Trafficking of PLP/DM20 and cAMP signaling in immortalized jimpy oligodendrocytes
Abstract
The synthesis, transport, and insertion of jimpy proteolipid protein and DM20 were studied in normal (158N) and jimpy (158JP) immortalized oligodendrocyte lines. Four different expression vectors encoding fusion proteins composed of native PLP and DM20 or jimpy PLP or DM20 were linked to enhanced green fluorescent protein (EGFP). All four transfected fusion proteins had similar distributions in the cell bodies and processes of the two cell types. Both normal and jimpy PLP-EGFP and DM20-EGFP were detected in both cell lines as far as 200 μm from the cell body, indicating synthesis and transport of mutated PLP and DM20 toward the plasma membrane. Immunocytochemistry of fixed normal and jimpy cells with the O10 antibody, which recognizes a conformationally sensitive PLP/DM20 epitope, confirmed that normal and jimpy PLP and DM20 were transported to the plasma membrane. Live staining of normal and jimpy cells transiently transfected with the native PLP showed positive staining, indicating PLP was correctly inserted into the membrane of both normal and jimpy oligodendrocytes. However, live staining of normal and jimpy cells transiently transfected with jimpy PLP showed no positive staining, indicating the mutated protein is abnormally inserted into the plasma membrane. Electrophysiological recordings of the resting membrane potential measured in the whole cell mode of the patch-clamp technique showed the absence of a developmentally regulated negative shift in the membrane potential in jimpy cells compared to normal native or immortalized oligodendrocytes. Treatment of 158N cells and native oligodendrocytes with dibutyryl cAMP (dbcAMP) caused morphological and biochemical differentiation, but failed to do so in 158JP cells, suggesting an abnormal signaling pathway in jimpy. The defect in cAMP signaling in jimpy oligodendrocytes was associated with the suppression of increase in mRNA level of the inducible cAMP early repressor (ICER). When the jimpy oligodendrocyte line was transfected with normal PLP or DM20 and exposed to dbcAMP, the cells failed to differentiate. This finding suggests that improper insertion of jimpy protein into the plasma membrane alters the membrane in such a way that certain signaling pathways are permanently altered. The abnormal insertion of jimpy PLP/DM20 into the plasma membrane may be the basis for the lack of cell signaling and abnormal resting potential in jimpy oligodendrocytes. GLIA 40:300–311, 2002. © 2002 Wiley-Liss, Inc.
INTRODUCTION
Jimpy mutant mice are characterized by severe hypomyelination in the central nervous system (CNS) (Skoff and Knapp, 1992; Campagnoni and Skoff, 2001). The mutation in PLP/DM20 gene produces a deletion in exon 5 and a modification in the C-terminus sequence of the jimpy PLP and DM20 proteins (Nave et al., 1986; Macklin et al., 1987). PLP and DM20, the most abundant proteins of myelin in the CNS, play a structural role in myelin sheath stability but other biological functions in the nervous system have been proposed (Knapp, 1996; Nadon and West, 1998; Yamada et al., 1999). However, it remains unclear how the jimpy PLP/DM20 affects a wide range of abnormalities in oligodendrocytes and in other cell types (Skoff, 1976; Hertz et al., 1980; Ghandour and Skoff, 1988; Knapp and Skoff, 1993; Feutz et al., 1995, 2001; Knapp et al., 1998, 1999). Moreover, the basic molecular event responsible for the jimpy abnormalities remains undefined. Sequestration of mutated PLP in endoplasmic reticulum (ER) has been proposed to be a primary mechanism that then leads to oligodendrocyte cell death (Gow et al., 1994, 1998; Jung et al., 1996; Thomson et al., 1997; Krämer at al., 2001; Southwood and Gow, 2001; Bauer et al., 2002). The impaired transport of jimpy PLP/DM20 in ER reported in the literature was mainly based on observations made on cell types not related to oligodendrocyte lineage and the synthesis and transport in these cell lines may not mimic that of oligodendrocytes. We used an immortalized jimpy oligodendrocyte line (Feutz et al., 2001) to reinvestigate PLP/DM20 trafficking and to dissect the jimpy phenotype in vitro. An immortalized jimpy oligodendrocyte line was used because the native jimpy oligodendrocytes in culture have a shortened survival that makes it difficult to maintain them in pure culture in significant number (Feutz et al., 1995). Also, the presence of contaminant astrocytes in primary cultures may alter oligodendrocytes response to chemical or environmental factors.
In the present study, the processing of jimpy and normal PLP/DM20 in the jimpy (158JP) and normal (158N) immortalized oligodendrocyte lines was studied using four different expression vectors encoding for fusion proteins of normal or jimpy PLP or DM20 and enhanced green fluorescent protein (EGFP): nPLP-EGFP, nDM20-EGFP, jpPLP-EGFP, and jpDM20-EGFP. The effect of the newly synthesized PLP and DM20 fusion proteins in immortalized normal and jimpy cells on cell differentiation induced by the dbcAMP was also described. The deficiency in the dbcAMP stimulation pathway was investigated by the detection of the expression of inducible cAMP early repressor (ICER) mRNA. ICER gene exerts a direct and efficient negative feedback in cAMP signaling pathway (Molina et al., 1993; Folco and Koren, 1997; Krueger et al., 1999). In addition, because resting membrane potential was described as an index of cell maturation and plasma membrane composition in oligodendroglia (Knutson et al., 1997), the resting potential of plasma membrane in jimpy oligodendrocytes was also recorded.
