The significance of aragonite in the interpretation of the microscopic archaeological record
Abstract
Aragonite is one of the metastable polymorphs of calcium carbonate and is commonly found at archaeological sites in the form of mollusk shells and speleothems. Other occurrences include corals, fish otoliths, seed endocarps, wood ash, and lime plaster. These materials contain microscopic embedded information of fundamental importance for the assessment of the state of preservation of the archaeological record, the reconstruction of paleoenvironments, the identification of pyrotechnological processes, and the establishment of absolute chronologies. However, this information can be used only if the aragonite found at archaeological sites preserves its pristine structure and chemistry. Aragonite is metastable at ambient conditions and tends to dissolve and recrystallize into secondary aragonite or calcite, a process that entails the loss of its original elemental and isotopic signature. Therefore, well-grounded archaeological interpretations depend on the correct identification of aragonite and the careful characterization of its basic properties. This article reviews the dissolution processes of different types of aragonite, the methods used for their identification, and the archaeological information that can be extracted. Particular emphasis is given to the discussion of key issues regarding absolute dating and postdepositional processes of archaeological sediments, and to a detailed overview of the recently observed pyrogenic aragonite.
1 INTRODUCTION
Aragonite is one of the metastable polymorphs of calcium carbonate (CaCO3) at ambient temperatures and pressures (Lippmann, 1973). It is formed by many organisms, and in marine, freshwater, and terrestrial environments, aragonite is mineralized mainly by mollusks and stony corals in their exoskeletons, and by teleost fish in otoliths (Lowenstam & Weiner, 1989). Some plant species mineralize aragonite in seed endocarps (Cowan, Gabel, Jahren, & Tieszen, 1997). In geologic contexts, aragonite precipitates out of boiling water at hot springs, where it occurs in some types of travertine (Jones, 2017), and it is a major constituent of speleothems that form from supersaturated solutions in caves (Frisia, Borsato, Fairchild, McDermott, & Selmo, 2002). In addition to the geogenic and biogenic pathways, aragonite is produced by humans through the deliberate use of fire, in the form of ash and lime plaster. Both materials usually nucleate as calcite, the stable polymorph of CaCO3, but recently it has been shown that aragonite may form as well (Toffolo & Boaretto, 2014). Considering the widespread distribution of aragonite, its formation and dissolution processes are relevant for several fields of research, such as biomineralization, geochemistry, environmental science, paleoclimate reconstruction, and archaeology (e.g., Beck et al., 1992; Lowenstam & Weiner, 1989; Morse, Arvidson, & Lüttge, 2007; Ridgwell & Zeebe, 2005; Weiner, 2010).
Aragonitic shells are materials commonly found at archaeological sites (e.g., Bar-Yosef Mayer, 2005). At the microscopic scale, they contain embedded information of considerable value for the study of the human past. Shells and corals are excellent paleoclimate recorders, as they incorporate the oxygen and carbon isotope signature of water at the time of growth (e.g., Gagan et al., 2000; Gillikin et al., 2005). Aragonitic speleothems can provide paleoenvironmental records (e.g., Finch, Shaw, Holmgren, & Lee-Thorp, 2003) and have been dated with the U-series method to establish absolute chronologies at several hominin sites (e.g., Pickering, Kramers, Partridge, Kodolanyi, & Pettke, 2010). In addition, U-series dates of speleothems and corals have improved part of the radiocarbon (14C) calibration curve (Reimer et al., 2013). Aragonite in speleothems may be used also for 14C dating (e.g., Brook, Scott, Railsback, & Goddard, 2010), as well as aragonitic shells if a correction exists for the local reservoir age (e.g., Reimer & McCormac, 2002), and hackberry endocarps (Quade et al., 2014). Pyrogenic aragonite has been observed for the first time only recently, but has been used to infer the presence of combustion features in the absence of visible structures (e.g., Asscher, Lehmann, Rosen, Weiner, & Boaretto, 2015) and has been successfully dated using 14C (Toffolo et al., 2017).
All of the abovementioned results may be achieved only if one fundamental condition is fulfilled: aragonite must be well-preserved. This is a crucial requirement because aragonite is a metastable CaCO3 polymorph, and under unfavorable settings, it recrystallizes into calcite, a process that changes its chemical composition and makes the resulting calcite unsuitable for many applications, or, if undetected, it introduces a major source of error in measurements. In addition, it is often tacitly assumed that the simple occurrence of aragonite in shells and speleothems is sufficient proof of their pristine state. However, it has been shown that these materials do suffer from aragonite recrystallization, which entails isotopic exchange (e.g., Buchardt & Weiner, 1981). Only detailed analysis of shell microstructure and local structural order of aragonite crystals may provide final evidence regarding their state of preservation and isotopic content (e.g., Seuss, Titshack, Seifert, Neubauer, & Nützel, 2012). It thus seems that the correct identification and characterization of aragonite in its different forms is of the utmost importance for well-grounded archaeological interpretations. In fact, a major requirement of archaeology is the evaluation of the degree of preservation of the archaeological record, to verify whether the microscopic information embedded in a material selected for analysis is actually representative of its formation process and whether observed patterns in the occurrence and distribution of artifacts, bones and minerals are the result of past human activities or are driven by changes in environmental conditions of archaeological importance (Toffolo, 2018; Weiner, 2010). Therefore, the aim of this article is to review the different types of aragonitic materials found at archaeological sites, their dissolution process, the methods used for their characterization and to extract embedded information, and their significance for the interpretation of the archaeological record, including key issues that require further understanding. More specifically, the review will focus on aragonite as a proxy of postdepositional processes, as a paleoenvironmental record, as a synthetic material, and as an archive of absolute time signatures, always taking into account its relation to the sedimentary context.
