SpL201: A conditionally immortalized Schwann cell precursor line that generates myelin
Abstract
Dramatic progress has been made over recent years toward the elucidation of the mechanisms regulating lineage determination and cell survival in the developing peripheral nervous system. However, our understanding of Schwann cell development is limited. This is partly due to the difficulties in culturing primary Schwann cell precursor cells, the earliest developmental stage of the Schwann cell lineage defined to date. Both the inability to maintain cultured Schwann cell precursor cells in an undifferentiated state and the technical difficulties involved in their isolation have hampered progress. We have conditionally immortalized rat Schwann cell precursor cells using a retrovirally encoded EGFR/neu fusion protein to circumvent these problems and to generate a source of homogeneous cells. The resulting SpL201 cell line expresses p75 and nestin, two proteins expressed by neural crest-derived cells, as well as peripheral myelin protein 22, protein zero, and Oct-6 as markers of the Schwann cell lineage. When cultured in EGF-containing medium, the SpL201 cells proliferate and maintain an undifferentiated, Schwann cell precursor cell-like state. The cell line is dependent on EGF for survival but can differentiate into early Schwann cell-like cells in response to exogenous factors. Like primary rat Schwann cells, SpL201 cells upregulate Oct-6 and myelin gene expression in response to forskolin treatment. Furthermore, the SpL201 cell line can form myelin in the presence of axons in vitro and is capable of extensively remyelinating a CNS white matter lesion in vivo. Thus, this cell line provides a valuable and unique tool to study the Schwann cell lineage, including differentiation from the Schwann cell precursor cell stage through to myelination. GLIA 36:31–47, 2001. © 2001 Wiley-Liss, Inc.
INTRODUCTION
Schwann cells of the peripheral nervous system (PNS) are derived from neural crest stem cells (NCSCs), which leave the dorsal neural tube around embryonic day 9–10 (E9–10) in the rat. As the neural crest cells migrate and reach their target tissues, they become specified to multiple cell types (reviewed by Anderson, 1997). Recent loss-of-function experiments indicate that the transcription factor Sox10 is required for peripheral gliogenesis (Britsch et al., 2001) and it has been suggested that neuron-derived neuregulin (Dong et al., 1995; Morrison et al., 1999; Shah et al., 1994) and Notch signaling (Morrison et al., 2000; Wakamatsu et al., 2000) may regulate development of the Schwann cell lineage. The Schwann cell lineage can be identified within developing peripheral nerves of rats starting at E12–14 with the appearance of Schwann cell precursor cells (SCPCs) (Jessen et al., 1994). SCPCs, like NCSCs, express the low-affinity NGF receptor p75. However, they can be distinguished from NCSCs by their expression of the growth-associated protein 43 (Jessen et al., 1994), protein zero (P0), peripheral myelin protein 22 (PMP22) (Hagedorn et al., 1999), and Oct-6 (SCIP) (Blanchard et al., 1996).
Between E15 and E18, rat SCPCs undergo a striking differentiation to early Schwann cells. Early Schwann cells are characterized by the increased expression of the small calcium binding protein S-100, glial fibrillary acidic protein (GFAP), and the O4 antigen (Jessen et al., 1990; Mirsky et al., 1990; Jessen et al., 1994). Early Schwann cells also differ from SCPCs in their dependence on axon-derived survival factors. Whereas most SCPCs die within 24 h under defined culture conditions (Jessen et al., 1994), early Schwann cells are able to survive in the same culture medium. The latter is probably due to a recently described autocrine survival-loop that includes PDGF, IGF, and NT-3 (Meier et al., 1999).
In vitro studies have identified several combinations of growth factors regulating the survival, differentiation, and proliferation of rat SCPCs (Gavrilovic et al., 1995; Jessen et al., 1994; Lobsiger et al., 2000; Morrison et al., 2000). Among these factors, β-neuregulin-1 is the sole factor that can induce all three responses from SCPCs in vitro (Dong et al., 1995). Animals with a null mutation in the neuregulin-1 gene or the loci encoding the erbB2 or erbB3 receptors are devoid of SCPCs along peripheral nerves (Lee et al., 1995; Meyer and Birchmeier, 1995; Riethmacher et al., 1997). Although these in vivo studies have shown that neuregulin-1 is important in embryonic Schwann cell development, they were unable to demonstrate unambiguously whether the phenotype stems from aberrant determination, migration, survival, or differentiation of SCPCs. Recent data show that Sox10 regulates the expression of erbB3 by neural crest cells and that Sox10-deficient mice fail to generate peripheral glial cells (Britsch et al., 2001). Hence, although some of the requirements for Sox10 could be explained by reduced expression of neuregulin signaling components, the complete lack of satellite cells in the dorsal root ganglia (DRG), as well as Schwann cells in Sox10-deficient animals contrasts with neuregulin mutants and suggests a function for Sox10 in glial fate determination (Britsch et al., 2001). Interestingly, the erbB3 mutants that survive until birth (Riethmacher et al., 1997), and rescued erbB2 mutants (Morris et al., 1999; Woldeyesus et al., 1999), show a concomitant loss of motor and sensory neurons similar to the Sox10-deficient mice (Britsch et al., 2001). This neuronal cell death is likely attributable to the reduction of Schwann cells in these animals. This suggests a bidirectional dialogue between neurons and immature Schwann cells of the embryonic peripheral nerve in that Schwann cells are required by PNS neurons for survival (Davies et al., 1998).
Early Schwann cells are mitotically very active (Stewart et al., 1993) and differentiate into immature Schwann cells of the neonatal nerve, where they associate with axons (reviewed by Jessen and Mirsky, 1999; Mirsky and Jessen, 1999). Large-caliber axons are ensheathed by Schwann cells that form a myelin sheath to facilitate saltatory conduction. By contrast, small-caliber axons remain as tight bundles surrounded by a Schwann cell but are not myelinated (reviewed by Taylor and Suter, 1997). Axon-derived signals are important for the regulation of later Schwann cell development (Morrissey et al., 1995; Grinspan et al., 1996; Syroid et al., 1996), but the identities of the specific signals are not completely understood. In addition to the formation of myelin, mature Schwann cells possess the ability to dedifferentiate after nerve injury and provide a supportive environment for axonal regeneration. This dedifferentiation of Schwann cells deprived of their axons results in a state reminiscent of embryonic Schwann cells, with the reexpression of genes active during early nerve development (reviewed by Stoll and Müller, 1999). However, although there are similarities between the two processes of regeneration and development, there are also significant differences (Kim et al., 2000).
In vitro approaches are necessary to analyze in detail the intricate interplay between developing neurons and Schwann cells that is critical for the temporal and spatial differentiation of peripheral nerves. The difficulty and low cell yield associated with isolating SCPCs and the lack of specific factors to maintain and expand SCPCs indefinitely in an undifferentiated state necessitates immortalization of embryonic SCPCs. Hence, a precursor cell line would be a valuable tool to analyze development of the early peripheral glial lineage. We describe the analysis of a rat SCPC line (SpL201) generated by conditional, factor-dependent immortalization. We have used a retrovirus to introduce a gene encoding a fusion protein consisting of the extracellular ligand-binding domain of the epidermal growth factor receptor (EGFR) and the signal-transducing intracellular tyrosine kinase domain of c-neu (Jung et al., 1995) into primary rat E14.5 sciatic nerve SCPCs. We show that this line is dependent on EGF and maintains characteristics of primary SCPCs. The cells differentiate to express early Schwann cell markers and upregulate myelin gene expression in response to forskolin, which mimics axonal signals in vitro. Furthermore, we show that SpL201 cells behave as Schwann cells in their ability to associate with and ensheathe axons in vitro and to generate myelin in neuronal coculture. Finally, we demonstrate that these cells are able to remyelinate denuded axons after transplantation into foci of demyelination in the adult rat spinal cord.