MATERIALS AND METHODS
Two immortalized oligodendrocyte lines were obtained as previously described (Feutz et al., 2001). Two-day-old oligodendrocytes in secondary cultures were prepared from 12-day in vitro (DIV) primary cultures derived from normal and jimpy 2- to 3-day-old brains according to the published procedure (Feutz et al., 1995). The plasmid pMTwt SV40 was used, including the SV40 large T-antigen gene under the control of the wild-type promoter of mouse metallothionein-I gene. Oligodendrocyte cultures in 35 mm petri dishes were used for immortalization. Transfection was performed using the calcium phosphate procedure (Profection kit, Promega, France) with 0.5 to 1 μg/dish of purified plasmids pMTwt SVneo. After 4-h incubation, cultures were washed twice with DMEM containing 10% calf serum and 150 μM zinc sulfate, cultured for 1 week, and then the cells were switched to the same medium complemented with 200 μg/ml G418 (Geneticin sulfate, Sigma). The medium containing G418 was changed every 3 days and the cells were exposed to G418 for 3 weeks. The surviving colonies were isolated and then cloned by limiting dilution (Feutz et al., 2001). The cells were then maintained in culture in DMEM supplemented with 5% calf serum (Invitrogen, France) and without G418.
Infection Procedure
Both 158N and 158JP oligodendrocytes were cultured on coverslips in 24-microwell plates at 3,000 cells/well. After 4 days, the cells were used for transfection. Transfection was performed by the calcium phosphate procedure as described above with 0.1–0.2 μg DNA/coverslip with one of four purified plasmids (nPLP-EGFP, nDM20-EGFP, jpPLP-EGFP, or jpDM20-EGFP). After 4-h incubation, cultures were washed twice with DMEM containing 5% calf serum and then incubated for 24, 48, and 72 h in the same medium. Cells were then fixed in 4% paraformaldehyde (PFA) for 15 min at room temperature (RT) and then stored at 4°C in phosphate buffer saline (PBS).
Generation of Vectors and Expression Constructs
PLP-EGFP and DM20-EGFP fusion constructs were made by initially cloning full-length PLP and DM20 cDNA (kindly provided by Dr. A. Fannon, Mount Sinai, NY) into the BamHI site of the expression vector pCMV, and then inserting the EGFP gene in frame with PLP or DM20 at the 3′ end. The constructs were isolated by transformation of LE392 competent cells, followed by Kanimycin selection, and confirmed by gel electrophoresis and restriction analysis. The sequence of the inserts and PLP-EGFP/DM20-EGFP fusion points were verified by DNA sequencing. The performance of the construct expressions was tested by transfection into NT2 cells and then by detection of the fluorescent PLP/DM20 products. The monoclonal antibody O10 (kindly provided by Dr. K. Nave) combined with rhodamine-conjugated secondary antibody was used to attest the correct insertion of PLP/DM20 by performing live staining. Both EGFP fluorescence and rhodamine O10 immunofluorescence confirmed that PLP-EGFP and DM20-EGFP were synthesized and transported to the cell surface. Jimpy PLP-EGFP and jimpy DM20-EGFP were constructed using the same protocols as their wild-type counterparts. Since jimpy PLP and DM20 cDNAs (kindly provided by Dr. A. Fannon, Mount Sinai, NY) differ from wild type at the 3′ end, a different set of primers were used for PCR amplification. The size and orientation of the constructs were tested after transformation into LE392 cells.
Immunodetection of Myelin Antigens and Other Cellular Components
Cells grown on 14 mm glass coverslips in 24-well plates were fixed in 4% paraformaldehyde (PFA) in PBS for 30 min. Cultures were then saturated by incubation for 1 h with 10% normal goat serum or in 3% blocking solution in PBS (Roche, France). After several washes in PBS, cells were then incubated with one of the following antibodies; rabbit anti-PLP directed to the sequence 117–129 peptide (Trifilieff et al., 1986) or to PLP C-terminal (Benjamins et al., 1989). Appropriate fluorescein Alexa 488 and Alexa 546-labeled secondary antibodies were used (Molecular Probes, The Netherlands). All incubations were performed at RT. Surface antigen detection was performed by incubation of the primary antibody with live cells for 30 min at 37°C followed by fixation with 4% PFA for 5 min. PLP-EGFP and DM20-EGFP expression in transfected cells was visualized with fluorescence microscopy either directly or after incubation with a rabbit polyclonal antibody to EGFP (Clontec, France) and then with Alexa-conjugated antirabbit IgG (Molecular Probes). Coverslips were mounted in Aquapolymount (Polysciences, Germany) for light microscopic observation (Leica DM and Olympus BX 60 microscopes). Transfected cells were also examined by confocal microscopy using the ACAS laser cytometer (Meridian Instruments).
Dibutyryl cAMP Treatment
After plating immortalized jimpy and normal cells at 3,000 cells/coverslip in 24-well plates for 4 days, transfected and nontransfected cultures were treated with N6,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate (dbcAMP; Sigma) at 1 mM for 72 h. Treated cultures were then rinsed and fixed with 4% PFA for 20 min for immunocytochemical staining.
