., when mutant mice were still indistinguishable from age-matched wildtype controls). Importantly, we compared mice of both genotypes with and without a standardized minor neurotrauma to the right parietal cortex (Sargin et al., 2009; Sirén et al., 2006), applied at the age of mouse puberty (P28), as shown in Fig. 1a.
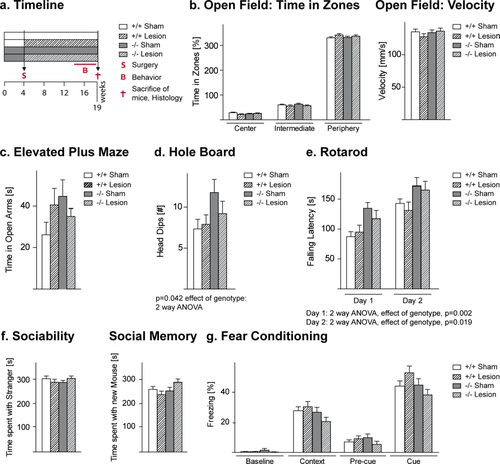
Effects of the Cnp1 null mutant genotype alone and in combination with a mild neurotrauma on readouts of basic behavioral functions. (a) Experimental setup. (b,c) Open field parameters and elevated plus maze do not show significant differences between groups. (d,e) Genotype effects are seen in hole board and rota-rod. (f,g) No differences are noticeable in social tests and fear conditioning. Mean ± SEM presented; −/− = Cnp1 null mutants; +/+ = wildtype controls; n = 21–24 per group. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
First, we established basic behavioral functions, from locomotor activity to anxiety. In these experiments, no abnormality in the time spent in the three zones of the open field was observed in any of the groups (Fig. 1b). Also, the time spent in open arms of the elevated plus maze did not show significant differences (Fig. 1c), suggesting comparable levels of anxiety among the experimental groups. Velocity, measured in the open field, was similar in all groups (Fig. 1b). However, independent of a prior lesion, we found that the Cnp1−/− genotype caused a mild hyperactivity in the hole board test of explorative activity (2-way ANOVA: effect of genotype F1,87 = 4.28, P = 0.042, Fig. 1d). Surprisingly, over 2 days of testing, Cnp1−/− mice showed longer latencies to fall from a rotarod (2-way ANOVA day 1: effect of genotype F1,86 = 9.98, P = 0.002; day 2: effect of genotype F1,86 = 5.69, P = 0.019) likely the result of motoric “overcompensation” (Fig. 1e). Social interaction/memory testing in the tripartite chamber and fear conditioning tests revealed no differences between the experimental groups (Fig. 1f,g).
In contrast, the 8-arm radial maze test, a specific cognitive task measuring working memory, uncovered an effect of the cortical cryolesion specifically on performance of the Cnp1−/− mice that was absent in all other groups. The lesion significantly influenced (1) the time required to finish the trial (3-way ANOVA: effect of lesion F1,84 = 4.83, P = 0.031) and (2) the number of errors (3-way ANOVA: effect of lesion F1,84 = 6.59, P = 0.012). Moreover, 3-way ANOVA revealed differences among the groups in the number of correct responses made before committing the first error (effect of genotype F1,84 = 6.52, P = 0.012 and the genotype*lesion interaction: F1,84 = 3.89, P = 0.052). To substantiate our observation that the cryolesion affected the two genotypes differently, we performed confirmatory post-hoc 2-way ANOVA for repeated measures, separately for wildtype and mutant mice. The effects of a lesion were only evident in Cnp1 mutant mice, but not in wildtype. In the mutant group, the prior cryolesion significantly increased the time needed to finish a trial (Fig. 2a; 2-way ANOVA: effect of lesion F1,43 = 6.86, P = 0.012), increased the number of errors (Fig. 2b; 2-way ANOVA: F1,43 = 5.71, P = 0.021), and decreased the number of correct responses before committing the first error (Fig. 2c; 2-way ANOVA: F1,43 = 5.57, P = 0.022). Taken together, these data indicate that the Cnp1 null mutation is behaviorally compensated for many months, unless combined with a second hit that is not sufficient to induce behavioral abnormalities on its own, thereby leading to a specific working memory deficit.