MATERIALS AND METHODS
Cell Culture Media
Standard medium consisted of Dulbecco's modified Eagle's medium (DMEM; (Gibco)) with 10% fetal calf serum (FCS; Sera-Tech), 50 μg/ml gentamycin (Sigma), and 10 ng/ml human recombinant epidermal growth factor (EGF; PeproTech). Serum/forskolin medium consisted of standard medium without EGF, but with 20 μM forskolin (Sigma). Defined SCPC medium: Prepared as described previously (Lobsiger et al., 2000). SM medium was prepared according to Hagedorn et al. (1999), but without EGF. SpL201 cells were cultured at 37°C in a humid atmosphere with 6% CO2 and on tissue culture plastic dishes (Corning).
Antibodies and Immunocytochemistry
Immunocytochemistry was performed as described previously (Lobsiger et al., 2000). In brief, cells were fixed for 15 min at room temperature in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS). For myelin staining (MBP/MAG), cells were postfixed for 5 min consecutively with acetone (100%; −20°C), acetone/water (50/50%; −16°C), and acetone (100%; −20°C). In addition to the antibody O4 (mouse monoclonal, IgM; a kind gift from Dr. M. Schwab, 1/10), primary antibodies against the following antigens were used: MAG (rabbit polyclonal; a kind gift from Dr. S.S. Scherer, 1/400), MBP (mouse monoclonal; Roche Diagnostics, 1/100), nestin (mouse monoclonal; Developmental Studies Hybridoma Bank, IA, 1/20), NF160 (mouse monoclonal; Sigma, 1/100), Oct-6 (rabbit polyclonal, a kind gift from Dr. D.N. Meijer, 1/200), p75ex (rabbit anti-mouse p75 extracellular domain; Calbiochem, 1/300), p75in (rabbit anti-human, p75 intracellular domain, No. 9992, a kind gift from Dr. M. Chao, 1/750), S-100 (rabbit anti-cow S-100; Dakopatts, 1/500), and SMA (mouse monoclonal; Sigma, 1/200). Blocking solutions consisted of 10% goat serum (GS) in PBS for O4 and p75ex; 10% GS/0.2% Tween20 (Sigma) for nestin, NF160, p75in, S-100, SMA; 1% GS/0.1% Triton X-100 (Sigma) for MAG and MBP; and 5% GS/1% TritonX-100 for Oct-6.
Isolation and Culture of Primary Rat Embryonic Schwann Cells
Cultures of rat (outbred OFA Sprague Dawley-strain; BRL, Switzerland) E14.5 sciatic nerve SCPCs and E19.5 early Schwann cells were prepared as described previously (Lobsiger et al., 2000). In brief, freshly isolated cells were plated in defined SCPC medium onto glass coverslips (Arnold Bott AG, Zurich, Switzerland) coated with poly-L-lysine/laminin (Sigma) and fixed after 3 h. Neuronal fate determination assays were performed by plating primary E14.5 sciatic nerve cells at low density (10–20% confluency) on poly-D-lysine/fibronectin-(Sigma) coated plastic dishes in SM-medium and treated as described for NCSCs by Hagedorn et al. (1999).
Generation and Culture of the Schwann Cell Precursor Line SpL201
Virus-producing GPE cells (Markowitz et al., 1988) containing the EGFR/neu MMLV-based replication-incompetent viral construct (Lehvaslaiho et al., 1989) were a kind gift from Dr. J. Trotter (Jung et al., 1995). The cells were cultured, and virus particles (virus-titer approx. 3 × 105 pfu/ml) were collected in DMEM and filtered (0.45 μm; Millipore) as described by Jung et al. (1995). Primary E14.5 rat sciatic nerve cells were isolated and resuspended in a 1:1 mixture of medium (DMEM, 10% FCS, 50 μg/ml bovine pituitary extract; Biomedical Technologies); EGFR/neu virus-containing supernatant. Polybrene (Sigma) was added to 8 μg/ml and the cells plated in 300 μl of medium onto coated glass coverslips. The cells were cultured for 16 h, after which the medium was exchanged to defined SCPC medium with 10 ng/ml of EGF. After 4 days, the medium was exchanged with the standard medium. Clones were picked and the cells passaged onto uncoated tissue plastic (Corning) and cultured for 3 weeks before selection in standard medium containing 0.8 mg/ml of geneticin (G418; ICN Biochemicals, Germany) for 3–5 weeks. Surviving colonies were passaged in standard medium and the putative SCPC lines (termed SpL) isolated.
Analysis of the Candidate SCPC Line SpL201
Cells of the SpL201 line were cultured on uncoated plastic dishes in standard or serum/forskolin medium (unless otherwise stated). SpL201 cells were reselected with G418 for 12 days after 30 days of culture in standard medium. In order to verify clonality of the SpL201 cells, the parent line was diluted to single-cell density and plated into wells of culture plates. The cells were allowed to adhere and each well was analyzed by phase optics. Wells containing single isolated cells were marked and expanded. Clonal cells were passaged and cultured under standard conditions. Southern blot analysis was performed on genomic DNA isolated from the SpL201 cells and three individual SpL201-derived subclones. Southern blots were performed as described by Jung et al. (1998) and hybridized with a [32P]-dCTP-labeled neomycin-resistance gene DNA-probe (NEO). Differentiation and survival assays were performed on subconfluent SpL201 cells, cultured for 5 days in standard or serum/forskolin medium. The cells were washed three times with DMEM, scraped from the plate, centrifuged for 5 min, and plated in defined SCPC medium at medium density (30–50% confluency) onto poly-L-lysine/laminin-coated 30-mm plastic dishes. After 3 h, cells were either fixed and immunostained (for the differentiation assays) or the medium exchanged to defined SCPC-medium with or without 10 ng/ml EGF, fixed, and immunostained after 48 h (for the survival assays). Proliferation assays were performed on low-density (10–20% confluency) cultures of SpL201 cells and BrdU incorporation and immunostaining was conducted according to manufacturer's instructions (BrdU Labeling and Detection Kit I; Roche Diagnostics; see Results). Quantification of the differentiation, survival and proliferation assays was performed by counting five arbitrarily chosen visual fields per separate condition from two representative experiments (with 200–300 cells per field; see Results). For all counting, pairwise statistical significance was calculated by the Wilcoxon two-sample test. Neuronal fate determination assays were performed as described above for the primary cells. Clonal analysis of differentiation was performed by plating SpL201 cells in standard medium at low density (5–10 single cells per 30 mm uncoated dish (as described by Morrison et al., 2000)), and single cells marked. The clones were expanded for 10 days and subsequently immunostained. Only marked clones that were derived from a single cell were analyzed (see Results).
