Epidermal growth factor receptor expression regulates proliferation in the postnatal rat retina
Abstract
Epidermal growth factor (EGF) is known to promote proliferation of both retinal progenitors and Muller glia in vitro, but several questions remain concerning an in vivo role for this factor. In this study, we investigated whether the EGF receptor (EGFR) is necessary for the maintenance of normal levels of progenitor and Muller glial proliferation in vivo. Here, we show that (1) mice with homozygous deletion of the Egfr gene have reduced proliferation in late stages of retinal histogenesis, (2) EGF is mitogenic for Müller glia in vivo during the first two postnatal weeks in the rodent retina, (3) the effectiveness of EGF as a Müller glial mitogen declines in parallel with the decline in EGFR expression as the retina matures, and (4) following damage to the retina from continuous light exposure, EGFR expression is up-regulated in Müller glia to levels close to those in the neonatal retina, resulting in a renewed mitotic response to EGF. Together with previous results from other studies, these data indicate that the downregulation of a growth factor receptor is one mechanism by which glial cells maintain mitotic quiescence in the mature nervous system. © 2006 Wiley-Liss, Inc.
INTRODUCTION
The proper regulation of retinal proliferation is important because the balance of cell numbers and types is critical to mature retinal function. This regulation is achieved through the action of both cytostatic and mitotic signaling molecules. For example, we have recently shown that transforming growth factor β 2 (TGFβ2) is expressed in the postnatal retina, and inhibits progenitor and glial proliferation in vitro and in vivo (Close etal., 2005). Fibroblast growth factor (FGF), epidermal growth factor (EGF), transforming growth factor α (TGFα), transforming growth factor β 3 (TGFβ3), and sonic hedgehog (Shh) have all previously been shown to promote retinal progenitor and/or glial proliferation in vitro (Anchan and Reh, 1995; Anchan et al., 1991; Jensen and Wallace, 1997; Levine et al., 1997; Lillien and Cepko, 1992; Moshiri and Reh, 2004; Wang et al., 2002). Some of these factors, such as FGF and Shh, have also proven to be mitogenic in vivo (Fischer and Reh, 2002; Fischer et al., 2002a, b; Wang et al., 2002; Wang et al., 2005). While previous cell culture studies have shown that postnatal retinal cells are more responsive to the mitogenic effects of EGF and/or TGFα than their embryonic counterparts, the effects of the EGF signaling pathway on postnatal retinal proliferation have not been investigated in vivo.
Following the normal period of histogenesis, neural progenitors and glial cells do not proliferate in most regions of the central nervous system (CNS), including the retina (see Close et al., 2005 for review). The maintenance of mitotic quiescence is likely to be under tight regulation in the mature nervous system, but little is known about the factors that inhibit glial proliferation. Previous studies have shown that when Muller glia are dissociated and placed in tissue culture, they respond to some of the same growth factors as retinal progenitors (Milenkovic et al., 2004; Reichelt et al., 1989; Roque et al., 1992; Scherer and Schnitzer, 1994). Thus, it is possible that these mitogens become limiting in their supply as the retina matures. Alternatively, the receptors for growth factors may become down-regulated as progenitors cease proliferation. Although Müller glia do not normally proliferate in adult animals, these cells can be stimulated to re-enter the mitotic cycle in posthatch chicks, by treatment with either neurotoxins or co-injections of FGF and insulin (Fischer and Reh, 2001, 2002; Fischer et al., 2002b); suggesting that the factors themselves may be limiting. Studies of the rodent retina suggest that mammalian Müller glia may be more resistant to cell cycle entry in response to damage or growth factor treatments. For instance, treatment with neurotoxins, including ouabain or NMDA, results in only a small number of Müller glia entering the cell cycle in the adult rodent retina (Dyer and Cepko, 2000; Ooto et al., 2004). In experiments with posthatch chickens and rats, some of the proliferating Müller glia generate cells that express markers and morphology of neurons (Fischer and Reh, 2002; Fischer et al., 2002a, b; Ooto et al., 2004), indicating that Müller glial re-entry intothe cell cycle may initiate a regenerative process. Thus, studies of the factors that stimulate Müller glial proliferation in the mammalian retina may contribute to the understanding of how retinas may be regenerated after damage or disease.
In this study, we have examined the role of epidermal growth factor receptor (EGFR) in the regulation of proliferation in the postnatal retina. Here, we report that EGFR is required to maintain normal levels of proliferation in the postnatal retina. We also find that EGF is an effective mitogen for Müller glia during the first two postnatal weeks. As the retina matures, this mitogenic response declines, with a coincident reduction in the expression of EGFR. However, after light-induced damage in the adult rat retina, EGFR expression increases, resulting in a return of the mitotic response of Müller glia to EGF. These data suggest that one mechanism by which cell proliferation may be regulated in glia is through changes in growth factor receptor expression.
MATERIALS AND METHODS
Animals
All animals used in this study were treated according to guidelines of the University of Washington IACUC. Rats were purchased from Charles River Laboratories. Rats and mice were killed by CO2 over-anesthesia and cervical dislocation. Eyes were removed and retinas were dissected in Hank's buffered salt solution. Mice heterozygous for Egfr deletion (Egfrtm1Mag) were obtained from Jackson laboratories (Threadgill et al., 1995). They were maintained on the CD-1 background and bred as heterozygotes. Retinas from littermate wild type animals were compared with Egfrtm1Mag Egfrtm1Mag mouse retinas.
