Regulation of L-type Ca++ currents and process morphology in white matter oligodendrocyte precursor cells by golli-myelin proteins
Abstract
The golli myelin basic proteins are expressed in oligodendroglial precursor cells (OPCs) where they play a role in regulating Ca2+ homeostasis. During depolarization, they influence process outgrowth and migration through their action on voltage-operated Ca2+ channels (VOCCs). To identify ion channels that are modulated by golli, we examined the electrophysiological properties of VOCCs in OPCs in the white matter of golli knock-out and control mice. OPCs exhibited two distinct Ca2+ channels, which were distinguished by their voltage dependence and pharmacological profiles and which exhibited many of the hallmarks of LVA/T-type and HVA/L-type Ca2+ channels. The density of high-voltage-activated (HVA) currents was reduced in OPCs recorded in golli-KO tissue, while low-voltage-activated (LVA) currents remained unaltered in these cells. These data indicate that golli exerts an exclusive influence on L-type Ca2+ channels in OPCs. Oligodendrocytes (OLs) also displayed LVA and HVA currents, although the density of these currents was much reduced at this developmental stage. These currents were not altered in golli-KO OLs showing the influence of golli on L-type Ca2+ channels is restricted to a specific time-window during the course of oligodendroglial development. The actions of golli on OPC L-type Ca2+ channels were accompanied by changes in process morphology, including a reduction in process complexity and the appearance of enlarged varicosities that decorated these cellular processes. These data on L-type Ca2+ channels and process development provide in situ evidence for the influence of golli on VOCCs, and offer an explanation for the hypomyelination observed in the brains of golli-KO mice. © 2010 Wiley-Liss, Inc.
INTRODUCTION
The myelin basic protein (MBP) gene produces two families of protein, the classic MBPs known for their structural role within the myelin sheath, and the golli-MBPs. Recent data show that golli-MBPs play a role in regulating Ca2+ homeostasis in cells of the immune system (Feng et al., 2006) and in oligodendroglial cells (Paez et al., 2007a, 2009a, b). Several lines of evidence indicate that increased expression of golli enhances voltage-dependent Ca2+ influx (Paez et al., 2007a, 2009a). Furthermore, golli modulates process extension and migration in oligodendroglial precursor cells (OPCs) (Paez et al., 2009a), both of which depend upon Ca2+ entry through voltage-operated Ca2+ channels (VOCCs), and which are integral to the eventual development of myelinating oligodendrocytes (OLs) (Miller, 2002).
Previous studies investigating the action of golli on OPC Ca2+ influx used ratiometric imaging to monitor intracellular [Ca2+] (Paez et al., 2007a, 2009a). Although these experiments demonstrated the involvement of VOCCs in golli-dependent modulation of Ca2+ signals, several issues are still unresolved. It is not known whether these actions of golli stem directly from an influence on VOCCs, or indirectly through other routes. For example, modulation of K+ channels by golli could alter membrane excitability thereby indirectly altering the activation of other voltage-dependent channels, including VOCCs. OPCs are known to express at least two subtypes of VOCC (von Blankenfeld et al., 1992), and it is yet unresolved whether one or both of these is influenced by golli. Also, in line with the apparent role of golli in shaping OPC developmental behaviors such as process outgrowth and migration, there is evidence that the action of golli on VOCC function is restricted to OPCs, and disappears upon differentiation (Paez et al., 2007b). Whether this developmental regulation occurs in vivo is not known. These issues can be resolved by performing patch-clamp recordings of OPC and OL Ca2+ currents in golli-KO tissue.
Golli overexpression induces morphological changes in oligodendroglial cells in vitro, including the elaboration of processes and membrane sheets (Paez et al., 2007a; Reyes and Campagnoni, 2002). Furthermore OLs cultured from golli-KO and overexpressing brains exhibit alterations in process complexity (Jacobs et al., 2005, 2009), suggesting that golli is important for the development of OLs in the intact brain. However, the morphological development of golli-KO OPCs has not been examined in situ. This issue could be addressed by examining individual dye labeled OPCs in slices prepared from golli-KO brains.
In this study, we have addressed all these issues and found that (1) OPCs exhibit low-voltage-activated (LVA) and high-voltage-activated (HVA) Ca2+ currents that exhibit the hallmarks of T and L-type Ca2+ channels, and (2) golli specifically influences L-type channels in OPCs, but does not influence these channels in OLs. OPCs in golli-KO brains exhibited reduced process branching, and these processes displayed enlarged varicosities. These alterations in the functioning of L-type channels and process morphology in OPCs in the golli-KO may underlie the deficits in myelination observed in the golli-KO and overexpressing animals (Jacobs et al., 2005, 2009), and provide further evidence of the importance of the golli-MBPs in oligodendroglial development.
MATERIALS AND METHODS
Animals
OPC Ca2+ channel currents were examined in situ by recording from fluorescently tagged cells in acute slices obtained from a transgenic mouse expressing EGFP under control of the proteolipid protein promoter (PLP-EGFP) (Mallon et al., 2002). To investigate the influence of golli-MBP on these currents, we recorded EGFP+ cells in a line generated by crossing the homozygous golli-KO line (Jacobs et al., 2005) with the PLP-EGFP mouse.
Acute Slice Preparation
Whole-cell recordings were made from coronal forebrain slices obtained from the PLP-EGFP and golli-KO/PLP-EGFP pups (P3-P7). Pups were anesthetized by isoflurane inhalation and the brain was removed and placed in oxygenated ice-cold slice solution (see solutions) for 5 min. Coronal forebrain slices containing the corpus callosum and lateral ventricles were cut at 300 μm and transferred into a holding chamber containing ice-cold oxygenated bicarbonate buffered recording solution (see solutions). The holding chamber was then placed into a water bath and incubated at 30°C for 30 min, after which slices were maintained at room temperature (RT).
Solutions
All external solutions were bubbled with 95% O2/5% CO2 and maintained at RT (20–23°C). Slice solution contained the following compounds (in mM): 26 NaHCO3, 1.25 NaH2PO4, 10 glucose, 125 NaCl, 3 KCl, 5 MgCl2, 1 CaCl2. Bicarbonate buffered recording solution (BBS) contained the following compounds (in mM): 26 mM NaHCO3, 1.25 mM NaH2PO4, 10 glucose, 125 NaCl, 3KCl, 2 MgCl2, 2 CaCl2. Ba++ currents were recorded in the following solution (in mM): 10 glucose, 5 Na-HEPES, 10 TEA (tetraethylammonium), 118 choline chloride, 10 BaCl2. All external solutions were prepared with pH and osmolarity adjusted to 7.3 and 300–310 mM/kg, respectively. Ba++ currents were recorded with a Cs-methanesulfonate based internal solution containing (in mM): 125 Cs-methanesulfonate, 4 NaCl, 3 KCl, 1 MgCl2, 5 Mg-ATP, 9 EGTA, 8 Na-HEPES, 1 tris-GTP, 0.1 leupeptin, 10 phosphocreatine (di-tris). Delayed rectifier K+ currents were measured with an internal solution containing (in mM): 112.5 K-gluconate, 4 NaCl, 17.5 KCl, 0.5 CaCl2, 1 MgCl2, 0.5 Mg-ATP, 0.05 EGTA, 1 Na-HEPES, 0.1 tris-GTP, 0.1 leupeptin, 0.5 phosphocreatine (di-tris). All internal solutions were prepared with biocytin (0.05%) and were adjusted to a final pH and osmolarity of 7.2 and 290 mM/kg, respectively.