Maturation Assay and Northern Blot Analysis
SpL201 cells were washed with DMEM and cultured for different times (see Results) in serum/forskolin medium before total RNA was extracted from subconfluent cultures for Northern blot analysis. RNA was isolated using Trizol (Sigma), followed by a modified acid phenol guanidinium isothiocyanate method (Chomczynski and Sacchi, 1987), as described by Lobsiger et al. (1996); 5 μg of total RNA was separated on a denaturing agarose-formaldehyde gel and transferred to a nitrocellulose membrane (Optitran BA-S83, reinforced NC; Schleicher & Schüll, Germany); the membrane was hybridized consecutively with [32P]-dCTP-labeled mouse Oct-6, P0, PMP22, and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) DNA probes as described by Lobsiger et al. (1996). The membrane was exposed for Oct-6 (42 h), P0 (25 h), PMP22 (42 h), and GAPDH (8 h) to PhosphorImager-screens and analyzed without background subtraction (PhosphorImager, Storm 820; Molecular Dynamics; see Results). Before each new hybridization step, the membrane was stripped.
In Vitro Myelination
Rat E15 DRG sensory neurons were isolated, plated onto glass coverslips coated with collagen, and treated three times with fluorodeoxyuridine (10 μM; Sigma) during a total culture period of 3 weeks in DRG medium as described in Bunge et al. (1991) to eliminate endogenous satellite glia. SpL201 cells, cultured in standard medium were labeled with a red fluorescent lipid-soluble dye according to the manufacturer's instructions (PKH26-GL, Red Fluorescent Cell Linker Kit; Sigma). Approximately 60,000 cells were plated per coverslip containing one dissociated DRG devoid of endogenous glial cells. The medium was exchanged every second day for 8 days (DMEM, 20% FBS, 50 ng/ml NGF (mNGF 2.5S; grade II; Alomone Labs, Jerusalem, Israel)) before myelination was induced for an additional 4 weeks with medium containing 50 μg/ml ascorbic acid (Sigma) and the cells being subsequently fixed and analyzed.
In Vivo Myelination
Focal demyelinating lesions were created in the spinal cord of 12 adult female Sprague Dawley rats (180–200 g; Harlan, UK) by injection of ethidium bromide. The rats were anaesthetized using halothane and oxygen and a dorsal laminectomy was carried out at the level of the thoracolumbar junction under sterile conditions; 1 μl of a solution of 0.1% ethidium bromide was injected into the dorsal funiculus using a glass micropipette-tipped Hamilton syringe and a micromanipulator. Tissues were then closed in routine fashion and rats were administered the analgesic carprofen (10 mg/kg s.c.; Zenecarp, C-VET, Leyland, Lancs, UK). In order to suppress inherent remyelination, the rats were restrained 24 h later using the neuroleptoanalgesic combination of fentanyl and fluanisone (0.4 ml/kg i.m.; Hypnorm; Jannsen Pharmaceuticals, High Wycombe, Bucks, UK) and a 4-cm section of spinal cord exposed to 40 Gy X-irradiation using a Pantak orthovoltage (255 kV) radiotherapy machine (Pantak Ltd, Reading, UK). Transplantation was carried out 48 h after X-irradiation. SpL201 cells (passages 8 and 10), cultured and scraped in standard medium, were washed and resuspended in Eagle's minimal medium containing 10 mM HEPES (1.5 × 105 cells/μl). Rats were anaesthetized using a combination of halothane and oxygen and the laminectomy site exposed. A 60-μm bore glass micropipette-tipped Hamilton syringe held by a micromanipulator was introduced into the demyelinating lesion in the dorsal funiculus to a depth of 1 mm below the pial surface and 1 μl of a SpL201 cell suspension injected. Tissues were closed in routine fashion, and animals were administered carprofen (10 mg/kg s.c.) and long-acting penicillin (0.5 ml/kg i.m.; Duphapen; Solvay Duphar Veterinary, Southampton, Hants, UK). Immunosuppression was maintained by daily injection of cyclosporin A (15 mg/kg s.c.; Sandimmun; Sandoz, Frimley, Surrey, UK), beginning the day before transplantation and continuing throughout the course of the experiment. Tissue processing and staining: The rats were sacrificed either 14 or 28 days after transplantation. Transplant recipients were deeply anesthetized with pentobarbital (i.v.; Sagatal; Rhone Merieux, Harlow, Essex, UK) and perfused via the descending aorta with 4% glutaraldehyde in phosphate buffer. The thoracic and lumbar spinal cord was removed and immersed for a minimum of 1 h in 4% glutaraldehyde. The section of spinal cord containing the lesion was then cut into 1-mm coronal sections and washed in phosphate buffer prior to immersion in osmium tetroxide at 4°C overnight. The tissue blocks were dehydrated through ascending ethanol washes and embedded in TAAB resin; 1-μm sections were cut from hardened resin blocks, stained using toluidine blue, and examined by light microscopy. In addition, selected blocks were trimmed and examined by electron microscopy (Hitachi, H600) or, after resin removal, processed for immunohistochemistry using anti-P0 antibody. Bound antibody was identified by applying biotinylated goat anti-mouse IgG (Sigma), followed by Vectastain ABC (Vector Laboratories) and 3,3′-diaminobenzidine (DAB; Sigma) to generate a colored reaction product. The lesions in transplanted animals were compared with control animals that either received no transplant or that were injected with saline.
RESULTS
Conditional Immortalization of Primary Rat Schwann Cell Precursors
In order to generate an immortalized Schwann cell precursor line, E14.5 rat sciatic nerves were isolated and dissociated; the cells were infected with a replication-deficient MMLV-retrovirus. The retrovirus carried a gene encoding a chimeric fusion protein consisting of the ligand-binding domain of the human EGFR and the rat c-neu tyrosine kinase under the transcriptional control of a thymidine kinase (Tk) promoter. Furthermore, a neomycin-resistance gene was expressed from the viral 5′-LTR (Fig. 1A). The infected cells were cultured in the presence of EGF and fetal calf serum (FCS), neither of which can support primary SCPCs in vitro (Jessen et al., 1994). Uninfected cells died by apoptosis because of lack of trophic support (data not shown). The infected cells were expanded in medium containing serum and EGF (standard medium) before being selected in the presence of G418. The surviving colonies, representing potential immortalized SCPCs, were passaged in standard medium. The clonality of the candidate SCPC-line SpL201 was analyzed by genomic DNA Southern blots hybridized with a probe for the neomycin-resistance gene of the viral vector (Fig. 1A,B). Three genomic DNA fragments hybridized with the probe, raising the possibility that the SpL201 cell line was not clonal. Therefore, we diluted the SpL201 line and expanded clones derived from a single cell (see Materials and Methods). Subsequently, Southern blot analysis was performed on three randomly chosen subclones (sCl1, 5, and 7) to confirm the clonality of the parental line. The same three DNA fragments detected by Southern blot analysis of DNA isolated from the parent line were recognized by the neomycin-probe in all three subclones (results for subclone sCl7 and the parental line SpL201 are shown in Fig. 1B; identical results were obtained with sCl1 and 5; data not shown). These results demonstrate that the parental line SpL201 is of clonal origin. Thus, the most likely explanation for the three independent genomic DNA loci identified by Southern blot analysis is the presence of multiple viral integrations.
