Cell cycle inhibitors p21 and p16 are required for the regulation of Schwann cell proliferation
Abstract
Regulated cell proliferation is a crucial prerequisite for Schwann cells to achieve myelination in development and regeneration. In the present study, we have investigated the function of the cell cycle inhibitors p21 and p16 as potential regulators of Schwann cell proliferation, using p21- or p16-deficient mice. We report that both inhibitors are required for proper withdrawal of Schwann cells from the cell cycle during development and following injury. Postnatal Schwann cells express p21 exclusively in the cytoplasm, first detectable at postnatal day 7. This cytoplasmic p21 expression is necessary for proper Schwann cell proliferation control in the late development of peripheral nerves. After axonal damage, p21 is found in Schwann cell nuclei during the initiation of the proliferation period. This stage is critically regulated by p21, since loss of p21 leads to a strong increase in Schwann cell proliferation. Unexpectedly, p21 levels are upregulated in this phase suggesting that the role of p21 may be more complex than purely inhibitory for the Schwann cell cycle. However, inhibition of Schwann cell proliferation is the overriding crucial function of p21 and p16 in peripheral nerves as revealed by the consequences of loss-of-function in development and after injury. Different mechanisms appear to underlie the inhibitory function, depending on whether p21 is cytoplasmic or nuclear. © 2005 Wiley-Liss, Inc.
INTRODUCTION
The development of Schwann cells requires the precise integration of controlled proliferation and regulated cell cycle exit. These processes are accompanied by morphological changes that culminate in the specialized nature of mature myelinating and nonmyelinating Schwann cells in adult nerves (Lobsiger et al., 2002). Impaired control of these fundamental processes in Schwann cell biology is associated with diseases and pathological states including inherited peripheral neuropathies (Atanasoski et al., 2002; Suter and Scherer, 2003), peripheral nerve tumors (Riccardi, 1991), and peripheral neuropathies secondary to diabetes, cancer chemotherapeutic agents, or toxins (Berger and Schaumburg, 1995).
Schwann cell proliferation reaches a peak around birth and gradually decreases when the cells begin to differentiate (Scherer et al., 1994; Mirsky et al., 2002). Following nerve injury, axons distal to the site of injury degenerate. As a consequence, Schwann cells dedifferentiate, accompanied by a wave of proliferation (Scherer et al., 1994). We and other investigators have shown that injury-induced Schwann cell proliferation requires cyclin D1 (Kim et al., 2000; Atanasoski et al., 2001), while developmentally regulated proliferation does not. Thus, the molecular controls of Schwann cell proliferation during nerve development and following injury differ significantly.
Cell proliferation is controlled by the activation of cyclin-dependent kinases (cdks), which associate with cyclins D and E, and drive cells through the G1 phase of the cell cycle (Sherr, 1993, 1994; Roberts, 1999). Two classes of cdk inhibitors modulate the activity of cdk–cyclin complexes. The Cip/Kip family affects cyclin E–cdk2 complexes, while the Ink family targets preferentially cyclin D–cdk4/6 (Ortega et al., 2002; Sherr and Roberts, 1999). Biochemical activities of the inhibitors and their expression patterns during development implicate these proteins in the regulation of cell proliferation and cell differentiation (Zindy et al., 1997).
There is a relative scarcity of available data on the regulation and function of cell cycle inhibitors in vivo. Peripheral nerve development and injury-induced Schwann cell proliferation provide excellent paradigms to assess this issue in different but related settings. In the present study, we have analyzed the functional roles of p21 as a representative of the Cip/Kip family and p16 for the Ink family. We found that p21 appears first in the cytoplasm of Schwann cells during late postnatal development when most cells have already ceased dividing. This expression and cellular localization of p21 is maintained into adulthood. After nerve injury, however, we found p21 mainly in nuclei of dedifferentiated Schwann cells. Consistent with the expression data, p21-deficient Schwann cells divide normally in early nerve development, but cytoplasmic p21 is necessary for proper growth arrest in later phases. After nerve injury, nuclear p21 is required for correct cell cycle control at the peak of Schwann cell proliferation and in later stages in denervated nerves. A similar function for p21 was shown for keratinocytes in wounded skin, demonstrating that the anti-mitotic effect of nuclear p21 after injury is not organ-specific. In comparison, the analysis of p16-deficient nerves indicated similar functions of p16 in the control of Schwann cell proliferation in development and after injury but the effects tended to be less prominent.
MATERIALS AND METHODS
Animals
Animal experiments were approved by the Veterinary Office of the Canton of Zurich. All mice analyzed were on the FVB background (Elevage Janvier, Le Genest, Saint Isle, France). p21-deficient (Deng et al., 1995), and p16-deficient (NCI Mouse Models of Human Cancers Consortium [MMHCC]) Frederick, MD have been described.
Sciatic Nerve Injuries
Sciatic nerves of anesthetized mice (100 mg/kg Ketaminol, 5 mg/kg Narcoxyl i.p.) were exposed at the sciatic notch. Nerves were transected and the two nerve stumps diverted to prevent axonal regeneration into the distal nerve stump. At varying times after nerve injury, distal nerve stumps were recovered for experimental analysis.