Drugs
TEA (Sigma-Aldrich, St. Louis, MO) at a final concentration of 10 mM was added to all external solutions in experiments examining Ba++ currents. TEA was not added to external solutions in experiments measuring K+ currents. Stock solutions of Ni++ (50 mM, Fisher Scientific, Pittsburgh, PA) and Nifedipine (20 mM, Sigma-Aldrich) were prepared in distilled water, and working solutions were obtained by dilution in Ba++ recording solution.
Electrophysiology
Whole-cell Ba++ current recordings were performed as follows. Tissue slices were maintained in a recording chamber mounted on an upright microscope (Olympus BX51, Olympus America, Center Valley, PA) and were perfused with oxygenated bicarbonate buffered solution. EGFP+ cells in subcortical white matter were identified under epifluorescent illumination. Target cells were then approached under visual control and giga ohm seals were obtained. Patch pipettes (3–6 MΩ) were fabricated from standard glass capillaries (World Precision Instruments, Sarasota, FL) and filled with Cs+-based intracellular solution (Junction potential of 3.5 mV). Because of the small size of this voltage offset Ba++ current data were not corrected for this junction potential. Signals were amplified by an Axopatch 1D amplifier (Molecular Devices, Sunnyvale, CA), digitized at 10 kHz and filtered at 5 kHz. Signals were grounded with a AgCl bath electrode. Data acquisition was performed with pClamp version 9.0 (Molecular Devices). After achieving whole-cell access cell membrane parameters (membrane capacitance, input resistance, access resistance, and holding current) were obtained from the recording software. These readings were monitored continuously throughout the experiment and cells that showed dramatic changes in these measures, or displayed access resistances ≥30 MΩ were excluded from the final analysis. Average access resistances for OPCs were: Control, 23 MΩ (±0.9); Golli-KO, 24.8 MΩ (±1.3). Average access resistances for immature OLs were: Control, 18.2 MΩ (±1.4); golli-KO, 16.9 MΩ (±1.5). Series resistance compensation was not applied. Voltage errors in Ba++ current recordings resulting from uncompensated series resistance were calculated to range from 0.9 to 1.3%. Leak and capacitance currents were subtracted digitally using a P/N protocol. In this procedure the averaged response to three, scaled, negative pulses, applied prior to each voltage step, were subtracted from the subsequent test sweep. Ba++ currents were recorded as follows: Upon achieving a stable recording, the perfusion system was switched to deliver the Ba++-based external solution. Experiments commenced at least 2 min into the perfusion of this solution. Ba++ currents were activated in voltage-clamp by a pulse protocol that moved the cell membrane through a series of depolarizing voltage commands (−70 to +50 mV, 10 mV steps with a duration of 150 ms). Delayed rectifier K+ currents were recorded using a K+ gluconate-based internal solution (Junction potential of 12 mV). K+ current data were corrected for this junction potential. For these recordings, cells were maintained at a holding potential of −52 mV to inactivate A-type K+ currents. Delayed rectifier K+ currents were activated by a pulse protocol (−92 to +68 mV, 10 mV increments, step duration of 150 ms).
Postrecording Immunohistochemistry and Histology
Methods for examining the expression of oligodendroglial stage-specific markers in patch-clamped cells were based on a protocol described by Karadottir and Attwell (2006). Cells were loaded with biocytin through the patch-pipette, and after completion of the recording the patch-pipette was removed carefully from the cell. Tissue slices were immediately transferred to a 24-well plate and fixed in 4% paraformaldehyde at 4°C. After fixation (1–3 d), slices were washed four times in phosphate buffered solution (PBS) containing 0.01% Triton-X100 (washing solution). Slices were then stored in PBS at 4°C for up to 3 weeks pending further processing. Subsequently, slices were blocked and permeabalized in PBS containing 10% normal goat serum (NGS) and 0.2% Triton-X100. This incubation was carried out for 4–5 h at RT under agitation. Slices were then incubated overnight (∼15 h) in primary antibodies diluted in carrier solution (PBS plus 10% NGS). OPCs were identified with polyconal rabbit anti-NG2 (1:300, Millipore, Billerica, MA), while immature OLs were distinguished with monoclonal mouse anti-adenomatus polyposis coli/CC1 (1:500, EMD chemicals, Gibbstown, NJ). Following primary incubations, slices were rinsed four times in washing solution before incubation with secondary antibodies (4–6 h, 1:800, RT under agitation). NG2 and CC1 labeling was detected with Alexa Fluor®-594 conjugated goat IgG antibodies raised against the appropriate host species (Invitrogen, San Diego, CA). Slices were then rinsed four times before incubation with streptavidin-AMCA (1:400, Vector Laboratories, Burlingame, CA) for 2 h (RT with agitation). Slices were rinsed four times, mounted on microscope slides (Superfrost®/Plus, 22 × 75 mm, Fisher Scientific, Pittsburgh, PA) and air-dried for 10–13 min. Slides were coverslipped (22 × 50 mm cover glass, VWR, Westchester, PA) with aqueous mounting media (Aquamount, Lerner Laboratories, Pittsburgh, PA). Immunolabeling was examined on an inverted microscope (Olympus IX81) equipped with a spinning disc confocal setup. Slices were imaged through a DAPI filter set (Chroma Technology Corp., Rockingham, VT) to detect biocytin-streptavidin AMCA signals, and through a Calcium Crimson™ filter set (Chroma Technology Corp.) for examination of NG2 and CC1-Alexa Fluor 594 signals. Optical sections were acquired at 0.5 μm using SlideBook™ 4.0 software (Intelligent Imaging Innovations, Denver, CO).
Camera Lucida Reconstruction of Biocytin-Filled OPCs and Analysis of Cell Morphology
Sections containing biocytin-filled cells were prepared for camera lucida reconstruction as follows. After fixation (as described above), tissue containing biocytin-filled cells was incubated in PBS containing 0.2% Triton X-100 and 10% NGS for 2 h (RT with agitation), incubated in 0.3% H2O2 for 15 min, and then incubated in an avidin biotin complex solution for 30 min (Vectastain ABC system, Vector Laboratories). Slices were rinsed four times and then treated with DAB substrate for 13 min (1:10 dilution, Roche Applied Science, Penzberg, Germany) to generate the reaction product. Slices were then rinsed three times in washing solution before mounting on slides. Labeled cells were drawn by camera lucida using a Zeiss Ultraphot IIIb microscope (Carl Zeiss Micro imaging, Thornwood, NY) equipped with a 40× planapochromat objective (n.a. = 0.95, brightfield transmission illumination). Drawings of biocytin-filled cells were examined for branch complexity and varicosity size. Morphological complexity was assessed by describing the maximum branch order for each process on each cell. Branch orders were determined by counting the number of division points, which preceded that particular branch (counting from the cell body outward). In total, 48 control and 48 golli-KO processes were examined. Primary process, and total process length were measured by hand using a Plan Measure (Alvin & Co., Windsor, CT). Varicosities were measured from one or two processes on each cell and these measurements were restricted to varicosities occurring on the first three branch orders of a process.
