cAMP-dependent reorganization of the Cajal bodies and splicing machinery in cultured Schwann cells
Abstract
It is well established that forskolin-induced elevation of cAMP results in activation of DNA synthesis in Schwann cell cultures. This promitotic response is partially mediated by the Cdk2, which is required for the transition from the G1 to the S phase of the cell cycle. In the present study, we analyze the effects of cAMP elevation in cultured Schwann cells on the transcriptional activity and on the organization of two nuclear compartments involved in pre-mRNA processing: Cajal bodies (CBs) and splicing factor compartments. Our immunofluorescence and quantitative studies show that forskolin treatment induces a 5.6-fold increase in the proportion of S phase Schwann cells, detected by a short pulse (20 min) of BrdU incorporation. This increase in DNA synthesis correlates with an activation of global transcription, as is indicated by the higher nuclear incorporation of BrU in nascent RNA. Forskolin treatment significantly increases the percentage of Schwann cells containing typical CBs, which concentrate spliceosomal snRNPs and the survival motor neuron (SMN) protein. This increase in the number of CBs closely correlates with the activation of transcription. Moreover, the occurrence of CBs is significantly higher in BrdU (+) cells than in BrdU (−) cells, indicating that entry in the S phase promotes the formation of CBs. During the S phase, Schwann cell nuclei display higher Cdk2 nuclear staining and concentrate this kinase in CBs. Forskolin also induces a redistribution of the pre-mRNA splicing factors in Schwann cells. Primary cultures of Schwann cells provide an excellent physiological model to demonstrate that the assembly of CBs is a transcription- and replication-dependent cellular event. Moreover, the S phase accumulation of Cdk2 observed in Schwann cells supports a functional link between CBs and DNA replication, which is mediated by the possible participation of CBs in the regulatory control of histone gene expression. GLIA 40:378–388, 2002. © 2002 Wiley-Liss, Inc.
INTRODUCTION
Schwann cells are glial cells of peripheral nerves that include two subtypes: the myelinating Schwann cell, which forms the myelin sheath around the single large diameter axon, and the nonmyelinating Schwann cell, which encloses several small-diameter axons. Schwann cells generate from the neural crest. Their development involves three main stages: the transition of crest cells to precursors, the transition of precursors to immature cells and, finally, the maturation of two Schwann cell subtypes (for review, see Jessen and Mirsky, 1999). In rodents, immature Schwann cells are present at birth. Thus, during the perinatal period, they can be isolated from the sciatic nerve and cultured. The proliferation and differentiation of immature Schwann cells is mediated by the adenyl cyclase-cyclic adenosine monophosphate (cAMP) second-messenger pathway, which synergistically potentiates the effect of several Schwann cell mitogens, such as platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), and IGF (for review, see Morgan et al., 1991; Stewart et al., 1991; Dong et al., 1997; Bermingham et al., 2001). In Schwann cell cultures, the elevation of cAMP induced by the adenylate cyclase activator forskolin (Seamon and Daly, 1986) mimics the effects of the axon-associated mitogens resulting in an activation of DNA synthesis (for review, see Morgan et al., 1991). Recent studies in Schwann cell cultures exposed to PDGF have demonstrated that the promitotic response to cAMP is mediated by the up regulation of cyclin D1, which is required for the progression through the G1 phase of the cell cycle (Kim et al., 2001). Moreover, the control of the cell cycle in Schwann cells appears to be regulated by the cyclin-dependent kinase-2 (Cdk2), an essential enzyme for the transition from the G1 to S phase (for review, see Ekholm and Reed, 2000). Levels of Cdk2 protein and mRNA increase in proliferating Schwann cells cultured in serum and forskolin, while the expression of Cdk2 declines dramatically during cell cycle arrest (Tikoo et al., 2000). All these data indicate that the transition from the G1 to S phase represents a critical cellular event of Schwann cell growth control.