Injections
Intraocular injections were performed as described previously (Close et al., 2005) for rats at postnatal day 10 (P10). For injections made in animals from postnatal day 14 to adulthood, rats were anesthetized using ketamine/xylazine and proparacaine topical anesthetic was applied to the eyes prior to injection. A scalpel blade was used to make a small incision at the nasal corneal/scleral junction, and growth factors were delivered in 5 μL volumes using a Hamilton Syringe with a 30.5 G needle attached. Growth factor injections were made using a solution composed of sterile phosphate-buffered saline (PBS) and 0.2% bovine serum albumin (BSA) for control, or human recombinant EGF (R&D systems). Following injection, topical antibiotic eye lubricant was applied to both eyes to prevent drying and infection. BrdU injections were delivered intra-peritoneally at a dose of 50 mg/kg.
Light Treatment
Albino rats over the age of 14 days were subjected to constant light from a 15 Watt fluorescent, full spectrum bulb positioned directly over the cage for 7 days. Food, water, and temperature of the environs were monitored daily.
Genotyping
Egfr−/− homozygous mice were genotyped by polymerase chain reaction (PCR) with the forward primer 5′-CTC CTC TTC TTC CCG CAC TGT G-3′, the reverse primer 5′-CAT TGG TTG TGG CAG CAG TCA CTG-3′, the nested forward primer 5′-GTC TGT CTC GGA TTA ATC CCG GAG-3′, and the nested reverse primer 5′-CTG CTC GGA TGG CTC TGT AAG TCC-3′ for the determination of the Egfr gene; the forward primer 5′-CTG GCG TTA CCC AAC TTA ATC GC-3′, the reverse primer 5′-GTA GGT AGT CAC GCA ACT CGC CG-3′, the nested forward primer 5′-CGA TCG CCC TTC CCA ACA GTT GC-3′, and the nested reverse primer 5′-CAA CGA GAC GTC ACG GAA AAT GC-3′ for the identification of the LacZ gene, using mouse brain or mouse tail prepared with TRIZOL (Invitrogen) following the manufacturer's protocol.
Immunohistochemistry
Tissues were rinsed in PBS, fixed in 4% paraformaldehyde, 4% sucrose in PBS for 1 h, and sunk in 30% sucrose prior to cryosectioning. Cryosections were mounted on Superfrost slides (VWR). Slides and cells stained with anti-BrdU were incubated for 10 min in 4N HCL prior to blocking, and then washed twice with PBS. All blocking steps were performed at room temperature for 1.5 h in 0.3% TritonX-100 (TX-100) and 5% goat serum in PBS except for those treated with the sheep anti-EGFR antibody, in which fetal bovine serum was substituted for goat serum in the blocking step. All primary antibody staining procedures were performed overnight at room temperature in 0.3% TX-100 in PBS, except for anti-EGFR incubations, which were performed overnight at 4°C. Following primary incubation, 4 × 15 min washes in PBS were performed. Secondary antibody incubations were performed for 1 h at room temperature in 0.3% TX-100 in PBS, followed by 4 × 15 min washes in PBS. Sections or coverslips were then allowed to dry, and mounted in Fluoromount-G (Southern Biotechnology Associates) medium for microscopy. Antibodies used in this study include mouse anti-BrdU (1:150; G3G4 Developmental Studies Hybridoma Bank), rat anti-BrdU (1:100; Accurate), mouse anti-Nestin (1:80; Developmental Studies Hybridoma Bank), rabbit antibovine cellular retinaldehyde binding protein (CRALBP) (1:500; UW55, gift of Jack Saari, University of Washington), rabbit anti-Phospho Histone H3 (1:750; Upstate), sheep anti-EGFR (1:50; Upstate), goat antirabbit Alexa 568 (1:500; Molecular Probes), goat antirabbit Alexa 488 (1:500; Molecular Probes), goat antimouse Alexa 488 (1:500; Molecular Probes), goat antimouse Alexa 568 (1:500; Molecular Probes), goat antirat 488 (1:500; Molecular Probes), donkey antisheep Alexa 568 (1:500; Molecular Probes), and goat antiguinea pig Cy-3 (1:700; Chemicon).
Quantitative RT PCR
-
GAPDH, 5′: AAG GTC ATC CCA GAG CTG AA
-
GAPDH, 3′: GTC CTC AGT GTA GCC CAG GA
-
EGFR, 5′: ACT CTG ACG GGC TTT GTC AC
-
EGFR, 3′: CAA GCG CCA TAG GTC TGT TT
Efficiency curves were performed to determine the number of cycles difference represented by a two-fold dilution of template. Template cDNA expressing all ligands and receptors (P10) was diluted 8-, 16-, 32-, and 64-fold and the average difference for all primers between each twofold dilution was found to be one. Therefore, a one-cycle difference represents a twofold dilution in these experiments. Error bars shown represent standard error of the mean of three trials.