Preparation of Teased Fibers and Cryosections
Animals were first anesthetized with Metofane (Schering-Plough Animal Health, Kenilworth, NJ), and were then decapitated and sciatic nerves removed. To obtain adequate immunoreactivity, nerves were fixed for 30 min in 4% paraformaldehyde. Fixed nerves were teased in phosphate-buffered saline (PBS) after careful removal of the perineurium, and were air-dried on SuperFrost/Plus glass slides (Menzel-Gläser, Braunschweig Germany). Alternatively, fixed nerves were embedded in OTC (Miles, Kankakee, IL), and 5-μm longitudinal sections were cut at −25°C and mounted on SuperFrost/Plus glass slides.
Skin Wounding and Preparation of Wound Tissue
Full-thickness excisional wounds were generated and processed as previously described (Werner et al., 1994; Kaesler et al., 2004).
Infection of Schwann Cells With Recombinant Adenovirus Vectors
Rat Schwann cells, isolated at postnatal day 3, were grown in Schwann cell medium (Dulbecco's modified Eagle's medium (DMEM, Gibco-BRL, Basel, Switzerland), containing 10% FCS, 50 μg/ml gentamicin (Sigma, Buchs, Switzerland), 100 μg/ml crude GGF; BioReba, Biotechnology Reinach, Switzerland), and 2 μM forskolin (Sigma) as described (Atanasoski et al., 2004). Infections with ADV-p21 and pMH4-p16 adenovirus were performed as described (Yang et al., 1996; Schreiber et al., 1999; Atanasoski et al., 2004). Adenovirus vectors were used at m.o.i. 1,000. At 24 h post-infection, the medium was changed, and the cells were maintained in Schwann cell medium for another 2 days before analysis. Infection experiments were repeated three times to ensure reproducibility of the results.
Immunofluorescence and Peroxidase/DAB Staining
Longitudinal nerve sections, teased fibers, rat Schwann cell cultures, and frozen sections of wounded skin were fixed and immunostained as described (Atanasoski et al., 2004). Primary antibodies: Rat monoclonal antibodies against MBP (1:50 dilution; Serotec, Düsseldorf, Germany), Ki-67 (1:20; DAKO, Zug, Switzerland), rabbit polyclonal antibodies against S-100 (1:300; DAKO), and p21 (1:100; Santa Cruz). The specificity of the anti-p21 antibody was tested on samples of p21-deficient animals. To assess proliferation in nerve samples, animals were injected intraperitoneally with BrdU (50 mg/kg) twice within 2 h before they were sacrificed. Nerve sections were first stained for S-100 and further processed by methanol-fixation for 20 min at 4°C and subsequent treatment with 1 M HCl for 20 min at 60°C. Sections were then incubated with biotinylated antibody against BrdU (1:10; Caltag Laboratories, San Francisco CA, no. MD5215), followed by incubation with streptavidin-FITC (1:400; Roche Diagnostics, Rotkreuz, Switzerland). For cells, BrdU labeling and detection were carried out according to the manufacturers instructions (Roche). BrdU labeling and peroxidase/DAB staining of skin wounds was performed as described (Werner et al., 1994; Kaesler et al., 2004).
Western Blotting
Protein lysates from sciatic nerve tissue and cells were prepared; Western blotting was performed as described (Atanasoski et al., 2004). Primary antibodies: p21 (Santa Cruz Biotechnology, Santa Cruz, CA; 1:1000), p16 (Neo Markers, 1:20), and β-actin (Sigma; 1:1,000). The blots were quantified using ImageJ software (http://rsb.info.nih.gov/ij/) normalizing the individual lanes to the β-actin signal.
Reverse Transcription and Real-Time PCR
RNA was isolated from sciatic nerve tissue and 200 ng were reverse transcribed with Superscript™ II (Invitrogen, Basel, Switzerland) according to the manufacturer's protocol (Atanasoski et al., 2004). mRNA for PMP22, MPZ/PO, and Periaxin were quantified as described (Atanasoski et al., 2004). P16 mRNA was quantified using a pre-Developed TaqMan® Assay Reagent kit from Applied Biosystems.
RESULTS
Postnatal p21 Expression in Mouse Sciatic Nerves Is First Detected at Day 7 and Localized in the Cytoplasm of Schwann Cells
As the first step to analyze the function of p21 in Schwann cells, we investigated expression and subcellular localization during postnatal development of sciatic nerves (Fig. 1). Immunofluorescence-based antibody stainings on longitudinal sections revealed no detectable p21 expression (red) at postnatal days 2 (P2; Fig. 1a) and 5 (P5; Fig. 1b). However, we detected cytoplasmic p21 expression (Fig. 1, arrowheads in c,d,g,h) during late development at P7 (Fig. 1c), as well as in adult nerves at P90 (Fig. 1d) as visualized by co-staining with the nuclear marker DAPI (blue, cigar-shaped Schwann cell nuclei in Fig. 1e–h).