Northern Blots of ICER and GAPDH
Total RNAs were isolated from confluent cultures in 100 mm individual petri dishes using Trizol (Invitrogen, Life Technologies, France). cDNA synthesis from the RNAs was performed using the Riboclone cDNA Synthesis System AMV RT kit (Promega). Total RNA (4 μg) in the presence of 2.5 μg oligo dT15 primers (Promega) in 15 μl H2O was heated at 70°C for 5 min. First-strand buffer and RNAsine ribonuclease inhibitor (40 units, Promega) were then added and incubated at 42°C for 5 min. Avian myeloblastosis virus (AMV) reverse transcriptase (30 U; Promega) was used at 42°C for 1 h. cDNAs were then subjected to PCR with specific primers to ICER (sense, 5′-AACATGGCTGTAACTGGAGA; antisense, 5′-TTACTCTGCTTTATGGCAAT) and GAPDH (sense, 5′-TCCACTCACGGCAAATTCAACG; antisense 5′-CAGGCGGCACGTCAGATCCACG). Amplification was performed in 10 mM Tris-HCl buffer, pH 9, containing 50 mM KCl, 1.5 mM MgCl2, 0.2 mM dNTPs, 0.1% Triton X-100, 0.2 mg/ml BSA, and 2 U Taq DNA polymerase (Appligene, France) for 35 cycles in thermocycler Progene (Techne, U.K.) at 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min, and then, at the end 10 min, at 72°C for ICER. GAPDH was amplified with 35 cycles, 94°C for 1 min, 69°C for 1 min, and 72°C for 1 min. The probes were purified using GFX columns (AmershamPharmacia Biotech, France) and then labeled with dCTP-P32 using Random Primed DNA kit (Roche). The labeled probes were then purified on Sephadex G25 gel (AmershamPharmacia Biotech). After electrophoresis on agarose gel, the RNAs (10 μg/sample) were transferred to hybridization membrane Hybond-N (AmershamPharmacia Biotech). Hybridization with ICER and GAPDH probes were performed at 42°C for 12 h. For autoradiography, the hybridized nylon membrane was exposed to Kodak film at −80°C for 4 days and then developed.
Electrophysiological Recordings
For electrophysiological experiments, cells were cultured as described above and plated in 35 mm noncoated dishes at low cell density (2,000 cells per dish). Recordings were performed on 2- or 5-day-old cells using solutions of the following composition (in mM): NaCl 132, KCl 2, HEPES 10, MgCl2 2, CaCl2 0.5, D-glucose 10 for the bath and KCl 125, NaCl 5, EGTA 5.5, CaCl2 1, MgCl2 2, HEPES 10 for the pipette medium. The pH was adjusted to 7.4 with NaOH and 7.2 with KOH for the bath and the pipette medium, respectively. Resting membrane potential was measured in the whole cell mode of the patch-clamp technique (Hamill et al., 1981) using an Axopatch 200B amplifier (Axon Instruments, CA) and the clampex routine of the pClamp software package (Axon Instruments). After gigaseal establishment with pipettes of 3 to 4 MΩ resistance, the breakthrough into whole cell was achieved in current-clamp mode, permitting resting potential determination before dialysis of the cell with the pipette medium. Records were filtered at 1 kHz and acquired (2 kHz sampling frequency) with a LabRack interface card (Scientific Instruments, OH).
RESULTS
O10 Antibody Immunoreactivity With PLP/DM20 in Nontransfected Oligodendrocyte Lines
Our previous study (Feutz et al., 2001), using antibody that detects only the PLP isoform, showed that PLP was distributed similarly in cell bodies and processes in both normal and jimpy oligodendrocyte lines and it is associated with plasma membrane. The monoclonal antibody O10, which detects a conformationally sensitive PLP/DM20 epitope, also showed positive immunoreactivity on paraformaldehyde-fixed cells from both normal and jimpy cell lines (Fig. 1A and C). This observation further confirms that both normal and jimpy PLP and DM20 are translated and transported to membrane. However, when normal and jimpy live cells were incubated with O10 antibody to detect the surface epitope, only normal cells were immunofluorescent (Fig. 1B), while the jimpy cells did not exhibit any significant specific immunofluorescence (Fig. 1D), indicating PLP/DM20 is not properly inserted into the plasma membrane.
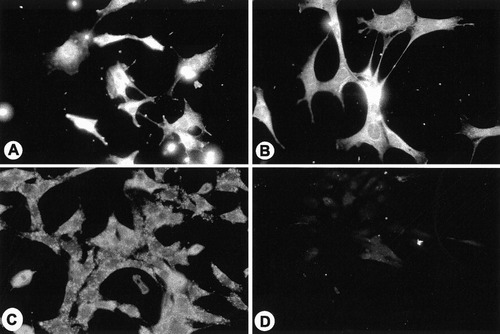
010 immunoreactivity detection by Alexa 546-tagged antimouse secondary antibody. 158N cells (A and B) and 158JP cells (C and D) at 5 days in culture after plating. Paraformaldehyde-fixed cells (A and C) and live cells (B and D) were incubated with O10 antibody. While both normal and jimpy cells were immunolabeled following fixation, only normal cells were immunofluorescent for live staining. ×300.