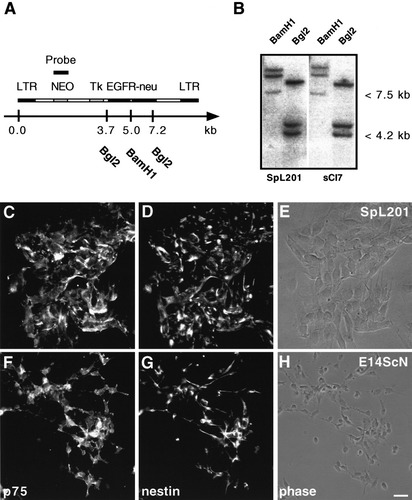
The SpL201 line was generated from neural crest-derived primary E14.5 rat sciatic nerve cells. A: The retrovirus used for infection of the E14.5 rat sciatic nerve cells contained the EGFR-neu fusion protein under the control of the herpes virus thymidine kinase (Tk)-promoter. Restriction sites are indicated with the corresponding distances in kilobases (kb) from the 5′-end of the construct. Neomycin resistance gene (NEO), viral long terminal repeats (LTR). B: Genomic Southern blot of BamHI and Bgl2-digested DNA isolated from the SpL201 line (SpL201) and a subclone (sCl7) hybridized with a neomycin probe indicated in A (see text for details). Cells of the SpL201 line, cultured in standard medium (C–E) and freshly isolated rat E14.5 sciatic nerve cells (E14ScN) (F–H), cultured for 3 h in defined medium and immunostained with antibodies directed against p75 (C,F) and nestin (D,G). p75 and nestin are markers of neural crest-derived cells within the embryonic rat sciatic nerve and expressed in E14.5 SCPCs. Primary Schwann cells have little cytoplasm and a disorganized cytoskeleton due to the incomplete spreading of the cells 3 h post-isolation (G). E,H: Corresponding phase views of C,D and F,G. Scale bar in H = 10 μm (for A–H).
The Spl201 Cells Are Neural Crest-Derived and Belong to the Schwann Cell Lineage
The embryonic nerves of rats contain neural crest-derived and non-neural crest-derived cells (Morrison et al., 1999). Therefore, we used antibodies against p75 and the intermediate filament protein nestin to establish whether the SpL201 cell line originated from the neural crest (Jessen et al., 1994) (Fig. 1C–H). SpL201 cells were strongly p75 and nestin-positive with a flat, epithelial-like morphology and a tendency to form epithelial-like layers (Fig. 1C–E). Hence, SpL201 cells resembled primary E14.5 SCPCs (E14 ScN) analyzed 3 h after plating (Fig. 1F–H).
The trunk neural crest gives rise to many cell types, including peripheral neurons, satellite glia, Schwann cells, smooth muscle cells, and melanocytes (reviewed by Anderson, 1997). Thus, we analyzed the expression of PMP22, P0 and the transcription factor Oct-6 to elucidate to which lineage the SpL201 cells belong (data not shown; and Fig. 2). PMP22, P0 and Oct-6 are coexpressed by SCPCs but not by NCSCs or embryonic DRG sensory neurons (Blanchard et al., 1996; Hagedorn et al., 1999). Furthermore, within the PNS glial lineage, Oct-6 is expressed by SCPCs, but not by embryonic DRG satellite glia (Blanchard et al., 1996; Hagedorn et al., 2000). SpL201 cells express Oct-6 comparable to primary E14.5 SCPCs (E14 ScN) (Fig. 2A,C) suggesting that they are within the PNS glial lineage and of Schwann cell, rather than satellite cell origin.
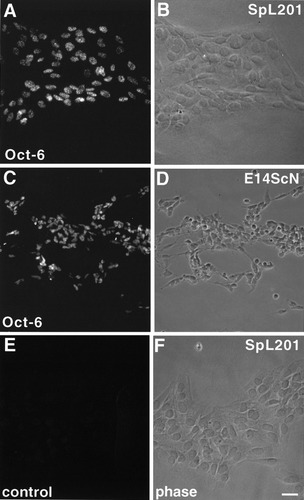
SpL201 cells belong to the Schwann cell lineage. A: Cells of the SpL201 line, cultured in standard medium express Oct-6 localized to the nucleus. B: Phase-contrast image of cells shown in A. C: Freshly isolated rat E14.5 sciatic nerve cells (E14ScN), cultured for 3 h in defined SCPC medium, also express nuclear Oct-6. D: Phase-contrast image of cells shown in C. E: Preimmune serum shows no specific staining of SpL201 cells or primary E14.5 sciatic nerve cells (data not shown). F: Phase-contrast image of cells shown in E. Scale bar in F = 10 μm (for A–F).
SpL201 Cells Upregulate S-100 and O4 in Response to Serum/Forskolin Treatment
We analyzed the expression of S-100 and the O4 antigen to define the developmental stage and potential of SpL201 cells. Both S-100 and O4 distinguish early Schwann cells from SCPCs (Jessen et al., 1994; Mirsky et al., 1990). SpL201 cells express low levels of S-100 and O4 antigen under standard culture conditions (SpL201/u in Fig. 3A–C). These findings are comparable to the low expression levels of S-100 and O4 of primary SCPCs (E14ScN) 3 h ex vivo (Fig. 3G–I). In contrast, early Schwann cells (E19ScN) isolated at E19.5 and identified by p75 immunostaining showed high expression of S-100 and O4 (3 h ex vivo; Fig. 3J–L). These data indicate that the SpL201 cells are more closely related to the SCPC state of the Schwann cell lineage than to early Schwann cells.
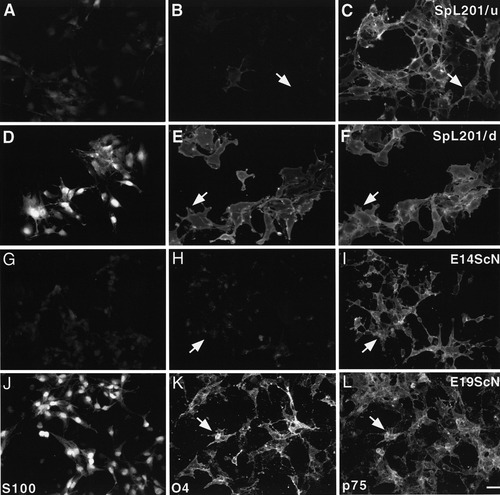
Cells of the SpL201 line can be differentiated from a precursor to an early Schwann cell-like stage. SpL201 cells cultured in standard medium (SpL201/u) show low levels of S-100 (A) and O4 antigen expression (B) but express p75 (C). In contrast, SpL201 cells cultured in serum/forskolin medium for 5 days (SpL201/d) show high levels of nuclear S-100 (D) and membrane-associated O4 antigen (E) and p75 expression (F). Freshly isolated rat E14.5 SCPC (E14ScN) also exhibit low levels of S-100 (G) and O4 antigen expression (H) but express p75 (I), whereas E19.5 (E19ScN) sciatic nerve cells have elevated levels of nuclear S-100 (J) and O4 antigen (K). In addition, E19.5 sciatic nerve cells retain expression of p75 (L). The single O4-positive cell seen in B likely represents an SpL201 cell having undergone spontaneous differentiation. Arrows in B,C,H,I indicate p75-positive, O4-negative cells; arrows in E,F,K,L indicate p75, O4 double-positive cells. Scale bar in L = 10 μm (for A–L).