In the present study, we analyze the effects of cAMP elevation in cultured Schwann cells on the organization of two nuclear compartments involved in pre-mRNA processing: the Cajal body (CB) and splicing factor compartments (Matera, 1999; Mintz et al., 1999; Gall, 2000). The CB is a nuclear organelle discovered by Cajal (1903) in several neuronal types, using silver staining procedures. Cajal bodies are transcription-dependent nuclear organelles that concentrate molecular constituents involved in the processing of three categories of nuclear RNA: pre-mRNA, pre-rRNA, and histone pre-mRNA. The main molecular components of the CB are the specific marker coilin; the spliceosomal small nuclear ribonucleoproteins (snRNPs), the U7 snRNA involved in histone pre-mRNA processing, the survival motor neuron (SMN) complex implicated in the biogenesis pathway of the snRNPs, and the nucleolar protein fibrillarin required for pre-rRNA processing (Liu et al., 1996; Carvalho et al., 1999; Matera, 1999; Gall, 2000; Pena et al., 2001; Carmo-Fonseca, 2002).
Concerning the splicing factor compartments, they are involved in the assembly of pre-mRNA splicing machinery into splicing-competent particles that are to be transferred to active sites of transcription and splicing (Spector, 1993; Sacco-Bubulya and Spector, 2002). The stored factors in the splicing factor compartments include spliceosomal snRNPs and non-snRNP splicing factors, such as the splicing regulatory (SR) proteins (for review, see Spector, 1993; Misteli et al., 1997; Mintz et al., 1999).
In the studies described in the present report, using in situ detection of nascent RNA transcripts, we show that forskolin treatment induces an overall transcriptional activation, concomitantly with the activation of DNA synthesis detected by the increase of BrdU incorporation. This cellular activation of the Schwann cells is associated with the progression through the G1-S phase of the cell cycle and involves a significant increase in the number of CBs, a transient expression of Cdk2 in CBs and a reorganization of the areas of splicing factors. This response to cAMP seems to reflect the accommodation of the pre-mRNA splicing machinery to the increased requirements of pre-mRNA splicing produced by transcriptional activation in Schwann cells.
MATERIAL AND METHODS
Culture of Schwann Cells and Treatments
Schwann cells were prepared from sciatic nerves of 3-day-old Sprague-Dawley rats. Schwann cell cultures were performed as described by Brockes et al. (1979) and maintained in DMEM supplemented with 10% fetal bovine serum (FBS) for 24 h. Cytosine arabinoside was added to the culture medium and was maintained for 48 h to obtain highly purified Schwann cell cultures. On the fourth day, Schwann cells were plated on glass coverslips coated with poly-L-lysine (Morgan et al., 1991). Cells were cultured in 1:1 DMEM and Ham's F12 medium containing 10% FBS. In experimental cultures, 2 μM forskolin (Sigma) was added to the medium. For S phase studies, bromodeoxyuridine (BrdU) was added to the medium at a concentration of 2.5 × 10−5 M for 20 min. The cells were then washed, fixed, and processed by immunofluorescence with the anti-BrdU antibody. For in situ detection of newly transcribed RNA, Schwann cells grown on glass coverslips were incubated for 1 h in culture medium containing 5 mM bromouridine (BrU, Sigma-Aldrich), washed three times with phosphate-buffered saline (PBS), fixed, and processed by immunofluorescence with the anti-BrU monoclonal antibody. RNase treatments were performed before immunofluorescence labeling at room temperature in PBS containing 200 μg/ml RNase A (Roche) for 30 min (Pellizzoni et al., 2001).
Antibodies
Antibodies used in these experiments were as follows: mouse monoclonal anti-SMN (2B1; Liu and Dreyfuss, 1996); mouse monoclonal anti-BrU (Sigma-Aldrich); mouse monoclonal anti-BrdU (Amersham) and anti-BrdU-Fluorescein (Roche); mouse monoclonal 4G3 directed against the U2B″ snRNP (Euro Diagnostica, the Netherlands); rabbit polyclonal serum 204.3 anti-coilin (Bohmann et al., 1995); rabbit polyclonal serum anti-histone H4 acetylated (Chemicon); rabbit polyserum anti-Cdk2 (Santa Cruz Laboratories); and a human serum C45 (kindly provided by W. van Venrooij) that recognizes the Sm complex of the spliceosomal snRNPs.