Distribution of Normal and Jimpy Fusion Protein PLP-EGFP and DM20-EGFP in Transfected Normal and Jimpy Cells
Normal 158N cells transfected with the vectors nPLP-EGFP (Fig. 2A) or with nDM20-EGFP (Fig. 2B) for 48 h expressed the fusion fluorescent protein in the cell body and its fine processes. Similarly, jimpy 158JP cells transfected with the same vectors express both normal PLP (Fig. 2C) and DM20 (Fig. 2D) in cell perikarya and processes. Both normal and jimpy transfected cells expressed high level of normal transfectant PLP or DM20 as judged by the immunofluoresence detection with anti-GFP antibody. When jimpy PLP or jimpy DM20 expression vectors were introduced into the normal cells, jimpy PLP (Fig. 3A) and jimpy DM20 (Fig. 3B) were detected in cell bodies as well as in distal cell processes. Also, high levels of jimpy PLP (fig. 3C) and jimpy DM20 (Fig. 3D) were detected in jimpy cells in both cell body and its extensions.
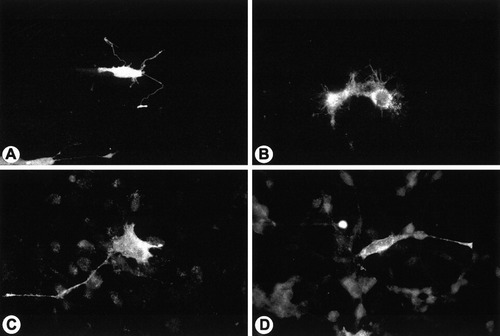
Normal 158N (A and B) and jimpy 158JP cells (C and D) were transfected with the vector nPLP-EGFP to express normal PLP (A and C) or with the vector nDM20-EGFP (B and D) to express normal DM20. The fusion protein detection was amplified using anti-EGFP antibody and Alexa 546-tagged antirabbit IgG. Immunofluorescence detection showed PLP and DM20 are present in the cell bodies and long, thin processes in both normal (A and B) and jimpy (C and D) immortalized oligodendrocytes. ×300.
Normal 158N (A and B) and jimpy 158JP cells (C and D) were transfected with the vector jpPLP-EGFP to express jimpy PLP (A and C) or with the vector jpDM20-EGFP (B and C) to express jimpy DM20. The fusion protein detection was amplified using anti-EGFP antibody and Alexa 546-tagged antirabbit IgG. Immunofluorescence detection showed mutated PLP and DM20 are present in the cell bodies and processes in both normal (A and B) and jimpy (C and D) immortalized oligodendrocytes. ×300.
Confocal microscopy using the Meridian laser cytometer confirmed the distribution of jimpy PLP (Fig. 4) and DM20 (not shown) in jimpy cells observed with whole cell fluorescence microscopy. Higher concentration (red color) of PLP was observed in the cell body surrounding the nucleus and also at the emergence of cell processes. Lower levels were present in the distal cell extensions (Fig. 4). Jimpy DM20 was also observed in the cell processes but the highest concentration was found in the cell perikaryon (not shown).
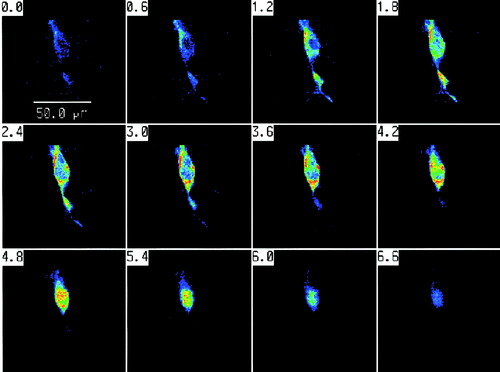
Confocal microscopy of jimpy PLP immunofluoresce in transfected 158JP cells with the vector jpPLP-EGFP. Selected 0.6 μm sections from one jimpy immortalized oligodendrocyte show the distribution of jimpy PLP. Higher concentration (red color) of PLP was observed in the cell body and also in cell processes. Lower levels were present in the distal ends of cell extensions.
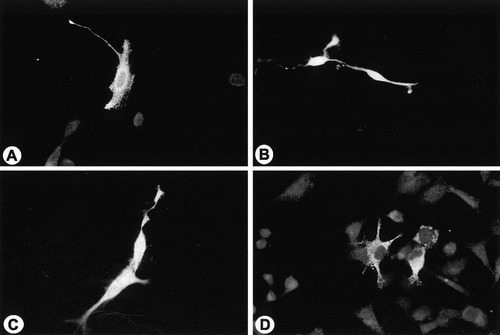
The normal cell line (Fig. 5A and C) transfected with normal PLP showed a bright immunofluorescence using O10 antibody on live cells (Fig. 5A) but when transfected with jimpy PLP they did not show any specific fluorescence (Fig. 5C). The background fluorescence observed in normal cells was due mainly to the presence of endogenous PLP in normal cells. The normal PLP can be detected on the cell surface of jimpy cells (Fig. 5B). In contrast, jimpy PLP was not detected on live jimpy cells with O10 antibody (Fig. 5D). Normal and jimpy DM20 showed similar behavior in both transfected cell lines (not shown). Ten to 20 cultures on coverslips obtained from three to five transfection experiments were usually used to generate the present data throughout the article. Each coverslip showed 20 to 30 fluorescent transfected cells.