We also assessed whether SpL201 cells could differentiate from the precursor state to an S-100, O4-positive early Schwann cell-like state in response to a simulated axonal signal by treatment with serum and forskolin (Lemke and Chao, 1988). SpL201 cells were cultured in medium lacking EGF and supplemented with 20 μM forskolin (serum/forskolin medium; see Materials and Methods) (SpL201/d in Fig. 3D–F). A 5-day treatment with serum/forskolin resulted in an upregulation of S-100, which showed a prominent nuclear localization in most cells (cf. Fig. 3A and D), and of O4 (cf. Fig. 3B and E). These data are consistent with the upregulation of S-100 (Fig. 3G,J) and O4 (Fig. 3H,K) seen in the transition of SCPCs to early Schwann cells ex vivo.
EGF Is a Mitogen for SpL201 Cells in Serum-Containing Medium
We used the EGFR/neu fusion protein to immortalize primary E14.5 SCPCs and to obtain cells that display SCPC characteristics. Therefore, we analyzed the mitotic action of EGF on the SpL201 cells in standard medium. We performed a dose response analysis with increasing concentrations of EGF (0–50 ng/ml) and assessed proliferation after 3 days by incorporation of bromodeoxyuridine (BrdU) (Fig. 4A). The percentage of cells that had incorporated BrdU was determined under different EGF concentrations by immunostaining with an anti-BrdU antibody. We observed that EGF acted in a concentration-dependent manner to significantly increase proliferation of the SpL201 cells up to 3-fold at the maximal EGF concentration used (Fig. 4A). The basal BrdU incorporation in the absence of exogenous EGF is due to other mitogens derived from serum. In medium containing 10 ng/ml EGF, the doubling-time was approximately 24 h as assessed by determining the total cell number per plate over a 3-day period. In addition, we have assessed the stability of the SpL201 cell line by continuous culture. After 35 passages, approximately 4.5 months in vitro, the cells remained homogeneous and showed EGF-dependent proliferation (data not shown). Treatment of these long-term cultured SpL201 cells with serum/forskolin resulted in the induction of O4 and S-100 expression similar to cells of earlier passages.
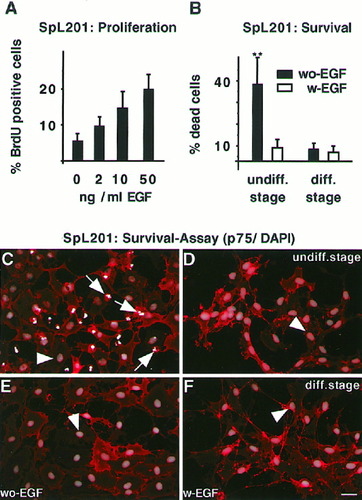
Epidermal growth factor (EGF) induces proliferation of the undifferentiated SpL201 line and is required for full survival of the precursor, but not the early Schwann cell-like stage of SpL201 cells. A: Dose response of EGF-induced proliferation of SpL201 cells cultured in standard medium with 0, 2, 10, and 50 ng/ml EGF assessed by BrdU incubation during the last 3 h (0 ng/ml: 4.3 ± 1.7%; 2 ng/ml: 10.4 ± 2%; 10 ng/ml: 14 ± 3.9%; 50 ng/ml: 19.3 ± 3.3%). Cells in standard culture conditions (undiff. stage) (C,D) and cultures treated for 5 days with serum/forskolin (diff. stage) (E,F) were replated and cultured for 48 h in defined medium without (without EGF) (C,E) or with EGF (with EGF) (D,F). Subsequently, the cells were fixed and immunostained with anti-p75 antibodies (C–F; in red). Nuclear integrity was determined by DAPI staining (1 μg/ml; Sigma) (C–F; in white/gray). White fragmented nuclei correspond to dead cells (arrows), while intact gray nuclei correspond to living cells (arrowheads). The percentage of dead cells was determined (see Materials and Methods) and expressed (B) as the standard means of percentages with standard deviations (undiff. stage/without EGF: 35.4 ± 12.4%; with EGF: 6 ± 3.4%; diff. stage/without EGF: 5.7 ± 2.6%; with EGF: 4.1 ± 2.3%). Significant differences between cultures with and without EGF are indicated by two asterisks (P < 0.001). Scale bar in F = 10 μm (for A–F).
EGF Is an Anti-Apoptotic Survival Factor for SpL201 Cells in Defined Medium
SCPCs are characterized by their dependence on axon-derived survival factors in vitro, in contrast to early Schwann cells (Jessen et al., 1994). Therefore, we assessed the effects of EGF on SpL201 cell survival under defined culture conditions prior to and following forskolin-induced differentiation. In the absence of EGF, a significant fraction (approx. 35%) of the SpL201 cells (undiff. stage; Fig. 4B) underwent programmed cell death within 48 h as determined by chromatin condensation and nuclear fragmentation visualized by DAPI-staining (arrows in Fig. 4C); 10 ng/ml of EGF was able to rescue the SpL201 cells from apoptotic cell death, as indicated by the reduction in the number of cells with condensed and fragmented nuclei (Fig. 4B,D). The dependence on EGF correlated with the differentiation state of the cells, only few cells (∼6%) showed signs of apoptotic cell death in the absence of EGF after 5 days of serum/forskolin treatment to induce differentiation (diff. stage; Fig. 4B,E,F).
SpL201 Cells Are Restricted to a Non-neuronal, Predominantly Glial Fate
Recently, it has been shown that NCSC-like cells can be isolated from E14.5 rat sciatic nerve. These cells give rise to sympathetic neurons upon treatment with bone morphogenetic protein-2 (BMP-2) (Morrison et al., 1999). Therefore, we analyzed whether the SpL201 cells retained the potential to generate neurons in response to BMP-2 treatment (Fig. 5). SpL201 cells and primary E14.5 sciatic nerve cells were plated at low density in SM medium (see Materials and Methods) in the absence of EGF and supplemented with 1.6 nM of BMP-2 after 3 h. The cells were subsequently cultured for 6 days in the same medium before being immunostained for p75 and neurofilament 160 (NF160) (Hagedorn et al., 1999). Approximately 60% of the primary E14.5 sciatic nerve cells generated clusters of p75/NF160 double-positive neurons in response to BMP-2 treatment (arrowhead in Fig. 5A–C). The other cells retained p75 expression but failed to express the neuronal marker and adopted a bipolar morphology reminiscent of early Schwann cells (arrows in Fig. 5A–C), confirming the observations of Morrison et al. (1999). In contrast, SpL201 cells did not upregulate NF160 in the presence of BMP-2 after 6 days and retained a flattened morphology (arrows in Fig. 5D–F).
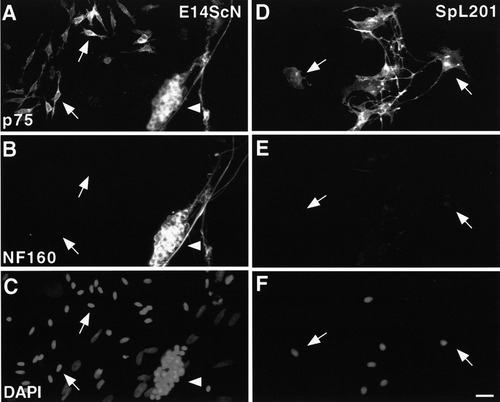
Glial fate restriction of the SpL201 line. Freshly isolated rat E14.5 sciatic nerve cells (E14ScN) (A–C:) and cells of the precursor stage of the SpL201 line (D–F) were cultured for 6 days in SM medium with BMP-2 (for neuronal fate induction) and subsequently immunostained with antibodies directed against p75 (A,D) and NF160 (B,E) along with the corresponding DAPI stainings (C,F). Cells of the SpL201 line are restricted to a non-neuronal, predominantly glial fate (arrows), while E14.5 sciatic nerve give rise to clusters of neurons (arrowhead) and non-neuronal cells (arrows in A,B). Scale bar in F = 10 μm (for A–F).