Data Analysis and Statistics
All electrophysiological data were analyzed offline with Clampfit 8.0 software (Molecular Devices). Before current measurement, traces were normalized to the average holding current measured in the period preceding the voltage step. Low-voltage-activated Ba++ currents were measured as the average current over a span of 16 ms occurring immediately after onset of the test voltage. High-voltage-activated Ba++ currents and delayed rectifier K+ currents were measured during the steady-state phase between 100 and 150 ms after onset of the sweep. Current densities were calculated by dividing membrane current by the membrane capacitance value recorded prior to each test. Single comparisons between two groups were made by unpaired t-tests. Comparisons of current density voltage relationships between drug and control groups, and golli genotypes were examined by two-way analysis of variance (ANOVA) with repeated measures. Intergroup differences were then identified by Bonferroni post hoc testing. These parametric tests were calculated using Prism 4.0 for Macintosh (Graphpad Software, El Camino Real, CA). The distribution of processes between categories was compared by chi-square analysis (two by two contingency table design). The cumulative probability plots for varicosity size were analyzed by two-sample Kolmogorov-Smirnov tests. A significance of P < 0.05 was used in all tests.
RESULTS
Membrane Capacitance Measurements of Oligodendrocytes in the PLP-EGFP Mouse Can Predict Maturation State
Oligodendroglial cells in the subcortical white matter were identified by expression of EGFP under control of the PLP-promoter (Mallon et al., 2002). This transgene is expressed at all developmental stages in the OL lineage including OPCs and immature differentiated OLs. The developmental status of oligodendroglial cells may be determined by examining membrane capacitance (Chittajallu et al., 2005). This method derives from the fact that the transition from OPC to OL is accompanied by a progressive increase in the extent of membrane surface area, which in itself is related to membrane capacitance. To confirm the identity of PLP-EGFP+ cells in early-postnatal (P3-P7) subcortical white matter, and to evaluate the use of membrane capacitance for diagnosing the developmental stage of these PLP-EGFP labeled cells, we recorded EGFP+ cells and performed postrecording immunohistochemistry (IHC) for NG2 and CC1, a marker for differentiated OLs (Bhat et al., 1996) (see Fig. 1). Of the EGFP+ cells recorded with a capacitance of ≤20 pF, 95.5% were found to exhibit NG2 immunoreactivity (21 of 22 cells), while none of the EGFP+ cells examined within this category displayed CC1 labeling (0 of 5 cells). Conversely 90% of cells with capacitance values between 30 and 90 pF displayed robust CC1 immunoreactivity (9 of 10 cells), while none of the cells within this category displayed NG2 labeling (0 of 6 cells). These data indicate that the vast majority of white-matter PLP-EGFP+ cells with capacitance levels ≤20 pF exhibit NG2 labeling and are therefore likely to represent oligodendroglial cells at the OPC stage, while the majority of PLP-EGFP+ cells with capacitance values ≥30 pF were immunoreactive for CC1, identifying them as mature OLs. When EGFP+ cells with intermediate capacitance values between 20 and 30 pF were probed with anti-NG2, 50% displayed strong NG2 labeling (2 of 4 cells) while 50% displayed weaker NG2 signal (2 of 4 cells). Additionally, investigation of CC1 immunoreactivity in cells within this category revealed two examples of cells with CC1 labeling (2 of 2 cells). These observations, when considered together with the fact that none of the cells examined with capacitance values <20 pF displayed CC1 labeling, suggest that cells within this category (20–30 pF) represent a transitional stage during which NG2 expression declines, and CC1 expression begins.
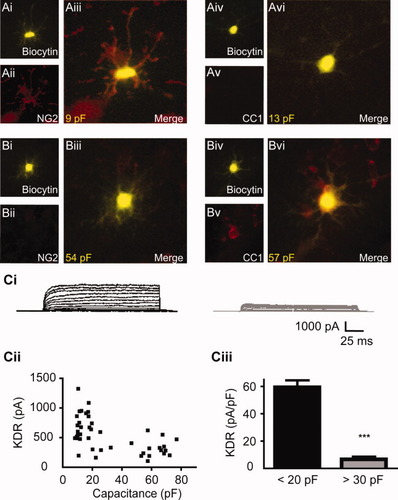
Membrane capacitance and oligodendrocyte development in situ. (A) PLP-EGFP Cells with membrane capacitance values ≤20 pF express the OPC marker NG2 (Ai–iii), but do not express the mature oligodendrocyte marker CC1 (Aiv–vi). Yellow numerals in (A) and (B) indicate membrane capacitance. (B) PLP-EGFP cells with membrane capacitance values ≥30 pF express the mature oligodendrocyte marker CC1 (Bi–iii), but are not immunoreactive for NG2 (Biv–vi). (Ci) Delayed rectifier K+ currents are downregulated in cells with membrane capacitance values >30 pF. Example traces for delayed rectifier K+ currents recorded from PLP-EGFP+ cells with capacitance values that were either <20 pF (left record), or >30 pF (right record). (Cii) Plotting KDR current against membrane capacitance reveals a negative relationship between these parameters. (Ciii) The average KDR current density (activated at a test potential of +68 mV) in cells with a membrane capacitance >30 pF (n = 16) is significantly lower than in cells with capacitance values <20 pF (n = 26) (5.74 pA/pF ± 0.68 vs. 55.19 pA/pF ± 5.28). *** significance at P < 0.001. Data are expressed as means ± SE.
Delayed rectifier K+ currents (KDR) are downregulated during OL maturation (Gallo et al., 1996). We exploited this feature of oligodendroglial physiology to further evaluate the use of membrane capacitance as a diagnostic test for developmental status. White matter PLP-EGFP+ cells were patch-clamped and KDR amplitude and membrane capacitance were measured. Examination of the relationship between peak KDR current amplitude and membrane capacitance (Fig. 1Cii) indicated that KDR current was smaller in cells with larger capacitance values. Interestingly this data revealed a transition in KDR amplitude between 20 and 30 pF that corresponds well with the apparent decrease in NG2 immunoreactivity, and increase in CC1 signal observed in cells within this category. When the data were divided according to these parameters, we found that cells exhibiting a membrane capacitance of <20 pF displayed a significantly greater peak KDR density (t = 8.28, df = 38, P < 0.001) (Fig. 1Ciii), and current amplitude (t = 5.07, df = 41, P < 0.001) (Supp. Info. Fig. 1). The reduced function of KDR in cells with capacitance values >30 pF is therefore in good agreement with our IHC data detecting the mature OL marker CC1 in cells of this type (Fig. 1B).