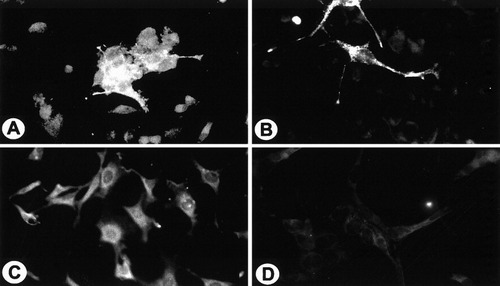
Transfected normal (A and C) and jimpy cells (B and D) to express normal PLP-EGFP (A and B) and jimpy PLP-EGFP (C and D) were photographed after live cell staining for PLP antibody and immunodetection with rhodamine-tagged secondary antimouse antibody. Both normal (a cluster of three to four cells; A) and jimpy (two cells; B) transfected cells showed a robust fluorescence denoting the correct insertion of normal PLP in oligodendrocyte plasma membrane in normal (A) and jimpy cells (B). Tansfected normal (C) and jimpy (D) cells with jimpy PLP-EGFP after live staining for O10 antibody did not show any significant immunofluorescence. The endogenous immunofluoresce in the rest of normal cells (A and C) correspond to the localization of the endogenous PLP/DM20. ×400.
Electrophysiological Recording
Because resting membrane potential was described as an index of cell maturation and plasma membrane composition in oligodendroglial progenitors (Knutson et al., 1997), we investigated whether failure to insert jimpy PLP in proper orientation into plasma membrane of 158JP cells influences this resting membrane potential. Recording pipette potential was recorded before and after breakthrough into the whole cell. After membrane breakdown, the potential signal showed a sharp negative shift, which generally peaked at a minimum before a slow evolution to more depolarized steady-state values. The peak value was considered as a good estimation of the resting potential (RP) before cell dialysis. Oligodendrocytes, used here as a control, showed as expected (Knutson et al., 1997) a statistically significant (t-test, P < 0.05) negative shift in RP according to the age of the cells in culture. The RP obtained at 2 and 5 days after plating were −31.3 ± 8.9 and −48.1 ± 4.5 mV, respectively (Fig. 6). The 158N cells that are, like the oligodendrocytes, sensitive to cAMP showed the same negative shift amplitude in RP values. The absolute values, however, were more depolarized (−10.1 ± 1.7 and −28.1 ± 2.6 mV for 2- and 5-day-old cells, respectively; P < 0.0005). In contrast, the 158JP cells did not exhibit any RP evolution during the 5 days of culture. A mean value of −14.2 ± 2.8 mV was obtained at 5 days of cell culture, which is not significantly different from the −12.5 ± 1.9 mV measured at 2 days.
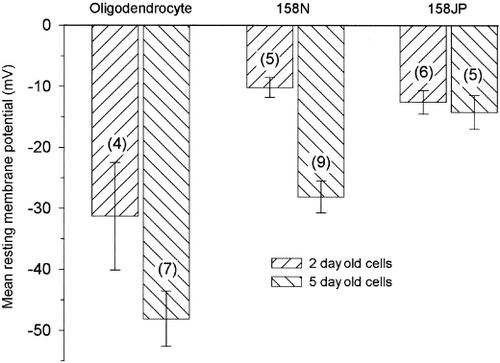
Comparison of resting membrane potential of oligodendrocytes, 158N and 158JP cells recorded at the culture age indicated in the diagram. The number of cells recorded in each case is shown in parentheses. Note the negative shift in the membrane potential for the native oligodendrocyte in enriched secondary cultures and the 158N cells but not for the 158JP cells. Bars represent ± SEM.
Expression of Transfectant PLP and DM20 and Differentiation of Oligodendrocyte Lines Induced by dbcAMP
Dramatic morphologic transformation of 158N cells from flat to process bearing cells after 72-h treatment with dbcAMP was clearly observed when examined under a phase contrast microscope or when stained for PLP (Fig. 7A and B). The cell bodies became round and smaller, had many emerging cell processes, and were more brightly stained with PLP, while jimpy cells remained insensitive to similar treatment (Fig. 7C and D).
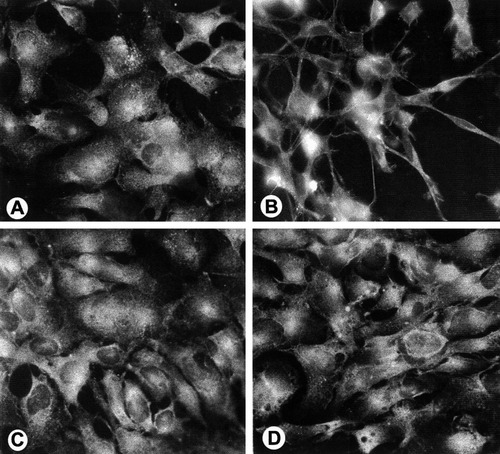
Immunofluorescent detection of endogenous PLP using the polyclonal antibody to the sequence 117–129 of PLP in normal 158N (A and B) and 158JP cells (C and D). Untreated cells (A and C) and 72-h dbcAMP-treated cells (B and D). Normal immortalized oligodendrocytes show a dramatic shift in their morphology after dbcAMP. The cell bodies became round and smaller with many emerging thin processes in addition to brighter staining with PLP (B), while jimpy cells remain insensitive to similar treatment (D). ×300.
