CNS axons retain their competence for myelination throughout life
Abstract
An important question relevant to developing remyelination therapies is whether axons that remain without myelin sheaths after an episode of demyelination retain myelination competence. To resolve this, we have developed a model of transplantation into the nerve fibre layer of the adult rat retina, where the axons are unmyelinated. In the adult, these axons can be myelinated by transplantation of both the oligodendrocyte progenitor cells (OPCs) and an OPC line (CG4). The extent of myelination achieved following transplantation of OPCs is the same in young adult recipients (2 months old) as that which occurs in old adult recipients (12–18 months old), indicating that there are no changes in axons remaining unmyelinated for many months that would prevent effective remyelination. This finding suggests that chronically demyelinated regions of axons such as those in seen in multiple sclerosis are likely to remain competent to be remyelinated. © 2003 Wiley-Liss, Inc.
A feature characteristic of chronic multiple sclerosis (MS) lesions is the failure of remyelination (Prineas et al., 2002). Loss of myelin may be an important cause of axonal damage contributing to the chronic progressive nature of this disease (Kornek et al., 2000; Bjartmar et al., 2003). Therapeutic strategies to enhance remyelination by increasing the number of oligodendroglial cells able to form myelin in the lesion, either by transplantation or by stimulation of endogenous cells, may therefore be beneficial in MS. These strategies obviously require that chronically demyelinated axon segments remain competent for myelination. Axons are competent for myelination during development, and also directly following demyelination in both acute experimental models of white matter injury (Bunge et al., 1961; Lampert, 1965; Blakemore, 1973; Dal Canto and Barbano, 1984), and in MS (Prineas and Connell, 1979). However, it is unknown whether axons that remain unmyelinated after an episode of demyelination retain this competence or lose it progressively with time. Any such loss would clearly limit the efficacy of remyelination strategies based on increasing cell numbers.
This important question can, in principle, be addressed by the use of transplantation. If competence for myelination is lost progressively, transplantation of myelin-forming cells into areas containing axons without myelin sheaths will produce less myelination the longer the time the axon has remained unmyelinated. However, the lack of appropriate animal models that provide consistent temporal and spatial patterns of chronic demyelination means that this is a difficult prediction to test experimentally. Transplantation of myelin-forming cells into the nerve fibre layer (NFL) of the normal retina may represent an alternative approach to this question. Axons in this layer remain unmyelinated throughout life as a consequence of the inability of oligodendrocyte precursor cells to migrate out of the optic nerve into the retina (Small et al., 1987; ffrench-Constant et al., 1988; Perry and Lund, 1990). Myelination of the NFL following transplantation of myelin-forming cells has been described previously in developing rats and mice (Huang et al., 1991; Laeng et al., 1996; Ader et al., 2000). These studies indicate that the usually unmyelinated portion of the retinal ganglion cell axon within the retina is competent for myelination at the time when these axons are being myelinated within the optic nerve. Thus, transplantation of cells into the NFL of older adult animals represents an in vivo model in which to ask whether those regions of the axons that remain unmyelinated progressively lose the competence to support the myelination process.
To establish the most effective method for delivery of cells into the adult NFL, the retinae of adult female Fischer rats were injected with fluorescent beads of 10μm in diameter (Molecular Probes, Eugene, OR). We compared a transvitreal approach, in which the needle was inserted through the corneal-scleral junction and the NFL approached through the vitreous, with a transscleral approach in which the needle was inserted through the sclera and the NFL approached through the outer retinal layers. Animals were sacrificed 3 days following transplantation, and cryostat sections were cut from injected eyes. Nuclei of the retinal layers were visualised using mounting media containing DAPI (Vector Laboratories, Burlingame, CA), and sections examined under fluorescence microscopy. Beads were more reliably delivered to the NFL with the transvitreal approach than with the transscleral approach (Fig. 1). In the latter approach, beads were often seen distant from the NFL in deeper retinal layers or behind the sclera. All subsequent transplants were therefore performed using the transvitreal approach.