2 BASIC PROPERTIES OF ARAGONITE
Several forms of CaCO3 exist in nature (Table 1). These are called polymorphs and include calcite, aragonite, vaterite, the hydrous forms calcium carbonate hemihydrate, monohydrocalcite and ikaite, and amorphous calcium carbonate (ACC; Beniash, Aizenberg, Addadi, & Weiner, 1997; Lippmann, 1973; Zou et al., 2019). Of all these, only calcite is thermodynamically stable at ambient temperatures and pressures, whereas other polymorphs are metastable and eventually, under unfavorable conditions, recrystallize into calcite according to Ostwald's Rule of Stages (Addadi, Raz, & Weiner, 2003; Lippmann, 1973; Ostwald, 1897). In addition, vaterite, calcium carbonate hemihydrate, monohydrocalcite, and ikaite are relatively uncommon in nature, and ACC is extremely unstable. Therefore, calcite and aragonite are the most relevant polymorphs for archaeology (Weiner, 2010). The following sections provide an overview of the basic properties of aragonite, the methods used for its identification, and its occurrence in biogenic, geogenic, and pyrogenic materials.
| Polymorph name | Chemical formula | Crystal system | Occurrence |
|---|---|---|---|
| Calcite | CaCO3 | Trigonal | Geologic spar, rocks, sediments, organisms, wood ash, lime plaster, and cement |
| Aragonite | CaCO3 | Orthorhombic | Geologic spar, rocks, sediments, organisms, wood ash, lime plaster, and cement |
| Vaterite | CaCO3 | Hexagonal | Geologic spar, organisms, and cement |
| Calcium carbonate hemihydrate | CaCO3⋅½H2O | Monoclinic | Synthesized in laboratory conditions; not observed in nature (caves?) |
| Monohydrocalcite | CaCO3⋅H2O | Trigonal | Geologic spar in caves, lacustrine sediments, and organisms |
| Ikaite | CaCO3⋅6H2O | Monoclinic | Marine tufa, marine sediments, sea ice, and geologic spar in caves |
| Amorphous calcium carbonate | CaCO3⋅H2O (stable) | - | Organisms |
| CaCO3 (transient) | - | Organisms |
2.1 The calcite–aragonite polymorphism
Polymorphs share the same chemical composition but are characterized by a different spatial arrangement of the atoms. Aragonite and calcite are both CaCO3, but exhibit major differences in their crystal lattice. The two polymorphs feature a layered structure, in which Ca2+ atoms alternate with (CO3)2− groups. Compared to calcite, Ca2+ in aragonite occupies similar positions, whereas (CO3)2− groups are staggered and slightly rotated with respect to each other (Figure 1). In addition, the distances between oxygen atoms belonging to different (CO3)2− groups in the aragonite CaO9 polyhedron are significantly shorter compared with the relative O–O distances in the calcite CaO6 octahedron, thus making aragonite (CO3)2− groups more tightly packed (Lippmann, 1973). As a result, aragonite is denser than calcite, and is characterized by higher specific gravity (2.93 g/ml, as opposed to 2.71 g/ml of calcite). This physical property is especially important when it comes to separating the two polymorphs in mixtures (see Sections 2.2 and 3.4).
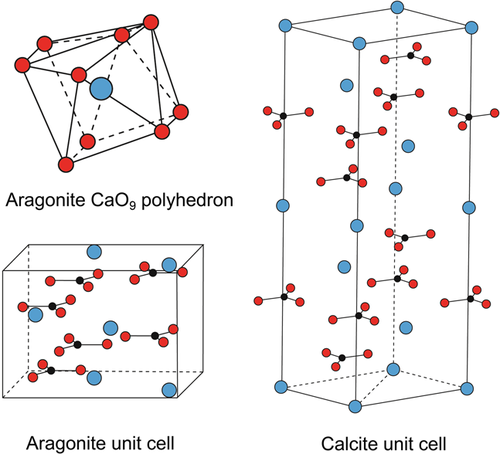
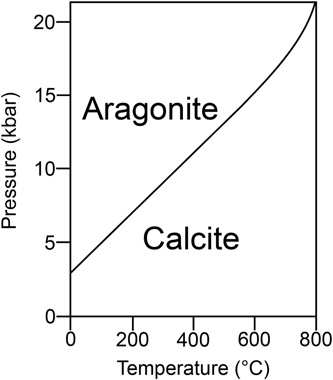
Looking at the respective stability fields, it is clear that aragonite is thermodynamically stable only at elevated pressures, and it is metastable relative to calcite at earth surface conditions (Figure 2). It follows that all forms of aragonite that exist at normal pressure will ultimately convert to calcite. The rate of conversion is slow and may take place over geologic time spans, although it is greatly affected by local conditions and especially by the action of water (Fyfe & Bischoff, 1965). This situation is also reflected by different solubility products at 25°C and 1 atm (kA = 10−8.22 and kC = 10−8.35). The particular atomic arrangement of aragonite, which produces a rather asymmetric CaO9 polyhedron (Figure 1), is conducive to greater solubility compared to calcite (Lippmann, 1973). Both polymorphs are stable at pH 8.2, but tend to dissolve with decreasing pH, which is a common condition at archaeological sites. Another consequence of the thermodynamic properties of calcite and aragonite is that Ca(HCO3)2 (calcium bicarbonate) in aqueous solution, which may originate from the dissolution of an aragonite substrate, usually precipitates as calcite at normal pressure. This is a crucial point for the study of diagenesis of archaeological deposits (see Section 3.1). Aragonite can convert to calcite also when heated to temperatures above ~300°C (e.g., Yoshioka & Kitano, 1985). This transition, which is not reversible, should be taken into account in the characterization of pyrogenic materials at archaeological sites (see Section 3.3). Notable exceptions to the phase diagram of aragonite discussed above involve the metastable occurrence of aragonite in aqueous solutions. More specifically, aragonite may precipitate out of boiling water as the only polymorph, although this process is still poorly understood and subject to a number of interlinked factors (Jones, 2017; Lippmann, 1973, and references therein). At ambient temperatures, aragonite precipitates from solutions rich in Mg2+, as is the case of sea water and salt lakes (Kitano & Hood, 1962; Lippmann, 1973).
2.2 Methods of identification
Before spectroscopy, calcite and aragonite were usually distinguished based on their physical properties and reaction with solutions. Rose (1837) exploited the different specific gravities of calcite and aragonite to discern the two, and this was the method used also in early works to identify CaCO3 polymorphs in carbonate rocks (e.g., Sorby, 1879). Meigen's test involves boiling aragonite and calcite crystals in a 0.1 M aqueous solution of Co(NO3)2, which gives aragonite a purple hue, whereas calcite remains white or becomes pale blue (Meigen, 1901). Another test by Feigl and Leitmeier (1933) exploits the reaction between aragonite/calcite and a solution of MnSO4 and Ag2SO4, which makes aragonite black and calcite pale gray. However, these staining tests provide low accuracy, especially in the case of mixtures in sediments. Thin-section petrography in crossed polarized light has also been used to differentiate the two polymorphs based on the range and intensity of their interference colors, cleavage shape, and type of extinction (Bragg, 1924; Johnston, Merwin, & Williamson, 1916). This approach lacks accuracy, as the refractive indices of calcite and aragonite are similar, and relatively large fragments in specific spatial arrangements are required for secure identification (Canti, 2017). In fact, petrographic assessment is often supported by staining tests (e.g., Murray, 1954). Thus, applying this method to archaeological sediment thin sections (micromorphology), where CaCO3 crystals are randomly oriented and mixed with several other phases, is rather difficult.