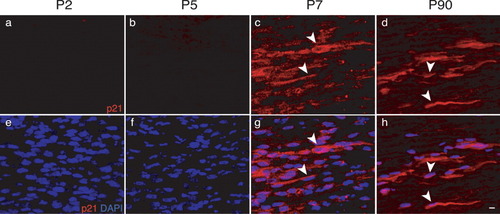
p21 expression in Schwann cells in vivo. Double-labeled longitudinal sections (p21, red; nuclear stain DAPI, blue) of mouse sciatic nerves during development at postnatal (P) days P2 (a,c), P5 (b,f), P7 (c,g), and P90 (d,h). Arrowheads indicate cytoplasmic p21. Note that expression of p21 in Schwann cells starts at P7. Scale bar = 10 μm in h (refers to a–h).
Growth Arrest of Schwann Cells Is Impaired in the Absence of Cytoplasmic p21 in Late Postnatal Development
To assess the functional significance of the observed late developmental cytoplasmic p21 expression, we compared the proliferation rates of Schwann cells in developing sciatic nerves of wildtype (wt) and p21-deficient mice (p21−/−; Fig. 2). Longitudinal sections of wt (Fig. 2A,a–f) and mutant (Fig. 2A,g–l) P2, P5, and P7 nerves were double-stained with the nuclear proliferation marker Ki-67 (red, Fig. 2A,a–l) and DAPI (blue, Fig. 2A,d–f,j–l). Consistent with the timing of p21 expression, the two genotypes differed only in the number of mitotically active Schwann cells at P7 (Fig. 2A, cf. c,f and i,l). For quantitative analyses, we determined the proliferation rates by calculating the number of Ki-67+ cells in relation to the total number of cells within a defined area (0.15 mm2) on different section levels (Fig. 2B). Schwann cells of both genotypes showed decreasing proliferation rates from P2 to P5 as previously reported for normal development (Scherer et al., 1994). At P7, however, the Schwann cell proliferation rate was about three times higher in p21-deficient nerves compared with wt (Fig. 2B). Additional experiments revealed that this effect was not accompanied by increased apoptosis (data not shown). We interpret these data as a reflection of an inhibitory function of cytoplasmic p21 in regulating proliferation during late Schwann cell development, possibly by influencing the timing of differentiation.
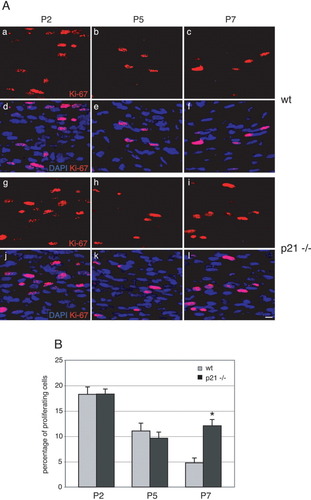
p21 is functionally required in the late development of Schwann cells. A: Longitudinal sections of developing wild-type (wt; a–f) and p21-deficient (p21−/−; g–l) mouse sciatic nerves at P2, P5, and P7. Sections were double-labeled with the proliferation marker Ki-67 (red) and DAPI (blue). Note the higher number of proliferating cells in mutant versus wt nerves at P7 (c,i). B: Quantitative analysis of Schwann cell proliferation in developing sciatic nerves of wt (gray bars) and p21-deficient (black bars) mice at P2, P5, and P7. Note the decrease in Schwann cell proliferation from P2 to P7 in controls, while proliferation is maintained in mutant animals at P7. Data are mean values obtained from three animals per time point and genotype. *P ≤ 0.05, Student's t-test. Scale bar = 10 μm in A,l (refers to a–l).
The effects of p21 on proliferation in the developing nerve are transient, as no Schwann cell proliferation is detected in adult nerves of p21-deficient or wt mice (Fig. 5A, cf. a,d and g,j). However, we found that mutant adult peripheral nerves are affected in the length of myelinated internodes and Schwann cell density. Myelin internodes of axons of comparable size (∼7 μm) were analyzed by myelin basic protein (MBP) staining using teased nerve fiber preparations (Fig. 3A, red). Myelinated segments in p21-mutant nerves (Fig. 3A,b and 3B) were markedly shorter compared with wt (Fig. 3A,a and 3B) and cell density was significantly increased in the mutant (Fig. 3C). These results are consistent with the previously observed prolonged Schwann cell proliferation during nerve development in the absence of cytoplasmic p21.