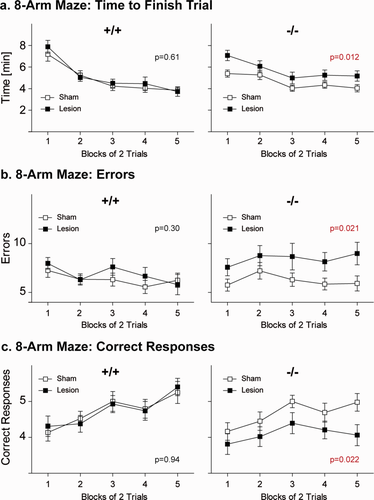
Working memory performance measured in the 8-arm radial maze is perturbed upon “dual hit,” i.e., only in Cnp1−/− mutant mice that encountered a juvenile parietal neurotrauma. Cnp1 null mutant mice exposed to a juvenile parietal cryolesion need significantly more time to finish the 8-arm radial maze task (a), make significantly more errors (b) and show significantly less correct responses before committing the first error (c) when compared with all other three experimental groups. Mean ± SEM presented; −/− = Cnp1 null mutants; +/+ = wildtype controls; n = 21–24 per group. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Gliosis, Inflammation, and Axonal Pathology
To correlate the behavioral difference of Cnp1 mutants (with and without mild neurotrauma) to histopathological changes, we analyzed by immunohistochemistry a subset of the mice from all four cohorts at the end of the behavioral analyses, i.e., at 4.5 months of age (Cnp1−/−, lesioned: n = 7; Cnp1−/−, sham operated: n = 7; Cnp1+/+, lesioned: n = 6; Cnp1+/+, sham operated: n = 6).
Neurodegeneration is associated with the activation of immune cells. Staining for CD3 (cluster of differentiation 3) T cell co-receptors demonstrated that the loss of CNP and subsequent axonal degeneration was sufficient to trigger the recruitment of T cells into the brain (Fig. 3a), similar to our findings in other myelin mutants (Ip et al., 2006; Kassmann et al., 2007). Upon quantification, T cells were accumulating selectively in white matter areas (hippocampal fimbria, corpus callosum, and striatal white matter tracts), but not in gray matter (cortex and hippocampus) (Fig. 3b). This finding was supported by flow cytometry (FACS) at 9 months of age, when increased numbers of CD8 T cells could be sorted from Cnp1 mutant spinal cord compared with wildtype control (Fig. 3c). Interestingly, the prior parietal injury at age P28 showed no significant effect on T cell number (Fig. 3b), suggesting that the T cell recruiting signals and the mechanisms that modify disease upon juvenile cryolesion in Cnp1 mutant mice are distinct.
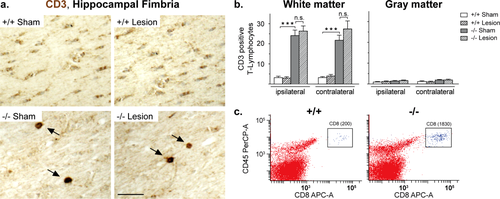
White matter infiltration by T-lymphocytes in Cnp1 null mutant brains. (a) Representative light microscopic images of the ipsilateral hippocampal fimbria of 4.5-month-old nonlesioned and lesioned mutants and controls (as indicated) immunostained for CD3. Scale bar, 20 μm. (b) Bar graphs give the genotype-dependent and lesion-dependent quantitation of the total number of CD3 positive T-lymphocytes in corpus callosum, hippocampal fimbria, and striatal white matter tracts (collectively referred to as white matter) and in hippocampus and cortex (collectively referred to as gray matter), in each case separately presented for the lesioned side (ipsilateral) and the nonlesioned side (contralateral) of the brain. (c) Flow cytometry (FACS) analysis depicting the total number of spinal CD8 T-cells sorted from a Cnp1 null mutant and an age-matched control mouse at 9 months of age. Mean ± SEM presented; two-sided Student's t-test used; ***P < 0.001; −/− = Cnp1 null mutants; +/+ = wildtype controls. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
We therefore turned to the innate immune system and quantified the number of microglial cells, as identified by expression of IBA-1 (ionized calcium-binding adapter molecule 1) and the number of activated microglial cells as determined by Mac3 expression. Because the 8-arm radial maze test had revealed a specific effect of the cortical lesion in Cnp1−/− mice and is known to be sensitive to hippocampal damage we concentrated our initial immunohistochemical analysis on hippocampal gray matter and hippocampal fimbria. In both regions, Cnp1−/− mutants contained a higher number of IBA-1 and Mac3 positive microglial cells when compared with controls (Fig. 4a–d). Importantly, the unilateral parietal cryolesion, performed more than 3 months earlier, led to a significant bilateral increase in the number of activated (Mac3 positive) microglia (Fig. 4d). This indicates that the juvenile lesion acted as a “second hit” that globally enhanced microglia activation in Cnp1−/− mutants. In contrast, in Cnp1+/+ control mice the same juvenile lesion did not lead to a persistent increase of activated microglia cells (Fig. 4d).