2.2.1 Crystallography, spectroscopy, and electron microscopy
Calcite and aragonite are characterized by different arrangements of the atoms in the crystal lattice, and therefore may be distinguished using analytical methods that address both long- and short-range atomic order. X-ray diffraction (XRD) determines crystal structures and may be applied to single CaCO3 crystals, powdered mixtures of different CaCO3 polymorphs and carbonate-bearing sediments. With an appropriate sample setup, thin sections may be analyzed as well (Berthold & Mentzer, 2017). Besides phase identification, it is possible to semi-quantify crystalline phases, even for relatively small amounts of sample (<50 mg; e.g., Berthold, Bjeoumikhov, & Brügemann, 2009). In addition, XRD can measure bond lengths and thus identify microstrain fluctuations and distortions in the crystal lattice of CaCO3, including samples of archaeological importance (e.g., Pokroy, Quintana, Caspi, Berner, & Zolotoyabko, 2004; Xu, Toffolo, Boaretto, & Poduska, 2016; Xu, Toffolo, Regev, Boaretto, & Poduska, 2015).
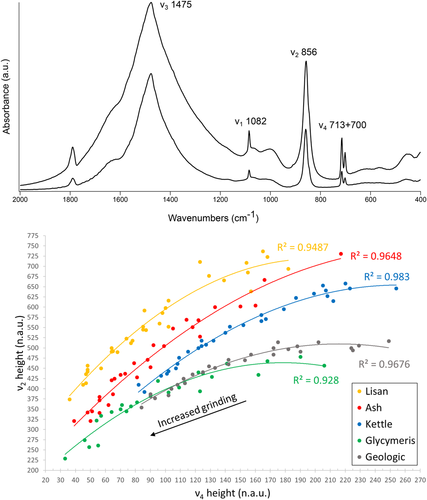
Vibrational spectroscopy allows accurate results for poorly ordered crystals due to its ability in characterizing the types of vibration of specific molecules, rather than the diffraction patterns of X-rays along macroscopic distances (e.g., Diem, 2015). Fourier transform infrared spectroscopy (FTIR) is thus particularly well-suited when it comes to determining the nature of minerals characterized by short-range atomic order (Toffolo & Berna, 2018). This is the case of pyrogenic carbonates (e.g., Chu, Regev, Weiner, & Boaretto, 2008). FTIR is usually performed in the mid-infrared range (4000–400 cm−1 or 2500–25,000 nm) in transmission mode on powders embedded in KBr pellets. By plotting changes in the intensity of the ν2 and ν4 absorptions of calcite (normalized to the ν3 absorption) upon repeated and increased grinding of the same KBr pellet, Poduska et al. (2011) showed that the trend lines thus obtained, called “grinding curves,” are specific to calcites characterized by different degrees of atomic order, which are a consequence of their formation path. This method has been used to distinguish geogenic calcite from its anthropogenic form (Regev, Poduska, Addadi, Weiner, & Boaretto, 2010). Similarly, geogenic aragonite exhibits a different grinding curve compared with biogenic aragonite and aragonite precipitated from boiling water (Suzuki, Dauphin, Addadi, & Weiner, 2011). Toffolo, Regev, Dubernet, Lefrais, and Boaretto (2019) extended the method to pyrogenic aragonite and developed a procedure based on FTIR and XRD to determine degrees of atomic order in calcite–aragonite mixtures (Figure 3). Powdered samples may be analyzed also in attenuated total reflectance (ATR) mode (e.g., Loftus, Rogers, & Lee-Thorp, 2015; Villagran, Strauss, Miller, Ligouis, & Oliveira, 2017). Thin sections and polished slabs may be scanned using an FTIR microscope in total reflectance and ATR modes (µFTIR), thus allowing the analysis of areas as small as 25 × 25 µm (Berna, 2017). Similar to FTIR, Raman spectroscopy can distinguish calcite from aragonite based on peak position (e.g., Diem, 2015). When this is done using a microscope (µRaman), the spot size is <5 µm and thus relatively small crystals may be targeted (e.g., Dauphin et al., 2019; Toffolo et al., 2017). Working on thin sections and polished slabs/casts with FTIR and Raman is fundamental to obtain information on the spatial distribution of polymorphs and crystals characterized by different degrees of atomic order (e.g., Poduska et al., 2012; Sibony-Nevo et al., 2019).
Scanning electron microscopy (SEM) on powdered samples allows a close-up view of crystal habit, which is a diagnostic parameter for several crystals. Habit may be measured in geogenic crystals using SEM images, but mixtures of different phases in archaeological sediments may prove too complex and/or poorly preserved for secure identification. In contrast, the microstructure of biogenic aragonite is easily recognized when preserved. A cathodoluminescence detector (SEM-CL) can differentiate the two polymorphs in pellets and thin sections based on the wavelength of the photons emitted after irradiation by an electron beam. Mn2+ substituting for Ca2+ in the CaCO3 lattice produces orange luminescence in calcite and green to orange luminescence in aragonite (e.g., Toffolo, Ricci, Caneve, & Kaplan-Ashiri, 2019).
Currently, FTIR, Raman and XRD are the most accurate and rapid methods available in archaeology to determine the presence of CaCO3 polymorphs, their quantity, and their degree of local structural order. If used in conjunction with thin sections and optical/electron microscopy, spatial information may be retrieved as well. Clearly, these methods achieve best results when integrated to contribute multiple independent lines of evidence (Weiner, 2010).
2.3 Biogenic aragonite
Despite the fact that aragonite is mineralized by many organisms in different kingdoms, only aragonite formed in mollusk shells, coral skeletons, fish otoliths, and seed endocarps has so far yielded information of archaeological relevance (Quade et al., 2014; Weiner, 2010).
All classes belonging to the phylum Mollusca, and in particular the Gastropoda and Bivalvia, include organisms that mineralize aragonite to produce exoskeletons, or shells (Lowenstam & Weiner, 1989). Mollusk shells feature a layered structure with aragonite crystals organized in different microstructures and characterized by various habits (Carter & Clark, 1985). If calcite is present as well, it always occurs in separate layers (e.g., Böhm, Demmert, Harris, & Fey, 2016). Crystals may show different degrees of atomic order in mollusks, as shown by FTIR grinding curves of nacre, myostracum, ligament, and crossed-lamellar aragonite (Suzuki et al., 2011). Figure 3 shows the grinding curve of a powdered Glycymeris insubrica (bittersweet clam) shell compared with other aragonite reference standards (Toffolo, Regev, et al., 2019). Stony corals (phylum Cnidaria, class Anthozoa, order Scleractinia) mineralize aragonite exoskeletons (Lowenstam & Weiner, 1989). Otoliths are mineralized bodies produced by all teleost fish to sense acceleration, sounds, and the gravitational field. Their structure is composed of radiating spherulites of aragonite crystals that nucleate from a planar organic substrate at their center (Lowenstam & Weiner, 1989). In seed endocarps, and especially in Celtis sp., aragonite is mineralized in a honeycomb structure of fibrous crystals (Cowan et al., 1997; Quade et al., 2014).