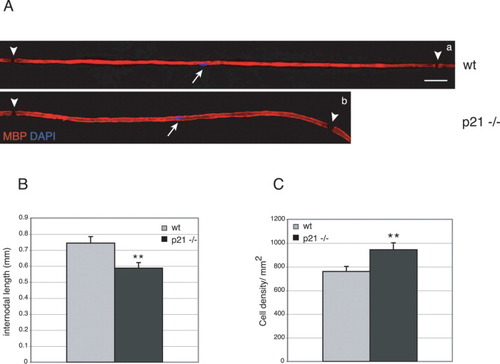
Internodal length is decreased in Schwann cells from p21-deficient mice. A: Double-labeling of teased myelinated wt (a) and mutant (b) nerve fibers of ∼7-μm thickness. The myelin marker MBP (red) marks internodes and DAPI (blue) Schwann cell nuclei (arrows). Arrowheads point to nodes of Ranvier. Note the shorter internode in mutant versus wt mice. B: Quantitative analysis of internodal lengths of myelinated wt (gray bar) and p21-deficient (black bar) nerve fibers. Twenty-one internodes from three animals per genotype were measured, and mean values were determined. Internodes are significantly shorter in p21-deficient animals **P ≤ 0.01, Student's t-test. C: Quantitative analysis of cell density in wt (gray bar) and p21-deficient (black bar) sciatic nerve fibers. Schwann cell numbers on nerve sections were determined per randomly chosen defined areas. Nerves of three animals per genotype were analyzed and the mean values determined. Cell density is significantly increased in mutant versus wt mice. **P ≤ 0.01, Student's t-test. Scale bar = 50 μm in A,a (refers to a,b).
P21 Is Nuclear in Schwann Cells Following Nerve Injury
To test the function of p21 in the proliferation of adult Schwann cells after nerve injury, we examined nerve sections of the distal stump of sciatic nerves 4 days after transection (4dpT; Fig. 4A,b,d,f), compared with the uninjured contralateral nerves (Fig. 4A,a,c,e), by triple staining with antibodies to p21 (red), the Schwann cell marker S-100 (green), and DAPI (blue). In injured nerves, p21 was found prominently in the nuclei of dedifferentiating Schwann cells (Fig. 4A, arrowheads in b,d,f), in sharp contrast to the exclusive cytoplasmic p21 found in unlesioned nerves during development (Fig. 1) and in adulthood (Fig. 4A, arrowheads in a,c,e).
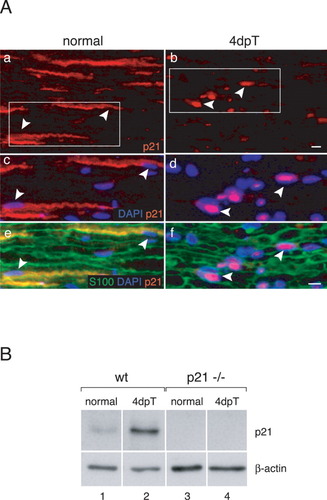
Nuclear p21 expression in Schwann cells after nerve injury. A: Triple labeling of uninjured normal nerve sections (a,c,e) and nerve sections 4 days after nerve injury (4dpT; b,d,f): p21 (red), DAPI (blue), and Schwann cell marker S-100 (green). c,e are magnifications of the box in a, whereas d,f represent magnifications of the box in b. Arrowheads in a,c,e point to cytoplasmic p21. Note the nuclear localization of p21 after nerve injury (arrowheads in b,d,f). B: Western blot analysis of sciatic nerve extracts of wt normal (lane 1) and injured (lane 2), as well as p21-deficient normal (lane 3) and injured (lane 4) samples (controls for specificity of antibodies). Blots were incubated with a polyclonal anti-p21 antibody. Note the three-fold increase in p21 levels 4dpT. Equal loading of protein extracts was controlled by β-actin detection. Scale bar = 20 μm in A,b (refers to a,b); 20 μm in A,f (refers to c–f).
The striking change of intracellular p21 localization after nerve injury was accompanied by an approximately threefold increase in p21 protein following nerve axotomy (Fig. 4B, compare lanes 1 to 2). This observation is unexpected if p21 is viewed as a pure cell-cycle inhibitor. Data obtained in vitro have suggested that p21 may play a dual role in regulating the cell cycle, first by stabilizing cyclin D1–cdk4 complexes in early phases to promote cell proliferation, and later acting as an inhibitor of proliferation (LaBaer et al., 1997; Cheng et al., 1999). To address whether this might relate to our findings, we determined the specific proliferation rate of p21-expressing Schwann cells compared with the overall proliferation rate, if all Schwann cells are considered. Longitudinal nerve sections derived from BrdU-pulsed mice 4dpT and 12dpT were double-stained for BrdU and p21 and the results quantified. At 4dpT, 38 ± 7% of the p21-positive Schwann cells were proliferating, while only 8 ± 1% of all Schwann cells were BrdU+, consistent with a potential proliferation-promoting effect of p21. At 12dpT, we found no p21/BrdU double-positive Schwann cells, while a small number (0.8€±€0.1%) of BrdU+ Schwann cells were present, suggesting that only the growth inhibitory function of p21 is retained at this time point.