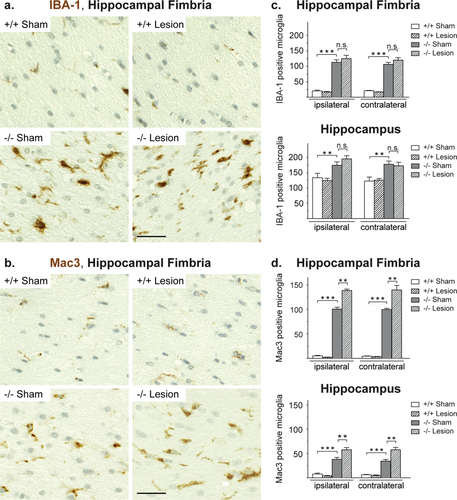
Microglia activation in mice with the Cnp1 null mutant genotype is intensified upon juvenile parietal neurotrauma. (a,b) Representative light microscopic images of the ipsilateral hippocampal fimbria of 4.5-month-old nonlesioned and lesioned mutants and controls (as indicated) immunostained for IBA-1 (a) and Mac3 (b). Scale bar, 20 μm. (c,d) Bar graphs give the genotype-dependent and lesion-dependent quantitation of the total number of IBA-1 positive cells (c) and Mac3 positive cells (d) in hippocampal fimbria and hippocampal gray matter (in each case presented for the lesioned side (ipsilateral) and the nonlesioned side (contralateral) of the brain). Mean ± SEM presented; two-sided Student's t-test used; **P < 0.01; ***P < 0.001; −/− = Cnp1 null mutants; +/+ = wildtype controls. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
A similar effect of the lesion was observed when we quantified GFAP positive astrocytes in the gray matter of the hippocampus and in the hippocampal fimbria. Again, inactivation of the Cnp1 gene was by itself sufficient to enhance GFAP immunoreactivity (Fig. 5a,b). However, the uniparietal lesion significantly accentuated astrogliosis, which was global in the forebrain but restricted to white matter tracts (Fig. 5b). Similar to microgliosis, parietal injury had by itself no long-lasting effect on astrocytes in wildtype mice (Fig. 5b).
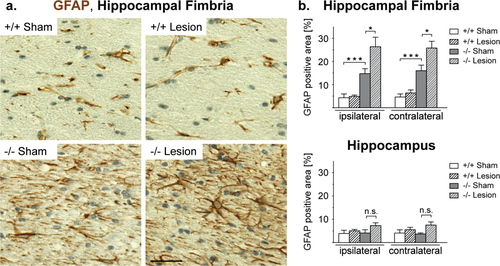
Astrogliosis present in white matter of Cnp1 null mutant mice is accentuated after juvenile parietal neurotrauma. (a) Representative light microscopic images of the ipsilateral hippocampal fimbria of 4.5-month-old nonlesioned and lesioned mutants and controls (as indicated) immunostained for GFAP. Scale bar, 20 μm. (b) Bar graphs give the genotype-dependent and lesion-dependent quantitation of the relative size of GFAP positive area in hippocampal fimbria (top panel) and hippocampal gray matter (bottom panel) for the lesioned side (ipsilateral) and the nonlesioned side (contralateral) of the brain. Mean ± SEM presented; two-sided Student's t-test used; *P < 0.05; ***P < 0.001; −/− = Cnp1 null mutants; +/+ = wildtype controls. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Finally, we assessed by immunohistochemistry of the amyloid precursor protein (APP) whether the early cryolesions influenced the known axonal transport defects in Cnp1 mutant mice. As expected, in (nonlesioned) Cnp1 mutants we quantified significantly more APP-positive axonal swellings (spheroids) than in (nonlesioned) control mice (Fig. 6a,b). Importantly, the unilateral lesion by itself did not cause any signs of persistent axonal pathology in wildtype mice. However, in Cnp1 mutants minor brain trauma was responsible for a significant and bilateral increase in the number of APP-positive spheroids in hippocampus and hippocampal fimbria, i.e., in both gray and white matter (Fig. 6b).