The oxygen isotopic composition of aragonite in many shells, corals, and otoliths is in equilibrium or near-equilibrium with water, and, therefore, these materials may be used to determine water paleotemperatures based on the ratio of the 18O and 16O isotopes of oxygen, which depends on the isotopic composition, salinity, and temperature of water (e.g., Epstein, Buchsbaum, Lowenstam, & Urey, 1953; see Section 3.2). Given their ubiquity at archaeological sites and higher solubility compared with calcitic shells, aragonitic shells offer a good indication of the state of preservation of the archaeological record (see Section 3.1).
2.4 Geogenic aragonite
Aragonite is usually absent from limestone and chalk (i.e., a form of limestone rich in carbonate and silicate fossils such as coccoliths and radiolarians), the main types of CaCO3 rocks, due to their old age. Over geologic time spans, aragonite fossils derived from the accumulation of the hard parts of marine organisms on the ocean's floor recrystallize into calcite during the lithification process, and thus it is rare to find pristine aragonite fossils in rocks older than the Triassic (Buchardt & Weiner, 1981; Dauphin, 2002; Lippmann, 1973; Seuss et al., 2012). Other rare forms include aragonite in metamorphic rocks produced by enormous pressures on calcite crystals (Coleman & Lee, 1962), and evaporites formed in Mg-rich water in lakes such as the Great Salt Lake (McGee et al., 2012; Spencer et al., 1984) and the Lake Lisan formation, a Pleistocene precursor of the Dead Sea (Bartov, Stein, Enzel, Agnon, & Reches, 2002). Lisan aragonite forms characteristic acicular needles that exhibit poor atomic order when examined using FTIR (Figures 3 and 4a). In some instances, it is present in archaeological deposits at sites located near the paleo-lake (Elisabetta Boaretto, pers. comm.), and thus it should be distinguished from biogenic and pyrogenic forms. Other aragonite occurrences in rocks are ooids in limestone (e.g., Karkanas, Berna, Fallu, & Gauß, 2019), and cement and rhizoliths in aeolianites (e.g., Erginal et al., 2013).

The more common geogenic forms of aragonite are travertine and speleothems. Large, single crystals grow slowly by the addition of molecules that originate from supersaturated solutions (e.g., Rodriguez-Navarro, Ruiz-Agudo, Harris, & Wolf, 2016; Figure 4b). As a result, they show the highest degree of local structural order as seen using FTIR grinding curves (Figure 3). Aragonitic travertine forms at hot springs, where aragonite precipitates out of boiling water and may occur together with calcite (Jones, 2017). This type of aragonite may nucleate as acicular or pseudohexagonal crystals (Figure 4c). Speleothems such as stalactites, stalagmites and flowstones may be entirely composed of aragonite, calcite or a mixture thereof. The accretion process is caused by the precipitation of CaCO3 out of supersaturated solutions over geologic time spans, and it is particularly common in dolomitic caves due to the presence of Mg (e.g., Frisia et al., 2002). Speleothems often exhibit a layered structure with relatively large crystals that represents subsequent additions through time, and thus are suitable for the establishment of paleoenvironmental and chronological sequences, provided that corrections exist for isotopic fractionation and reservoir age effect (e.g., Brook et al., 2010; Lachniet, 2015; see Sections 3.2 and 3.4).
2.5 Pyrogenic aragonite
In a recent study, Toffolo and Boaretto (2014) showed that aragonite may form at earth surface conditions upon the carbonation of Mg-free CaO and Ca(OH)2 obtained by heating a CaCO3 starting material to temperatures above 600°C . This type of aragonite has been called “pyrogenic” because the CaO substrate required for its nucleation can be obtained only by burning CaCO3. While this is consistent with Ostwald's Rule of Stages, according to which the less stable polymorphs should nucleate first (Ostwald, 1897), the formation of aragonite at normal pressure is at variance with the phase diagram shown in Figure 2. To date, this anomaly remains unexplained, but holds considerable potential for the study of ancient pyrotechnology and for the chemistry of CaCO3. Toffolo and Boaretto (2014) showed that clues regarding the kinetics of the reaction may be sought in environmental factors such as temperature, relative humidity (RH), and CO2 partial pressure, which can affect the rate of aragonite nucleation and growth. In particular, low temperature and elevated CO2 pressure favor the occurrence of aragonite over calcite in quicklime. Early experiments involving the dissolution of CaCO3 in carbonated water have shown that aragonite may precipitate out of Ca(HCO3)2 solutions through slow evaporation, although at that time the occurrence of Mg was never verified (Michel, 1905; Warth, 1902). This is the main source of uncertainty also in more recent studies where the occurrence of aragonite cannot be unequivocally linked to the carbonation of Ca(OH)2. In a study based on the calcination of CaCO3 and subsequent hydration and carbonation of CaO, Dubina, Korat, Black, Strupi-Šuput, and Plank (2013) showed that aragonite forms at 80°C and 40–60% RH. These results are consistent with aragonite precipitation from boiling water.
Pyrogenic aragonite nucleates as acicular crystals a few hundred nm in length, often in clusters radiating from a center (Figure 4d). Toffolo et al. (2017) showed that with time, these needles can grow up to ~50 µm. At the level of local structural order, determined with FTIR, pyrogenic aragonite is poorly ordered; only Lisan aragonite is less ordered (Figure 3). Though pyrogenic aragonite nucleates from Ca(OH)2 obtained from several different CaCO3 substrates, all its occurrences may be grouped under two categories of anthropogenic materials relevant to archaeology, namely lime plaster and wood ash.
2.5.1 Lime plaster
Lime plaster is a synthetic form of CaCO3 that can only be produced through pyrotechnological activities (Weiner, 2010). To produce lime plaster, carbonate rocks such as limestone, chalk, marble, travertine, or dolomite are calcined at 850°C or higher temperature to obtain CaO, called quicklime. This highly reactive material is slaked with water to form Ca(OH)2, or hydrated lime, which can be applied to surfaces as coating or may be mixed with a carbonate or silicate aggregate to increase its volume and enhance mechanical properties. Upon setting, hydrated lime incorporates atmospheric CO2 while water evaporates, and thus it turns back to CaCO3 (Boynton, 1980). This pyrogenic form may nucleate as aragonite (see Section 3.4). In addition, the incorporation of CO2 from air implies that the newly formed crystals have the same 14C content as the atmosphere at the time of nucleation and may provide accurate 14C dates (see Section 3.4.2).