After 72 h, all the fluorescent cells from the normal cell line 158N transfected with the EGFP alone showed transformation in their morphology after treatment with dbcAMP (Fig. 8). Interestingly, most of the normal cells transfected with nPLP-EGFP or nDM20-EGFP plasmids and consequently overexpressed PLP or DM20 lost their potential of differentiation (Fig. 8). Only 20% to 25% of the fluorescent cells exhibited moderate transformation. In contrast, after similar treatment, jimpy 158JP cells transfected with the control plasmid EGFP remained insensitive to dbcAMP (Fig. 8), as did jimpy cells transfected with nPLP-EGFP or nDM20-EGFP (Fig. 8). The expression of normal fusion protein PLP-EGFP or DM20-EGFP in jimpy cells did not restore the sensitivity to dbcAMP treatment.
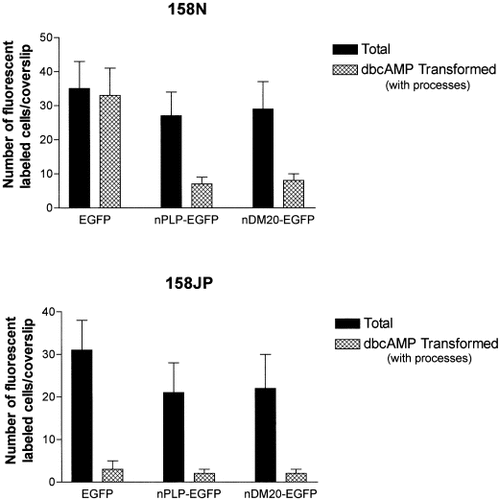
Effect of the expression of transfectant EGFP alone, normal PLP-EGFP and DM20-EGFP on oligodendrocyte lines treated with dbcAMP. Stimulation of morphologic transformation of 158N cells was observed only when transfectant EGFP alone was expressed. In contrast, when the fusion proteins PLP-EGFP or DM20-EGFP were expressed, only 20% to 25% of 158N transfected cells showed moderate transformation while the rest remained without showing significant transformation. This observation shows that the production of high levels of PLP or DM20 may inhibit the intrinsic respond to cAMP in normal oligodendrocytes. The expression of normal PLP or DM20 in jimpy 158JP cells did not restore the deficit of jimpy cell response to dbcAMP as shown by the absence of morphologic transformation. All the transfected fluorescent EGFP+, PLP-EGFP+, and DM20-EGFP+ cells remained flat without any significant changes in their morphology or in their staining intensity.
The activation of ICER gene by dbcAMP in the normal immortalized line was detected by northern blots. After agarose gel electrophoresis of the RNAs (10 μg/sample) from dbcAMP-treated and nontreated 158N and 158 JP cell, the Hybond-N membrane was hybridized with the ICER and GAPDH probes. ICER mRNA level was increased substantially by 2 h after dbcAMP treatment and then completely downregulated after 12 h (Fig. 9A). In contrast, jimpy immortalized cell line did not show any significant transient increase in ICER transcriptional level following the same treatment with dbcAMP (Fig. 9B). The housekeeping gene GAPDH mRNA levels were detected as a control experiment (Fig. 9C and D).
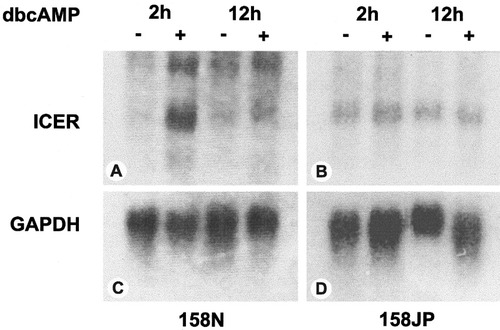
Northern blots of ICER and GAPDH. ICER and GAPDH probes were synthesized by RT-PCR as described in text. The probes were then labeled with dCTP-P32 using Random Primed DNA kit. The labeled probes were then purified on Sephadex G25 gel. After electrophoresis on agarose gel, the RNAs (10 μg/sample) from dbcAMP-treated and nontreated 158N and 158JP cell lines were transferred to hybridization membrane Hybond-N. Hybridization with ICER and GAPDH probes was performed at 42°C for 12 h. Hybridized nylon membrane was exposed to Kodak film at −80°C for 4 days and then developed. DbcAMP induced stimulation of ICER transcription within 2 h of dbcAMP treatment in normal cell line 158N (A), whereas no significant induction of ICER transcription was observed in jimpy 158JP cell line (B). ICER transcription was completely downregulated in 158N cells in 12 h after dbcAMP treatment (A), while it remained unchanged in jimpy 158JP cells (B). GAPDH mRNA detection after hybridization with GAPDH probes to 158N (C) and 158JP (D) total RNAs.