Western Blotting
Western blots were performed as previously described (Close et al., 2005), using the following antibodies: sheep anti-EGFR (1:1,000; Upstate), mouse anti-p27 (1:1,000; Transduction Labs), mouse anti-β-Actin (1:5,000; Abcam), goat antimouse horseradish peroxidase (HRP) conjugate (1:20,000; BioRad), and rabbit anti-sheep HRP conjugate (1:20,000). Bands were quantified by scanning the blots. The values were compared with and adjusted for the levels of β-actin protein for loading control, and then these adjusted EGFR expression levels were normalized to P4 protein levels, and averaged over two separate trials with two separate protein sample series.
RESULTS
EGFR-Deficient Mice Demonstrate a Decrease in Retinal Proliferation
Previous studies have shown that EGF and related ligands can stimulate proliferation of retinal progenitors in vitro (Anchan and Reh, 1995; Anchan et al., 1991; Lillien, 1995; Lillien and Cepko, 1992; Lillien and Wancio, 1998). To determine whether EGFR is required to maintain normal levels of proliferation in vivo, we analyzed the retinas of neonatal mice deficient in the receptor (Threadgill et al., 1995). These mice are born at normal Mendelian ratios, and there is no evidence for embryonic lethality on the CD-1 background. We genotyped each litter, sacrificed animals at various postnatal ages, and analyzed retinal sections for differences in mitotic activity when compared with littermate wild type animals. Mitotic activity was assessed using phospho-Histone-H3 immunoreactivity (PH3), a marker of M-phase cells.
In both the wild type and Egfr deficient mouse retinas, the PH3 cells were largely confined to the outer (scleral) surface of the retina, equivalent to the ventricular surface of the CNS. In the retinas of newborn mice, there was no obvious difference between the wild type and Egfr deficient mice in either central or peripheral regions of the retina (Fig. 1A). By postnatal day 3, however, the central retina had fewer PH3-labeled cells in the Egfr−/− mouse retina (Figs. 1A,B) although the peripheral regions of the retinas from the two genotypes had similar numbers of PH3 immunoreactive cells. By postnatal day 6, the proliferation of progenitors in central regions of retina is finished; however, in the peripheral retinal regions, many PH3 cells are still present (Fig. 1A). We found a significant difference in the number of PH3 cells in peripheral retina between the wild type and Egfr deficient mice at P6 (Figs. 1A,C). We also examined the expression of Chx10 in late stages of postnatal histogenesis (day 7). We also found a decline in the number of Chx10 expressing progenitor cells in the Egfr−/− mouse retinas (Figs. 1D,E). These data indicate that while the early stages of progenitor proliferation are not significantly affected by the loss of the Egfr, there is progressively less proliferation in the retinas of Egfr−/− mice at later stages of histogenesis.
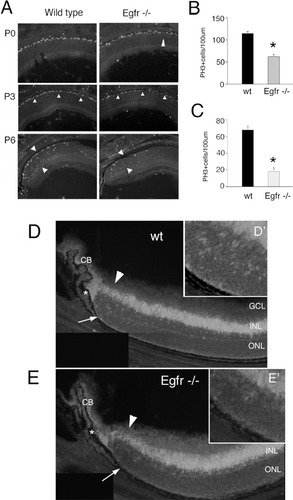
EGFR is required for normal levels of retinal progenitor proliferation. Egfr deficient mice and wild type littermates were killed on the day of birth (P0), or at postnatal day 3 (P3) or postnatal day 6 (P6). (A) Micrographs of sections through the central retina at P0 and P3 and of peripheral retina at P6 showing PH3 labeling of mitotic cells in the retinas. (B) Quantitation of PH3+ cells in P3 central retina of Egfr−/− mice (black bar) and wt littermate control animals (light gray bar). Error bar shows SEM (asterisk: P < 0.005, t test; five animals per group). (C) Quantitation of PH3+ cells in P6 peripheral retina of Egfr−/− mice (black bar) and wt littermate control animals (light gray bar). Error bar shows SEM (asterisk: P < 0.002, Students' t test; six animals per group). (D, E) Chx10 immunofluorescence of wild type and Egfr−/− mouse retina at postnatal day 7 (P7) shows both bipolar cell labeling (arrowhead in INL) and progenitor cells in the ONL (arrow, and insets). There are many Chx10+ progenitor cells in the ONL of the wild type (D, arrow and inset D′ at higher magnification), but few in the Egfr−/− retina (E, arrow, and inset E′ at higher magnification). ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer; CB, ciliary body. Asterisk indicates the retinal margin, where a few retinal progenitors are concentrated at this age.