Nuclear p21 Is Required for Proliferation Control of Mature Dedifferentiated Schwann Cells After Injury
Next, we examined the requirement for nuclear p21 in injured adult nerves by comparing the proliferation rates of Schwann cells of wt and p21-deficient mice (Fig. 5). Mitotically active Schwann cells were labeled by pulsing mice with BrdU 4dpT (Fig. 5A,b,e,h,k) or 12dpT (Fig. 5A,c,f,i,l). In uninjured adult nerves, wt and mutant Schwann cells have ceased to proliferate (Fig. 5A, cf. a,d and g,j). After nerve injury, wt Schwann cells in the distal stump restarted DNA synthesis 4dpT (Fig. 5A,b,e), followed by a sharp decline by 12dpT (Fig. 5A,c,f). Similarly, p21-deficient Schwann cells also proliferated upon axotomy (Fig. 5A,h,i,k,l). Quantitative analyses revealed a significant increase in Schwann cell proliferation rates in mutant (black bars) compared with control (gray bars) mice at both, 4dpT and 12dpT. We conclude that the function of p21 as an inhibitor of the cell cycle is the dominant feature lost in the nerves of p21-deficient mice at early stages of Schwann cell proliferation during Wallerian degeneration as well as at 12dpT.
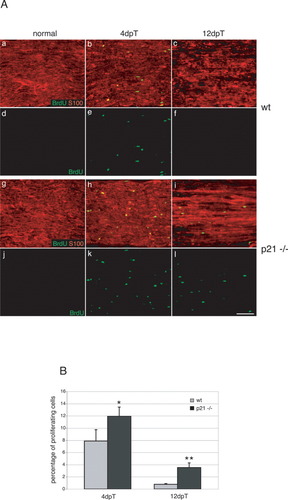
p21 affects injury-induced proliferation of adult Schwann cells. A: Panels a–f show longitudinal sections of wt, panels g–l longitudinal sections of p21-deficient nerves. Axotomy was performed and nerves collected 4 days (4dpT) and 12 days (12dpT) post-transection and compared with uninjured nerves (normal). Animals were injected with BrdU 2 h before sacrifice. Sections were double-labeled with an anti-BrdU antibody (green) (a-l) and antibodies against S-100 (red) (a–c and g–i). B: Quantitative analyses of Schwann cell proliferation after nerve injury in wt (gray bars) and p21-deficient (black bars) mice 4dpT and 12dpT. The number of S-100/BrdU+ cells was counted ∼3 mm distal to the site of injury on different nerve sections covering an area of 0.25 mm2. Note the significant increase in Schwann cell proliferation 4dpT and 12dpT in mutant versus wt mice during Wallerian degeneration. Data are mean values obtained from three animals per time point and genotype. *P ≤ 0.05; **P ≤ 0.01, Student's t-test. Scale bar = 100 μm in A,l (refers to a–l).
Our results suggest an inhibitory effect of both cytoplasmic and nuclear p21 on Schwann cell proliferation. To corroborate these findings, we examined the consequences of forced p21 overexpression in cultured rat Schwann cells (Fig. 6). Cells were infected with control EGFP- or p21-expressing adenovirus, and 48 h later, proliferating cells were labeled with BrdU for 16 h (Fig. 6A). Triple immunostainings with p21 (red), BrdU (green), and DAPI (blue) revealed nuclear localization of p21 (Fig. 6A,b), as seen in vivo following nerve injury. p21-infected cells no longer proliferated, in contrast to EGFP-infected cells (Fig. 6A, cf. c and d). Western blot analysis revealed that Schwann cells, cultured under the growth-promoting conditions used, do not express detectable endogenous p21 but overexpress the protein strongly after infection with the p21-expressing virus (Fig. 6B). To examine the influence of p21 on Schwann cell differentiation, we infected primary Schwann cells with an EGFP- or a p21-expressing adenovirus and measured the expression of myelin genes. Compared with control EGFP-infected cells (Fig. 6C, gray bars), qRT-PCR experiments revealed no induction of the myelin-related genes PMP22, MPZ/PO, and periaxin in p21-infected Schwann cell cultures (Fig. 6C, black bars), indicating that p21 does not promote differentiation of Schwann cells in vitro.
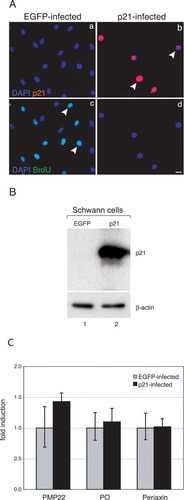
p21 inhibits Schwann cell proliferation in vitro. A: Triple immunostaining of a representative control EGFP-infected (a,c) and p21-infected (b,d) culture. (a,b) DAPI, blue; p21, red; DAPI/p21, purple; (c,d) DAPI, blue; BrdU, green; DAPI/BrdU, bluish green. Arrowheads in (b) indicate p21-expressing, growth-arrested cells; the arrowheads in (c) point to p21-negative, proliferating cells. The infection rate was 100%. Note that overexpression of p21 leads to a complete growth-arrest of Schwann cells. B: Immunoblot analyses of lysates from control EGFP- and p21-infected Schwann cells. The blot was probed with a polyclonal anti-p21 antibody. Note that proliferating Schwann cells in vitro do not express endogenous p21. Equal amounts of protein extract were controlled by β-actin detection. C: qRT-PCR was performed to determine the abundance of various myelin-related markers (PMP22, MPZ/P0, and periaxin) in control EGFP (gray bars) versus p21-infected (black bars) primary rat Schwann cells. cDNA from three different cultures per paradigm were generated. Each bar represents the mean value of three independent PCR experiments ± SD. Student's t-test was applied to test for significance. Values of control-infected samples were arbitrarily set to 1. None of the tested myelin-related molecules was upregulated by p21 overexpression. Scale bar = 10 μm in A,d (refers to a–d).