Immunofluorescence
Cells grown on glass coverslips were washed with PBS, fixed in 3.7% paraformaldehyde in PBS, pH 7.4, for 12 min at room temperature, and permeabilized with 0.5% Triton-X-100 for 20 min. Alternatively, cells were fixed in methanol for 10 min at −20°C. Cells were blocked in PBS containing 3% bovine serum albumin (BSA) for 30 min at room temperature. For double-labeling experiments, the two primary antibodies were incubated sequentially, followed by incubation with its specific secondary antibody coupled to FITC or Texas Red. The samples were mounted with the antifading Vectashield (Vector, Burlingame, CA). Samples were examined with a laser confocal microscope (Bio-Rad MRC-1024), using argon ion (488-nm) and HeNe (543-nm) lasers to excite FITC and TxR, respectively. Images from each channel were recorded independently and then merged.
For BrdU incorporation studies, cells were incubated for 20 min in a culture medium containing BrdU and were washed in PBS, fixed in 3.7% paraformaldehyde in PBS, and treated with 2 N HCl for 20 min at 37°C to denature DNA, followed by treatment with 0.1 M sodium borate buffer, pH 8.5, for 10 min. After washing in PBS, the cells were processed for immunofluorescence with the anti-BrdU antibody. Double labeling experiments combining BrdU detection with either coilin or Cdk2 were also performed.
Statistical analysis of the proportion of BrdU-positive and -negative cells and of the percentage of cells containing CBs was performed with the Statview program (Abacus Concepts). Quantitative results are the mean values resulting from a minimum of three separate experiments.
RESULTS
Transcriptional Activation Induced by Elevation of cAMP Can Be Detected In Situ and Correlates With the Increase in DNA Synthesis
We have used a simple procedure for in situ detection of nascent transcripts in Schwann cells using the addition of 5 mM BrU to the culture medium for 1 h, followed by fixation and detection with anti-BrU antibodies (Koberna et al., 2000; Pellizzoni et al., 2001). Schwann cells treated with forskolin for 24 h show a higher level of transcription than those cultured in a forskolin-free medium (Fig. 1A,B). Diffuse labeling of variable intensity is observed through the nucleoplasm. Also noteworthy is the strong staining of the nucleolus, which is not surprising because it represents the most active transcription site in the cell nucleus (Fig. 1B,F). Incorporation of BrU into nascent RNA was clearly demonstrated by the sensitivity of the staining to RNase digestion (Fig. 1C). As a quantitative approach to estimate the transcription level in the Schwann cell population, we counted the cells that displayed low (Fig. 1D), moderate (Fig. 1E), and high (Fig. 1F) intensity of BrU labeling. The proportion of cells with high and moderate intensity staining increased from 29% to 49% after forskolin treatment (Table 1).
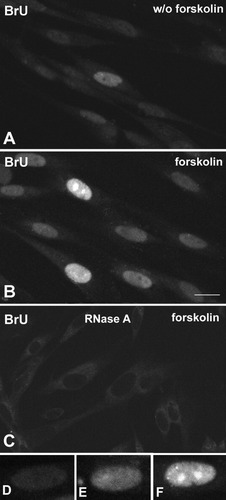
Indirect immunofluorescence detection of nascent RNA by laser confocal microscopy using anti-BrU antibodies on Schwann cells labeled in the presence of 5 mM BrU. A: Without forskolin treatment. B: Cells treated with 2 μM forskolin. Forskolin-treated cells exhibit higher nuclear staining than untreated cells. C: Effect of RNase treatment before immunostaining. Note the absence of nuclear staining. D–F: Representative examples of the different intensity levels of BrU incorporation estimated for the quantitative analysis: low, BrU+ (D), moderate, BrU++ (E), and high, BrU+++ (F). B,F: Strong BrU staining of the nucleoli. See Table 1. Scale bar = 10 μm.
| Schwann cells | Without forskolin (%) | With forskolin (%) |
|---|---|---|
| BrU+ | 71 | 51 |
| BrU++ | 13 | 24 |
| BrU+++ | 16 | 25 |
- BrU, bromouridine.
- * χ2 P < 0.01.
The quantitative analysis of BrdU-positive Schwann cells showed that the forskolin-induced activation of transcription was accompanied by a 5.6-fold increase in the proportion of cells in the S phase of the cell cycle, detected by a short pulse (20 min) of BrdU incorporation (5%, in forskolin-untreated cells vs 28%, in forskolin-treated cells; P < 0.001). Early and late S-phase Schwann cells were clearly identified by the different pattern of BrdU staining (Hozak et al., 1994; Wei et al., 1998). Thus, early S-phase cells showed a diffuse BrdU staining, corresponding to the replication of the euchromatin, while late S-phase cells exhibited large nuclear foci of BrdU staining, which coincides with the replication of heterochromatin masses at the nuclear periphery (Fig. 2).