EGF is a Müller Glial Mitogen In Vivo
Previous studies have implicated EGF in Müller glial differentiation and proliferation (Lillien, 1995; Milenkovic et al., 2004; Reichelt et al., 1989; Roque et al., 1992; Scherer and Schnitzer, 1994). To determine whether EGF promotes Müller glial proliferation in vivo, we made injections of EGF in progressively older animals and analyzed the response to EGF. The proliferation of multipotent retinal progenitor cells is complete in the rat retina at postnatal day 10 (Close et. al., 2005), and the Müller glia have developed characteristics of their differentiated phenotype. Thus, intraocular injections of EGF at this age allow us to separate effects of EGF on progenitor cells from those on Müller glia. We made intraocular injections of EGF into rat pups starting at P10 for two consecutive days, followed immediately by 24 h of BrdU administration. Injections of vehicle alone did not result in significant Müller glial proliferation (Figs. 2A–C). By contrast, we found that at this stage, EGF was a potent Müller glial mitogen. Many cells were immunoreactive for both BrdU and the Müller glial marker CRALBP in the inner nuclear layer of the central retina following intraocular EGF injections (Figs. 2D–I). Some double-labeled cells were observed in the outer nuclear layer as well (Figs. 2G–I, asterisks). When the number of CRALBP/BrdU double-labeled cells was quantified at the different doses of EGF, we found that the effect of EGF was dose dependent (Fig. 2J). Control injections of PBS and 0.2% BSA (PBS/BSA) resulted in an average of 10.8 (±3.8) CRALBP/BrdU double-positive cells per square millimeter of retina, while injections of 250 ng EGF resulted in 383 (±91.9) CRALBP/BrdU double-positive cells per square millimeter of retina (Fig. 2G). Injections of 500 ng and 1 μg EGF resulted in 765 (±16.8) and 695 (±77) CRALBP/BrdU double positive cells per square millimeter, respectively (Fig. 2J). These data indicate that EGF is a potent Müller glial mitogen at postnatal day 10, and that its effects depend on dosage.
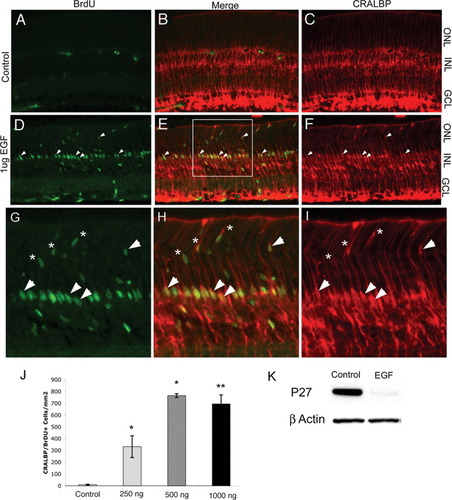
EGF is a Müller glial mitogen. Rat pups treated with EGF or vehicle control for two consecutive days starting at P10. (A–C) Few BrdU-labeled cells (A, green) are also CRALBP-labeled (C, red) in control retinas. (D–F) EGF-treated retinas contained many BrdU-labeled (D, green), CRALBP-labeled cells (F, red, arrows mark double-labeled cells). (G–I) Higher magnification micrographs of boxed region in (E) showing examples of double-labeled cells in the ONL (asterisks). (J) Quantitation of BrdU/CRALBP double-positive cells in the various EGF treatment conditions; the response appears to saturate at 500 ng (*P < 0.02; **P < 0.01 Students' t test; error bars = SEM). (K) Western blot analysis of protein from animals injected with PBS/BSA or 1 μg EGF starting on P10, lower bands indicate β-actin loading control.
The cyclin-dependent kinase inhibitor (cdki) p27kip1 is an important negative regulator of both retinal progenitor and Müller glial proliferation at the G- to S-phase transition of the cell cycle (Dyer and Cepko, 2000, 2001; Levine et al., 2000). To determine the effects of EGF injection on p27kip1 expression, we injected 1 μg EGF for two consecutive days starting at P10. Protein was extracted from treated retinas 4 h after the final EGF injection. Following a protein assay, we performed Western blots to determine the levels of p27kip1 expression in control and EGF-treated retinas. Although p27kip1 expression is robust in control-treated retinas, we find that in retinas treated with 1 μg EGF, p27kip1 protein levels are greatly reduced (Fig. 2K). Thus, one of the molecular mechanisms through which EGF promotes proliferation in Müller glia may be via the downregulation of p27kip1.
To determine whether the mitogenic response to EGF persists at later ages, we performed intraocular injections of the factor in postnatal day 14 (P14) rats. Rats were injected with either PBS/BSA for control or 1 μg EGF for two consecutive days, followed by 24 h of BrdU injections. In vehicle-injected eyes, few or no Müller glia enter the cell cycle; very few CRALBP/BrdU double-labeledcells were present in the retina (Figs. 3A–C). In P14 EGF-treated animals, some CRALBP/BrdU double-positive cells are present in the retina (Figs. 3D–F, white arrows), though significantly fewer than in P10 injected animals. We found that on average, there are 5.33 (±0.3) CRALBP/BrdU double-labeled cells per mm2 in P14 control-injected retinas, and 43.26 (±14.8) BrdU-positive Müller glia in P14 EGF-treated retinas (Fig. 3G). Thus, the number of BrdU labeled Müller glia in animals injected with EGF at P14 was only about a tenth of that of animals treated at P10 (P < 0.05, pairwise comparison Student's t test). When we made similar injections at P21, only an occasional cell was labeled with BrdU. Thus, there is a dramatic decline in the mitogenic response of Müller glia to EGF as the retina matures.