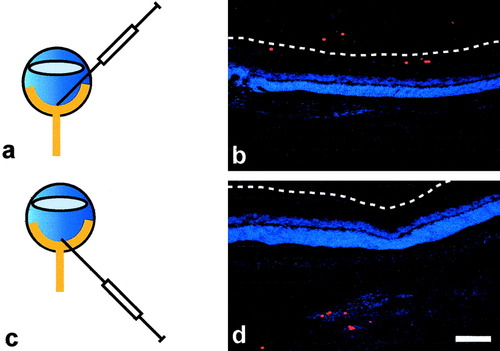
Retinal localisation of red fluorescent beads 3 days after transvitreal (a,b) or transscleral (c,d) injection; 10-μm cryostat sections were mounted in DAPI-containing media, to visualise nuclei of the retinal layers. With a transvitreal injection (a), beads were more often located within the vicinity of the nerve fibre layer (NFL) (indicated by the dashed line) (b). A transscleral approach (c) often resulted in beads located behind the NFL (d). Scale bar = 100μm.
We next established that it is possible to myelinate the adult NFL following transplantation of oligodendrocyte lineage cells. We transplanted CG-4 cells, an oligodendrocyte progenitor cell (OPC) line originally isolated from neonatal rat brains (Louis et al., 1992). These cells have been shown previously to remyelinate axons both in toxin models of demyelination (Franklin et al., 1995) and in spontaneously occurring myelin mutant rats (Tontsch et al., 1994). For transplantation, cells grown and passaged in DMEM/Sato medium supplemented with 30% B104 conditioned medium, as described previously (Louis et al., 1992) were resuspended at a density of 30,000 cells/μl; 1 μl of this suspension was injected into the NFL of three young adult (8-week-old) Fischer rats. Potential rejection of cells was averted by daily injections of Cyclosporin A (15 mg/kg, subcutaneously) beginning on the day before transplantation. Animals were sacrificed 4 weeks following transplantation by cardiac injection of pentobarbital (Pentoject, Animalcare Ltd.), and eyes were immersion-fixed in 4% glutaraldehyde for 24 h before being processed into resin. Semi-thin sections were cut and analysed by light microscopy, and selected sections were also cut for electron microscopy. In two of the three transplanted animals, myelinated axons could be identified within the NFL by light and electron microscopy (Fig. 2a,b).
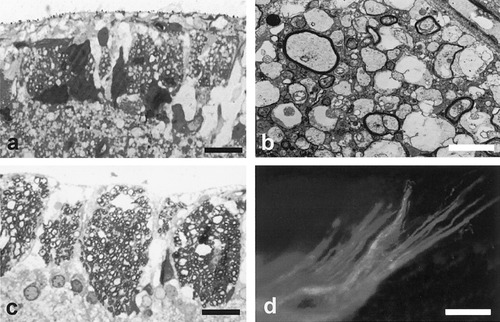
Myelination of nerve fibre layer (NFL) axons following transplantation of CG-4 cells (a,b) and primary oligodendrocyte progenitor cells (OPCs) (c,d). Occasional myelinated axons were observed following transplantation of CG-4 cells by both light microscopy (a), and electron microscopy (b) (transverse sections). Extensive myelination was seen following transplantation of primary oligodendrocyte precursor cells by light microscopy (c) (transverse section) and by immunohistochemistry with an antibody against myelin basic protein on flat-mounted retinae (d). Scale bars = 20μm in a,c; 1 μm in b; 200 μm in d.
We also examined myelination by primary (OPCs) transplanted into the NFL of young adult Fischer rats. The goals of these experiments were first, to show that primary cells as well as an immortalised cell line will myelinate adult NFL axons, and second, to demonstrate the feasibility of using cells derived from inbred rat strains so obviating the need for posttransplant immunosuppression. OPCs were obtained by mechanical dissociation from neonatal Fischer rat cortical cultures, using techniques described previously (Milner and ffrench-Constant, 1994). For transplantation, OPCs were resuspended in DMEM/Sato at 30,000 cells/μl, and 1 μl of this solution injected. The animals were sacrificed 4 weeks (n = 5) and 8 weeks (n = 5) following transplant, and the eyes were prepared for resin sectioning as described above. Alternatively, in two of the 4-week survival animals, retinae were dissected away from the sclera and were flat-mounted; immunohistochemistry was performed with an antibody against myelin basic protein. Extensive myelination of axon fascicles in the NFL was seen both in resin sections (Fig. 2C) and under fluorescent microscopy (Fig. 2D). Confocal microscopy of flat-mounts revealed areas of myelin extending radially from the point of injection close to the optic nerve head, following the path of axon fascicles toward the retinal margin.