Subcellular Localization of p21 In Developing Schwann Cells After Injury
To test whether the nuclear p21 localization observed in proliferating Schwann cells after injury is a feature restricted to mature Schwann cells, we axotomized sciatic nerves at P5 to induce an injury-dependent proliferation of immature Schwann cells (Atanasoski et al., 2001) (Fig. 7). Two days later, longitudinal nerve sections were immunostained to examine the subcellular localization of p21. Some Schwann cells contained p21 protein (red) in the nucleus (DAPI, blue; arrow in Fig. 7b,d), similar to our findings in mature Schwann cells after injury (Fig. 4A) but different from noninjured nerves during development (Fig. 7a,c). Thus, p21 localization in proliferating Schwann cells reflects a difference between the injured and intact nerve regardless of the developmental stage.
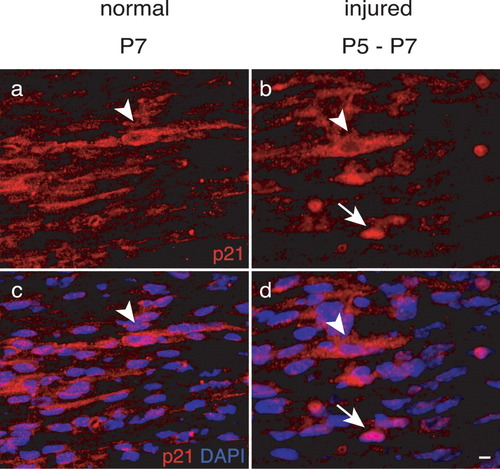
p21 localization in developing Schwann cells after nerve injury. Double-labeling of longitudinal sections of developing normal and injured mouse sciatic nerves [p21 (red); DAPI (blue)]. Nerves were lesioned at P5 and analyzed 2 days post axotomy. Note that p21 is exclusively cytoplasmic in unlesioned nerves at P7 (arrowheads), while upon injury, p21 is also found in Schwann cell nuclei (arrows). Scale bar = 5 μm in d (refers to a–d).
Nuclear p21 Mediates Growth Arrest of Keratinocytes Upon Skin Injury
To assess whether p21 has similar critical functions in other injury models associated with cell proliferation, we investigated wound healing of the skin as an additional model, and studied the proliferation rate of keratinocytes. In nonwounded epidermis of adult mice, we could not detect p21 (data not shown). In 5-day full-thickness excisional wounds, however, p21-positive nuclei of keratinocytes were abundant within the hyperproliferative epithelium (HE; Fig. 8a). Histological analysis of hematoxylin and eosin (H&E)-stained sections from the center of 5-day wounds revealed no obvious differences between wt and p21-deficient mice (Fig. 8c,d). Furthermore, the area of the hyperproliferative epithelium was similar in wt and p21-deficient mice. However, when the effect of the loss of nuclear p21 on keratinocyte proliferation was analyzed by counting BrdU+ cells per HE area, we found a significantly higher number of proliferating cells in p21-deficient mice compared with wt animals (Fig. 8b). The proliferating keratinocytes were located mainly in the basal cell layer of the epidermis in both, wt (Fig. 8c,e) and p21-deficient mice (Fig. 8d,f). However, BrdU+ suprabasal keratinocytes were more numerous in the HE of p21-deficient mice (Fig. 8f, arrowheads), corresponding to the expression pattern of p21 in wt wound epidermis (Fig. 8a). Thus, nuclear p21 is inhibiting keratinocyte proliferation in wounded skin, comparable to the situation with Schwann cells after nerve injury.
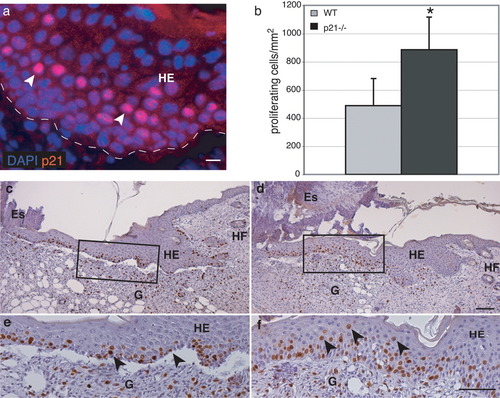
Enhanced keratinocyte proliferation in 5-day wounds of p21-deficient mice. a: Immunolocalization of p21 (red) in the nuclei (DAPI, blue) of suprabasal keratinocytes of the hyperproliferative epithelium (HE) of wt mice 5 days after injury. The basement membrane is indicated with a dotted line. Cross-sections from the middle of 5-day excisional wounds. c,d,e,f: Mice were injected with BrdU and were sacrificed 2 h after injection. Ethanol/acetic acid-fixed paraffin sections of control mice (c,e) and p21-deficient mice (d,f) were incubated with a peroxidase-conjugated antibody against BrdU, stained with diaminobenzidine and counterstained with hematoxylin. Keratinocytes in the tongue of the hyperproliferative epithelium (HE) migrate between the granulation tissue (G) and the eschar (Es) starting from the uninjured side, which can be identified by intact hair follicles (HF). b: Quantitative analysis of BrdU+ keratinocytes in the hyperproliferative epithelium. Significantly more BrdU+ keratinocytes were found in p21-deficient mice. Data are mean values obtained from 7 mice per genotype (wt: 11 wounds, p21-deficient mice: 8 wounds). *P ≤ 0.05, Student's t-test. e,f: Detail of c,d: BrdU+ keratinocytes of control mice (e) were mainly found in the basal cell layer (arrowheads), whereas in p21-deficient mice (f) more BrdU+ cells were present in the suprabasal cell layers (arrowheads). Scale bars = 20 μm in a (refers to a); 100 μm in d (refers to c,d); 100 μm in f (refers to e,f).