SpL201 Cells Can Differentiate into Myofibroblast-like Cells
NCSC-like cells from the E14.5 rat nerve also have the potential to generate smooth muscle actin (SMA)-positive myofibroblast-like cells in vitro (Morrison et al., 1999). We assessed whether SpL201 cells could generate SMA-positive/p75-negative myofibroblasts by clonal analysis. SpL201 cells were plated at clonal density and cultured in standard medium for 6 days followed by removal of EGF from the medium and culturing for an additional 6 days. The clones were fixed and immunostained with anti-p75 and anti-SMA antibodies. An average of 50% of the clones contained both p75-positive and SMA-positive cells (p75/SMA-mixed clones) (Fig. 6A,B,G). Approximately 40% of the remaining clones contained only p75-positive cells (Fig. 6C,D,G) and 10% consisted of p75-negative, SMA-positive cells (Fig. 6E,F,G). Analysis of the parental line in standard medium 3 h after plating showed less than 5% of the cells expressed SMA (data not shown). Thus, most of the SMA-expressing cell clones generated in the clonal analysis were likely to have been derived from p75-positive, SMA-negative cells. These results suggest that SpL201 cells can differentiate into myofibroblast-like cells. Preliminary experiments indicate that the percentage of mixed p75/SMA-positive cell clones is markedly reduced in the presence of EGF (data not shown).
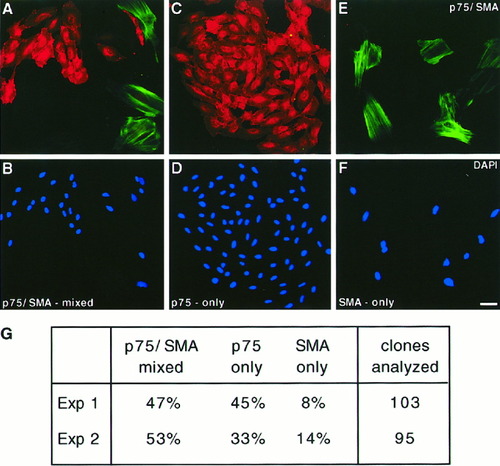
SpL201 cells can differentiate into myofibroblasts. A–F: SpL201 cells were cultured at clonal density for 6 days in standard medium, followed by 6 days in standard medium lacking EGF and immunostained with antibodies directed against p75 (A,C,E; red) and smooth muscle actin (SMA) (A,C,E; green), with the corresponding DAPI stainings (B,D,F). G: Quantification of single clones analyzed in two independent experiments and the percentages of clones with p75-positive and SMA-positive cells (p75/SMA-mixed), only p75-positive cells (p75-only), or only SMA-positive cells (SMA-only). Scale bar in F = 10 μm (for A–G).
SpL201 Cells Upregulate the Schwann Cell Marker Oct-6 and the Myelin Genes P0 and PMP22 in Response to Forskolin
To determine whether SpL201 cells retained the potential to differentiate into mature Schwann cells, we analyzed their ability to upregulate expression of Schwann cell differentiation markers (Fig. 7). Oct-6, P0, and PMP22 are upregulated by postnatal Schwann cells in response to serum/forskolin treatment that mimics axon-derived signals (Lemke and Chao, 1988). Therefore, we cultured SpL201 cells in serum/forskolin containing medium for increasing periods of time (0 h to 5 days) and subsequently isolated total RNA for Northern blot analysis. Blots were hybridized consecutively with cDNA probes corresponding to Oct-6, P0, and PMP22 and finally GAPDH to determine the amount of mRNA per lane (Fig. 7A). The resulting hybridization patterns were quantified by PhosphorImager analysis and the relative expression levels of Oct-6, P0, and PMP22 normalized to the GAPDH hybridization signal (Fig. 7B). SpL201 cells upregulated Oct-6 to 2.7-fold above basal expression levels in response to 5 days of forskolin treatment. P0 expression was increased to a maximum of 3.8-fold, while PMP22 showed a marginal upregulation of 1.7-fold after 24 h of forskolin treatment (Fig. 7B). A similar regulation has also been reported for postnatal rat Schwann cells (Lemke and Chao, 1988; Monuki et al., 1989; Taylor et al., 1995; and our own observations, data not shown).
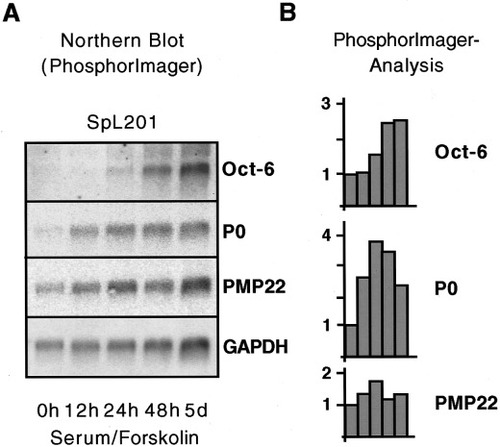
Forskolin induces differentiation of the SpL201 line along the Schwann cell lineage. A: Northern blot analysis of the Schwann cell differentiation markers Oct-6, P0, and PMP22. Cells of the precursor stage of the SpL201 line were treated for 0 h to 5 days with serum/forskolin, before total RNA was extracted and the corresponding Northern blot hybridized sequentially with probes specific for the premyelin gene Oct-6, the myelin genes P0 and PMP22, and GAPDH as a loading control. B: Hybridization signals were quantified using PhosphorImager analysis and signals were normalized to GAPDH and expressed as fold change (calculated as the standard mean of two Northern blots using the same RNA) of the expression levels relative to the expression level at 0 h (see text).
SpL201 Cells Recognize and Myelinate Neurites in Cocultures
Since SpL201 cells express Schwann cell markers and can upregulate Oct-6, P0, and PMP22 in response to a simulated axonal signal in vitro, we tested their ability to recognize neurites and their capacity to myelinate axons (Fig. 8). Therefore, we cocultured SpL201 cells in the precursor stage with purified E15 rat DRG sensory neurons (Fig. 8A). We labeled the cell line with a red-fluorescent lipid-soluble dye (PKH26, Sigma) prior to coculture, in order to distinguish the SpL201 cells from potentially contaminating residual satellite glia. After 12 h of coculture, many of the SpL201 cells had associated with and bound to neurites, forming pearl-like strings (Fig. 8B). After 8 days of coculture, the alignment of the SpL201 cells with the neurites was more pronounced and most of the SpL201 cells within this coculture retained the lipid-soluble dye (Fig. 8C). Sister plates of DRG neurons without SpL201 cells contained only neurons and were devoid of endogenous satellite glia (Fig. 8A). To analyze the physiological potential of the SpL201 line further, we supplemented the medium with ascorbic acid to induce myelination in vitro (reviewed by Wood et al., 1990). We analyzed the cultures for myelin formation after an additional 4 weeks. In order to identify dye-positive myelinating cells in the coculture, we used a two-step fixation procedure, since the lipid dye is sensitive to acetone fixation. PFA-fixed myelinated fibers were identified using phase optics (arrow in Fig. 8D), analyzed for the presence of the lipophilic dye (arrow in Fig. 8D), and marked. Subsequently, the cultures were postfixed with acetone to permeabilize the myelin and were immunostained with antibodies directed against the myelin markers, myelin basic protein (MBP; red in Fig. 8E) and myelin-associated glycoprotein (MAG; green in Fig. 8E,F). Dye-labeled SpL201 cells (arrow in Fig. 8D–F) could be found associated with myelin sheaths that were immunostained with both the MBP and MAG antibodies (arrowheads in Fig. 8D,E,F).
