Microglial activation of the NLRP3 inflammasome by the priming signals derived from macrophages infected with mycobacteria
Abstract
The inflammasome is a multimolecular complex that orchestrates the activation of proinflammatory caspases and interleukin (IL)-1β, which is generally increased in the cerebrospinal fluids of patients with tuberculous meningitis. However, it has not been clarified whether mycobacteria can activate the inflammasome and induce IL-1β maturation in microglia. In this study, we found that the priming of primary murine microglial cells with conditioned media from cultures of macrophages infected with Mycobacterium tuberculosis (Mtb) led to robust activation of caspase-1 and IL-1β secretion after Mtb stimulation. Potassium efflux and the lysosomal proteases cathepsin B and cathepsin L were required for the Mtb-induced caspase-1 activation and maturation of IL-1β production in primed microglia. Mtb-induced IL-1β maturation was also found to depend on the nucleotide binding and oligomerization of domain-like receptor family pyrin domain containing 3 protein (NLRP3) and apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), as well as the generation of mitochondrial reactive oxygen species (ROS). Notably, the priming of microglia with tumor necrosis factor-α or oncostatin M resulted in caspase-1 cleavage and IL-1β secretion in response to Mtb. Moreover, dexamethasone, as an adjunctive therapy for patients of tuberculous meningitis, significantly reduced the Mtb-induced maturation of IL-1β through inhibition of mitochondrial ROS generation. Collectively, these data suggest that Mtb stimulation induces activation of the microglial NLRP3 inflammasome (composed of NLRP3, ASC, and cysteine protease caspase-1) through microglia–leukocyte interactions as a priming signal, and that dexamethasone decreases inflammasome activation through inhibition of ROS of mitochondrial origin. © 2012 Wiley Periodicals, Inc.
INTRODUCTION
Central nervous system tuberculosis (CNS-TB), which is caused by infection with Mycobacterium tuberculosis (Mtb), is a major public health problem in developing regions, with high mortality and high neurological morbidity (Rock et al., 2008). Tuberculous meningitis (TBM) is the main type of CNS-TB and is frequently associated with miliary or disseminated tuberculosis (Horne, 1951). The resident CNS macrophages, microglial cells, are the principal target cells in CNS-TB, and they are productively infected with Mtb (Rock et al., 2004). Activated microglia release numerous cytokines and chemokines that have both neurodegenerative and neuroprotective effects through interactions with other cell types, including astrocytes, lymphocytes, and neurons (Rock et al., 2004, 2008). The cerebrospinal fluids of TBM patients usually contain elevated levels of interleukin (IL)-1β, tumor necrosis factor (TNF)-α, IL-8, chemokine (C-C motif) ligand 2 (CCL) 2, and macrophage inflammatory protein-1α (Drevets et al., 2004). The activated microglial cells not only secrete these products but also exert important functions in innate immunity during CNS infection or inflammation through the expression of a number of immune-recognition molecules (Rock et al., 2004, 2008).
The inflammasome, which is an intracellular multimolecular complex for the activation of inflammatory caspases, orchestrates the cleavage and secretion of IL-1β, IL-18, and IL-33, thereby generating a potent inflammatory response (Franchi et al., 2009; Lamkanfi and Dixit, 2009). The nucleotide binding and oligomerization domain-like receptor family pyrin domain containing 3 (NLRP3) inflammasome, which is composed of NLRP3, the adaptor molecule apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and the cysteine protease caspase-1, is one of the best characterized inflammasomes with responses to various endogenous and exogenous danger signals (Cassel et al., 2009). Inflammasome activation and secretion of active IL-1β are tightly regulated through two distinct steps, the production of biologically inert precursor pro-IL-1β (Signal 1), and the processing and maturation of IL-1β (Signal 2), although the mechanisms of activation of the inflammasome differ between various cell types (Netea et al., 2010). Significant insight into the modulating roles of the NLRP3 inflammasome in neuroinflammatory conditions has come from recent studies showing that inflammasome activation and innate inflammatory responses in the CNS potentiate or inhibit neurodegenerative processes (Chakraborty et al., 2010). Recent studies have revealed a role for reactive oxygen species (ROS) of mitochondrial origin in the promotion of NLRP3 inflammasome activation (Nakahira et al., 2011). However, the triggering mechanisms during mycobacterial infection of inflammasome activation in microglia, which are the main macrophage-type cells in the CNS, remain to be clarified. Moreover, it is not known whether interactions between leukocytes and microglial cells are important for the activation of inflammasomes by microglia upon mycobacterial infection or whether dexamethasone (Dexa), an adjunctive therapy for TBM, inhibits inflammasome activation.
In this study, we examined the mechanisms that trigger inflammasome activation by microglia after Mtb stimulation. We show that the priming of primary murine microglial cells with conditioned media from cultures of Mtb-infected macrophages (CoMtb) is required for the activation of caspase-1 and IL-1β maturation in microglia during Mtb infection. Furthermore, we demonstrate that potassium efflux, lysosomal proteases cathepsin B and cathepsin L, and mitochondrial ROS contribute to CoMtb-mediated NLRP3 inflammasome activation in response to Mtb stimulation. We found that Mtb-induced host factors [oncostatin M (OSM) or TNF-α] primed microglial cells to activate NLRP3 inflammasome in response to Mtb, and that Dexa significantly inhibited mitochondrial ROS generation to control IL-1β secretion and maturation by microglia after Mtb stimulation.