Although CC1 immunoreactivity in PLP-EGFP+ cells indicates an oligodendroglial cell that has advanced beyond the precursor stage, it does not allow discrimination between the different developmental stages (premyelinating vs. myelinating) that accompany maturation. However, the cells with capacitance values >30 pF recorded in this study lack the symmetrical inward and outward membrane currents (Fig. 1Ci) exhibited by myelinating OLs in situ (Berger et al., 1991; Fuss et al., 2000). Additionally, biocytin filling of these cells did not reveal the stereotypical pattern of processes aligned in parallel to axonal fibers exhibited by myelinating OLs (Fuss et al., 2000) (Fig. 1B). Overall, the properties exhibited by these cells (P3 to P7 tissue, >30 pF) are consistent with an immature OL, perhaps similar to the premyelinating OLs described by Trapp et al. (1997). In the present report we will distinguish OPCs from immature OLs according to their capacitance values, such that cells with values ≤20 pF are identified as OPCs, while cells with values ≥30 pF are recognized as immature OLs. In practice, immature OLs were readily distinguished from OPCs by their greater expression of the PLP-EGFP transgene. Thus cells could be selected for recording on the basis of their appearance (bright vs. dim EGFP), and this choice could then be confirmed after gaining whole-cell access by measurement of the cells membrane capacitance.
Isolating Low-Voltage and High-Voltage Activated Ca2+ Channels in PLP-EGFP+ OPCs
White matter PLP-EGFP+ OPCs (Fig. 2A) were patch-clamped with a CS+-based pipette solution and their intrinsic membrane currents were examined in voltage-clamp experiments. While recording in regular BBS, depolarization of OPCs activated a transient inward current (Fig. 2Aii). This current activated at −30 mV, peaked at 10 mV, disappeared in the absence of Na+ (Fig. 2Aiii) and was sensitive to TTX (data not shown), indicating that this is a voltage-dependent Na+ current. This conductance shows similar properties to the Na+ currents previously described in white matter OPCs (Berger et al., 1992). Ba++ currents were isolated by switching to a recording solution containing Ba++ and lacking Na+ and K+ (Fig. 2Aiii). Following this solution, exchange depolarizing voltage steps (−70 to +50 mV) activated a lower-amplitude current that persisted throughout the voltage-command. During the first 30 ms this Ba++ current displayed an inactivating component, which was followed by a more sustained phase. Previous studies of VOCCs in oligodendroglial cells identified the presence of both LVA and HVA Ca2+ currents (Berger et al., 1992; von Blankenfeld et al., 1992; Williamson et al., 1997). Aside from activating and inactivating at lower membrane voltages, LVA channel activity typically appears as a fast inactivating current (Ertel, 2004) similar to the initial component we observed in OPCs (Fig. 2Aiii, 2Bi). To investigate the presence of LVA and HVA channels in these cells, OPCs were maintained at a holding potential (Vh) of either −120 or −50 mV, and then stepped to various test potentials (−70 to +50 mV) (Fig. 2B). Examination of the current traces obtained from these experiments revealed two distinct currents: one, activated from Vh −120 mV, displayed a prominent fast inactivating component; while the second, activated from Vh −50 mV, was sustained and noninactivating (Fig. 2Bi). To isolate the fast inactivating Ba++ current, traces from the two holding potentials were subtracted (Vh-120 − Vh-50). Similar to the transient current obtained at Vh −120 mV, this isolated current displayed a prominent initial inactivating component (Fig. 2Bii). Analysis of the current density/voltage properties of these two currents showed that the subtraction current exhibited an activation voltage (−40 mV) and peak density voltage (−10 mV) that were hyperpolarized with respect to those displayed by the sustained current (−30 mV and 0 mV respectively) (Fig. 2Biii). These voltage-dependent properties are typical of LVA and HVA Ca2+ currents, and so we will refer to the isolated (Vh-120 − Vh-50) and sustained (activated from Vh-50 mV) currents as LVA and HVA, respectively.
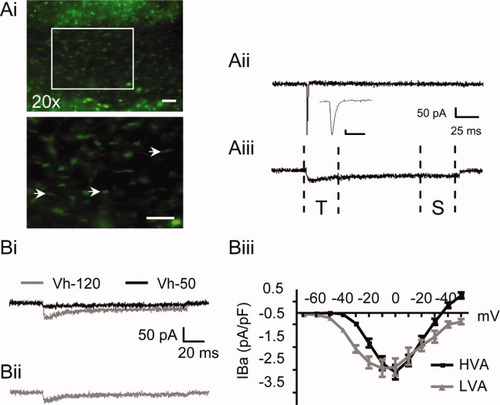
Low-voltage and high-voltage-activated Ca2+ channel currents in PLP-EGFP+ OPCs. (Ai) Fluorescent images of a coronal section prepared from a P3 PLP-EGFP brain. Top panel shows the corpus callosum at the level of the lateral ventricles (20× objective). Lower panel shows an enlarged image of the corpus callosum (area enclosed in white box in top panel). Recordings were made from PLP-EGFP+ OPCs. These cells typically exhibited a faint EGFP signal and an elongated cell body (arrow heads). Scale bars: top and lower panel 50 μm. (Aii) Depolarization of the OPC membrane to 0 mV activates a fast inward Na+ current. Inset shows Na+ current on an expanded time base. Scale bar for inset indicates 50 pA and 5 ms. (Aiii) Recording in solutions containing 10 mM Ba++ and lacking Na+ and K+ reveals a slower sustained inward current containing distinct transient (T) and sustained (S) phases (main scale bar applies to Aii and Aiii). (Bi) The transient current component is present in recordings made from a holding potential (Vh) of −120 mV (gray), but is inactivated when currents are recorded from a Vh of −50 mV (black). Subtraction of these traces (Vh-120 − Vh-50) reveals a transient fast-inactivating current (Bii) (Scale bar applies to Bi and Bii). (Biii) Summary graph displaying current density/voltage plots constructed from the isolated current (LVA) (n = 9) and the noninactivating sustained current (HVA) (n = 9). Data are expressed as means ± SE.
Pharmacological Characterization of LVA and HVA Channels in OPCs
A wide variety of neuronal, muscle, and cardiac cell types exhibit LVA currents that are mediated by the activity of T-type Ca2+ channels (Perez-Reyes, 2003). T-type channels have been suggested to exhibit sensitivity to low concentrations of the inorganic Ca2+ channel blocker Ni++. To assess the contribution of T-type channels to the LVA current in OPCs, we examined the influence of Ni++ (50 μM) on the magnitude of these responses. Ni++ reduced the density of LVA currents at both −30 and −10 mV test potentials (ANOVA F(10,1) = 106.5, P < 0.001, post hoc comparison of Ni++ vs. vehicle P < 0.05 at each test, Fig. 3A). As a further test of the effect of Ni++ on the LVA current, we examined sensitivity of the transient component (recorded at Vh-120) before and after application of Ni++. For this purpose, we compared the ratio of the transient and sustained current components (transient/sustained) in cells recorded in the presence or absence of Ni++ (Fig. 3B). This ratio remained stable throughout four cycles of testing in nontreated control cells (Fig. 3Bii). However, cells exposed to Ni++ displayed a marked reduction in this ratio. Statistical analysis of data in control and Ni++-treated OPCs showed that the ratio of transient to sustained current was reduced after 5 min of treatment with Ni++ (ANOVA F(28,1) = 8.81, P < 0.01, post hoc comparison of Ni++ vs. vehicle P < 0.05). Consistent with these results examination of current traces revealed the loss of the transient component following application of Ni++ (Fig. 3Bi). HVA currents are relatively insensitive to low concentrations of Ni++ (Ertel, 2004). In agreement with this, we found that Ni++ did not reduce the density of HVA currents in OPCs (Fig. 3C). Overall this data indicate that the LVA current contains a Ni++ sensitive component, which is absent from the OPC HVA current. Together, the sensitivity to Ni++, coupled with the voltage and time-dependent properties described above, suggest an involvement of T-type channels in the LVA currents of OPCs.