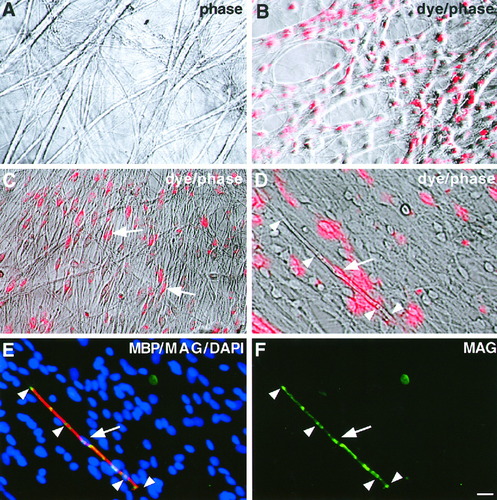
SpL201 cells can generate myelin in vitro. A: Rat E15 dorsal root ganglion (DRG) neurons were cultured in the presence of fluorodeoxyuridine to eliminate proliferating Schwann cells and fibroblasts. After 3 weeks. the neurons had built fascicles of axons covering the culture dish. B: Dye-labeled SpL201 cells (precursor stage) were cocultured with purified rat E15 DRG neurons. The dye-positive SpL201 cells (red) attached to the naked neurites after 12 h. C: After 8 days of coculture many dye-positive SpL201 cells aligned to neurites (arrows), while sister plates without SpL201 cells were glia-free (A). D–F: After an additional 4 weeks in ascorbic acid-containing medium, cultures were fixed and dye-positive SpL201 cells (D; arrow) forming phase-contrast rich myelin (D; arrowheads) identified. Cells were acetone-postfixed and immunostained with antibodies against MBP (E; red) and MAG (E,F; green) and nuclei visualized by DAPI-staining (E; blue). Scale bar in F = 10 μm (for A–F).
SpL201 Cells Extensively Remyelinate a Demyelinated CNS Lesion In Vivo
In order to investigate the ability of SpL201 cells to establish a normal myelinating phenotype in vivo, we transplanted these cells into experimentally induced demyelinated lesions in the spinal cord white matter of immunosuppressed adult rats (Fig. 9). Lesions were induced by injecting a small volume of ethidium bromide into the dorsal funiculus of the spinal cord. This procedure creates a discrete region of demyelination extending up to 4 mm rostral and caudal to the injection site. Under normal circumstances, demyelination induced in this manner is rapidly followed by remyelination (Blakemore, 1982). However, this intrinsic remyelination can be prevented by exposure of the spinal cord to 40 Gy of X-irradiation. This leaves a focal area of white matter pathology in which demyelinated axons exist in a glia-free environment (Blakemore and Crang, 1985). Since host glial cells can not contribute to the repair of the X-irradiated ethidium bromide (X-EB) lesion, any remyelination following transplantation must be performed by donor cells which, therefore, do not require labeling (Crang and Blakemore, 1991).
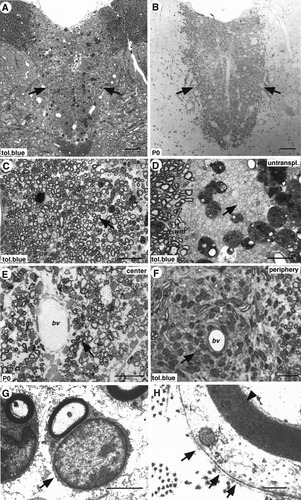
SpL201 cells can remyelinate a demyelinated CNS lesion. SpL201 cells in the precursor stage were transplanted into X-EB lesions in the spinal cord of immunosuppressed adult rats. A,B: Cross section of the spinal cord showing a transplanted lesion occupying the dorsal funiculus 4 weeks after transplantation, stained with toluidine blue (A; tol.blue) and anti-P0 antibodies (B; P0) to confirm widespread formation of peripheral-type myelin throughout the lesion (arrows mark lesion border). C,D: At higher magnification, remyelination is obvious (C; arrow) and easily distinguishable from both demyelinated axons (D; arrow) and oligodendrocyte myelinated axons of surrounding, unlesioned white matter (D; wm) in an untransplanted animal (D). E: Immunostaining demonstrated the presence of P0 in the myelin sheaths of differentiated Schwann cells (E; arrow), surrounding a blood vessel (E; bv) in the center of the lesion. F: In addition, foci of undifferentiated cells could be identified, commonly surrounding blood vessels at the periphery of the lesion (F; arrow). G,H: Ultrastructural analysis of the transplanted lesion confirmed the one-to-one association of Schwann cell and axon (G; arrow) and the presence of a (fragmented) basal lamina around the Schwann cell membrane (H; arrows). The distance between adjacent lamellae of the myelin sheath was 12.5 nm (H; arrowhead), a typical value for osmium-fixed tissue. Scale bars = 100 μm in A,B; 25 μm in C,D,E,F; 2 μm in G; 200 nm in H.
SpL201 cells in the precursor stage were transplanted into lesions in 12 animals (group A, n = 6; group B; n = 6). Animals from both groups were sacrificed 2 weeks (n = 2; data not shown) and 4 weeks (n = 4; Fig. 9) posttransplantation. Examination of toluidine blue-stained resin sections revealed large numbers of cells within the lesion area in all animals of both groups after 2 (data not shown) and 4 weeks (Fig. 9). At 2 weeks, transplanted SpL201 cells were dispersed throughout the area of demyelination, with a high density in the central region surrounding the injection site and relatively few cells in the rostral and caudal most areas of the lesion. Thin myelin sheaths were identified in the center of the lesion but were uncommon in more peripheral regions where most of the axons remained demyelinated. However, transplanted cells could be seen engaging demyelinated axons, and clusters of cells that had not associated with axons were also present in these peripheral regions. These cells had an undifferentiated appearance with irregular shaped nuclei and relatively scant cytoplasm. They were often found surrounding blood vessels at the junction between the lesion and the normal gray matter of the dorsal horns.
After 4 weeks, the distribution of transplanted SpL201 cells throughout the lesion was more uniform than at 2 weeks. Transplanted cells were present throughout the lesion at both the rostral and caudal extremities (Fig. 9A). Remyelination was more extensive than at 2 weeks, and in many areas nearly all the axons were remyelinated (Fig. 9C). Individual SpL201 cells formed a single myelin sheath and were usually surrounded by a fragmented basal lamina (Fig. 9G,H), morphological features characteristic of myelinating Schwann cells (reviewed in Arroyo and Scherer, 2000). Furthermore, the myelin sheaths formed by the transplanted cells expressed the PNS myelin-specific protein P0 (Fig. 9B,E). In addition, small clusters of undifferentiated cells remained around blood vessels at the periphery but not in the center of the lesion (cf. Fig. 9E,F). It is possible that these undifferentiated cells were responding to serum-derived trophic factors.