P16 Is Required for Schwann Cell Growth Arrest During Late Postnatal Development and During Late Wallerian Degeneration
Next, we investigated whether other cell cycle inhibitors are similarly important for the control of Schwann cell proliferation. We chose to analyze p16 as a representative member of the Ink family in a comparative study to p21. We were hampered in this analysis by the lack of reliable antibodies to p16 for use in mouse tissues. Thus, we applied quantitative real-time reverse-transcriptase polymerase chain reaction (qRT-PCR) to gain an insight into the expression levels of p16 in Schwann cells during development and following nerve injury. We found p16 mRNA expressed in nerves of developing animals at P5, P7 and in the adult (P90; Fig. 9A). Furthermore, a significant increase in the amount of p16 transcripts was observed after nerve injury 4dpT, while levels returned to normal at 12dpT (Fig. 9A).
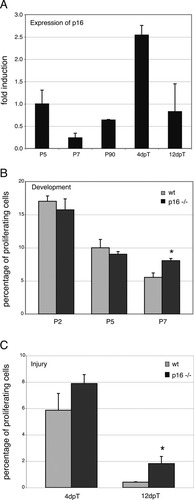
P16 is functionally required in Schwann cells at later stages of development and affects injury-induced proliferation of adult Schwann cells. A: qRT-PCR was performed to determine p16 mRNA levels in mouse sciatic nerves during postnatal development (P5–P90) and following nerve injury 4dpT and 12dpT (black bars) in adult mice. cDNA from nerves of at least three animals was generated per time point and pooled. Each bar represents the mean value of PCR experiments performed in triplicate. Results are presented as ratios of p16 to 18S rRNA and normalized to the levels at P5. Note that p16 mRNA is slightly lower in later development and is induced upon nerve injury. B: Quantitative analyses of Schwann cell proliferation in developing sciatic nerves of wt (gray bars) and p16-deficient (black bars) mice at P2, P5, and P7. Note the decrease in Schwann cell proliferation from P2 to P7 in wt, while the proliferation phase is prolonged in mutant animals at P7. Data are mean values obtained from three animals per time point and genotype. *P ≤ 0.05, Student's t-test. C: Quantitative analyses of Schwann cell proliferation after nerve injury in wt (gray bars) and p16-deficient (black bars) mice 4dpT and 12dpT. Significantly increased proliferation was found 12dpT in mutants compared with wt. Only a tendency of the same effect was observed 4dpT. Data are mean values obtained from three animals per time point and genotype. *P ≤ 0.05, Student's t-test.
The availability of p16-deficient mice allowed us to test the requirement for p16 by direct loss-of-function analysis. As with p21-deficient animals, we stained longitudinal sections of developing nerves from wt and p16-deficient mice with Ki-67 and DAPI (data not shown). Proliferation rates did not differ significantly between wt (gray bars) and p16-deficient (black bars) nerves at P2 or P5 (Fig. 9B). However, we counted slightly but significantly more proliferating Schwann cells at P7 in the absence of p16, similar to the results obtained in p21-deficient mice (cf. Figs. 5B and 9B). Thus, growth arrest of Schwann cells in late postnatal development is similarly dependent on members of the Ink and the Cip/Kip families of cell cycle inhibitors.
We also examined the proliferation rate of Schwann cells of wt and p16-deficient mice following nerve injury (Fig. 9C). Quantitative analyses revealed increased Schwann cell proliferation in p16-deficient mice compared with wt. The changes were significant at 12dpT, but not at 4dpT, as seen in p21-deficient nerves, suggesting that p21 might be a more potent inhibitor compared with p16. Preliminary data from p16-overexpression experiments in cultured Schwann cells are consistent with this hypothesis (data not shown).