2.5.2 Wood ash
Wood ash is a byproduct of the combustion of plant material, and it is mainly composed of CaCO3 (Weiner, 2010). When wood species that produce the calcium oxalates CaC2O4∙H2O (whewellite) or CaC2O4∙2H2O (weddellite) are burnt to temperatures above 400°C, these decompose to CaCO3 in the form of calcite (Frost & Weier, 2003, 2004). This is loosely termed here “low-temperature ash.” If the temperature of combustion exceeds 600°C, CaCO3 will decompose to CaO, in a process similar to quicklime production. After the fire is extinguished, unstable CaO molecules react with humidity in air to form Ca(OH)2 (as in hydrated lime), which subsequently reacts with atmospheric CO2 and yields CaCO3. As stated, the latter may nucleate as aragonite under specific environmental settings (Toffolo & Boaretto, 2014). This is called here “high-temperature ash.” Therefore, pyrogenic aragonite can be used to identify combustion features (see Section 3.3.1). As in lime plaster, pyrogenic aragonite crystals bear the same 14C content as the atmosphere and, therefore, can be used for 14C dating (see Section 3.4.2).
3 ARCHAEOLOGICAL SIGNIFICANCE
The following sections review the occurrence of biogenic, geogenic and pyrogenic aragonite in archaeological contexts and the embedded information that may be extracted from well-preserved crystals to better understand the archaeological record.
3.1 Preservation of the archaeological record
The preserved archaeological record represents only a small portion of what was deposited at a site during its occupation. The ability to determine what components have disappeared during burial in sediments is of paramount importance in the reconstruction of past human activities, especially at the microscopic scale (Finkelstein et al., 2012; Karkanas, 2010; Karkanas, Bar-Yosef, Goldberg, & Weiner, 2000). Since minerals are the main component of archaeological deposits (including artifacts), most of the information about the missing record can be obtained from minerals. Aragonite is the most unstable mineral found at archaeological sites and it may readily undergo diagenesis under certain conditions (Weiner, 2010). The occurrence of aragonite is thus a good indicator of the degree of preservation of the archaeological record.
Aragonite is usually found in its biogenic form at archaeological sites, that is, shells of land snails or marine mollusks. Their preservation is greatly affected by the pH of groundwater and the hydrologic regime of the site. Typically, groundwater derived from rain contains carbonic acid, which lowers the pH to 5.7 and promotes aragonite dissolution (Karkanas, 2016). Groundwater in archaeological deposits that rest on carbonate bedrock or that are rich in geogenic/pyrogenic calcite is buffered to pH 8 and is thus conducive to aragonite preservation (Weiner, 2010). Similar conditions are achieved at sites where geogenic carbonates are absent, but groundwater is alkaline due to the presence of dissolved sodium carbonate, as at some brackish springs (e.g., Toffolo, Brink, & Berna, 2015). In contrast, plant root respiration and the decomposition of organic matter by bacteria and fungi produce CO2 and organic acids that decrease the pH of groundwater, ultimately leading to aragonite dissolution. For instance, if at a specific locale static groundwater is slightly acidic and undersaturated with respect to aragonite, it reacts with aragonitic shells embedded in sediment, which dissolve until the solution attains equilibrium. In solution, C and O isotopes from the shell are mixed with the same isotopes originated from groundwater movement. The same applies to minor elements such as Mg and U. At this stage, only part of the shell has dissolved. When the same groundwater migrates and is replaced by new undersaturated water in a constant flow, more aragonite dissolves until the entire shell disappears. It follows that this process takes place more rapidly in porous sandy sediments, whereas clay-rich deposits slow down the reaction. When groundwater reaches saturation or evaporates, and assuming that no other soluble mineral is involved in the diagenetic process, calcite precipitates preferentially at ambient conditions because it is more stable than aragonite (Figure 2). This calcite contains foreign isotopes that were present in solution, and the original composition of the shell is lost. If phosphate ions are present in solution, calcium phosphate will precipitate. It should be kept in mind that dissolution and recrystallization processes start at the nanoscale and affect the surface of single aragonite crystals. Therefore, a very small quantity of water is required to trigger the reaction (Pingitore, 1976).
The occurrence of aragonitic shells at a certain locale within an archaeological site thus implies that all of the less soluble carbonate and phosphate minerals, such as calcite, dolomite, and carbonate hydroxyapatite (bone mineral), should be well-preserved at the same spot. This is particularly important, considering that diagenesis can alter the original distribution and concentration of archaeological materials and features such as bones, teeth, lithic tools, burials, and hearths, and can disrupt the stratigraphic sequence and specific depositional contexts and microcontexts (Karkanas, 2016). Weiner, Goldberg, and Bar-Yosef (2002) tracked with FTIR the three-dimensional distribution of aragonite within prehistoric sediments at Hayonim Cave, Israel, to exclude bone dissolution in places where aragonite was preserved but bones were not found. A similar approach was used at other prehistoric sites such as Kebara Cave, also located in Israel (Weiner et al., 2007), Grotte XVI in France (Karkanas, Rigaud, Simek, Albert, & Weiner, 2002), Pinnacle Point 5-6 in South Africa (Esteban et al., 2018), and at Iron Age Tell es-Safi/Gath, Israel (Namdar et al., 2011). Aldeias, Goldberg, Dibble, and El-Hajraoui (2014) characterized the degree of preservation of biogenic aragonite at Contrebandiers Cave (Morocco) using micromorphology thin sections. If land snail shells are used as proxy, it should be verified that they are not intrusive. Hackberry endocarps, identified with FTIR, µFTIR, and XRD, are an indicator of good preservation (Quade et al., 2014; Shillito, Almond, Nicholson, Pantos, & Matthews, 2009). Another important implication of the preservation of aragonite is that dosimeters for luminescence and electron spin resonance dating will not be affected by the ionizing radiation of secondary 40K released upon the dissolution of wood ash, which is mainly comprised of unstable pyrogenic calcite and siliceous aggregates rich in potassium (Mercier et al., 1995; Weiner et al., 2002). Conversely, where biogenic aragonite recrystallizes partially or totally into calcite, diagenesis has taken place and therefore the preservation of ash or lime plaster should not be expected due to their low degree of atomic order (Figure 3). Since changes in hydrology and sedimentation at a site are often caused by changes in climatic conditions, the alteration of aragonitic shells and less soluble minerals may be used to reconstruct the evolution of the local paleoenvironment (Karkanas, 2016). Pyrogenic aragonite can provide an even more accurate proxy. Its poor degree of structural order and the high surface-to-volume ratio of acicular crystals make it extremely prone to dissolution. When it is found preserved, it can be expected that the preservation of the archaeological record will be very good. Therefore, it is suggested here that a more subtle way of assessing diagenesis is the application of XRD (Pokroy et al., 2004) and FTIR grinding curves (Toffolo, Regev, et al., 2019) to the characterization of local structural order in aragonite crystals (Figure 3).