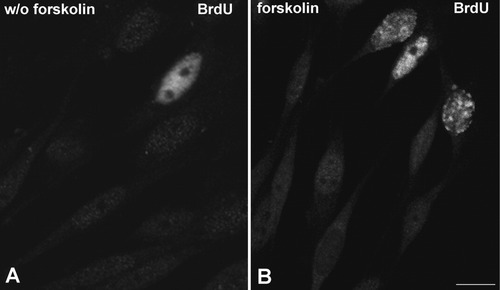
Indirect immunofluorescence detection of BrdU incorporation after a short pulse of 20 min in forskolin-untreated (A) and -treated Schwann cells (B). Forskolin induces the entry of Schwann cells in the S phase. B: Three different stages of the S phase. Scale bar = 10μM.
Elevation of cAMP Induces a Proliferation of Cajal Bodies in Schwann Cells
The immunofluorescence study with an antibody that recognizes the CB marker coilin revealed this nuclear organelle in some Schwann cell nuclei (Fig. 3A). Double immunolabeling experiments demonstrated that coilin colocalizes with both the Sm protein complex of the snRNPs and with the SMN protein in CBs (Fig. 3A–C). This finding indicates that the CBs observed in cultured Schwann cells concentrate typical splicing factors found in mature CBs from other cell types (Matera, 1999; Gall, 2000; Sleeman et al., 2001).

Double-label laser confocal microscopy using anti-coilin (A) and anti-SMN (B) antibodies on a Schwann cell. Both molecular components colocalize in the CB (arrowheads). C: Immunofluorescence illustrating the concentration of the Sm complex of spliceosomal snRNPs in a bright nuclear focus that corresponds to a CB. Scale bar = 10 μm.
Quantitative analysis of CBs in coilin immunostaining samples revealed that forskolin induces a twofold increase in the percentage of cells containing CBs in comparison with the untreated cells. Moreover, forskolin induced an increase in the proportion of cells containing more than one CB (Table 2). To establish whether the increase in the number of CBs might be related to the entry of Schwann cells in the S phase of cell cycle, double labeling experiments of BrdU incorporation (20 min) and coilin immunostaining were performed. Figure 5A–C illustrates the presence of CBs in BrdU-immunostained cells. In addition, quantitative analysis demonstrated a higher percentage of cells containing CBs in BrdU (+) cells, in both forskolin-exposed and -untreated cultures, than in BrdU (−) cells, indicating that the entry in the S phase promotes the formation of CBs (Table 3).
| % of cells with CBs | ||||
|---|---|---|---|---|
| CBs | 1 CB | 2 CBs | >2 CBs | |
| Without forskolin | 30 | 20 | 8 | 3 |
| With forskolin | 60 | 26 | 19 | 15 |
- CBs, Cajal bodies.
- * χ2 (P < 0.001). The proportion of cells containing CBs, and the percentage of cells containing two or more CBs per nucleus, significantly increases after forskolin treatment.
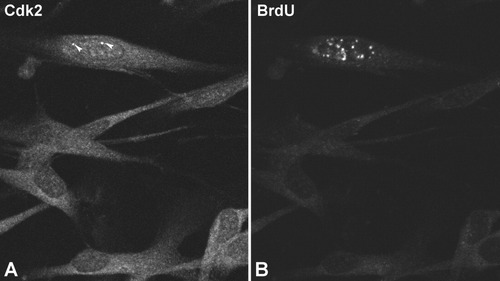
Double-label laser confocal microscopy using anti-Cdk2 (A) and anti-BrdU (B) antibodies on Schwann cells incubated for 20 min with BrdU. The BrdU (+) cell in a late S phase exhibits higher nuclear Cdk2 staining than the BrdU (−) cells. In addition, the BrdU (+) cell shows two intensely fluorescent nuclear foci (arrowheads, A). Scale bar = 10 μm.