DISCUSSION
In this study, we have characterized the expression of the cdk-inhibitors p21 and p16 in Schwann cells and assessed their functions in different proliferative settings in intact and injured peripheral nerves. We show that toward the end of the proliferative phase in postnatal development, p21 appears in the cytoplasm of Schwann cells. Consistent with this observation, ablation of cytoplasmic p21 leads to a prolonged proliferation period. In injury models, p21 localizes to the nuclei of dedifferentiated Schwann cells as well as keratinocytes early after the insult. Elimination of nuclear p21 causes an increase in proliferating cells at the peak of the proliferative phase in injured nerves and skin. P16-deficient mice exhibit proliferation defects during development and following nerve injury that are comparable to those in p21-deficient animals. Thus, both p21 and p16 are individually required, crucial regulators of Schwann cell proliferation in peripheral nerves.
P21 inhibits cell cycle progression by binding to cyclin–cdk complexes and to proliferating cell nuclear antigen (PCNA) (Zhang et al., 1994; Sherr and Roberts, 1995). The cell-cycle inhibitory activity of p21 correlates with its nuclear localization (el-Deiry et al., 1994). In agreement, we found that injury-induced proliferation is associated with nuclear p21 and, in the absence of p21, cell proliferation is increased in Schwann cells of the injured sciatic nerve and in keratinocytes of the injured skin. Intriguingly, we found strongly increased p21 levels following nerve injury and skin wounding when cells reentered the cell cycle. This finding contrasts, at first glance, with the widely accepted role of p21 as an inhibitor of cyclin–cdk complexes. However, most quiescent cells, including cultured Schwann cells, do not express p21, but may induce the inhibitor when some mitogen-activated signaling pathways are elevated (Jaumot et al., 1997; Harrisingh et al., 2004). A commonly suggested explanation for these findings relates to a reported dual role of p21 in the regulation of the cell cycle. p21 can stabilize cyclin D1–cdk4 complexes in early phases of the cell cycle to promote cell proliferation, while later on, p21 acts as an inhibitor (Cheng et al., 1999; LaBaer et al., 1997). Consistent with a proliferation-promoting component in the function of p21, we observed a higher rate of p21-expressing, proliferating Schwann cells compared with the overall Schwann cell proliferation rate after sciatic nerve injury. However, lack of nuclear p21 caused an overall increase in cell proliferation suggesting that the loss of the inhibitory function is dominating in a loss-of-function paradigm.
Our results also demonstrate a crucial function of cytoplasmic p21 expression, at least in late stages of developing peripheral nerves. In peripheral blood monocytes, cytoplasmic p21 acts as an inhibitor of apoptosis (Asada et al., 1999). In Schwann cells, however, independent of the subcellular localization of p21, we did not detect alterations in cell survival in the absence of p21 (data not shown). Instead, during nerve development, cytoplasmic p21 appears to control Schwann cell cycle exit, which is a novel function attributed to cytoplasmic p21. Cytoplasmic p21 is also associated with increased neurite outgrowth in developing neurons (Tanaka et al., 2002), suggesting a role in differentiation. Similarly, p21 promotes oligodendrocyte differentiation in the central nervous system (Zezula et al., 2001) and early in life, the brain of p21-deficient mice is hypomyelinated (Deng et al., 1995). In a preliminary attempt to test for altered Schwann cell differentiation, we observed a tendency to reduced myelin thickness at P9 in p21-deficient peripheral nerves but this effect was transient and did not reach statistical significance (data not shown).
Effects of forced p21 expression on differentiation are cell-type specific. While p21 overexpression accelerates differentiation of PC12 cells (Erhardt and Pittman, 1998) and C2C12 myoblasts (Skapek et al., 1995), it inhibits the differentiation of mouse keratinocytes (Di Cunto et al., 1998). This differentiation-inducing potential of p21 may provide an explanation for our finding that the area of the hyperproliferative wound epidermis was identical in wild-type and p21 knockout mice despite the higher mitogenic activity of p21 null keratinocytes. Thus, the enhanced proliferation may be compensated by concomitant stimulation of differentiation, thereby retaining the balance between both processes. We found that overexpression of p21 in Schwann cells very efficiently stopped proliferation, without inducing the differentiation program. These findings are consistent with the strong effect of p21 as a proliferation inhibitor that we observed in vivo.
We conclude that p21 and p16 regulate Schwann cell proliferation. The underlying mechanisms are likely to be multi-faceted with specific functions attributable to cytoplasmic p21 and nuclear p21. These functions clearly reflect differences between intracellular signaling mechanisms involved during normal development and in regenerative processes, similarly to our previous findings for cyclin D1 (Atanasoski et al., 2001). The identity of the cell cycle inhibitors that are used by immature Schwann cells to control Schwann cell proliferation during early phases of postnatal development remains an interesting open issue.
Acknowledgements
The authors thank Drs. Elizabeth Nabel and Frank Graham for recombinant adenovirus vectors, and Joke Nowitzki for technical assistance. We are grateful to Drs. Philip Leder and Robert DePinho for mice deficient in p21 and p16, and to Dr. Ned Mantei for comments on the manuscript. This work was supported by grants from the Kommission Innovative Forschung of the University of Münster (YO103231) (to P.Y.), the Swiss National Science Foundation (to U.S. and S.W.), and the NCCR Neural Plasticity and Repair (to U.S.).