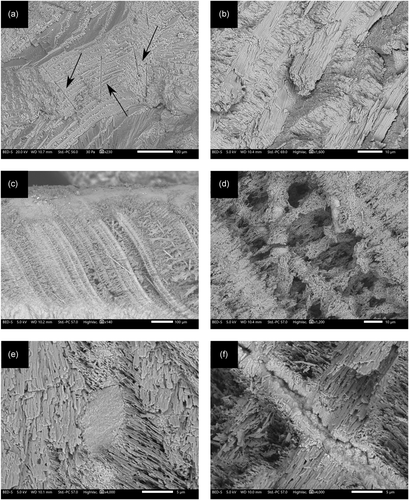
The fact that the mineralogy of aragonitic shells is preserved does not imply that their microstructure, isotopic signature, and trace-element composition are unaltered. Secondary aragonite crystals may nucleate in primary biogenic and geogenic aragonite materials. This uncertainty regarding the preservation of primary aragonite requires characterization at the microscopic scale. Figure 5 shows SEM images of a G. insubrica shell collected from the shore at Ashkelon, Israel, and a G. insubrica shell from an Iron Age context in ancient Ashkelon (Stager, Schloen, & Master, 2008), located about 100 m from the shore. According to FTIR, both specimens are entirely made of aragonite, and therefore could be considered pristine. However, a closer look using SEM shows a different situation. The beach shell exhibits the typical crossed-lamellar microstructure with well-preserved bundles of lath-like aragonite crystals grooved with microtubuli (Böhm et al., 2016; Figure 5a,b). The archaeological shell instead shows high porosity, disrupted bundles, secondary aragonite crystals, and dissolution features that betoken isotopic alteration (Figure 5c–e). Microtubuli probably favor the penetration of water (Figure 5f). Therefore, the archaeological shell is likely not suitable for paleoenvironmental reconstruction or absolute dating.
3.2 Paleoenvironments
The trace-element and isotopic signatures of aragonitic speleothems, corals, shells and otoliths can be used to reconstruct water paleotemperatures and rainfall records and thus contribute to a better understanding of paleoenvironments at specific sites (e.g., Gagan et al., 2000; Gillikin et al., 2005; Lachniet, 2015; Peacock et al., 2016). Several organisms mineralize aragonite whose oxygen isotopic composition is in equilibrium or near-equilibrium with water, and these species are used for paleoclimate studies (Campana, 1999; Epstein et al., 1953; Weiner & Dove, 2003). In contrast, carbon isotopes are affected by metabolic processes and environmental factors that determine fractionation (e.g., Campana, 1999; Grossman & Ku, 1986; McConnaughey & Gillikin, 2008). Some aragonitic speleothems require a correction for the fractionation of oxygen isotopes (Lachniet, 2015). Otoliths feature growth layers that are deposited every day during the life of the fish, and thus can be used to assess the age and season of death of the specimen (Disspain, Ulm, & Gillanders, 2016). Similarly, some mollusk species grow seasonal or annual aragonite layers that provide a chronological sequence of geochemical data (Andrus, 2011; Milano, Schöne, & Witbaard, 2017). Seed endocarps can yield valuable δ18O data sets (Quade et al., 2014). Water paleotemperature curves, rainfall records, and geochemical information obtained from speleothems and shell middens are particularly important in the study of cave and coastal sites, provided that aragonite is not affected by diagenesis. This becomes especially apparent when shells, otoliths and corals are buried in archaeological deposits, and speleothems are exposed to hydrologic regimes that favor aragonite recrystallization. Minor diagenesis cannot be detected visually, yet it may significantly alter isotopic and trace-element compositions.
3.2.1 Biogenic aragonite
Buchardt and Weiner (1981) showed using SEM that aragonitic ammonites from bituminous shales in Greenland, dated to the Cretaceous, suffered from mild dissolution and recrystallization of aragonite crystals into more compact secondary aragonitic structures and secondary calcite. These processes resulted in altered Mg/Ca ratios and δ13C values. In a similar study, Seuss et al. (2012) analyzed aragonitic cephalopods from the Late Carboniferous Buckhorn Asphalt Quarry in Oklahoma using XRD and SEM-EDS, and showed that secondary calcite formed in localized areas of the specimens. Diagenetic alteration determined higher Mg contents and δ13C–δ18O values in disagreement with existing data for the Late Carboniferous. Both studies focused on extremely old and exceptionally well-preserved specimens embedded in depositional contexts protected from water. This means that diagenesis took place immediately after the death of the organism and halted after inclusion within the rock formation. More important, these examples highlight the disruptive action of water and provide a term of comparison for biogenic aragonite in archaeological sediments, which are much more exposed to groundwater.
Several studies pointed out the problematic occurrence of secondary aragonite in mollusk shells, beside secondary calcite. Guzmán, Dauphin, Cuif, Denis, and Ortlieb (2009) analyzed modern and archaeological Concholepas concholepas (Chilean abalone) shells from the hyperarid Atacama region (Chile) using FTIR, SEM-EDS, and atomic force microscopy, and showed that the inner aragonitic layers of Holocene and Pleistocene specimens are affected by dissolution and recrystallization processes that result in depletion of the organic matrix and altered elemental composition. The authors conclude that polymorph assessment alone cannot be considered a sufficient screening criterion for paleoenvironmental analysis, and that the preservation state of each specimen should be thoroughly characterized. Faylona et al. (2011) reached similar conclusions regarding the use of archaeological Tridacna maxima (small giant clam) and Hipponus hipponus (bear paw clam) shells. Using µFTIR and SEM, they showed that these species of giant clams from the Balobok Neolithic midden (Philippines) are significantly altered by dissolution and nucleation of secondary aragonite as a result of exposure to meteoric freshwater in archaeological deposits, and thus cannot provide reliable paleoenvironmental information. Bayer et al. (2013) found that Holocene and Pleistocene shells of Amiantis purpurata (venus clam) collected from rock outcrops in Patagonia exhibit alteration features of the aragonite microstructure (based on XRD and SEM), which are linked to changes in elemental composition. Webb, Price, Nothdurft, Deer, and Rintoul (2007) identified secondary aragonitic cement of geologic origin in freshwater Velesunio ambiguus (floodplain mussel) shells using µRaman and SEM, and concluded that its nucleation has been caused by meteoric groundwater rich in Mg.