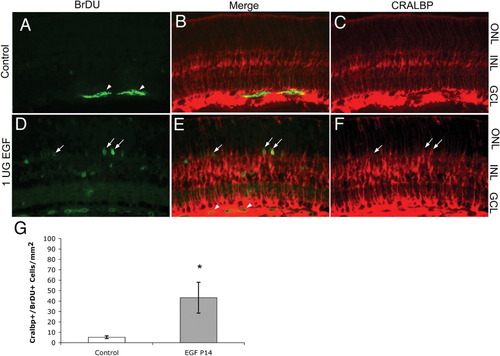
The Müller glial response to EGF declines by postnatal day 14. Rat pups were injected with EGF or vehicle control for two consecutive days starting postnatal day 14. (A–C) Few BrdU-labeledcells (A, green) are CRALBP-labeled(C, red) in control retinas. (D–F) EGF-treated retinas contained some BrdU-labeled (D, green), CRALBP-labeled cells (F, red, arrows mark double-labeled cells, arrowheads mark nonspecific labeling). (G) Quantitation of BrdU/CRALBP double-positive cells shows an increase in EGF-treated retinas over control (*P < 0.05; Students' t test; error bars = SEM), but far fewer labeled cells were present when compared with P10 treated animals (compare with Fig. 2J).
EGF Receptor Expression Declines as the Retina Matures
A decline in the expression of the EGFR could underlie the decline in the response of Müller glia to intraocular EGF injections. To determine whether EGFR levels decline quantitatively with the age of the animal, we extracted protein and mRNA from rat retinas at various ages. Protein levels were quantified, and 30 μg of protein from each of the timepoints was analyzed for EGFR expression via Western blot. EGFR protein levels were highest at postnatal day 4 in the retina, declining gradually until little or no EGFR protein could be detected at P21 and later (Fig. 4A). Quantitation confirms this trend (Fig. 4B). When compared with P4 levels of EGFR protein, P10 EGFR levels drop to 57.1% (±3.37), and P10 EGFR levels drop to 41.9% (±8.36). In retinas of animals P21 or older, EGFR levels are just 6.8% (±5.0) of P4 EGFR levels. Quantitation of EGFR mRNA transcripts via Q-RT-PCR reveals a similar decline in expression. Compared with adult levels of EGFR mRNA, embryonic day 18 (E18) retinas contained on average 11.7- (±1.24) fold more EGFR transcripts (Fig. 4C, white bar), while P4 retinas contained 17.1- (±1.16) fold more EGFR transcripts (Fig. 4C, light grey bar). P10 retinas contained an average of 5.3- (±1.37) fold more EGFR transcripts than adult retinas (Fig. 4C). These results show that at both the protein and mRNA levels, retinal EGFR expression declines with the age of the animal.
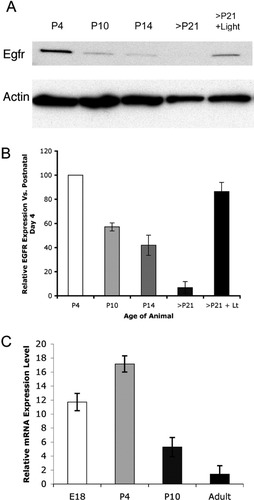
EGFR expression levels decline with age in the retina. Protein and mRNA for EGFR decline in the postnatal period. (A) Western blot shows decline in EGFR protein with the age of the animal; EGFR levels are undetectable in animals over P21. EGFR receptor expression increases in P21 animals after 1 week of light treatment. (B) Quantitation of Western blot results normalized to P4 EGFR levels (white bar, 100%) shows EGFR expression at P10 is down to (57.1 ± 3.4)% when compared with P4 levels, while P14 levels are down to (41.9 ± 8.4)%, and in animals older than P21, EGFR levels are just (6.8 ± 4.9)% of P4 levels. Following 1 week of light treatment, P21 EGFR levels are elevated to (86.5 ± 7.5)% when compared with P4. (*ManU: P < 0.001). (C) Q-RTPCR results show that EGFR mRNA levels are highest at earlier ages, and lowest in the adult retina. Compared with adult EGFR mRNA levels, EGFR transcripts are 11.7- (±1.24) fold more abundant in embryonic day 18 (E18) and 17.1- (±1.16) fold more abundant in P4 retinal tissue. At P10, EGFR mRNA is 5.3- (±1.37) fold more highly expressed than in adult rat retinal tissue. (*ManU: P < 0.001).
To determine which cell types express EGFR in the postnatal retina, we performed immunofluorescence using antibodies directed against EGFR and the Müller glial marker CRALBP. In the P4 retina, EGFR is expressed primarily by the nestin+ progenitor cells (data not shown), but by P10, the EGFR immunoreactivity overlaps well with CRALBP-positive Müller glial cell processes and cell bodies (Figs. 5A–F). Consistent with the Western analysis, there is a decline in the level of EGFR expression in the retina at postnatal day 21 (compare with Figs. 6A–E). The decline in EGFR expression correlates with the reduction in the numbers of proliferating Müller glia in older animals after EGF treatment.
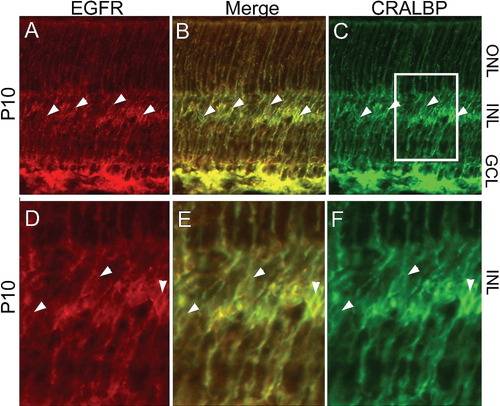
EGFR is expressed in CRALBP positive Müller glia. Rat retinas from P10 were co-labeled with CRALBP and EGFR antibodies to localize the expression of the receptor. (A–C) P10 retinas show EGFR labeling (A, red) in the cell bodies and processes of CRALBP-positive Müller glia (C, green), (B, merge, arrowheads point to examples of double-positive cell bodies). (D–F) Higher magnification view of boxed area indicated in C. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer.