MATERIALS AND METHODS
Primary Murine Microglial Cells
Primary adult microglial cell cultures were obtained from 8- to 10-week-old C57BL/6 mice, as described previously (Jin et al., 2012; Moussaud and Draheim, 2010), with some modification. Briefly, the cerebral cortices were dissected, carefully stripped of the meninges, and digested with enzymatic solution (116 mM NaCl, 5.4 mM KCl, 26 mM NaHCO3, 1 mM NaH2PO4, 1.5 mM CaCl2, 1 mM MgSO4, 0.5 mM EDTA, 25 mM glucose, 1 mM cysteine, and 20 U/mL papain) for 90 min at 37°C. The digested fraction pellet was resuspended in DNase I (0.5 mg/mL) for 5 min at room temperature. Then, filtered suspension cells were applied to a discontinuous density gradient using Percoll (GE healthcare, Freiburg, Germany) and centrifuged at 200g for 20 min. Finally, the pellet was resuspended in culture media (DMEM/F12 medium, Invitrogen, Carlsbad, CA) with GlutaMAXTMI (Invitrogen) supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin (Lonza, Walkersville, MD), and 5 ng/mL of murine recombinant granulocyte macrophage-colony stimulating factor (GM-CSF; R&D systems, Minneapolis, MN). The microglial cells were cultured for 2 weeks and the medium was changed twice per week. We confirmed that purified cell cultures consisted of >95% microglia (data not shown). All experimental procedures using animals were approved by the Chungnam National University Institutional Animal Care and Use Committee. All mice were maintained under standard laboratory conditions on a 12-h light/12-h dark cycle and food and water were provided ad libitum.
Reagents
Dimethylsulfoxide [DMSO; added to the cultures at 0.1% (v/v) as a solvent control] and Dexa were obtained from Sigma-Aldrich (St Louis, MO). z-YVAD-fmk was from Tocris Bioscience (Ellisville, MO). BAY11-7082, cathepsin B (CA-074Me), and cathepsin L (Z-FF-fmk) were from Calbiochem (San Diego, CA). MitoQ10 methanesulfonate (MitoQ) and decyl-TPP bromide (dTPP) were from Antipodean Pharmaceuticals (Auckland, New Zealand). For the experiments using elevated potassium levels (150 mM; Sigma-Aldrich), sodium was replaced with potassium at an equivalent molar concentration, as previously described (Lee et al., 2012).
Bacteria
The virulent Mtb strain H73Rv was cultured in Middlebrook 7H9 broth supplemented with 0.2% glycerol, 0.05% Tween-80, and 10% OADC, and frozen aliquots were stored at −70°C. Before use, each vial of Mtb was assayed for the number of viable organisms (colony-forming units) in triplicate on Middlebrook 7H10 agar (Yang et al., 2007).
Cultures of BMDMs and Preparation of CoMtb/CoU
Primary bone marrow-derived macrophages (BMDMs) were isolated from mice and differentiated for 5–7 days in medium that contained M-CSF (R&D Systems). BMDMs were infected with Mtb at a multiplicity of infection (moi) of 10 or were used uninfected. After 24 h of incubation, cell culture supernatants were harvested and filtered through a 0.2-μm sterile filter (Nalgene, Hereford, UK) (Green et al., 2010). The conditioned medium from Mtb-infected BMDMs was termed CoMtb, and conditioned medium from Mtb-uninfected BMDMs was termed CoU. The CoU and CoMtb (1:10 dilution) were used to prime microglial cells for 4 h in all of the experiments.
RNA Extraction and Reverse Transcription-Polymerase Chain Reaction Analysis
Reverse transcription-polymerase chain reaction (RT-PCR) for RNA extraction was performed as previously described (Lee et al., 2012). Briefly, total RNA was extracted from microglial cells using the TRIzol reagent (Invitrogen), according to the manufacturer's protocol. Extracted RNA was reverse-transcribed into cDNA with oligo (dT) primers (Bioneer, Korea). Semiquantitative PCR was performed with the Veriti Thermal Cycler (Applied Biosystems, Carlsbad, CA) for 35 cycles of annealing for 45 s at the following temperatures: 55°C for TNFα, IL6, and β&!hyphen;actin; 57°C for IL1β; and 58°C for ASC and NLRP3. Quantitative real-time RT-PCR was performed using power SYBRgreen PCR master mix (Applied Biosystems) on a StepOnePlus™ Real-Time PCR Systems (Applied Biosystems). The primer pairs used for PCR are listed in Table 1.
| Gene name | Sense primer | Antisense primer | Reference |
|---|---|---|---|
| IL-1β | CTCCATGAGCTTTGTACAAGG | TGCTGATGTACCAGTTGGGG | Ghosh et al., 2007 |
| TNF-α | CAGCCGATGGGTTGTACCTT | TGTGGGTGAGGAGCACGTAGT | Lyn-Kew et al., 2010 |
| IL-6 | GACAAAGCCAGAGTCCTTCAGAGAG | CTAGGTTTGCCGAGTAGATCTC | Perlman et al., 2003 |
| NLRP3 | CTGTGTGTGGGACTGAAGCAC | GCAGCCCTGCTGTTTCAGCAC | González-Benítez et al., 2008 |
| ASC | ATGGGGCGGGCACGAGATGCCATCCT | GCTCTGCTCCAGGTCCATCACCAAGT | González-Benítez et al., 2008 |
| OSM | GATGTACCCACTAAGCCGCC | GAGGACCGTTGAGGTCAAGC | Palmqvist et al., 2002 |
| β-Actin | GTGGGGCGCCCCAGGCACCA | CTTCCTTAATGTCACGCACGATTTC | Lee et al., 2009 |
Western Blot and Enzyme-Linked Immunosorbent Assay Analyses
Western blot analyses of total protein extracts and culture supernatants were performed as previously described (Lee et al., 2012). After infection, total protein extracts were harvested in 1× sample buffer. For detection of IL-1β maturation, the supernatants were collected and added to StrataClean resin (Stratagene, La Jolla, CA), and further incubated for 30 min, according to the manufacturer's protocol. The resin-bound proteins were washed with phosphate-buffered saline (PBS), resuspended in 1× sample buffer, and subjected to Western blotting. Equivalent amounts of the samples were resolved in SDS-PAGE gels and electrophoretically transferred to a polyvinylidene difluoride membrane. The membranes were blocked with 10% nonfat milk in PBS and then incubated with the following primary antibodies: anti-caspase-1 p45 (sc-56036; Santa Cruz Biotechnology, Santa Cruz, CA); anti-p10 (sc-514; Santa Cruz Biotechnology); anti-IL-1β (sc-7884; Santa Cruz Biotechnology); anti-OSM (R&D Systems); and anti-TNF-α (R&D Systems). To control for protein loading, β-actin (I-19; Santa Cruz Biotechnology) was used. After washing, the membranes were incubated with the corresponding peroxidase-conjugated secondary antibodies, and the label was visualized using the ECL system (Millipore, Billerica, MA). Cytokines were assayed by enzyme-linked immunosorbent assay [ELISA; for IL-1β, TNF-α, and IL-8 (BD Pharmingen, San Diego, CA)], as described previously (Lee et al., 2012).