Corals may undergo underwater diagenesis and recrystallize into secondary aragonite or calcite regardless of depth, due to a number of factors including water flow, temperature, salinity, pressure, undersaturation with respect to aragonite, low Mg concentration, and secondary biomineralization processes (Haley, 1984; McGregor & Gagan, 2003; Webb, Nothdurft, Kamber, Kloprogge, & Zhao, 2009). If corals are found embedded in archaeological sediments, diagenesis might be more severe and lead to the complete recrystallization of aragonite into calcite. Secondary aragonite may nucleate on pre-existing aragonite crystals, especially in an Mg-rich environment such as sea water (Enmar et al., 2000; Perrin, 2004). Hendy, Gagan, Lough, McCulloch, and deMenocal (2007) showed that the occurrence of secondary aragonite in Porites corals determined anomalies in their elemental and isotopic composition.
Little is known about the diagenetic alteration of fish otoliths, and secondary aragonite or calcite have not yet been reported in archaeological specimens (Disspain et al., 2016). However, Dufour, Cappetta, Denis, Dauphin, and Mariotti (2000) showed that Pliocene otoliths entirely composed of aragonite exhibit altered microstructure and anomalous δ18O values. Long et al. (2018) postulated a diagenetic alteration of Pleistocene otoliths from Lake Mungo (Australia) based on high Ba/Ca ratios obtained from areas close to the surface, although the mineralogical composition was not verified.
While the occurrence of secondary calcite in shells, corals and otoliths may be determined based on bulk mineralogical analyses such as XRD and FTIR, the studies described above all point out that mineralogical assessment alone is not sufficient to exclude the presence of secondary aragonite. µFTIR can identify aragonite in relatively small areas (25 × 25 µm), but in most cases crystals are much smaller. In addition, as of today there is no way of distinguishing aragonite crystals characterized by different degrees of local structural order based on reflectance spectra. The higher resolution of µRaman allows to overcome this limit, and the study of peak broadening in biogenic aragonites has shown that it is possible to distinguish degrees of structural order in single crystals (e.g., Sibony-Nevo et al., 2019). This characterization approach, coupled with micro-drilling or laser ablation sampling methods, may provide more accurate paleoenvironmental data (e.g., Jochum et al., 2012).
3.2.2 Geogenic aragonite
Aragonitic speleothems are affected by changes in the pH of cave water and by undersaturation with respect to aragonite, which determine its complete dissolution or its recrystallization into calcite with loss of elemental and isotopic composition (e.g., Hopley, Marshall, & Latham, 2009; Railsback, Dabous, Osmond, & Fleisher, 2002). Martín-García, Alonso-Zarza, and Martín-Pérez, (2009) and Martín-García et al. (2019) showed using XRD, thin-section petrography, SEM and electron microprobe analysis that primary aragonite in speleothems from Castañar Cave (Spain) exhibits dissolution features, secondary calcite growths on aragonite needles, and aragonite relics embedded in secondary calcite. These structural and mineralogical changes have been linked to discrepancies observed in δ18O and δ13C values. In a similar study of a speleothem from Eagle Cave (Spain) and including particle-induced X-ray emission analysis and electron backscatter diffraction, Dominguez-Villar et al. (2017) showed that the recrystallization process altered Mg and Sr contents and δ18O–δ13C values. Based on SEM images and µRaman, Perrin et al. (2014) determined that isomineral aragonite–aragonite recrystallization occurs in speleothems as part of the process that leads to the formation of secondary calcite. Acicular aragonite crystals grow in size and change their morphology via Ostwald ripening to ray-shaped crystals, which are characterized by slightly different elemental composition (Mg and Sr). It is not known whether secondary aragonite in speleothems inherits the isotopic signature of the parent phase, and further research in this direction is needed, possibly via laser ablation (Jochum et al., 2012; Wassenburg et al., 2012).
3.3 Pyrotechnology
3.3.1 Combustion features
The combustion of organics to obtain fire has played a key role in the biological and cultural evolution of the genus Homo, as evidence of controlled use of fire dates back to ∼1 million years ago (Berna et al., 2012). Finding that kind of evidence, even in relatively recent periods, is rather difficult, especially in the absence of combustion structures such as hearths and ovens. It follows that only the analysis of the microscopic archaeological record may provide clues about the occurrence of combustion features and whether these originate from deliberate use of fire (Goldberg, Miller, & Mentzer, 2017; Weiner, Xu, Goldberg, Liu, & Bar-Yosef, 1998). Wood ash is the most common indicator of the presence of burnt material, as it is a major byproduct of the combustion of plants (Weiner, 2010). As stated, calcite is the only polymorph in low-temperature ash, whereas high-temperature ash may contain calcite and aragonite (Toffolo & Boaretto, 2014).
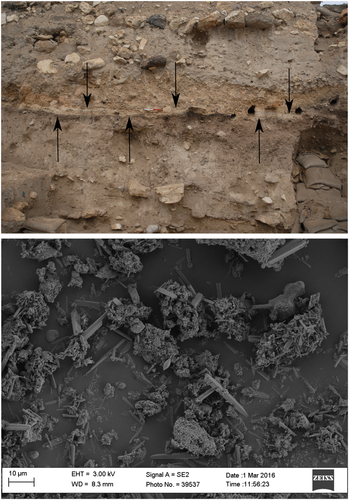
Pyrogenic aragonite has been reported in combustion features ranging from the Upper Paleolithic to the Iron Age, mainly in Israel, and in experimental ashes as well (Asscher et al., 2015; Gur-Arieh et al., 2014; Regev et al., 2015; Toffolo et al., 2017; Toffolo & Boaretto, 2014, table 1). One occurrence has been found by the author in an Iron Age hearth at Lefkandi, Greece (unpublished results). Dunseth et al. (2019) obtained aragonite by burning archaeological dung pellets. Weiner (2010, p. 283) noted the presence of aragonite at Hayonim Cave in sheep dung burned in historical times. Pyrogenic aragonite always nucleates together with pyrogenic calcite and usually forms in association with heat-altered clay minerals and melted phytoliths, which are consistent with high-temperature burning, and therefore can be regarded as an indicator of fire-related human activities when structures are not visible. Since pyrogenic aragonite requires CaO to nucleate, and CaO is obtained at temperatures above 600°C, aragonite can be used to estimate the temperature reached by a fire.