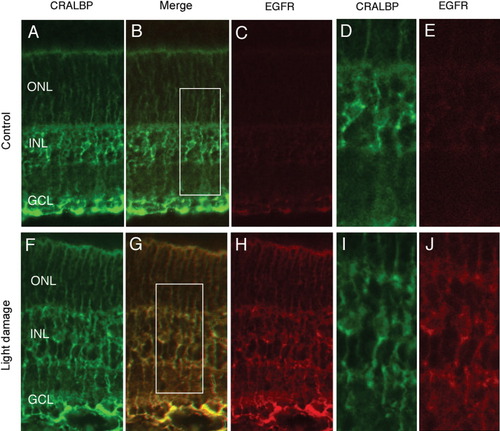
Light treated retinal Müller glia express EGFR. Retinas from animals over P21 that had either been housed in normal conditions or in 24 h of light treatment were fixed and processed for immunohistochemistry to determine EGFR expression patterns. Settings for confocal imaging were identical for both control and light-damaged retinas, so intensity of the EGFR labeling reflects level of EGFR immunofluorescence. (A–E) P21 retinas show low level of EGFR labeling (C and E, red), at low levels in CRALBP-positive cells (A and D, green). (D, E) Higher magnification of boxed area in B. (F–J) Light-treated animals show increased EGFR labeling (H and J, red) in CRALBP-positive cells (F and I, green). (I, J) Higher magnification of boxed region in G.
Light-Induced Retinal Damage Causes an Increase in EGFR Expression
Treatment of albino rats with light continuously for 1week results in degeneration of the outer nuclear layer, rendering Müller glia reactive and more likely to survive long-term in culture (Close et al., 2000; Sarthy, 1985). The fact that light damage allows Müller glia to proliferate in vitro suggests that there may be an up-regulation in the levels of the EGFR. To test this possibility, we compared the levels of EGFR protein in retinas from control and light-treated animals by Western blot. We found that EGFR expression levels increased significantly after rats were given light treatment (Fig. 4A). When this effect is quantified, EGFR expression levels appear to be restored to 86.5% (±7.4) of P4 levels in light-treated animals (Fig. 4B). Immunoflourescent labeling of retinal sections from light-treated rats confirmed that an increase in EGFR expression occurred in CRALBP+ Müller glia. Figure 6 shows the EGFR-immunoreactivity in P21 animals treated for 1 week with continuous light when compared with the normal P21 retina. The settings for the confocal microscope were kept the same for the EGFR micrographs from both the control and light-treated retinas. EGFR levels were consistently higher in light-treated rats (Figs. 6F–J), when compared with control P21 animals (Figs. 6A–E); the primary cells labeled with anti-EGFR antibody were the CRALBP+ Müller glia in the light-treated retinas as well as the control.
Combined EGF and Light-Treatment Stimulates Müller Glial Proliferation
To determine whether the changes in EGFR expression after light treatment at P21 can affect the Müller glial response to EGF, we performed EGF injections on animals older than postnatal day 21 that had either been housed in a normal 12 h light cycle, or had been subjected to 24 h light treatment in the 7 days prior to injection. Animals were given intraocular injections of either PBS/BSA as a control or 1 μg EGF for two consecutive days starting after P21, followed by 24 h of BrdU injections. We found that control injections elicited little or no proliferative response from Müller glia (Figs. 7A–C, and data not shown). However, animals that were subjected to light treatment prior to EGF injection showed a substantial number of BrdU-CRALBP double-labeled Müller glia present in the inner nuclear layer (Figs. 7D–F). When this data is quantified, we found that animals not treated with light and receiving intraocular PBS/BSA injections contained just 3.8 (±0.2) CRALBP-BrdU double-labeled cells per mm2 retina, while EGF injected animals contained 12.3 (±5) double-labeled cells per mm2 (Fig. 7G). We find that after light treatment, control-injected animals contained 5.8 (±1.9) CRALBP-BrdU+ cells per mm2, while those animals that received EGF injections, as well as light treatment, had on average 238 (±71.4) CRALBP-BrdU+ cells per mm2 in their retinas (Fig. 7G). These data support the hypothesis that the Müller glial response to EGF is sensitive to the levels of EGFR expression, and further suggest that the proliferative potential of Müller glia can be restored to nearly the levels of late-staged progenitor cells with an increase in EGFR expression at later stages.