Having demonstrated the feasibility of cell transplantation to the adult NFL as a model for studying myelination, we then asked whether the extent of myelination varied dependent on the duration of time the axons have been without a myelin sheath. In parallel with the experiments above using OPCs transplanted into 2-month-old Fischer rats, an identical population of primary OPCs was also transplanted into the NFL of much older adult (12–18-month-old) Fischer rats. The animals were sacrificed at either 4 weeks (n = 3) or 8 weeks (n = 4) posttransplantation, and the eyes were processed into resin as described above. Transplanted retinae were sectioned at 100-μm intervals throughout the myelinated area in both the young and old age groups. Myelination of the NFL was observed in both young and old animals at 4 and 8 weeks posttransplantation. In both age groups, more myelin was seen at 8 weeks than at 4 weeks following transplant. In all animals, the myelin formed a distinct patch, which was maximal at the optic nerve head, where the NFL is thickest and followed the fascicles radially toward the periphery. Near the point of injection, almost every axon within a fascicle was myelinated, although farther from the optic nerve head, the ratio of myelinated to unmyelinated axons decreased.
The results were quantified using an Microcomputer Imaging Device (Imaging Research Inc.) image analysis system, with a point grid overlaid on the myelin containing sections, and the numbers of points falling on myelinated axons counted. For each retina, a volume of myelination was estimated using the Cavalieri method (Howard and Reed, 1998), with the estimated volume of myelin being equal to the number of points counted × area associated with each point × distance between sections. There was no significant difference in the mean volume of myelination between the different age groups at either 4 or 8 weeks posttransplantation (Fig. 3).
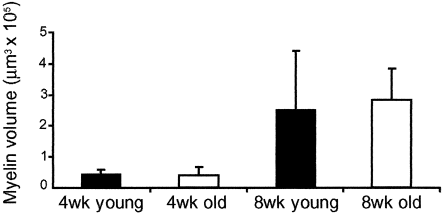
Mean volume of myelination following transplantation of primary oligodendrocyte precursor cells (OPCs) into young adult (2-month-old) and old adult (12–18-month-old) female Fischer rats at 4- and 8-week survival times. In both age groups, more extensive myelination occurred at 8 weeks survival. Statistical analysis of the means was performed with the Mann-Whitney test. There was no significant difference in the mean volume of myelination between the two age groups at either survival time. Young adult: 4 week, n = 3, 8 week, n = 5; old adult: 4 week, n = 3; 8 week, n = 4.
In this study, we present a novel in vivo model for the study of myelination biology with which it is possible to study the myelination of long-term unmyelinated axons. In an extension to the work of others in neonatal animals (Huang et al., 1991; Laeng et al., 1996; Ader et al., 2000), we show that a transplant can be delivered effectively to the NFL of the adult rat retina, and also that widespread myelination of the adult NFL is observed following transplantation of myelinating cells. These results indicate that, despite remaining unmyelinated into adulthood, the axons of the adult rat NFL remain capable of supporting myelination. Our results also demonstrate no difference between mean myelin volume in the NFL of young or old adult animals.
This indicates that the competence of a retinal ganglion cell axon to be myelinated is independent of its age and shows that cell surface or secreted axonal factors that promote myelination remain throughout life, while factors that inhibit myelination are not expressed with time. In support of this, we have found that polysialylated–neuronal cell adhesion molecule (PSA-NCAM) and jagged, two axonal cell surface molecules shown to inhibit myelination (Oumesmar et al., 1995; Charles et al., 2000; Givogri et al., 2002) and implicated in the persistent demyelination within MS lesions (Charles et al., 2002; John et al., 2002) are not expressed on the surface of adult NFL axons (unpublished observations). It has been reported that chronic MS lesions contain pre-myelinating oligodendrocytes (Chang et al., 2002); our results suggest that the failure of these cells to progress to a myelinating phenotype is not a consequence of the length of time the axons have remained unmyelinated. Although the extent to which the axolemma of an unmyelinated axon differs from that of a chronically demyelinated axon is an important issue requiring further investigation, we conclude from this work that there are no changes in portions of axons remaining unmyelinated for many months that would prevent effective remyelination. This suggests that chronically demyelinated regions of axons such as those in seen in MS are likely to remain competent to be remyelinated, and that therapeutic strategies designed to promote remyelination need not be hindered by an inevitable decrease in chronically demyelinated axons to support myelination.
Acknowledgements
The authors thank Dr. Jeremy Skepper for his help with quantification of myelination. This work was funded by a grant from the Multiple Sclerosis Society of Great Britain and Northern Ireland (to C.ff.C. and R.J.M.F.) and by a supporting studentship (to A.S.), by the Medical Research Council, and by a grant from Research into Aging (R.J.M.F.). C.ff.C. holds a Wellcome Trust Research Leave Fellowship for clinical academics.