Lentiviral Transduction of Primary Microglial Cells
To silence murine NLRP3 and ASC expression in mouse primary glial cells, pLKO.1-based lentiviral NLRP3 (NM_001079821; CCGGGCGTTAGAAACACTTCAAGAACTCGAGTTCTTGAAGTGTTTCTAACGCTTTTTG) and ASC (NM_023258; CCGGGAAGGAAATACATCCCTACTTCTCGAGAAGTAGGGATGTATTTCCTTCTTTTTG) constructs, respectively, were obtained as glycerol stocks from Open Biosystems (Huntsville, AL). Lentivirus was generated by transfection of HEK293T cells with pLKO puro.1 or target shRNA plasmids (for NLRP3 and ASC) and packaging plasmids (pMDLg/pRRE, pRSV-REV, and pMD2.VSV-g; Addgene, Cambridge, MA) using Lipofectamine 2000 (Invitrogen). After 72 h, the virus-containing culture supernatants were collected and filtered. Lentivirus titration was carried out as previously described (Lee et al., 2012). Infection of glial cells with the NLRP3-lentivirus or ASC-lentivirus (moi = 10) was performed in the presence of 8 μg/mL Polybrene (Sigma-Aldrich). After 2 days, the efficiency of transduction was determined by semiquantitative RT-PCR.
Nuclear Factor-κB Luciferase Reporter Assay
Luciferase reporter assays for nuclear factor (NF)-κB were performed as previously described (Lee et al., 2011). Briefly, microglial cells were transduced for 36 h with the Ad5HSVNF-κB luciferase construct (Gene Transfer Vector Core, University of Iowa, Des Moines, IA). The cells were lysed using luciferase lysis buffer (Promega, Madison, WI), and luciferase activity was measured according to the manufacturer's protocol.
Flow Cytometric Analysis of Mitochondrial ROS
The mitochondrial ROS levels in microglial cells were measured by staining with MitoSOX (Invitrogen) at 5 μM for 30 min at 37°C, followed by flow cytometry in a FACSCanto II (BD Biosciences, San Jose, CA), as previously described (Lee et al., 2011). The data for at least 30,000 cells per sample were acquired with the CellQuest Pro acquisition software (BD Biosciences) and analyzed with the FlowJo Cytometry Analysis software, version 7.5 (Tree Star, Ashland, OR).
Statistical Analyses
All data are presented as mean ± standard deviation (SD) of independent experiments. For statistical analyses, paired t-tests with Bonferroni adjustment or ANOVA for multiple comparisons were performed. Differences were considered significant at P < 0.05.
RESULTS
Priming of Primary Murine Microglial Cells with CoMtb Significantly Induces the Expression of IL-1β, TNF-α, and IL-6 in an NF-κB-Dependent Manner
Conditioned medium from cultures of Mtb-infected human monocytes enhances the expression of matrix metalloproteinase (MMP)-1, -3, and -9 in microglial cells (Green et al., 2010). Initially, we examined whether treatment of murine microglia with CoMtb induced the expression of pro-IL-1β, TNF-α, and IL-6. The control levels of IL1β, TNFα, and IL6 mRNA expression in unstimulated microglia were very low. Treatment of primary microglial cells with CoMtb induced significant increases in the mRNA levels of IL&!hyphen;1β, TNF&!hyphen;α, and IL&!hyphen;6 in a time-dependent manner (Fig. 1A). No such increases were observed in unprimed microglia or microglia that were treated with control supernatants from uninfected macrophages (CoU; Fig. 1A).
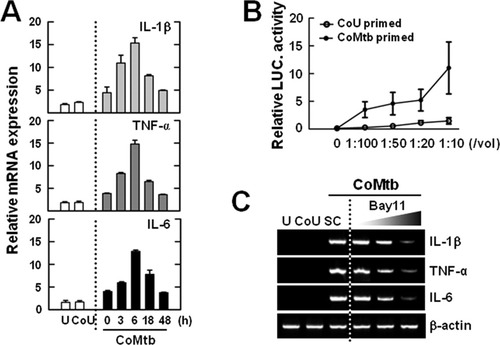
CoMtb induces the expression of mRNA for pro-IL-1β, TNF-α, and IL-6 in an NF-κB-dependent manner in primary microglial cells. (A and C) Primary microglial cells were primed with CoMtb for the indicated periods (0–48 h; A) or primed with CoMtb for 4 h in the presence or absence of BAY11-7082 (Bay11; 1, 5, and 10 μM; C). The cell lysates were subjected to quantitative real-time RT-PCR analysis. Data shown are representative of three independent experiments that gave similar results. (B) Primary microglial cells were transduced with Ad5HSVNF-κB-luc for 36 h and then treated with CoMtb at various dilutions (1:100, 1:50, 1:20, and 1:10) for 4 h. Cells were collected and assayed for NF-κB luciferase activity. U, uninfected; CoMtb, conditioned media from cultures of Mtb-infected BMDMs; CoU, conditioned media from cultures of control uninfected BMDMs; SC, solvent control (0.1% DMSO).