DISCUSSION
Distribution of Endogenous Normal and Jimpy PLP
We previously reported that both normal and jimpy PLP were detected at low levels with the antibody to the sequence 117–129AA of PLP in the immortalized normal and jimpy cell lines (Feutz et al., 2001). This antibody recognizes both normal and jimpy PLP but not DM20 because this sequence is deleted from DM20 and is before the jimpy mutation in intron 4 (Trifilieff et al., 1986). The 010 monoclonal antibody, which recognizes an extracellular domain of PLP related to the large loop of normal PLP/DM20 (Jung et al., 1996), showed that the live jimpy immortalized cells did not react with this antibody. In contrast, live normal cells and both normal and jimpy permeabilized cells were positive for O10. The present observation confirms the previous conclusion regarding the abnormal insertion of jimpy PLP/DM20 into the plasma membrane (Jung et al., 1996; Thomson et al., 1997; Feutz et al., 2001). This abnormal insertion of jimpy PLP/DM20 into the plasma membrane may underlie the abnormal cell signaling in jimpy. Neither bFGF nor cAMP induced stimulation of cell proliferation and differentiation respectively (Fetuz et al., 1995, 2001). The defective membrane resting potential reported in this study and other defects such as elevated levels of intracellular Ca2+ (Knapp et al., 1999) and progesterone (Le Goascogne et al., 2000) may be also related to the abnormal insertion of jimpy PLP/DM20 into plasma membrane of oligodendrocytes.
Expression of Transfectant PLP/DM20
When normal PLP and DM20 were expressed after transfection in both immortalized normal or jimpy oligodendrocytes, the two proteins showed roughly similar distributions in the cell bodies and processes. However, more intense fluorescence for PLP/DM20 was observed in the cell body around the cell nucleus where the protein is processed through the endoplasmic reticulum (Schwob et al., 1985; Roussel et al., 1987; Lees, 1998). It is interesting to note that jimpy immortalized cells were able to translocate normal PLP and DM20 to the cell processes distant from the cell bodies. This observation suggests that the protein transportation machinery in jimpy immortalized oligodendrocytes allowed synthesis and translocation of these two proteins. Our observation is in agreement with previous observations in vivo that showed the presence of MBP in the scattered myelin sheaths in jimpy brain (Duncan et al., 1989) and uniform distribution of CA II in jimpy oligodendrocytes (Ghandour and Skoff, 1988).
The expression of jimpy PLP and jimpy DM20 after transfection of 158N cells or 158JP cells was detected in the whole cell body and in cell processes. Previous observations showed that jimpy PLP is transported from the cell body to the cell processes in vivo and in vitro. In jimpy brain, jimpy PLP was detected in some myelin sheaths present in jimpy brain using the same PLP-specific antibody used in the present study (Roussel et al., 1987) and also in jimpy oligodendrocyte processes in culture (Feutz et al., 1995). Our observations differ from several studies that report jimpy and other mutated PLP and DM20 proteins are abnormally transported and completely sequestered within cells, which may cause cell toxicity (Gow et al., 1994, 1998; Jung et al., 1996; Thomson et al., 1997). These studies were done in cell lines like Cos7 that are unrelated to oligodendrocytes and that do not normally process PLP. They showed that after cell transfection, abnormal accumulation of jimpy PLP gene products occurred in the perinuclear area and particularly in the endoplasmic reticulum. We cannot exclude the possibility of partial accumulation of jimpy PLP/DM20 in oligodendrocyte perikarya but, more importantly, detectable amounts are transported to the plasma membrane. This association with the plasma membrane most likely affects maturation of the plasma membrane as shown with electrophysiological properties. The capacity of both immortalized normal and jimpy oligodendrocytes to transport the jimpy PLP and DM20 may be explained by the oligodendrocytic origin of the cell lines. Oligodendrocytes are quite unique, compared to the other cell types, characterized by very efficient myelin protein transportation (Kim and Pfeiffer, 1999; Simons et al., 2000; Krämer et al., 2001). Even though the oligodendrocytes are immortalized with the large T-antigen, transportation of myelin proteins in immortalized oligodendrocytes has more significance to the biology of oligodendrocytes than cell lines not derived from oligodendrocytes. The cellular distribution of the fusion proteins confirms the transport of jimpy PLP/DM20 towered the plasma membrane while the live staining for O10 antibody of transfected normal and jimpy cells confirmed the abnormal insertion of this protein (Fig. 10).
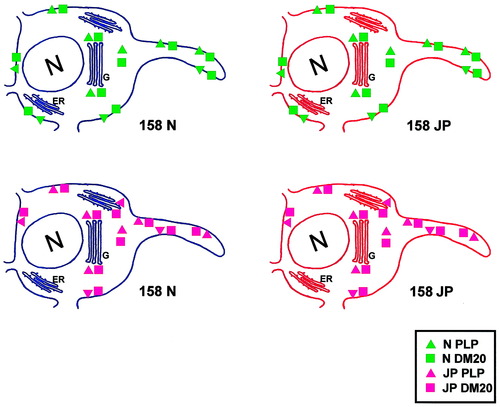
Schematic representation of imaging the expression of fusion normal or jimpy PLP-EGFP and DM20-EGFP in immortalized normal and jimpy oligodendrocyte lines. Both normal and jimpy PLP/DM20 are similarly distributed in the cell bodies and processes. At extracellular level, only normal PLP and DM20 represent the correct insertion into plasma membrane. Green triangles and rectangles represent normal PLP and DM20. Pink triangles and rectangles represent jimpy PLP and DM20, respectively. ER, endoplasmic reticulum; G, Golgi apparatus; N, cell nucleus.