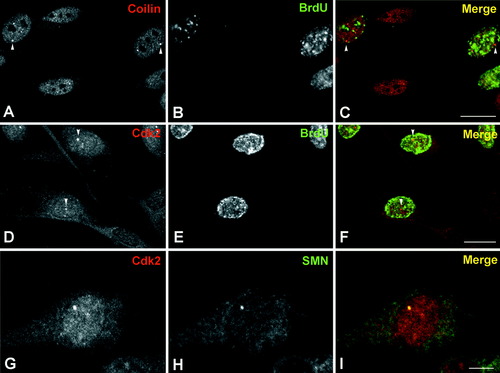
Double labeling experiments using anti-coilin and anti-BrdU (A–C), anti-Cdk2 and anti-BrdU (D–F), and anti-Cdk2 and anti-SMN (G–I) on forskolin-treated Schwann cells. A–C: Typical CBs stained with the anti-coilin antibody (arrowheads) appear in BrdU (+) cell nuclei. D–F: Similarly the Cdk2-positive nuclear foci are also localized in BrdU (+) cells. Note in D and E the higher Cdk2 nuclear staining of the BrdU (+) cells in comparison with the BrdU (−) cells. G,H: Double-label immunofluorescence illustrating colocalization of Cdk2 and SMN in a CB. Scale bar = 10 μm.
| % of cells with CBs | ||||||
|---|---|---|---|---|---|---|
| BrdU+ | BrdU− | |||||
| CBs | 1 CB | ≥2CBs | CBs | 1 CBs | ≥2CBs | |
| Without forskolin | 60 | 44 | 16 | 18 | 16 | 2 |
| With forskolin | 63 | 43 | 20 | 33 | 28 | 5 |
- CBs, Cajal bodies; BrdU, bromodeoxyuridine.
- * χ2 (P < 0.001). The proportion of cells containing CBs, and the percentage of cells containing one or more CB per nucleus, is significantly higher in BrdU (+) cells.
Having established that forskolin induces a replication-dependent formation of CBs, we then investigated whether the cellular distribution of these nuclear organelles was related to the transcription rate detected in situ by BrU uptake. Double labeling experiments of BrU and coilin immunostaining on forskolin-exposed cells (Fig. 6A–C) showed that CBs were visible in more than 80% of cells with a high intensity of BrU staining. In contrast, CBs appeared in less than 40% of cells with low intensity of BrU staining, indicating a transcription-dependent formation of CBs in response to cAMP elevation.
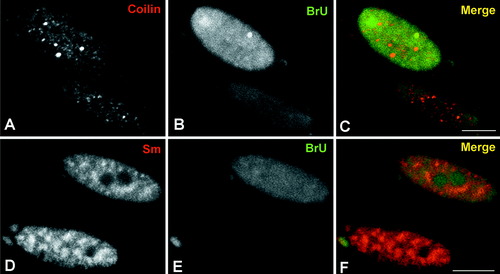
Double labeling experiments using anti-coilin and anti-BrU antibodies (A–C), and anti-Sm and anti-BrU antibodies (D–F) on forskolin-treated Schwann cells. A–C: The cell showing a higher level of BrU incorporation in the nascent RNA contains several CBs immunostained with the anti-coilin antibody. D,E: Similarly the cell with the higher transcriptional activity (BrU incorporation) exhibits a more diffuse distribution of the snRNP splicing factors detected with the anti-Sm antibody. Scale bar = 10 μm.
S Phase in Schwann Cells Increases the Nuclear Expression of Cdk2 and Induces Its Localization in Cajal Bodies
Since previous biochemical studies have demonstrated a forskolin-induced upregulation of both protein and mRNA Cdk2 in cultured Schwann cells (Tikoo et al., 2000), we next investigated the immunocytochemical distribution of this kinase during the transition from G1 to S phase. In Schwann cell cultures, Cdk2 showed a diffuse cytoplasmic staining of moderate intensity (Fig. 4A). At the nuclear level, Schwann cells with moderate and weak Cdk2 expression were clearly identified in both forskolin-treated and -untreated cultures. In Schwann cells displaying higher nuclear immunostaining, Cdk2 was diffusely distributed throughout the nucleoplasm, excluding nucleoli, in addition to being concentrated in small nuclear foci (Figs. 4A, 5D,G). These nuclear foci never appeared in Schwann cells exhibiting low nuclear staining for this kinase (Fig. 4A).