We then examined whether the NF-κB pathway was involved in the expression of pro-IL-1β in microglia treated with CoMtb. Treatment of microglial cells with CoMtb significantly induced NF-κB luciferase activities in a dose-dependent manner (Fig. 1B). In addition, the induction of IL&!hyphen;1β by CoMtb was abrogated in microglial cells pretreated with Bay11-7082, which is a specific inhibitor of NF-κB signaling (Fig. 1C). Consistently, the CoMtb-induced expression of mRNA for IL6 and TNFα was impaired by the NF-κB inhibitor (Fig. 1C). These results indicate that NF-κB signaling is essential for pro-IL-1β induction by CoMtb in microglia.
Mtb Stimulation Induces Caspase-1 Activation and IL-1β Secretion in CoMtb-Primed Microglia
We investigated whether Mtb could induce caspase-1 activation and IL-1β maturation in microglia. Infection of CoU-primed microglial cells with Mtb failed to induce secretion of IL-1β (Fig. 2A). However, priming of the microglial cells with CoMtb for 4 h significantly activated IL-1β production after Mtb stimulation (Fig. 2A). We next examined whether Mtb activated caspase-1 and IL-1β maturation in microglial cells at various time points after infection. After 3 h of Mtb infection, the supernatants of the cultures of primary microglial cells showed clear activation of caspase-1, as evidenced by increased levels of the cleaved p10 subunit (Fig. 2B, bottom). Consistent with this finding, the processed active form of IL-1β was evident in the culture supernatants after 3 h of Mtb infection (Fig. 2B, bottom). Furthermore, IL-1β secretion after Mtb infection was considerably reduced when CoMtb-primed microglial cells were pretreated with the caspase-1-specific inhibitor z-YVAD-fmk (Garcia-Calvo et al., 1998) (Fig. 2C). Taken together, these data suggest that Mtb robustly activates caspase-1 and induces IL-1β maturation in CoMtb-primed microglial cells.

Mtb induces the synthesis of mature IL-1β through caspase-1 activation in CoMtb-primed, but not in CoU-primed, microglia. (A) CoMtb-primed or CoU-primed microglial cells were infected with Mtb (moi = 0.1, 0.5, and 1) for 18 h (A). (B) CoMtb-primed microglial cells were infected with Mtb for the indicated times (3–18 h). The cells were subjected to Western blot analysis for caspase-1 and IL-1β (Cell, caspase-1 p45, 31-kDa pro-IL-1β; SN, cleaved caspase-1 p10, 17-kDa mature IL-1β). (C) CoMtb-primed microglial cells were infected with Mtb for 18 h in the absence or presence of z-YVAD-fmk (10, 20, and 50 μM). (A and C) Supernatants were collected and subjected to IL-1β ELISA. Data shown are representative of three independent experiments that gave similar results. ***P < 0.001, versus control cultures. U, uninfected; CoU, conditioned media from cultures of control uninfected BMDMs; CoMtb, conditioned media from cultures of Mtb-infected BMDMs; M, Mtb; SC, solvent control (0.1% DMSO); SN, supernatants; Casp1, caspase-1.
Mtb-Induced IL-1β Secretion Is Dependent on Potassium Efflux and the Release of Cathepsin B or Cathepsin L in Microglia
Potassium efflux is involved in activation of the inflammasome, because NLRP3 inflammasome activation can be inhibited by growth in media with high levels of potassium (Pétrilli et al., 2007). To investigate the role of potassium efflux in Mtb-dependent IL-1β maturation in microglia, we examined the Mtb-induced IL-1β levels in CoMtb-primed microglial cells grown in high-potassium media. As shown in Fig. 3A, a high level of potassium significantly attenuated the level of IL-1β, but not that of IL-8, in microglial cells after Mtb stimulation. Furthermore, mature IL-1β secretion and caspase-1 cleavage in response to Mtb infection were significantly inhibited by treatment of microglial cells with high concentrations of potassium (Fig. 3B). These data suggest that potassium efflux is required for Mtb-induced caspase-1 activation and IL-1β maturation in microglial cells.
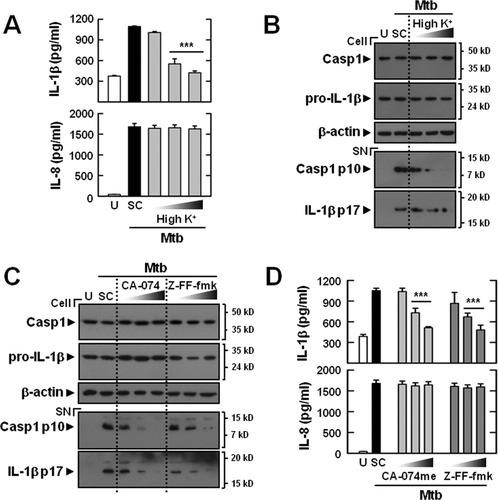
Mtb-induced IL-1β secretion is dependent on potassium efflux and the release of the lysosomal proteases cathepsin B and cathepsin L in primary microglial cells. CoMtb-primed microglial cells were incubated in high K+ buffer (10, 50, and 150 mM; A and B), cathepsin B (CA-074me; 1, 10, and 20 μM; C and D), or cathepsin L (Z-FF-fmk; 1, 10, and 20 μM; C and D) for 45 min before Mtb infection. (A and D) Supernatants were harvested 18 h after Mtb (moi = 1) infection and subjected to ELISA for IL-1β and IL-8. (B and C) Western blot analysis for caspase-1 and IL-1β (Cell, caspase-1 p45, 31-kDa pro-IL-1β; SN, cleaved caspase-1 p10, 17-kDa mature IL-1β). Data shown are representative of three independent experiments that gave similar results. ***P < 0.001, versus control cultures. U, uninfected; SC, solvent control (0.1% DMSO); SN, supernatants; Casp1, caspase-1.