Defect in cAMP Signaling and Expression of Normal and Jimpy PLP/DM20
The lack of dbcAMP stimulation in jimpy oligodendrocytes was previously reported (Feutz et al., 2001). After dbcAMP treatment, the normal oligodendrocyte line enhanced several-fold the levels of oligodendrocyte markers. In contrast, the jimpy oligodendrocyte line remained insensitive to similar treatment. The increased levels in oligodendrocyte markers is also associated with morphologic changes reflected by many emerging thin cell processes and rounded cell bodies (Feutz et al., 2001). The morphologic transformation of immortalized 158N normal oligodendrocytes and the absence of such transformation in 158JP jimpy cells were confirmed by the present data.
Cellular effects of cAMP elevation are multiple. In oligodendrocytes, the elevation of cAMP level resulted in acceleration of cell maturation (McMorris, 1983; Raible and McMorris, 1989; Sato-Bigbee and DeVries, 1996). Since the cAMP binding protein (CREB), an activator of cAMP inducible genes, did not show significant difference between normal and jimpy cells after stimulation (not shown), the ICER gene was selected because it exerts a direct and efficient negative feedback in cAMP signaling pathway (Molina et al., 1993; Folco and Koren, 1997; Krueger et al., 1999). ICER gene transcription plays a key role in this pathway and it is stimulated by the cAMP (Molina et al., 1993) but not by Ca2+ (Krueger et al., 1999) or by the membrane depolarization (Folco and Koren, 1997). The transient elevation of the transcription level of ICER may function to attenuate the cAMP response in normal immortalized oligodendrocytes but it fails to function in jimpy cells. The role of PLP in this pathway needs to be elucidated. Since jimpy immortalized cells showed higher levels of intracellular Ca2+ (Knapp et al., 1999), this high level might attenuate the stimulatory effect of cAMP on ICER. Thus, jimpy PLP/DM20 may modify the function of Ca2+ pumps leading to abnormal function of cAMP stimulation pathway. Alternatively, PLP may act itself as a membrane channel (Helynck et al., 1983) and jimpy PLP may alter the membrane functions directly.
The expression of normal PLP or DM20 after transfection of immortalized 158JP oligodendrocytes did not allow immortalized jimpy oligodendrocytes to restore their deficiency in cAMP stimulation. The jimpy mutation is dominant as previously reported (Nadon et al., 1994; Schneider et al., 1995), but the dominant negative character of the jimpy mutation did not prevent the expression of normal PLP or DM20 in jimpy cells. The weakly fluorescent jimpy cells as well as the strongly fluorescent ones remained insensitive to dbcAMP. Thus, the jimpy cells remained deficient for dbcAMP stimulation despite the presence of low and high level of normal PLP/DM20. More interestingly, when a high level of normal PLP and DM20 was induced by transfection with the vectors nPLP-EGFP and nDM20-EGFP, the differentiation of normal cells by the dbcAMP was reduced consequently. The highest level of PLP-EGFP or DM20-EGFP was found in cells that completely lost the differentiation potential. This observation at cellular level is in agreement with previous observations on the negative effects of PLP overexpression in oligodendrocytes in mice (Readhead et al., 1994; Ikenaka and Kagawa, 1995; Kagawa et al., 1995; Bradl et al., 1999).
Finally, the electrophysiological recordings of the resting plasma membrane potential showed the absence of a developmentally regulated negative shift in the membrane potential in jimpy cells compared to the normal native or immortalized oligodendrocytes. This result suggests that the absence of sensitivity of 158JP cells to cAMP is associated with the failure of jimpy cells to mature, most likely due to altered structure of the plasma membrane proteins in jimpy cells. The abnormal insertion of jimpy PLP/DM20 into plasma membrane may be the primary cause of such alterations. Preliminary results on membrane currents displayed by 158JP cells indicate that these cell types exhibit different ionic current pattern reported in native oligodendrocytes (Attali et al., 1997) or 158N cells. Moreover, because the death of oligodendrocytes may involve misfolded proteins and interactions of extracellular components with plasma membrane proteins (Casaccia-Bonnefil, 2000), our study shows an abnormal plasma membrane in jimpy oligodendrocytes that may also trigger and/or contribute to the apoptotic cascade.
Acknowledgements
Supported by National Multiple Sclerosis Society (NMSS) grant RG2579 (to R.P.S. and L.C.) and National Institutes of Neurological Diseases and Stroke (NINDS) 38236 (to R.P.S.). The authors thank Dr. E. Trifilieff for the generous gift of anti-PLP antibody, Dr. A. Fannon for PLP/DM20 cDNAs, Dr. K.A. Nave for O10 antibody, Professors G. Vincendon and D. Grucker for their support and encouragement, Dr. J. Benjamins for careful reading of the article, R. Lerch for assistance with the laser cytometer, and Y. Huss, E. Scherrer, and N. Munch for technical assistance.