To determine the relationship between the nuclear expression of Cdk2 and the S phase of the cell cycle in Schwann cells, we performed double labeling experiments of BrdU incorporation (20 min) and Cdk2 immunostaining. As expected, BrdU incorporation was detected only in cells that showed higher nuclear expression of Cdk2 (Fig. 5D,F). Furthermore, the presence of nuclear foci enriched in this kinase was restricted to BrdU (+) cells (Figs. 4A,B, 5D–F). Double immunostaining experiments revealed that the nuclear foci enriched in Cdk2 are CBs in which this kinase colocalizes with the SMN protein (Fig. 5G–I).
Forskolin Induces Reorganization of the Splicing Factor Compartments
To investigate the dynamics of the splicing factor compartments, also called “nuclear speckles,” in response to forskolin-induced metabolic changes we analyzed the distribution of pre-mRNA splicing factors by immunofluorescence. We used antibodies that recognize the Sm complex of the spliceosomal snRNPs and the U2B″ protein, which is specifically associated with the U2 snRNP. With both antibodies, the splicing factors appeared concentrated in several nuclear speckles, in addition to being diffusely distributed throughout the nucleoplasm, excluding the unstained nucleolus (Figs. 6D, 7C). However, forskolin treatment induced a moderate reorganization of splicing factors in Schwann cells. Thus, in the absence of forskolin, nuclear speckles appear well defined and intensely stained, while splicing factors tend to be more diffusely distributed in the nucleoplasm and aggregated in smaller speckles in cells exposed to the drug (Fig. 7A,C). Moreover, double labeling experiments to detect splicing factors and BrU or H4 acetylated histone revealed that the change in the distribution pattern of the splicing machinery occurs in actively transcribing cells. Thus, a reorganization of nuclear speckles was visible in Schwann cells having a high expression level of acetylated H4 histone (Fig. 7), which is associated with active chromatin domains (Struhl, 1998), and a moderate or high level of BrU incorporation (Fig. 6D–F).
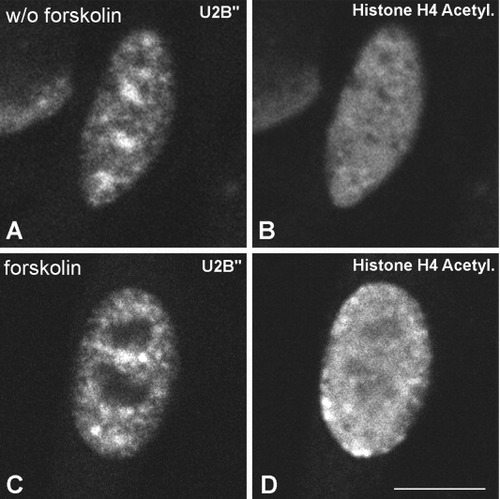
Double labeling experiments using anti-U2B″, which recognizes the spliceosomal snRNPs, (A and C) and anti-histone H4 acetylated (B,D) in forskolin-treated (C,D) and -untreated (A,B) Schwann cells. A,B: Without forskolin this cell nucleus shows three prominent nuclear speckles of U2B″ protein and low nuclear expression of acetylated H4 histone. C,D: After forskolin treatment, this cell nucleus exhibits a more diffuse distribution of U2B″ staining with smaller nuclear speckles, whereas the expression level of acetylated H4 histone is higher than that observed in untreated cells. D: Accumulation of this acetylated histone at the nuclear periphery. Scale bar = 10 μm.
DISCUSSION
Our results indicate that the well-known effects of cAMP elevation on the DNA synthesis in Schwann cells cultured in the presence of serum (Raff et al., 1978; Sobue et al., 1986; Porter et al., 1986; Davis and Stroobant, 1990; Eccleston et al., 1990; Morgan et al., 1991) are associated with an overall activation of transcription detected in situ by the increase of BrU incorporation in nascent transcripts. The stimulation of both DNA and RNA synthesis in the Schwann cell cultures seems to be related to the onset of the G1/S transition of the cell cycle and involves proliferation of CBs, nuclear expression of Cdk2 and reorganization of the splicing factor compartments.