DISCUSSION
The mechanisms that regulate early stages of Schwann cell development, their survival, proliferation, and differentiation are poorly understood (reviewed by Mirsky and Jessen, 1999). This is partly due to the lack of available trophic factors to maintain SCPCs in an undifferentiated state during prolonged cell culture (Dong et al., 1995; Gavrilovic et al. 1995; Jessen et al., 1994; Lobsiger et al., 2000, Brennan et al., 2000). Therefore, we have generated a conditionally immortalized rat SCPC line from E14.5 sciatic nerves. We have shown that the SpL201 cells retain characteristics of primary SCPCs including the expression of p75, nestin, and Oct-6, markers that identify the embryonic Schwann cell lineage. Furthermore, SpL201 cells can generate cells of the Schwann cell and myofibroblast lineages but, unlike the neural crest, are unable to produce autonomic neurons in response to BMP-2. This finding is in agreement with the recent results of Morrison et al. (1999) showing that a population of fate restricted PNS progenitors can generate glial cells and myofibroblasts, but not autonomic neurons, in vitro.
In addition to the expression of specific markers and restricted developmental potential, SpL201 cells retain dependence upon exogenous trophic support, one of the key properties of SCPCs. As a result of the introduction of the EGFR/neu fusion protein, SpL201 cells are dependent on EGF, which acts as a mitogen and maintains the cells in an undifferentiated state. Furthermore, removal of EGF from the defined culture medium results in cell death. This indicates that EGF is a survival factor for SpL201 cells and displays some of the properties of axon-derived signals required by primary SCPCs. This is intriguing, since EGF presumably acts through the EGFR/neu fusion protein to activate the erbB2 signaling cascade but, unlike neuregulin-1 acting on primary SCPCs via the heterodimeric receptor complex of erbB2 and erbB3 (Dong et al., 1995), has no apparent differentiation effect.
It is possible that the EGFR/neu fusion protein isolates the erbB2 (neu) from the erbB3-dependent signaling pathway in SpL201 cells treated with EGF. This would suggest that erbB2 signaling-associated intracellular proteins direct survival and mitogenic signals whereas erbB3 intracellular binding proteins may mediate differentiation signals induced by neuregulin-1. Indeed, previous studies have shown that the erbB signaling network mediates its diverse effects through a combinatorial interaction between the different receptor units forming both homodimers and heterodimers (reviewed in Alroy and Yarden, 1997). This interpretation is also consistent with the finding that naturally occurring amplification or overexpression of the human neu/HER2 proto-oncogene is responsible for uncontrolled cell proliferation in certain breast cancers (reviewed in Peles and Yarden, 1993). Similarly, N-ethyl-N-nitrosourea-induced rat schwannomas appear to have a specific activating mutation in the transmembrane region of c-neu (erbB2) (Nikitin et al., 1991). In this context, it is interesting that Dong et al. (1999) have demonstrated that the effects of neuregulin-1 on primary mouse SCPCs need both the MAPK (mitogen-activated protein kinase) and the PI3K (phosphoinositide-3-kinase) pathways, whereas only the PI3K pathway is required for neuregulin-1 survival effects on early Schwann cells. Thus, SpL201 cells provide a potential tool to analyze the contribution of the different components of the erbB signal transduction pathways throughout the Schwann cell lineage.
Treatment of the SpL201 cells with serum/forskolin resulted in the loss of EGF dependence, suggesting differentiation of the cells to an axon-independent early Schwann cell state. Indeed, serum/forskolin treatment of cultured perinatal Schwann cells has been suggested to mimic axonal-derived signals that regulate Schwann cell development (Lemke and Chao, 1988). We found that serum/forskolin treated SpL201 cells upregulate S-100, O4, and Oct-6, mirroring the pattern observed during the differentiation of SCPC to early Schwann cells in vivo. In addition, the forskolin-mediated upregulation of these late embryonic Schwann cell markers is accompanied by an induction of the myelin genes P0 and PMP22. These findings are similar to those made with perinatal Schwann cells in vitro (Lemke and Chao, 1988; Monuki et al., 1989; Taylor et al., 1995). However, the kinetics of myelin gene induction differ between SpL201 cells and Schwann cells, and the overall expression levels are lower in the line than in primary cells (data not shown). This may be explained by the earlier developmental stage of the SpL201 cells and that additional signals may be required for these cells to attain a fully differentiated Schwann cell state.
SpL201 cells also have the functional competence to respond to axon-derived signals in that they undergo differentiation, not only to late embryonic early Schwann cells but also to fully myelinating Schwann cells. Coculture with axons of DRG neurons resulted in Schwann cell–axon interactions, the ensheathment of a proportion of the neurons, and the deposition of myelin. Although we extended the period of coculture under myelination inducing conditions (ascorbic acid) to 4 weeks in an attempt to increase myelin formation, the in vitro myelination was inefficient. Thus, we performed transplantation studies and introduced SpL201 cells directly into demyelinated lesions in adult rat spinal cord. In this paradigm, SpL201 cells showed a remarkable ability to associate with and remyelinate axons within the lesion. The cells formed a one-to-one relationship with axons, deposited a basal lamina, and the myelin formed by the transplanted cells was characteristic of Schwann cell myelin. Importantly, the SpL201 cells distributed more widely throughout the lesion and underwent more extensive differentiation into myelinating Schwann cells than is the case after transplantation of Schwann cells derived from neonatal peripheral nerve into similar lesions (Iwashita and Blakemore, 2000).
These observations bear some resemblance to previous studies of transplanting cells of the oligodendrocyte lineage into lesions in that cells of earlier stages of the oligodendrocyte lineage were capable of more extensive remyelination than those of later stages (Groves et al., 1993; Keirstead et al., 1999). Thus, immature cells appear to be more effective at transplant-mediated remyelination. It will be interesting to analyze the ability of our embryonic SCPC-derived cells to migrate through normal adult CNS to demyelinated lesions following transplantation. Furthermore, transplanting SpL201 cells into animals with genetic abnormalities that result in hypomyelination throughout the CNS will indicate the true extent of their remyelination potential, and whether transplant-mediated repair by these cells is sufficient to overcome the clinical signs associated with widespread absence of myelin.
In summary, we have generated a SCPC line that retains the potential for differentiation and myelination in vitro and in vivo. The EGF dependence of the line circumvents the inability to maintain primary SCPCs in vitro and provides a constant source of cells to study various aspects of SCPC biology. In addition, SpL201 cells represent an excellent system to analyze mechanisms that may regulate Schwann cell differentiation and myelination by cell biological, genetic, and biochemical means. The elucidation of such developmental processes is likely to have wide-reaching implications for our understanding of diseases involving aberrant myelin formation and maintenance.
Acknowledgements
The authors thank Dr. J. Trotter for providing the EGFR/neu virus and Dr. M. Cesar for introducing us to the Schwann cell neuron coculture system. We thank Dr. S.S. Scherer for assistance with the analysis of the in vitro myelination assay and helpful discussions. We are grateful to Dr. N. Mantei and Dr. L. Sommer for critical reading of the manuscript. We also thank Drs. M. Schwab, J.J. Archelos, S.S. Scherer, D.N. Meijer, and M. Chao for kindly providing reagents. This work was supported by a Myelin Project grant (to R.J.M.F.) and the Swiss National Science Foundation.