Pharmacological analysis of LVA and HVA currents in PLP-EGFP+ OPCs. (Ai) Example traces showing LVA currents activated at a test potential of −30 mV in the absence (black) and presence (gray) of 50 μM Ni++. (Aii) Current density/voltage plots generated from LVA currents recorded in the absence (black, n = 3) and presence (gray, n = 3) of 50 μM Ni++. Application of Ni++ reduced LVA currents activated between −30 and −10 mV. For example, the mean current density at −30 mV was reduced from −2.78 pA/pF (±0.27) to −1.54 pA/pF (±0.22) following application of Ni++. (Bi, Bii) Ni++ selectively impairs the transient current component (i) as indicated by the reduced ratio of transient (T) to sustained (S) components in Ni++ treated cells (ii). For example, the average T:S ratio in OPCs treated with Ni++ for 5 min was 1.6 (±0.07, n = 4) as compared with 2.21 (±0.16, n = 5) in nontreated control cells. (Ci) Example traces of HVA currents activated at 0 mV before and after the application of Ni++. (Cii) The average current density for HVA currents activated before (n = 3) and in the presence of Ni++ (n = 3) was not found to differ at any of the test potentials examined (e.g., 0 mV: −2.60 pA/pF ± 1.50 vs. −2.27 pA/pF ± 1.22). (Di) Sample traces showing HVA currents activated at 0 mV recorded before (black) and after (gray) the application of nifedipine (10 μM). (Dii) HVA current density/voltage curves constructed from data recorded before and during perfusion of solution containing nifedipine. (Diii) Graph displaying the % inhibition produced by nifedipine on transient (gray) and HVA (black) current densities. HVA currents (n = 14) were inhibited to a significantly greater degree than the transient currents (n = 14). For example, at a test potential of 0 mV nifedipine blocked 81.54% (±4.65) of HVA current, and only 41.2% (±3.08) of the transient current. * significance at P < 0.05, ** significance at P < 0.01, *** significance at P < 0.001. Data are expressed as means ± SE.
To determine the contribution of L-type Ca2+ channels to the HVA current in OPCs, we recorded HVA currents before and after the application of nifedipine (10 μM) (Fig. 3D). Application of nifedipine produced a dramatic reduction in HVA current density (Fig. 3Dii) that was statistically significant at all test potentials positive to −20 mV (ANOVA F(26,1) = 36.31, P < 0.001, post hoc comparison for nifedipine vs. vehicle P < 0.001 at each test). The extent of this inhibition was investigated by calculating the % blockade at the four test potentials that represent the main activation window for this current (−20, −10, 0, and 10 mV). HVA current was reduced by 68.6% (±4.2), 73.3% (±4.4), 81.5% (±4.7), and 88.3% (±8.7), respectively at these potentials (Fig. 3Diii). T-type channels are considered less sensitive to nifedipine than L-type channels (Ertel, 2004). Consistent with this, we found the transient current (activated at Vh −120) to be less sensitive to nifedipine than the HVA current, being reduced by just 28.7% (±4.7), 37.5% (±3.1), 41.2% (±4.7), and 43.2% (±3.6) at test potentials of −20, −10, 0, and 10 mV, respectively (Fig. 3Diii). The greater sensitivity of HVA currents to nifedipine was confirmed by statistical analysis (ANOVA, F (26,1) = 73.0, P < 0.001, post hoc comparison for HVA vs. transient current P < 0.0001 at each test). Overall these pharmacological experiments show that T-type Ca2+ channels contribute to the LVA current, while L-type Ca2+ channels mediate the majority of HVA current in white matter OPCs.
LVA and HVA Currents Are Not Enhanced During Oligodendroglial Lineage Progression
Inward currents were also activated when immature OLs were depolarized in the presence of Ba++ (see Fig. 4). Similar to currents activated in OPCs, these currents displayed both inactivating and sustained components that could be separated by recording from different holding potentials (Fig. 4Ai). Subtraction of currents recorded at Vh-50 mV from those recorded at Vh-120 mV isolated a current activating at ∼−50 mV. In contrast, the currents recorded from Vh-50 mV showed a more depolarized activation potential of −30 mV. These voltage-dependent properties are similar to those exhibited by the LVA and HVA currents in OPCs (Fig. 2B). Interestingly, neither the LVA or HVA current amplitudes increased in step with the increased membrane capacitance in these immature OLs (average capacitances were 9.1 ± 0.6 pF and 40.0 ± 6.2 pF for OPCs and immature OLs, respectively) (Supp. Info. Fig. 2). Indeed a comparison of absolute HVA currents in OPCs and immature OLs indicated a reduction in the more mature cells (ANOVA, F (16,1) = 5.29, P < 0.05, post hoc comparison for OPC vs. immature OL HVA current P < 0.05 at 10 mV). In agreement with these results, the current densities for both LVA and HVA channels were significantly smaller in immature OLs (Fig. 4B) (LVA ANOVA, F(16,1) = 12.94, P < 0.01, post hoc comparison at test potentials −10 mV P < 0.05, 0, and 10 mV P < 0.001; HVA ANOVA, F(18,1) = 55.11, P < 0.001, post hoc comparison at test potentials −10, 0, and 10 mV P < 0.0001). Overall, these data indicate that the amplitude and density of LVA and HVA currents do not rise in parallel with the increased membrane capacitance that accompanies the transition from OPC to immature OL.

LVA and HVA currents in immature oligodendrocytes. (Ai) Sample recordings of Ca2+ channel currents in immature OLs. Top traces show currents activated from a holding potential of −120 mV (gray) and −50 mV (black). Note inactivation of the transient component when currents are activated from −50 mV. Bottom traces show the isolated LVA current (gray) superimposed on the HVA current activated from −50 mV (black). (Aii) The isolated LVA current (gray) activates at lower voltages than the HVA current (black). (Bi, Bii) Summary graphs of current density/voltage data for LVA (i) and HVA (ii) currents indicate that densities of both types of current are dramatically reduced in immature OLs. The average LVA current density in OPCs (n = 8) and immature OLs (n = 8) activated at 0 mV was significantly different (−1.46 pA/pF ± 0.24 vs. −0.40 pA/pF ± 0.11). The difference in HVA current density between OPCs (n = 12) and immature OLs (n = 8) at 0 mV also reached significance (−1.83 pA/pF ± 0.17 vs. −0.28 pA/pF ± 0.08). * significance at P < 0.05, ** significance at P < 0.01, *** significance at P < 0.001. Data are expressed as means ± SE.