The use of the BrU incorporation method to estimate the transcription rate in situ has been recently developed (Koberna et al., 2000; Pellizzoni et al., 2001). Unlike the current methods that required microinjection of BrU triphosphate or the use of permeabilized cells (Jackson et al., 1993), the simple addition of BrU allows its efficient incorporation into nascent transcripts and shows greater labeling in the nucleolus, the highest transcription compartment of the cell nucleus. The sensitivity of the BrU immunofluorescence to RNase treatment observed in the present study in Schwann cells and previously in HeLa cells (Pellizzoni et al., 2001) demonstrates that BrU is incorporated into RNA.
The overall increase in the transcription rate of Schwann cells reported here may result from the forskolin-induced elevation of cAMP. In most mammalian cells forskolin promotes growth arrest and cell differentiation, by cAMP inhibition of ras-dependent activation of raf (Cook and McCormick, 1993). However, in Schwann cells, forskolin activates the expression of growth factor receptors (Weinmaster and Lemke, 1990) and initiates and sustains the progression through the G1/S transition of the cell cycle (Kim et al., 2001). This peculiar relationship between cAMP and cell proliferation in Schwann cells provides the opportunity to study the nuclear changes associated with the promitotic response to cAMP. Forskolin is a co-mitogen for several growth factors, including PDGF, bFGF, IGF, and Reg-1 (Davis and Stroobant, 1990; Stewart et al., 1991; Livesey et al., 1997); it dramatically upregulates the expression of the PDGF receptor (Weinmaster and Lemke, 1990). Activation of transcription is also required for the increase in cell mass, which is especially important in controlling the progression through the G1 checkpoint to entry into the S phase (Mitchison, 1971; Polymenis and Schmidt, 1999; Stocker and Hafen, 2000). In the case of Schwann cells, Conlon et al. (2001) have proposed that extracellular growth signals, such as the IGF-I, control cell size by increasing protein synthesis and decreasing protein degradation.
In this context, the observed increase in both the proportion of cells containing CBs and the number of CBs per cell in Schwann cells in response to cAMP elevation clearly correlates to the stimulation of transcription. In fact, our previous experimental studies in neuronal models have demonstrated that the sustained upregulation of transcription in supraoptic neurons induces a significant increase in the number of CBs (Lafarga et al., 1991). In contrast, the inhibition of transcription with actinomycin D treatment or cellular stress disassembles CBs (Carmo-Fonseca et al., 1992; Lafarga et al., 1998). Similarly, a positive correlation between cell mass and number of CBs has been established in diploid trigeminal neurons (Pena et al., 2001). Regarding peripheral nerve Schwann cells, it is noteworthy that in pathological conditions we have previously reported (Berciano et al., 1996) the induction of CBs in reactive Schwann cells of the acute Guillain-Barré syndrome, a neuropathological process characterized by severe segmental demyelination (Arnason and Soliven, 1993). In agreement with the present results, these reactive Schwann cells show cytological features of enhanced transcription, such as an increase in nuclear size and chromatin decondensation, presumably related to the onset of a regenerative process.
Concerning the functional significance of the increase in the number of CBs in Schwann cells, it may reflect a cellular response to the elevated demand of mature pre-mRNA splicing factors induced by the transcriptional activation. In fact, in addition to coilin, the CBs found in Schwann cells concentrate spliceosomal snRNPs and the SMN protein, which are essential factors for the splicing of pre-mRNA (for review, see Gall, 2000; Mourelatos et al., 2001). Although the CBs are not sites of splicing that occurs co-transcriptionally at the transcription sites (for review, see Proudfoot, 2000), they represent a fundamental nuclear compartment in which some maturing steps of spliceosomal snRNPs take place, before their distribution to splicing sites (Carvalho et al., 1999; Sleeman et al, 2001). In conclusion, the proliferation of CBs in response to cAMP elevation may increase the efficiency in the supply of mature spliceosomal snRNPs to sites of splicing under conditions of transcriptional activation.
Also of note is the increase in the number of CBs in Schwann cells undergoing the S phase of cell cycle. Our results show that both the proportion of cells containing CBs and the number of these organelles per cell increase in BrdU (+) Schwann cells after a short pulse (20 min) of BrdU incorporation, which primarily detects cells in the S phase (Bravo and MacDonald-Bravo, 1987). The presence of CBs during the S phase of the cell cycle has also been reported in cultured HeLa cells (Andrade et al., 1993; Jordan et al., 1997; Liu et al., 2000). The higher frequency of CBs reported in the present study in Schwann cells undergoing the S phase correlates with the nuclear overexpression of Cdk2 and its accumulation in CBs, and may be associated with the particular transcription events that occur during this phase of the cell cycle.