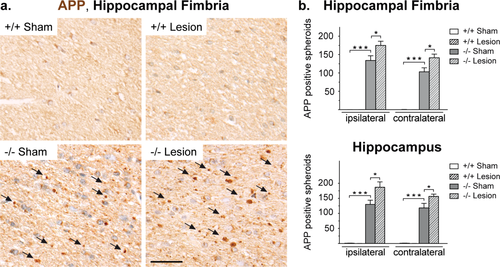
Neuronal pathology present in Cnp1 null mutants is accentuated after juvenile parietal neurotrauma. (a) Representative light microscopic images of the ipsilateral hippocampal fimbria of 4.5-month-old nonlesioned and lesioned mutants and controls (as indicated) immunostained for APP. Scale bar, 20 μm. (b) Bar graphs give the genotype-dependent and lesion-dependent quantitation of the total number of APP positive spheroids in hippocampal fimbria (top panel) and hippocampal gray matter (bottom panel) for the lesioned side (ipsilateral) and the nonlesioned side (contralateral) of the brain. Mean ± SEM presented; two-sided Student's t-test used; *P < 0.05; ***P < 0.001; −/− = Cnp1 null mutants; +/+ = wildtype controls. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Next, we quantified Mac3 positive signals and APP positive spheroids in the anterior commissures, i.e., in another white matter tract more distant from the lesion site, with similar results as obtained for hippocampal gray and white matter. Whilst the juvenile minor brain trauma did not cause persistent pathology in wildtype mice, lesioned Cnp1 mutants displayed a significant and bilateral increase of Mac3 positive microglia cells and APP-positive spheroids when compared with nonlesioned mutants (Fig. 7). We conclude that the effect of the lesion as a “second hit” is not restricted to hippocampus and hippocampal fimbria. This suggests that in genetically predisposed Cnp1 null mutant animals, a transient injury-derived signal inducing neurodegeneration and inflammation can spread within the entire brain.
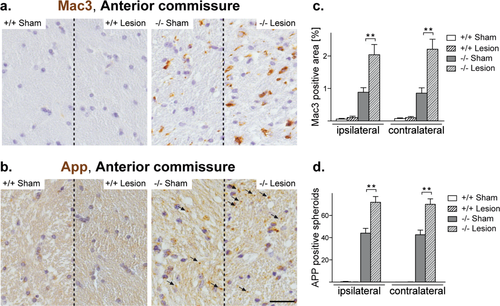
Microglia activation and neuronal pathology are accentuated in the anterior commissures upon juvenile neurotrauma. (a,b) Representative light microscopic images of the ipsilateral anterior commissure of 4.5-month-old nonlesioned and lesioned mutants and controls (as indicated) immunostained for Mac3 (a) and APP (b). Scale bar, 50 μm. (c,d) Bar graphs give the genotype-dependent and lesion-dependent quantitation of Mac3 positive signals (c) and APP positive spheroids (d) in the anterior commissures for the lesioned side (ipsilateral) and the non-lesioned side (contralateral) of the brain. Mean ± SEM presented; two-sided Student's t-test used; **P < 0.01; −/− = Cnp1 null mutants; +/+ = wildtype controls. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Both, genetic defects and traumatic injuries are “primary” events with respect to the clinical and histopathological readouts. To analyze, whether the cryolesion triggers an earlier manifestation of the Cnp1 mutant phenotype, or whether it accelerates the entire course of disease we scored APP positive spheroids and Mac3 positive microglial cells in brain sections at P28, i.e., at the time point, when the before analyzed cohorts of mice were lesioned. Here, in line with previous findings in Cnp1 mutant optic nerves (Edgar et al., 2009), Cnp1−/− brains already revealed a slight but significant increase in the number of axonal abnormalities and activated microglial cells, when compared with wildtype littermates (Fig. 8). This argues for a disease acceleration by the cryolesion.