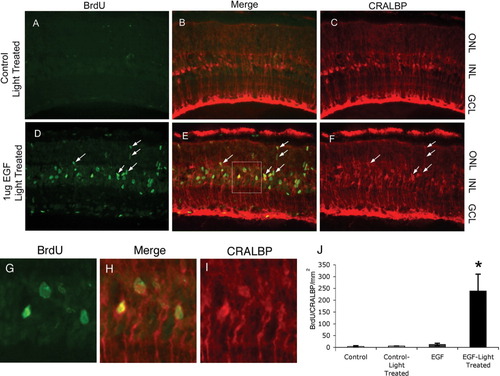
EGF treatment results in Müller glial proliferation after P21 following light treatment. (A–C) In light-treated, control-injected (PBS/BSA) animals, no BrdU+ (A, green), CRALBP+ (C, red) cells are present in the retina. (D–F) In light-treated, EGF injected animals, many BrdU+ (D, green), CRALBP+ (F, red) cells are present in the retina (E, merge, arrows indicate double-positive cells). (G–I) Higher magnification view of region boxed in (E) to show that the BrdU-labeled cells are also labeled for Cralbp. (J) Quantitation of effects of combined light treatment and EGF injections showing a large increase in the number of proliferating Muller glia in the combined treatment, but few double-labeled cells in EGF-treated or light-damaged conditions. (*P < 0.01, pairwise comparison to light-treated control, Student's t test; error bars = SEM).
DISCUSSION
Here, we show that EGFR expression levels are important in the regulation of postnatal retinal progenitor proliferation; the retinas of postnatal animals lacking Egfr contain fewer mitotic cells. We also show that EGFR is expressed in Müller glia and that EGF is a mitogen for Müller glia in vivo. The effects of EGF diminish with the age of the animal, and correlating with a decline in the expression of EGFR protein and mRNA in the retina. EGFR expression can be restored to Müller glia by damaging the retina with continuous exposure to light, and this increase in EGFR expression results in an increase in the mitotic response of Müller glia to EGF injection at later ages.
The results of our analysis of the Egfr deficient mice show that this receptor is required for normal retinal histogenesis. Several previous studies have shown that EGF and related ligands can stimulate proliferation of retinal progenitors (Anchan and Reh, 1995; Anchan et al., 1991; Lillien, 1995; Lillien and Cepko, 1992; Lillien and Wancio, 1998). Retinal progenitors express EGFR (Anchan et al., 1991; Lillien, 1995;) and the level of receptor expression and response to EGF increases in late-staged retinal progenitors, when compared with progenitors from early embryonic stages of development (Anchan and Reh, 1995; Anchan et al., 1991; Lillien, 1995; Lillien and Cepko, 1992). Given the high level of mitotic stimulation caused by EGF in these earlier studies, it is somewhat surprising that we find the loss of this receptor causes a relatively minor phenotype. The severity of an Egfr deficiency in mice is known to be very dependent on the background (Threadgill et al., 1995), and therefore it is possible that another molecular pathway compensates for the loss of this receptor.
Previous over-expression studies of the EGFR have shown that this factor can regulate cell fate decisions in the developing retina. For example, over-expression of EGFR using a retrovirus directs the progenitors to a Muller glial fate (Lillien, 1995). Therefore, we predicted that the Egfr−/− mice would show an absence of Muller glia. We did not find this to be the case, however, possibly because of compensation from another signaling pathway. As noted earlier, since we only found differences in proliferation near the end of histogenesis, it may be that only the final round of proliferation is absent in the mutants and therefore only a small change in the relative numbers of Muller glia would occur. In general, it appears that those factors that regulate proliferation or differentiation of retinal progenitors early in development, such as Shh (Moshiri and Reh, 2004; Dakubo and Wallace, 2004) and GDF11 (Kim et al., 2005), have more dramatic effects on the relative proportions of the different retinal cell types, while those factors that affect proliferation relatively late in histogenesis, such as p27kip (Dyer and Cepko, 2000; Levine et al., 2000), have more subtle, or undetectable, effects on relative cell-type proportions.
It is also possible that the reduction in proliferation we observed in the Egfr−/− mice was due to a specific reduction in the proliferation of Muller glia. Similarly, mice deficient in the cell cycle regulator p27kip display a prolongation of proliferation, and most of the late generated cells adopt a Muller glial fate (Dyer and Cepko, 2000; Levine et al., 2000). Therefore, it is possible that in the periphery of the retina, there is a specific Muller glia progenitor that is lost in the Egfr−/− mice. Although we cannot rule this out, we do not favor this possibility because (1) we find that the number of Chx10+ progenitor cells is reduced in the mutants, and Chx10 is not expressed in the Muller glia (see Fig. 1); and (2) retroviral lineage studies have shown that Muller glia are normally generated by a common precursor with the other retinal cell types (Turner and Cepko, 1987).
We also report that EGF is a potent mitogen for Müller glia in vivo, and their response to this factor parallels the level of expression of the EGFR. It has been known for many years that EGF can stimulate proliferation of Müller glial cells, though all of the evidence for this has come from in vitro studies (Milenkovic et al., 2004; Reichelt et al., 1989; Roque et al., 1992; Scherer and Schnitzer, 1994). Two previous in vivo studies have provided evidence that EGF might stimulate proliferation of progenitors and Müller glia, though neither directly demonstrated this; Henck et al. (2001) administered large, systemic doses of EGF to rats aged P1-P6, and observed rosette formation and cellular disruption of the outer nuclear layer of the retina; Sagar et al. (1991) have found that injection of adult rabbits with either EGF or TGFα results in the upregulation of c-fos expression by Müller glia.