The lysosomal damage and rupture that leads to inflammasome activation depends on the release of active cathepsin B or cathepsin L into the cytoplasm (Duewell et al., 2010; Hornung et al., 2008). Therefore, we examined whether inhibition of cathepsin B or cathepsin L activity affected Mtb-induced activation of IL-1β processing and release by CoMtb-primed microglia. To examine whether the release of lysosomal cathepsin B or cathepsin L into the cytoplasm is required for NLRP3 activation, Mtb-infected microglial cells were treated with increasing concentrations of the cathepsin B inhibitor CA-074me or the cathepsin L inhibitor Z-FF-fmk for 45 min, before being infected with Mtb for 18 h. The release of IL-1β, but not IL-8, was inhibited in a dose-dependent manner in cells that were pretreated with CA-074me or Z-FF-fmk (Fig. 3C). In addition, Mtb-induced activation of caspase-1 and IL-1β maturation in microglia was significantly inhibited in a dose-dependent manner by pretreatment with CA-074me or Z-FF-fmk (Fig. 3D). These data suggest that the catalytic activity of cathepsin B or cathepsin L is required for Mtb-induced inflammasome activation in microglia.
Mtb Stimulation Induces NLRP3 Protein Expression, Which Is Required for IL-1β Maturation in Microglia
The NLRP3 inflammasome is involved in Mtb early-secreted antigenic target 6-induced caspase-1 activation and IL-1β secretion in human macrophages (Mishra et al., 2010).We first examined whether CoMtb priming affected the expression of NLRP3 or ASC by microglia. Treatment of microglia with CoMtb led to robust induction of NLRP3 and ASC expression (Fig. 4A), indicating that enhanced expression of NLRP3 is required for inflammasome activation. To determine the effects of NLRP3 and ASC on Mtb-induced IL-1β secretion in microglia, we generated shRNA lentiviral vectors against NLRP3 and ASC. Knockdown of NLRP3 or ASC in microglia using lentiviral shRNA vectors decreased the expression levels of each gene by >95%, as measured by RT-PCR (Fig. 4B, inset). Analysis of the supernatants by ELISA revealed that Mtb-dependent secretion of IL-1β, but not IL-8, was significantly reduced by knockdown of NLRP3 or ASC, in CoMtb-primed microglial cells (Fig. 4B). Moreover, lentiviral shRNA specific for NLRP3 (shNLRP3) or ASC (shASC) markedly inhibited the secretion of mature IL-1β by microglia after Mtb infection, when compared with the secretion levels in microglia that were transduced with nonspecific control shRNA lentiviral particles (shNS; Fig. 4C). These results suggest that microglial induction of caspase-1 activation and IL-1β maturation in response to Mtb depends on NLRP3 and ASC expression.
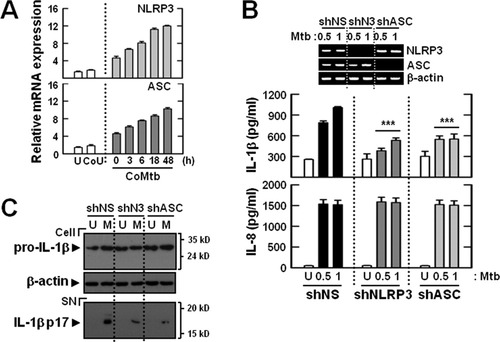
NLRP3 and ASC are required for Mtb-induced caspase-1 activation and IL-1β maturation in CoMtb-primed microglia. (A) Primary microglial cells were primed with CoMtb for the indicated periods (0–48 h). (B and C) The cells were transduced for 2 days with nonspecific control shRNA lentiviral particles (shNS) or lentiviral shRNA specific for NLRP3 (shNLRP3; shN3) or ASC (shASC). The microglial cells were then primed with CoMtb (4 h) and further incubated with Mtb (moi = 0.5 or 1) for 18 h. The cell lysates were subjected to quantitative real-time RT-PCR analysis (A), ELISA analysis (B), and IL-1β (Cell, 31-kDa pro-IL-1β; SN, 17-kDa mature IL-1β) detection by Western blot analysis (C). (B, top) RT-PCR analysis to assess transduction efficiency. The data shown are representative results from three independent experiments that gave similar results. ***P < 0.001, versus control cultures. U, uninfected; CoU, conditioned media from cultures of control uninfected BMDMs; CoMtb, conditioned media from cultures of Mtb-infected BMDMs; M, Mtb; SN, supernatants.
Mitochondrial ROS Generation Is Critical for Mtb-Mediated Activation of Caspase-1 and Secretion of IL-1β in Primed Microglia
Recently, it has been reported that NLRP3 inflammasome activation is triggered via mitochondrial ROS (Nakahira et al., 2011). We measured the production of mitochondrial ROS in microglia by flow cytometry with the MitoSOX Red dye (Fig. 5A). Compared with unprimed or CoU-primed microglia, the CoMtb-primed microglia displayed markedly increased mitochondrial ROS generation (Fig. 5A). To investigate whether the generated mitochondrial ROS are involved in Mtb-mediated pro-IL-1β cleavage, primary microglial cells were pretreated with or without scavengers for mitochondrial ROS. Pretreatment with MitoQ, which is a coenzyme Q10 analog that acts as a mitochondrial ROS scavenger (Chernyak et al., 2006), or dTPP, which is an inactive analog of MitoQ, before infection with Mtb, showed that the secretion of IL-1β was significantly inhibited by pretreatment of CoMtb-primed microglial cells with either MitoQ (Fig. 5B). Furthermore, Mtb-induced cleavage of IL-1β was significantly inhibited in MitoQ-pretreated microglial cells but not in cells treated with dTPP or a solvent control (Fig. 5C). These data suggest that intracellular and mitochondrial ROS are essential for Mtb-mediated IL-1β maturation in CoMtb-primed microglial cells.