Golli Regulates HVA Ca2+ Channels in OPCs
To determine if golli exerts a specific influence on OPC Ca2+ channels, we measured the density of the mixed OPC Ba++ current activated from Vh-70 mV (see Fig. 5) in slices obtained from golli-KO/PLP-EGFP and control PLP-EGFP animals. A transient inactivating component, while still apparent in currents activated from this holding potential, appeared to be less pronounced than in responses activated from Vh-120 mV (cf. Figs. 2Bi and 5Ai). Examination of the current density/voltage curves from these recordings suggested that the mean current density in golli-KO OPCs was smaller than in control cells (Fig. 5Aii). Statistical examination revealed that the apparent reduction in the mixed current in golli-KO OPCs approached significance (ANOVA, F(28,1) = 3.42, P = 0.075). Examination of the average amplitude of the mixed current in golli-KO and control OPCs provided a similar result (Fig. 5Aiii), although in this instance the difference between these groups reached statistical significance (ANOVA for currents recorded at −10, 0, and 10 mV, F(21,1) = 5.37, P < 0.05, post hoc comparisons at 0 mV (P < 0.05).
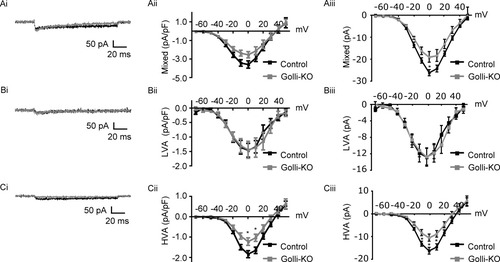
Golli specifically regulates HVA channels in OPCs. (Ai) Sample recordings showing mixed currents in control (black) and golli-KO (gray) OPCs. Summary graphs reveal reduced current density (Aii) and current amplitude (Aiii) for the mixed current in golli-KO OPCs. For example, the average current amplitude activated at 0 mV in golli-KO (n = 11) and control OPCs (n = 12) were −19.0 pA (±2.4) vs. −26.1 pA (±1.4), respectively. (Bi) Example traces showing LVA currents recorded from control (black) and golli-KO (gray) OPCs. Summary graphs showing average current densities (Bii) and current amplitudes (Biii) for LVA currents in golli-KO and control OPCs. LVA current density and amplitude did not differ between golli-KO and control OPCs, for example the average LVA current amplitude activated at 0 mV in golli-KO (n = 10) and control OPCs (n = 10) was −12.7 pA (±2.0) and −12.8 pA (±2.2) respectively. (Ci) HVA currents recorded from control (black) and golli-KO (gray) OPCs. Summary graphs indicate reduced HVA current density (Cii) and current amplitude (Ciii) in golli-KO OPCs. The average HVA amplitude was significantly smaller in golli-KO OPCs (n = 10) than the equivalent currents recorded from control OPCs (n = 12) (average current amplitudes at O mV: −10.5 pA (±1.6) vs. −16.2 pA (±1.5)).* significance at P < 0.05. Data are expressed as means ± SE.
Previous in vitro Ca2+ imaging data indicate that golli-dependent changes in Ca2+ uptake occur primarily through nifedipine sensitive channels (Paez et al., 2007b), and are less sensitive to Ni++ (Paez et al., 2007a). These results suggest that golli may exert a differential influence over HVA and LVA type Ca2+ channels. Recording from a holding potential of −70 mV allowed sampling of both LVA and HVA channels, which may have lead to a dilution of the effect of golli if its actions are directed specifically at HVA Ca2+ channels. To examine this issue, we isolated LVA and HVA currents in golli-KO and control OPCs. Both the current density and amplitude for the isolated LVA current appeared to be similar in the golli-KO and control OPCs (Fig. 5B). These observations were supported by statistical analysis, which indicated that neither LVA current density or amplitude differed between these groups (ANOVA for currents densities at −10, 0, and 10 mV, F(18,1) = 0.002, P = 0.97. ANOVA for currents recorded at −10, 0, and 10 mV, F(18,1) = 0.002, P = 0.96). In contrast to these results, OPCs lacking golli showed reductions in the density and amplitude of the HVA current (Fig. 5C). The reduction in HVA current density reached significance at the 0 and 10 mV test potentials (ANOVA for current densities at −10, 0, and 10 mV, F(21,1) = 7.34, P < 0.05, post hoc comparisons at 0 (P < 0.05) and 10 mV (P < 0.05)), while the reduction in HVA current amplitude was significant at the −10, 0, and 10 mV test potentials (ANOVA for currents recorded at −10, 0, and 10 mV, F(21,1) = 8.34, P < 0.01, post hoc comparisons at −10 (P < 0.05), 0 (P < 0.05), and 10 mV (P < 0.05)). The effects of golli on HVA density are unlikely to stem from an influence on membrane capacitance since both HVA current amplitude and density were significantly altered in golli-KO OPCs. In agreement with this membrane capacitance measured from golli-KO (9.22 ± 1.0 pF, n = 11) and control OPCs (9.08 ± 0.6 pF, n = 12) was not statistically different (t= 0.09, df = 20, P = 0.9). Overall these data indicate that golli exerts a specific influence on currents carried by HVA channels, while LVA channels remained unaffected.
The Influence of Golli on HVA Ca2+ Channels Disappears upon Differentiation
In vitro data show a reduction in voltage-dependent Ca2+ influx in oligodendroglial cells isolated from golli-KO animals (Paez et al., 2007b). This influence appeared to be developmentally regulated, since the effects of golli were restricted to OPCs and were not observed in more mature cells (Paez et al., 2007b). To determine whether golli's influence on Ca2+ channels is developmentally regulated in situ, we recorded VOCC currents in immature OLs in golli-KO/PLP-EGFP and control PLP-EGFP mice. LVA and HVA currents were recorded and current density/voltage plots were constructed. Statistical analysis of these data indicated that both the LVA (ANOVA, F (17,1) = 1.4, P = 0.25) and HVA currents (ANOVA, F (15,1) = 0.31, P = 0.59) were similar in these two genotypes (Fig. 6A,B). Overall, these data show that golli doesnot influence LVA and HVA Ca2+ channels in immature OLs. Moreover, these findings, in conjunction withthe effects we have observed on HVA currents in OPCs (Fig. 5C) show that golli is able to regulate specific ion channels in situ, and that these actions are restricted to the precursor stage of oligodendroglial development.