Cdk2 is an essential enzyme for the transition from the G1 to the S phase (for review, see Ekholm and Reed, 2000). In the case of Schwann cells, the levels of Cdk2 protein and mRNA increase in proliferating Schwann cells cultured in serum and forskolin, while the expression of Cdk2 declines dramatically during cell cycle arrest (Tikoo et al., 2000). Interestingly, recent studies in HeLa cells have shown a cell cycle-dependent localization of Cdk2 and cyclin E in CBs during the G1/S transition and early S phase (Liu et al., 2000). Biochemical studies have demonstrated that the cyclin E/Cdk2 complex phosphorylates the protein p220, an activator of the histone H2B promotor, which is also concentrated in CBs (Ma et al., 2000). On the basis of these data, it has been proposed that the complex cyclinE/Cdk2, in cooperation with the protein p220, coordinates the S-phase-specific activation of histone gene transcription (Zhao et al., 2000; Ma et al., 2000).
The link between the transcription of histone genes and CBs has emerged from the observation that a subset of CBs are physically associated with histone gene clusters on human chromosomes 1 and 6, and also with the histone loci of Xenopus lampbrush chromosomes (Frey and Matera, 1995; Schul et al., 1999; Abbott et al., 1999). In this context, the increase in the number of CBs, and also the specific accumulation of Cdk2 in these nuclear organelles during the S phase, might be related to the activation of histone gene transcription in Schwann cells and provide additional support to the view that CBs play an important role in the regulation of transcription during the S phase (Liu et al., 2000; Ma et al., 2000).
Our immunofluorescence studies of the distribution of snRNP splicing factors demonstrated that forskolin also induces a reorganization of the splicing factor compartments, which closely correlates with the activation of transcription. These splicing factors reorganize in smaller speckles, in addition to being more diffusely distributed in the nucleoplasm, in Schwann cells with a high level of BrU incorporation. It is well known that the splicing factor compartment participates in the assembly and storage of pre-mRNA splicing factors (for review, see Spector, 1993; Lamond and Earnshaw, 1998; Mintz et al., 1999). Our results are consistent with previous experimental data indicating that splicing factors are predominantly dispersed in actively transcribing cells, indicating that a great proportion of splicing factors are transported from the splicing factor compartments to sites of active transcription and splicing (Spector, 1993; Zeng et al., 1997; Misteli et al., 1997). In contrast, we have previously reported that the inhibition of transcription in Schwann cells undergoing cell death redistribute the splicing factors in a few very large compartments (Berciano et al., 1999).
In conclusion, our results indicate that the forskolin-induced activation of DNA synthesis in cultured Schwann cells is associated with a transcriptional activation visualized in situ by the incorporation of BrU in nascent RNA. These metabolic changes appear to be required for the G1/S transition and the S phase progression in Schwann cells and are associated with a dynamic reorganization of two nuclear compartments, CBs and nuclear speckles, involved in pre-mRNA processing. The primary culture of Schwann cells provides an excellent physiological model to demonstrate that the nuclear assembly of CBs is a transcription- and replication-dependent cellular event. Moreover, the S phase accumulation of Cdk2 observed in Schwann cells supports a functional link between CBs and DNA replication, which is mediated by the possible participation of CBs in the regulatory control of histone gene expression. Since an activation of transcription is required for the G1/S progression and both Cajal bodies and nuclear speckles are transcription-dependent nuclear organelles (Carmo-Fonseca et al., 1992; Zeng et al., 1997; Lafarga et al., 1998; Polymenis and Schmidt, 1999), the nuclear reorganization reported during the G1/S transition may reflect a general cellular response to cellular activation, independent of the signaling pathway that induces the promitotic response.
Acknowledgements
The authors thank Mrs. Raquel Garcia Ceballos for technical support. We also thank Professor G. Dreyfuss (University of Pennsylvania) for generously providing 2B1 (Anti-SMN) antibody, and Professor Angus I. Lamond (University of Dundee, UK) for anti-coilin rabbit serum 204.3.