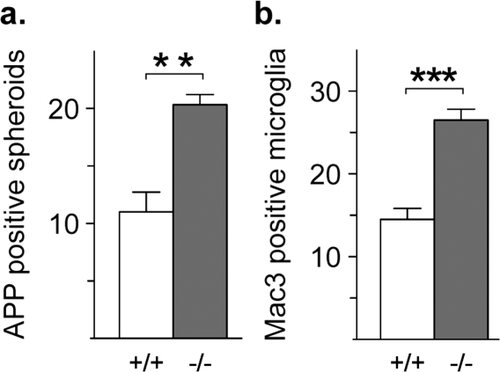
Early onset neuroinflammation and axonal pathology in Cnp1 null mutants. (a,b) Bar graphs give the genotype-dependent quantification of the total number of immunostained APP positive spheroids (a) and Mac3 positive cells (b) in Cnp1 null mutants and wildtype controls at P28 (quantified in two brain hemisphere sections per animal; n = 3 per genotype). Mean ± SEM presented; two-sided Student's t-test used; **P < 0.01; ***P < 0.001; −/− = Cnp1 null mutants; +/+ = wildtype controls.
This study comprises the direct comparison of Cnp1 null mutants with wildtype controls. We have previously shown that also heterozygous (Cnp1+/−) mice develop a mild neurodegenerative disorder associated with a unique behavioral phenotype (Hagemeyer et al., 2012). However, this marks a very late-onset (>18 months) disease, presumably caused by enhanced white matter aging, which is widely separated in time from the age at which we have performed the cryolesion in the present study. We therefore also analyzed heterozygous male Cnp1 mutant mice (Cnp1+/−) in comparison to wildtype controls for the effect of a juvenile cortical cryolesion in the same behavioral test battery (at 3.5 months of age) and for the same histopathological markers (at 4.5 months of age) as used for the analysis of the Cnp1 null mutants. However, we found no differences compared to wildtype controls (data not shown) and thus no interaction of Cnp1 heterozygosity and juvenile brain injury on CNS integrity at young age. These negative findings support our conclusion that neurotrauma (as a “second hit”) can only amplify ongoing inflammatory neurodegeneration, which is readily detected in Cnp1 null mice, but not in Cnp1 heterozygotes at the time of lesioning.
DISCUSSION
We have systematically combined two different lines of research on the mechanism of CNS neurodegeneration, which have so far only been studied in isolation. On one hand, this is the role of genetic predisposition to neurodegeneration, modeled in a mouse mutant of the myelin-associated Cnp1 gene, which develops a chronic-progressive degeneration of white matter tracts causing premature death (Edgar et al., 2009; Lappe-Siefke et al., 2003). On the other hand, this is the contribution of non-genetic (environmental) risks for long-term CNS integrity, hypothesized to act as modifiers (or “second hits”) for the clinical course of genetic diseases. Here, we have utilized juvenile mice and the model of a small (self-healing) cryolesion applied stereotactically to only one cortical hemisphere at a vulnerable period of postnatal brain development, i.e., mouse puberty (Sirén et al., 2006; Sargin et al., 2009).
The results of our study demonstrate a clear interaction of genetic and environmental factors on CNS integrity. It is possible to identify clinical features in these mice that appear specific to the combination of both factors. Indeed, at the age of 3.5 months (i.e., before perturbed Cnp1 expression dominates the motor phenotype), only cryolesioned mutants showed abnormalities of higher cognitive functions but not uninjured mutants or injured wildtype mice. The here applied 8-arm radial maze task serves as a test for working memory. This sensitive measure of cortical and subcortical network integrity requires not only intact hippocampal function but also a complex interaction of mainly frontal and parietal cortical areas, anterior cingulate as well as basal ganglia. It depends on intact executive functioning and spatial memory performance. The compromised function in this task, found exclusively in lesioned mutants, correlates at the histopathological level with a remarkable bilateral increase in neuroinflammation (microgliosis, astrocytosis) and axonal degeneration in all areas analyzed here, i.e., hippocampal gray and white matter as well as anterior commissures, white matter tracts far apart from the right parietal cryolesion site. Other brain areas would likely show similar pathology. Apparently, a ‘critical threshold’ of inflammation exists that ultimately leads to the observed cognitive decline. This may explain why non-lesioned mutants, despite their considerable histological abnormalities, still had normal working memory performance.
Both, genetic defect and traumatic injury are “primary” to the clinical readout. However, our findings suggest that the cryolesion at P28 accelerates rather than triggers the Cnp1 mutant phenotype. By the time of lesioning, already a small but significant number of axonal abnormalities and activated microglial cells were present in Cnp1 null mutant brains. In contrast there was no such interaction (possible) in Cnp1 heterozygous mice in which neuroinflammation and neurodegeneration begins much later in life (Hagemeyer et al., 2012), which supports the idea that neurotrauma as a “second hit” can only amplify an ongoing neurodegeneration associated with low-grade inflammation.