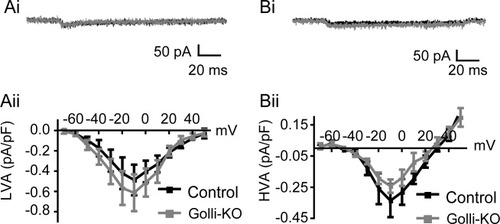
Golli does not influence Ca2+ channels in immature oligodendrocytes. (Ai) Sample LVA currents recorded from control (black) and golli-KO (gray) OLs. (Aii) Current density/voltage curves in golli-KO and control OLs are similar. The average LVA current density at −10 mV was −0.61 pA/pF (±0.18) and −0.48 pA/pF (±0.15) in golli-KO (n = 11) and control cells (n = 8) respectively. (Bi) Example traces showing HVA currents in control (black) and golli-KO (gray) OLs. (Bii) HVA Current densities in Golli-KO and control OLs are not different. The average HVA current density in golli-KO cells (n = 8) at −10 mV was −0.24 pA/pF (±0.04), while the corresponding value recorded from control cells (n= 10) was −0.33 pA/pF (± 0.11). Data are expressed as means ± SE.
OPCs Lacking Golli Show Reduced Process Complexity and Enlarged Membrane Varicosities
Although considerable in vitro data exists indicating a role for golli in the regulation of OPC process elaboration (Jacobs et al., 2005; Paez et al., 2007a, 2009a; Reyes and Campagnoni, 2002), less is known regarding the influence of golli in situ. To determine how the absence of golli influences the physical development of OPCs in situ, we examined the morphology of OPCs filled with biocytin during patch-clamp recording. PLP-EGFP+ white matter OPCs were patched in control and golli-KO tissue and these slices were then fixed and prepared for histological analysis. Biocytin-filled OPCs were subsequently reconstructed by camera lucida allowing a detailed examination of their morphology (Fig. 7A). Analysis of the maximum branch order reached by golli-KO and control processes revealed that significantly fewer golli-KO processes exhibited a complexity of five or more branch orders (χ2 = 22.89, df =1, P < 0.001), indicating that the absence of golli leads to a reduction in branch formation (Fig. 7B). Process outgrowth was examined by measuring both the length of primary branches, and the total length of each process for golli-KO and control OPCs. We then determined the number of primary branches that were ≥ two cell bodies in width, and the number of process that displayed a total length that was ≥ five cell bodies in width. Importantly cell body size was not found to differ between control and golli-KO OPCs, which displayed average cell body widths of 6.19 μm (± 0.51) and 6.54 μm (± 0.59), respectively (t = 0.45, df = 18, P = 0.65). Statistical analysis revealed that a greater number of golli-KO primary processes were ≥ two cell bodies in width (χ2 = 4.98, df = 1, P < 0.05, data not shown). Similarly, a greater number of golli-KO processes showed a total length that was ≥ five cell bodies in width (χ2 = 3.95, df = 1, P < 0.05, data not shown). The increased length of primary processes indicates that the first branch division occurs further from the cell body in golli-KO processes. Together the data on distance to first branch point, and total process length are consistent with the apparent decrease in process branching described above in golli-KO OPCs (Fig. 7B).
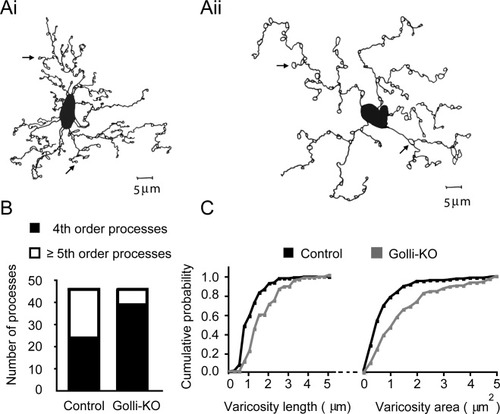
Reduced morphological complexity and enlarged membrane varicosities in OPCs lacking golli. (A) Camera lucida drawings of biocytin-filled OPCs taken from control (i) and golli-KO (ii) slices. Example membrane varicosities are indicated by arrows. (B) Golli-KO OPCs exhibit reduced process complexity. Processes were assigned to one of two classes depending on the maximum branch order reached. The number of processes assigned to the higher complexity (≥5th branch order) group differed significantly (22 out of 45 control processes vs. 7 out of 45 golli-KO processes). (C) Golli-KO OPCs exhibit larger membrane varicosities. Cumulative probability distributions for varicosity length (left) and area (right) measured from control (n = 115) and golli-KO (n = 109) varicosities.
Examination of the processes of golli-KO OPCs also revealed the presence of enlarged membrane varicosities (arrows Fig. 7Aii). These swellings were also present on the processes of control OPCs, although they appeared to be considerably smaller in these cells. To investigate potential differences in varicosity number and size, we counted and measured all of the varicosities present on the processes of control and golli-KO OPCs. Although the average number of varicosities per branch division was similar (1.43 varicosities/control branch vs. 1.69 varicosities/golli-KO branch), we found significant differences in the distribution of varicosities according to size (Fig. 7C). Cumulative probability plots for varicosity length and area revealed a rightward shift in the distribution of golli-KO varicosities. Analysis of these data showed that the distribution of varicosity length and area were shifted toward larger values in the golli-KO processes (two-sample Kolmogorov-Smirnov tests: length: χ2 = 30.64, P < 0.001; area: χ2 = 25.88, P < 0.001). Together these data indicate that processes in golli-KO OPCs exhibit a reduction in morphological complexity, and an enhancement in the size of membrane varicosities.
DISCUSSION
The present data expand our understanding of OPC physiology in two ways. First, they identify the functional expression of T- and L-type Ca2+ channels in developing white matter OPCs. Second, they advance our understanding of golli-MBP function in situ by showing that these proteins exert a specific modulatory influence on L-type Ca2+ currents. Interestingly, the density of both subtypes of Ca2+ current, and the influence of golli on the L-types, decline during maturation underlining the important role played by these channels during OPC development. OPCs in golli-KO tissue also display alterations in process morphology including a reduction in process complexity, and the appearance of enlarged varicosities. Both of these features suggest a deficit in process development, which may be related to the reduced entry of Ca2+ via L-type channels in OPCs lacking golli proteins.
Expression of LVA and HVA Channels in OPCs and Oligodendrocytes of the Developing White Matter
The activation of Ca2+ channels in white matter OPCs generated inward currents containing transient and sustained components. These components, when separated by recording at different holding potentials, displayed voltage-dependent properties typical of LVA (transient) and HVA (sustained) type Ca2+ currents (Perez-Reyes, 2003). Pharmacological analysis showed that the LVA current was significantly reduced by low concentrations of Ni++, and was relatively insensitive to nifedipine suggesting the involvement of T-type channels (Ertel, 2004; Perez-Reyes, 2003). It is important to note that the properties of this LVA current, i.e., fast kinetics, negative activation threshold and sensitivity to Ni++, have been associated with the activity of R-type Ca2+ channels (Ertel, 2004). However, while the α1E subunit, which may form an R-type Ca2+ channel pore, has been detected in myelinating OLs (Chen et al., 2000) the presence of this channel protein during earlier stages of OL development has not been determined, so the participation of R-type channels to this LVA current remains an open question. Conversely, the HVA current showed little sensitivity to Ni++, and was substantially reduced by nifedipine, which together provide strong evidence that this current is generated by the activation of L-type Ca2+ channels.