We have also found that Müller glia express the EGFR in vivo, and that the level of expression declines during postnatal development. As noted earlier, several studies have analyzed EGFR in developing retina and shown that the response to this factor increases from early to late embryonic development. Prior to our study, it was not known whether the level of this receptor remained high throughout life, or whether it was subjected to additional developmental regulation. We have found that the EGFR is significantly down-regulated during the second postnatal week, and that this has a profound effect on the response of the cells to EGF. Although Roque et al. (1992) reported a high level of EGFR expression on cultured Müller glia, our results suggest that the receptor was upregulated in vitro, since in vivo, these cells express only very low levels of receptor. Since light damage is frequently used as a method to enable efficient culture of Müller glia, it is possible that the up-regulation of EGFR, triggered by light damage, contributes to this phenomenon. A study by Powers and Planck (1997) also reported EGFR expression in Müller glia in the developing rat retina. However, there was no quantitation in their study, and the localization to Muller glia was not demonstrated with co-labeling for Müller glial-specific markers.
Our results, together with those of previous studies, indicate that the proliferation of retinal progenitors and Müller glia are both regulated by EGFR. It is known that retinal progenitors and Müller glia have similar gene expression profiles (Blackshaw et al., 2004), and that Muller glia are among the last cells generated in the developing retina (Turner and Cepko, 1987). While recent evidence indicates that the cessation of proliferation during development of the retina involves a TGFβ2 mediated signal (Close et al., 2005), the present results support the hypothesis that regulation of the EGFR also plays a role in the maintenance of glial mitotic quiescence in the mature retina. The end of retinal histogenesis is apparently mediated by a combination of at least two factors; in the first postnatal week, TGFβ2, released by neurons within the retina, inhibits the proliferation of the progenitors (prior to P7) and the Müller glia (after P7); in the second postnatal week, and thereafter, Müller glia are maintained in a mitotically quiescent state by the down-regulation of EGFR. Treatment of mature retina with inhibitors of the TGFβ signaling pathway and/or EGFR ligands can stimulate their proliferation in vitro, but in mature rats, damage to the retina that leads to an up-regulation in EGFR is required for EGF treatment to be mitotically active in vivo.
There are at least two possible ligands for the EGFR that are known to be expressed in the developing and mature retina. TGFα (Anchan et al., 1991) is expressed throughout the period of retinal histogenesis, and both TGFα (Fassio et al., 1989) and heparin binding epidermal growth factor (HB-EGF) are expressed in the mature retina. HB-EGF is mitogenic for Müller glia (Hollborn et al., 2005; Milenkovic et al., 2004), and is upregulated in patients with proliferative vitreoretinopathy (Hollborn et al., 2005). In addition, several other mitogenic factors are known to act through the EGFR (Milenkovic et al., 2004), and therefore it may be more efficient to regulate the expression of this key receptor than a diversity of ligands to limit histogenesis in the developing retina and Müller glial proliferation in the mature retina.
We found that in those animals where EGF stimulated proliferation of the Muller glia, not all of the BrdU-labeled Muller cells remained in the inner nuclear layer. Instead, many migrated to either the outer nuclear layer, or more rarely, into the ganglion cell layer. A similar phenomenon has been observed in the avian retina, following neurotoxin treatments or growth factor injections, and this proliferative response was followed by a limited production of new neurons (see Fischer and Reh, 2001). We therefore examined the BrdU-labeled cells in this study for evidence of neuronal gene expression. We failed to find evidence for neuronal production after EGF treatment in the rat retina, using antibodies against rhodopsin, recoverin, βIII-tubulin, or Hu (Close and Reh, unpublished observations). However, it has been previously shown that conditions that promote Muller glial proliferation past the normal phase of histogenesis lead to an accumulation of the over-produced Muller glia in the outer nuclear layer (Levine etal., 2000).
The response of Müller glia to EGF is similar to the mitogenic response of astrocytes (Leutz and Schachner, 1981) in other regions of the nervous system and progenitors of the developing mammalian CNS (Craig et al., 1996; Fallon et al., 2000; Kuhn et al., 1997; Reynolds and Weiss, 1996; Reynolds et al., 1992). Astrocytes of the mature mammalian brain respond to EGF by re-entering the cell cycle, both in vitro and in vivo (Doetsch et al., 2002; Huff et al., 1990; Kornblum et al., 2000; Leutz and Schachner, 1981). EGFR expression also declines with the age of the animal in other parts of the CNS. For example, EGFR is expressed at relatively high levels until postnatal day 19 in both astrocytes and Purkinje cells, with levels of expression declining thereafter (Gomez-Pinilla et al., 1988). By contrast, EGFR persists into adulthood in neurogenic regions, such as the SVZ (Doetsch et al., 2002; Seroogy et al., 1995; Weickert etal., 2000). Together with our data, these results from other brain regions indicate that EGFR expression levels in postnatal glia and progenitors may be a limiting factor for proliferation throughout the CNS.
Acknowledgements
The authors thank Branden Nelson, Paige Etter, and Olivia Berminghan-McDonogh for their critical review of the manuscript, and members of the Reh laboratory for constructive comments. They also thank Melissa Phillips and Paige Etter for their technical assistance.