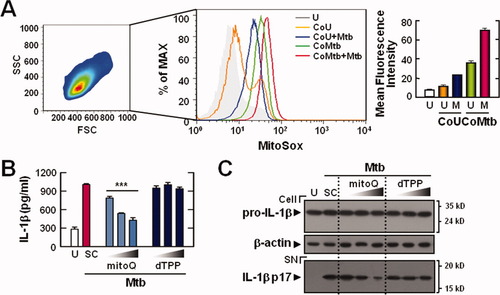
Mitochondrial ROS can trigger IL-1β maturation in CoMtb-primed microglia. (A) CoMtb- or CoU-primed microglial cells were infected with Mtb (moi = 1), and then stained with MitoSOX for 30 min and analyzed by flow cytometry. In total, 30,000 cells were analyzed per sample. The mean fluorescence intensities are shown as mean ± SD of three independent experiments (right). (B and C) CoMtb-primed microglial cells were pretreated with the mitochondrial antioxidant MitoQ (50, 100, and 200 nM) and the control compound dTPP (50, 100, and 200 nM) for 1 h. The cells were then infected with Mtb (moi = 1) and incubated for a further 18 h in the absence or presence of the mitochondrial antioxidant. (B) Supernatants were collected and subjected to ELISA. (C) Western blot analysis for caspase-1 and IL-1β (Cell, 31-kDa pro-IL-1β; SN, 17-kDa mature IL-1β). Data shown are representative of three independent experiments that gave similar results. ***P < 0.001, versus control cultures. U, uninfected; CoU, conditioned media from control uninfected BMDMs; CoMtb, Conditioned media from Mtb-infected BMDMs; M, Mtb; SC, solvent control (0.1% DMSO); SN, supernatants.
Oncostatin M or TNF-α Can Prime Microglia to Activate the Inflammasome in Response to Mtb
Although our data suggest that CoMtb primes the inflammasome in murine microglial cells by activating NF-κB, the manner of CoMtb activation of the NLRP3 inflammasome remains undefined. OSM, an IL-6 family cytokine, is involved in the pathogenesis of extrapulmonary tuberculosis through induction of MMP-1/3 secretion (O'Kane et al., 2008) and regulation of gene expression through activation of several signaling pathways, including NF-κB (Baker et al., 2010). The proinflammatory cytokine TNF-α is secreted by microglia in response to an Mtb component through NADPH oxidase-dependent ROS generation (Yang et al., 2007). Although these cytokines have been implicated in the potent activation of inflammatory responses, it is not clear whether these cytokines are involved in the induction of inflammasome activation in microglial cells.
We examined whether OSM or TNF-α is induced in BMDMs after stimulation with Mtb, and whether these factors may prime microglial cells to activate the inflammasome upon Mtb stimulation. Mtb significantly increased the levels of mRNA and protein expression for OSM and TNF-α in BMDMs (Fig. 6A,B). When BMDMs were incubated either with or without Mtb (moi of 1, 3, or 10) for 3, 6, 18, and 48 h, significant increases in OSM mRNA expression were observed, when compared with the values determined for uninfected cells (Fig. 6A). To exclude the potential effects of lipopolysaccharide (LPS) on OSM production, the BMDMs were preincubated with polymyxin B to block LPS activity. Preincubation of macrophages with polymyxin B did not affect the ability of Mtb to stimulate OSM production (data not shown). In addition, Mtb stimulation significantly induced the protein synthesis of OSM and TNF-α by BMDMs in a time-dependent manner (Fig. 6B).
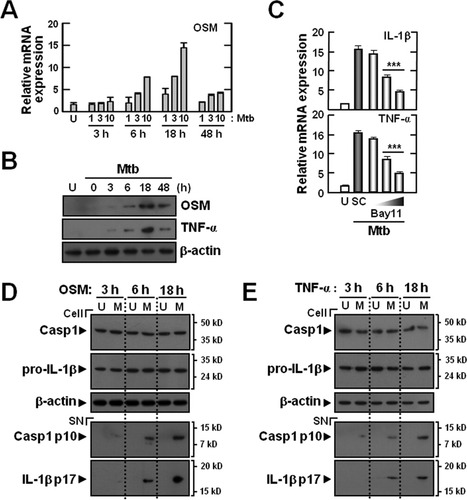
Oncostatin M or TNF-α can prime primary microglial cells to activate the inflammasome in response to Mtb. (A and B) BMDMs were infected with Mtb (moi = 1, 3, and 10 for A; moi = 10 for B) for the times indicated (0–48 h). (C) OSM-primed microglial cells were infected with Mtb (moi = 10) for 6 h in the absence or presence of BAY11-7082 (BAY11; 1, 5, and 10 μM). The cell lysates were subjected to real-time RT-PCR (A and C) or Western blot (B) analysis. (D and E) OSM (10 ng/mL)- or TNF-α (10 ng/mL)-primed microglial cells were infected with Mtb (moi = 1) for the times indicated (0–18 h). (D and E) Western blot analysis for caspase-1 and IL-1β (Cell, caspase-1 p45, 31-kDa pro-IL-1β; SN, cleaved caspase-1 p10, 17-kDa mature IL-1β). Data shown are representative of three independent experiments that gave similar results. ***P < 0.001, versus control cultures. U, uninfected; M, Mtb; SC, solvent control (0.1% DMSO); SN, supernatants; Casp1, caspase-1.
We further examined whether OSM induced the priming of microglia through the NF-κB signaling pathway. Pretreatment of microglia with Bay11-7082 significantly attenuated OSM-induced expression of mRNA for proIL1β and TNFα (Fig. 6C), suggesting an essential role for NF-κB signaling in OSM-induced pro-IL-1β and TNF-α synthesis in microglia. We also examined whether OSM or TNF-α priming of microglial cells followed by Mtb stimulation led to activation of caspase-1 and IL-1β processing. As shown in Fig. 6D,E, Mtb stimulation significantly induced the activation of caspase-1 and IL-1β maturation in OSM- or TNF-α-primed microglial cells. Taken together, these data suggest that macrophage-derived OSM and/or TNF-α resulting from Mtb stimulation can prime microglial cells to effectively activate the inflammasome.
Dexamethasone Inhibits Pro-IL-1β Synthesis and IL-1β Secretion in Microglia
Dexa, which is widely used for treating patients with CNS-TB, suppresses the production of MMP-1 and MMP-3 in CoMtb-stimulated human microglia (Green et al., 2010). We examined whether Dexa affected Mtb-induced IL-1β synthesis (Signal 1) and activation of caspase-1 (Signal 2) in CoMtb-primed microglial cells.