We also studied LVA and HVA channels in immature OLs. Although the amplitude of HVA currents was significantly reduced in immature OLs, the reduction in LVA currents was found to be less dramatic. None-the-less, these results indicate that these currents do not increase in amplitude in step with the growing membrane capacitance exhibited by maturing OLs. This observation is mirrored in the dramatic reduction in LVA and HVA current densities recorded from immature OLs. Overall, these data are consistent with a previous study of VOCCs in white matter oligodendroglial cells (Berger et al., 1992). In this article, the expression of Ca2+ currents declined during the first postnatal week, and were completely absent in cells recorded during the second. This developmental regulation of Ca2+ channels was not restricted to a specific subtype since both LVA and HVA currents decreased in a similar fashion. This indicates a preferential requirement for VOCCs during the earlier stages of oligodendroglial development, an idea that is supported by recent work showing a requirement for these channels during OPC migration (Paez et al., 2009a).
Specific Regulation of L-Type Ca2+ Channels by Golli-MBP
Previous work using OL cell lines revealed a role for golli-MBP in the regulation of OL Ca2+ channels (Paez et al., 2007a). Elevated external [K+] stimulated a rise in intracellular [Ca2+] that was enhanced in cells transfected with golli, and which was blocked by the application of Cd++. Subsequent studies using OPCs isolated from golli-KO and overexpressor animals have confirmed this finding showing that Ca2+ entry is enhanced in cells overexpressing golli, and reduced in cells lacking this protein (Paez et al., 2007b). Golli also influences OPC Ca2+ channels under basal conditions (Paez et al., 2009a). In this study, spontaneous fluctuations in the level of intracellular Ca2+ that depend on the activity of VOCCs were regulated by golli such that Ca2+ cycle frequency was enhanced by its overexpression, and reduced by its absence. Our whole-cell recordings extend these findings by revealing a reduced density of HVA currents in golli-KO OPCs. Importantly, these effects can be attributed to a direct action on HVA channels since all K+ and Na+ conductances were abolished under the conditions used to obtain these recordings.
Several lines of evidence suggest that golli selectively influences the function of L-type Ca2+ channels. First, golli regulation of spontaneous Ca2+ entry in vitro is abolished by the L-type channel blockers verapamil and nifedipine (Paez et al., 2009a). Second, the effects of golli on K+ stimulated Ca2+ entry show greater sensitivity to Cd++ than to Ni++ (Paez et al., 2007a). These results simultaneously argue against the involvement of T-type Ca2+ channels, which show a relatively high sensitivity to Ni++, and indicate an involvement of L-type channels, which are typically less sensitive to Ni++ (Mogul and Fox, 1991; Ozawa et al., 1989). The present data showing reduced L-type, but not T-type channel function in OPCs lacking golli are therefore in good agreement with the idea that golli selectively regulates L-type Ca2+ channels in OPCs.
Ca2+ entering through VOCCs has the ability to influence multiple signaling pathways, so the regulation of these ion channels confers golli with the potential to influence many cellular events. One recently described example of this involves the regulation of process outgrowth and OPC motility rates through mechanisms involving L-type Ca2+ channels (Paez et al., 2009a). Golli/Ca2+ channel interactions may also influence OPC development through the regulation of gene expression. Neuronal L-type Ca2+ channel activity stimulates a number of different transcriptional programs, and in particular is associated with the expression of genes that are responsive to CREB (cyclic-AMP response element binding protein) (Piedras-Renteria et al., 2004). The products of CREB-dependent transcription include ion channels, signaling molecules and structural proteins, all of which could potentially contribute to OL development, and which could be regulated through the influence exerted by golli on L-type Ca2+ channels. Interestingly, CREB plays an important role during OL differentiation and myelination (Bhat et al., 2007; Sato-Bigbee and DeVries, 1996; Shiga et al., 2005a, b), and so this signaling pathway represents a promising route by which golli/L-type channel interactions may exert their influence on OPC development.
The Influence of Golli on OPC Process Morphology
Considerable evidence indicates an important role for golli in the morphological development of OLs. Experiments using oligodendroglial cell lines and primary cultures show that overexpression of golli leads to dramatic increases in process outgrowth (Paez et al., 2007a, 2009a; Reyes and Campagnoni, 2002), while the absence of golli results in an opposing reduction in process elaboration (Jacobs et al., 2005). These morphological effects might be expected influence the generation of myelinating OLs. Indeed, this appears to be the case as is demonstrated by the pronounced hypomyelination in the brains of golli-KO and overexpressing mice (Jacobs et al., 2005, 2009). To seek direct evidence of the influence of golli on OPC morphology in situ, we examined individually labeled OPCs in the brains of golli-KO animals. Golli-KO OPCs exhibited reduced process branching, and an increase in the length of primary processes, indicating that the first branch division occurs further from the cell body. This reduced level of branching would likely to lead to the elaboration of a more diffuse network of processes, which could have consequences for the potential of these OPCs to establish contacts with potential targets for myelination.
In vitro experiments show that golli modulates oligodendroglial morphology through mechanisms involving VOCCs (Paez et al., 2007a, 2009a). For example, work in cell lines show that golli-dependent enhancements in process outgrowth are blocked by Cd++ (Paez et al., 2007a), indicating that the mechanisms by which golli stimulates this restructuring require the activity of VOCCs. The present observations of altered OPC L-type Ca2+ currents and process branching in golli-KO white matter strongly suggest a similar mechanism operates in situ. Also an involvement for L-type channels in this regulation is consistent with earlier findings that L-type channel blockers disrupt enhanced Ca2+ transients and migration rates in OPCs overexpressing golli (Paez et al., 2009a).
The absence of golli also leads to the appearance of enlarged membrane varicosities. Earlier work described similar swellings along the cellular processes of immature OLs in the corpus callosum (Berger et al., 1991). Here, an examination of labeled processes by electron microscopy revealed that these swellings contained large mitochondria. These swellings may therefore represent sites of increased metabolic activity, as would be expected at sites of active membrane expansion or retraction. It follows then that these varicosities may represent points of process outgrowth, and that the enlarged swellings present in the golli-KO may be related to the reduced degree of branching seen in these cells. Together, the reduced level of branching and enlarged varicosities present in golli-KO OPCs could reflect a general delay in oligodendroglial maturation. Indeed, the brains of adult golli overexpressing mice contain increased numbers of premyelinating cells, and both golli-KO and overexpressor animals exhibit delayed myelination (Jacobs et al., 2005, 2009). Taken together, these and the present data suggest that alterations in the level of golli expression lead to a delay in the maturation of oligodendroglial cells.
Overall, the data described here are consistent with earlier work highlighting the importance of voltage-dependent Ca2+ entry during oligodendroglial process elaboration (Paez et al., 2007a), and offers an explanation for the reduced level of myelination observed in the brains of golli-KO mice (Jacobs et al., 2005).
Acknowledgements
The authors thank Dr. Erin Jacobs for assistance with immunohistochemistry, Dr. Cristina Ghiani for supplying anti-CC1 reagents, Donna Crandall for assistance in preparing camera lucida figures, and Dr. Felix Schweizer and Michael Condro for technical advice on electrophysiology.