We therefore suggest a model, in which the disruption of Cnp1 expression in oligodendroglia causes, in addition to oligodendroglial abnormalities, secondary axonal injury with microglial activation. Activated microglia synthesize a variety of toxic molecules that may harm axons, i.e., proinflammatory mediators and reactive oxygen and nitrogen species (Lassmann, 2008, 2010). The latter may attenuate mitochondrial functions and induce a subsequent energy failure in axons (as a “tertiary” effect), which further reduces function and physical integrity of myelinated axons (Nave, 2010). Since this has features of a positive feedback loop, we suggest that the perturbation of Cnp1 expression in the CNS has the potential to trigger an undesirable self-energizing cycle of neurodegeneration and inflammation, which amplifies the primary pathology (Fig. 8).
Moreover, we suggest that this cycle can be accelerated by an “environmental” factor that independently triggers neuroinflammation, i.e., the locally administered cryolesion, which by itself is insufficient to maintain a pathologically relevant vicious cycle of inflammation and neurodegeneration in wildtype mice (Fig. 8). The nature of the underlying injury-derived signal, the mechanism of its spread in the CNS, and its apparent activity long after the primary lesion site has self-healed remain unknown. It is plausible that microglial cells in white matter tracts and diffusible messengers (such as NO) play a critical role in this spread.
For the subcortical white matter of Cnp1 mutant mice, we have reported an injury-independent activation of microglial cells, which may greatly enhance the global spread of such cortical injury signal. The mechanism by which Cnp1 mutant oligodendrocytes cause inflammation in the first place is unknown and may be solely the consequence axonal degeneration. However, it has been recently suggested that CNPase participates in the CNS in metabolizing 2′3′-cAMP, which is derived from cellular mRNA breakdown (Thompson et al., 1994) leading to the release of adenosine (Verrier et al., 2012), a known anti-inflammatory metabolite that might inhibit microglial response after traumatic brain injury (Haselkorn et al., 2010). Thus, activation of microglial cells emerges as a likely cellular mechanism by which distinct genetic and environmental factors interact and amplify a slowly progressive neurodegeneration phenotype.
The demonstration that minor neurotrauma modulates a genetic disease, and thus becomes an “environmental” risk factor, at least for the degree of myelin-associated neuropathology in Cnp1 mutant mice, has implications for other inflammatory white matter disorders. In human leukodystrophies, such as X-linked adreno-leukodystrophy and vanishing white matter disease, there have been anecdotal reports of head injuries causing disease or deteriorating its progression (Damon-Perriere et al., 2008; Espay et al., 2002; Raymond et al., 2010), and also the risk of multiple sclerosis may be increased following brain injury (Kang and Lin, 2012). Likewise, head trauma has been identified as an associated risk factor for psychiatric diseases, such as Alzheimer's disease and schizophrenia (Bryant et al., 2010; Molloy et al., 2011; Sastre et al., 2011; Van Den Heuvel et al., 2007; Whelan-Goodinson et al., 2009). It is intriguing that these diseases have been associated, by MRI and upon postmortem analysis, in part with white matter abnormalities, axonal degeneration, and low-grade inflammation9.
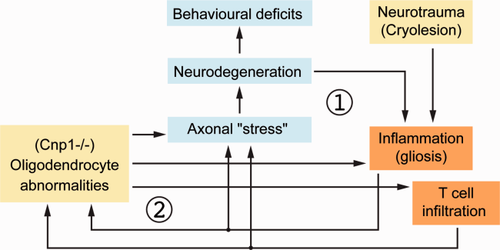
Schematic model depicting disease-relevant consequences of a minor cortical neurotrauma in mice with genetically induced oligodendroglial dysfunction. According to our model a minor cortical neurotrauma causes a transient inflammatory response (1), which is normally below threshold to cause pathogically relevant brain damage. However it leverages a “vicious cycle” (2) of low-grade inflammation and axon degeneration in white matter tracts of Cnp1 null mutant mice with primary genetically induced perturbation of oligodendrocyte functions in axonal support. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Acknowledgments
The authors thank members of the Nave lab and Rudolf Martini (Würzburg) for helpful discussions.