RT-PCR analysis revealed that Dexa treatment of microglial cells significantly reduced CoMtb-induced synthesis of mRNA for proIL1β and TNFα in a dose-dependent manner (Fig. 7A). In addition, it significantly inhibited IL-1β and TNF-α protein synthesis induced by Mtb stimulation in CoMtb-primed microglial cells (Fig. 7B). Notably, Mtb-induced generation of mature IL-1β, as well as pro-IL-1β, was significantly decreased in microglial cells that were preincubated with Dexa (Fig. 7C). Moreover, Mtb-induced cleavage of caspase-1 in microglial cells was significantly inhibited in a dose-dependent manner by Dexa treatment (Fig. 7C).
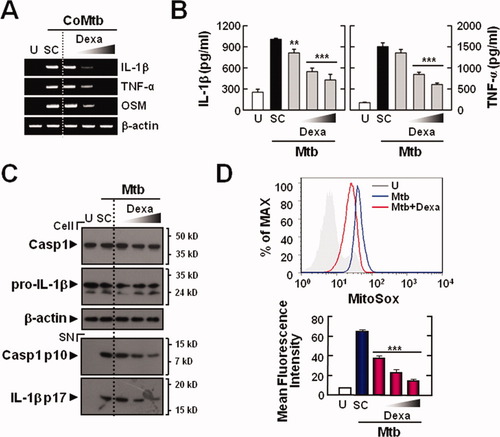
Dexamethasone suppresses IL-1β maturation through modulation of mitochondrial ROS in CoMtb-primed microglia. The primary microglial cells were pretreated for 45 min with dexamethasone (Dexa; 50, 100, and 200 nM), followed by incubation with CoMtb (A) or Mtb stimulation after CoMtb priming (B–D). The cells were then infected with Mtb (moi = 1) for 18 h (B–D). The cell lysates were subjected to semiquantitative RT-PCR analysis (A), ELISA (for IL-1β and TNF-α; B), and caspase-1 and IL-1β (Cell, caspase-1 p45, 31-kDa pro-IL-1β; SN, cleaved caspase-1 p10, 17-kDa mature IL-1β) detection by Western blot analysis (C). (D) The cells were stained with MitoSOX for 30 min and then analyzed by flow cytometry. In total, 30,000 cells were analyzed per sample. The mean fluorescence intensities are shown as mean ± SD of three independent experiments (bottom). Data shown are representative of three independent experiments that gave similar results. **P < 0.01, ***P < 0.001, versus control cultures. U, uninfected; SC, solvent control (0.1% DMSO); SN, supernatants; Casp1, caspase-1.
We previously reported that Dexa treatment significantly inhibits the Toll-like receptor-dependent inflammatory responses in BMDMs through modulation of ROS generation (Cuong et al., 2009). In this study, we further examined whether Dexa treatment regulated the Mtb-dependent generation of mitochondrial ROS in microglial cells. As shown in Fig. 7D, Dexa treatment of microglial cells significantly abrogated Mtb-induced mitochondrial ROS generation in CoMtb-primed microglial cells. Collectively, these data indicate that Dexa affects both pro-IL-1β synthesis (Signal 1) and IL-1β maturation (Signal 2) in primary microglial cells by inhibiting mitochondrial ROS generation and caspase-1 activation.
DISCUSSION
Inflammasomes have emerged as important innate immune-signaling elements that sense microbial motifs and damage-associated signals, so as to direct the secretion of inflammatory cytokines, such as IL-1β and IL-18 (Cassel et al., 2009). There has been significant progress in our understanding of how inflammasomes are activated and how dysregulated inflammasome activation affects the progress of inflammatory and autoimmune diseases. In the brain, the innate immune players, i.e., microglia and astrocytes, express pattern recognition receptors and activate the inflammasome, which seems to be involved in the pathogenesis of neuroinflammatory and neurodegenerative disorders (Chakraborty et al., 2010; Terada et al., 2010). However, it remains largely unknown as to whether Mtb activates the inflammasome in microglial cells, and it is not clear how this process is regulated in the context of innate immune regulatory pathways. In this study, we found that the NLRP3 inflammasome is activated by Mtb stimulation in CoMtb-primed microglial cells, but not in unprimed or CoU-primed conditions.
In a CNS with pathologic status, peripheral mononuclear cells circulate and migrate into the brain parenchyma through interactions with the endothelial cells of the blood–brain barrier (Hickey, 2001). Previous studies have shown that peripherally derived monocytes, microglia, and astrocytes are critically required for the regulation of MMP expression, secretion, and activity, suggesting a key role in the pathologic processes during CNS-TB (Green et al., 2010; Harris et al., 2007a, b). Currently, it seems that the induction of inflammasome activation requires a priming step for the production of pro-IL-1β (Signal 1) and that this is linked to the actual activation signal (Signal 2) (Hornung and Latz, 2010). However, the priming step/signals for inflammasome activation in microglial cells and during neuroinflammation have not been fully characterized. Therefore, we hypothesized that CoMtb driven from macrophages infected with Mtb may provide priming signal(s) for microglial inflammasome activation. Our present data demonstrate that CoMtb acts as an inducer of proIL1β mRNA in microglia, and that this induction is regulated by the NF-κB signaling pathway. CoMtb, which contains TNF-α, IL-6, IL-10, CCL-2, CCL-5, and CXC chemokine ligand 8 (Harris et al., 2007a), increases expression of the genes for MMP-1, -3, and -9 in microglia, under the regulation of NF-κB- and activator protein 1 c-Jun/FosB-dependent pathways (Green et al., 2010).
Our data also show that signals induced in the host cell by CoMtb, e.g., OSM and TNF-α, can induce the priming of inflammasome activation. Our data provide strong evidence that these macrophage-derived signals can activate NF-κB signaling pathways, which govern proinflammatory cytokine and pro-IL-1β production by microglial cells. OSM levels are elevated in several CNS inflammatory diseases, including multiple sclerosis (Ensoli et al., 2002) and HIV-associated neurocognitive disorder (Vecchiet et al., 2003). Additionally, our data partially correlate with the findings of Baker et al. (2010), who reported that OSM is required for the production of proinflammatory cytokines, such as TNF-α and inducible nitric oxide synthase, in microglia through NF-κB-dependent pathways. We also found significant induction of OSM mRNA and protein in microglial cells after stimulation with Mtb (data not shown). These data suggest that Mtb infection can directly induce OSM expression in microglia, thus causing inflammasome activation through autocrine OSM-mediated priming of microglial cells during CNS Mtb infection. Previous studies reported that OSM can be induced in microglia and macrophages in response to prostaglandin E2 through PKA-dependent pathways (Repovic and Benveniste, 2002). Thus, OSM is produced by both microglia and macrophages after Mtb stimulation, contributing to autocrine priming of microglia for inflammasome activation and a pathological inflammatory reaction during CNS-TB.
In terms of the pathogenic processes involved in neurodegenerative diseases, inflammasome activation (Signal 2) is known to be evoked by β-amyloid in Alzheimer's disease, superoxide dismutase 1 in amyotrophic lateral sclerosis, and perhaps huntingtin in Huntington's disease (reviewed in Masters and O'Neill, 2011). However, it is not known whether or by what mechanisms pathogens can initiate Signal 2 for inflammasome activation by microglia. To the best of our knowledge, this is the first report to show that Mtb can activate NLRP3 inflammasome via primed microglia. In the other immune cell types involved in inflammasome activation during mycobacterial infection, a double-edged role has been suggested for the inflammasome in inducing host-protective effects and detrimental effects with respect to the regulation of homeostasis. In murine macrophages, the Mtb zmp1 gene prevents inflammasome activation, which is involved in the host defense through enhancement of mycobacterial phagosomal maturation (Master et al., 2008). In contrast, the Esx-1 secretion system is required for inflammasome activation and has a detrimental effect on host responses and disease progression (Carlsson et al., 2010). Studies using an experimental TB model have shown that NLRP3 is not essential for the induction of protective immunity to Mtb, as NLRP3-deficient mice are able to restrict Mtb loads within well-differentiated granulomas (Walter et al., 2010). Moreover, previous studies have suggested potentially pathogenic and cytotoxic roles for IL-1β in neuronal cells in response to CNS insults; these effects depend on the release of free radicals (Thornton et al., 2006). Therefore, microglial inflammasome activation may lead to detrimental effects in the host and may contribute to neuroinflammatory pathogenesis during mycobacterial infection of the CNS.
Our current data also show that Mtb-induced inflammasome activation is regulated through mitochondrial ROS release, potassium efflux, and cathepsin B or cathepsin L. Our data partly corroborate the results of a recent study demonstrating that the formation of mitochondrial ROS is necessary for NLRP3 inflammasome activation by macrophages (Nakahira et al., 2011). Dysfunctional mitochondria, especially those with defective autophagy status, generate mitochondrial ROS, which are required for inflammasome-dependent inflammatory responses triggered by a variety of danger and infection signals (Tschopp, 2011). Potassium efflux and lysosomal cathepsin B protease are required for β-glucan-mediated NLRP3 inflammasome activation (Kankkunen et al., 2010). In addition, soluble schistosomal egg antigen-mediated inflammasome activation and IL-1β secretion are inhibited by blocking potassium efflux (Ritter et al., 2010). Duewell et al. also showed that crystalline cholesterol induces NLRP3 inflammasome and inflammatory responses in vivo, in a manner dependent on cathepsins B and L (Duewell et al., 2010). In the dextran sulfate sodium colitis model, macrophage IL-1β secretion depends on lysosomal maturation and cathepsins B and L (Bauer et al., 2010). Because Mtb-induced IL-1β maturation is modulated by blockade of potassium efflux, cathepsins B and L, and mitochondrial ROS, our findings imply that common mechanisms governing inflammasome activation contribute to microglial inflammasome activation in response to Mtb.
Earlier reports indicated that IL-1β, TNF-α, and chemokine levels are elevated in the cerebrospinal fluids of TBM patients (Drevets et al., 2004). These elevated IL-1β levels are often correlated with TNF-α concentration in cases of TBM (Akalin et al., 1994) and tend to decline during the prednisone 4-week regimen (Donald et al., 1995). Additionally, Dexa is involved in the inhibition of microglial activation and has beneficial effects in the treatment of acute bacterial meningitis (Hinkerohe et al., 2010). Our data show that Dexa treatment markedly regulates microglial IL-1β secretion and inhibits mitochondrial ROS, indicating a novel mechanism of Dexa-induced modulation of inflammasome activation, presumably through the regulation of mitochondrial ROS. Previous studies have shown that the modulation of IL-1β production occurs at the transcriptional level, but is not involved in inflammasome activation, in human peripheral blood mononuclear cells (Crişan et al., 2011). Mitochondrial ROS are thought to be essential for IL-1β maturation mediated by NLRP3 inflammasome activation (Nakahira et al., 2011). Our findings suggest that Dexa activity regulates IL-1β production in microglia at both the transcriptional and processing levels, suggesting that Dexa controls microglial IL-1β production in both inflammasome-dependent and independent manners. Thus, Dexa may prevent pathologic destruction of host tissue and improve the outcome of CNS-TB by multiple mechanisms.
Given the emerging role of the NLRP3 inflammasome activation in many inflammatory and autoimmune diseases, further studies are urgently required to clarify the exact roles and mechanisms of microglial inflammasome induction by various pathogenic stimuli. These efforts may contribute to the optimal development of therapeutics against CNS-TB and/or other inflammatory and infectious diseases of the CNS.
Acknowledgements
The authors are very grateful to J. J. Kim and J. M. Yuk for discussions and technical suggestions.