The relatively low occurrence of aragonite compared with calcite in ash layers whose composition has been characterized is due to two possible factors: (a) the temperature reached by short-lived fires did not exceed 600°C, a necessary step for the decomposition of CaCO3 to CaO; (b) aragonite completely recrystallized into calcite. The most effective method to identify pyrogenic aragonite is FTIR spectroscopy, which is sensitive to small amounts of poorly ordered phases (Toffolo & Berna, 2018). However, FTIR is not routinely used to analyze archaeological sediments, and, therefore, there are doubts as to whether aragonite in ash has been missed several times in the past, or confused with biogenic aragonite from shells that did not completely turn into calcite upon heating. To discern the two, Toffolo, Regev et al. (2019) characterized different types of aragonite reference standards using FTIR grinding curves. They showed that biogenic aragonite from G. insubrica, one of the most common bivalves found at archaeological sites in Israel (e.g., Sivan et al., 2006), has a grinding curve markedly different from the curve of pyrogenic aragonite (Figure 3). Therefore, it is possible to assess whether aragonite found within combustion features is of biogenic or pyrogenic origin (e.g., Albert et al., 2008, table 2; Esteban et al., 2018). This method should be calibrated based on the type of biogenic aragonite available at the site, since different organisms mineralize aragonite crystals with various degrees of atomic order (Suzuki et al., 2011). In addition, pyrogenic aragonite offers a clear-cut assessment of heat-induced alteration compared with pyrogenic calcite, as the latter can easily be mixed with geogenic calcite from sediments resting on top of chalk or limestone bedrock (thus yielding an uncertain FTIR signature; Regev et al., 2010: 3027), whereas geogenic aragonite bedrock is extremely rare.
Evidence of combustion can be found also when aragonitic shells are exposed to elevated temperatures, for instance in hearths. When the shells of organisms that mineralize aragonite are found in presumed combustion features and turn out to be made of calcite, it is safe to assume that they were exposed to temperatures >300°C (Yoshioka & Kitano, 1985). If they are entirely made of aragonite, either they were not burnt at all or the temperature might have been lower than 300°C. If other lines of evidence suggest the presence of ash but shells are unaltered, then the latter might well have been introduced at a later stage. Toffolo, Martin, Master, and Boaretto (2018) used calcitic (i.e., heat-altered; analyzed with FTIR) G. insubrica shells to confirm the occurrence of ash layers within a Late Bronze Age grain silo at Ashkelon, Israel. Villagran, Balbo, Madella, Vila, and Estevez (2011) burned Mytilus edulis (common mussel) shells to different temperatures to build a micromorphology reference collection of shells for the study of archaeological middens in South America. A similar approach was used by Villagran (2014) with Anomalocardia brasiliana (venus clam) shells. Aldeias, Gur-Arieh, Maria, Monteiro, and Cura (2019) characterized the recrystallization of aragonite into calcite upon heating in different species to investigate shellfish roasting from an experimental standpoint and with the aid of FTIR, µFTIR, XRD, and micromorphology. Karkanas et al. (2019) characterized with µFTIR the mineralogy of fossil aragonitic oolites embedded in the construction material of a Late Bronze Age pottery kiln in Greece to distinguish unaltered areas (aragonite) from areas affected by elevated temperatures (calcite). Finally, it should be borne in mind that low-temperature cooking of shells may alter their isotopic signature but not their mineralogy. Milano et al. (2018) showed using µRaman and SEM that heating Phorcus sp. shells to 300°C does not result in aragonite recrystallization into calcite, although the temperature is high enough to determine microstructural changes and anomalies in δ18O values. Müller et al. (2017) used carbonate clumped-isotope thermometry to show that boiling shells in sea water does not affect carbon and oxygen isotopes, whereas roasting at ∼175°C induces changes in the clumped-isotopic composition of aragonite. Similarly, Andrus and Crowe (2002) showed that boiling and roasting at ∼150°C may alter the elemental composition of otoliths. This information is particularly important for the paleoenvironmental study of shell middens and coastal sites. Visual control of these alterations via SEM in experimental samples would add more lines of evidence to the evaluation of archaeological specimens, together with the characterization of the degree of structural order of aragonite crystals.
3.3.2 Lime plaster
Controlled use of fire in prehistory eventually resulted in high-temperature burning of carbonate rocks to obtain quicklime for lime plaster production (Artioli, Secco, & Addis, 2019; Kingery, Vandiver, & Prickett, 1988). Optical microscopy and FTIR spectroscopy can securely identify pyrogenic carbonates in plaster (e.g., Friesem, Abadi, Shaham, & Grosman, 2019; Karkanas, 2007). Pyrogenic aragonite has been reported in lime plasters from the Pre-Pottery Neolithic B in Israel, as well as more recent periods such as the Middle Ages, always in association with pyrogenic calcite, which is the main polymorph found in plasters (Ricci et al., 2020; Toffolo & Boaretto, 2014, table 1; Toffolo, Regev, et al., 2019). Therefore, aragonite is another proxy that can be used in the identification of lime plaster products, especially when mixed with calcitic sediments of geologic origin. One of the few documented examples of Iron Age lime kiln featured the presence of pyrogenic aragonite in lime plasters residues found at the bottom of the structure (Eliyahu-Behar, Yahalom-Mack, & Ben-Shlomo, 2017). Aragonite was also detected in lime plaster-like material identified in slags from iron production at Iron Age sites in Israel (Eliyahu-Behar, Yahalom-Mack, Gadot, & Finkelstein, 2013).
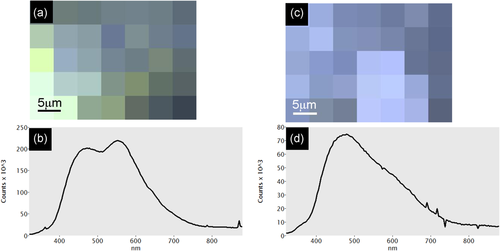
In the absence of experimental studies focused on the carbonation of Ca(OH)2 under different conditions, it is not possible to state whether the nucleation of aragonite is caused by the presence of Mg at the level of single Ca(OH)2 crystals or by kinetics only, and thus to a specific production technology. Research in this direction is much needed, including the assessment of the spatial distribution of different polymorphs based on µFTIR and µRaman (e.g., Poduska et al., 2012). A study by Eliyahu-Behar et al. (2009) showed that aragonite formed in plaster coatings applied to ceramic casting molds of Roman age, and that its occurrence might be linked to the presence of Mg in the underlying ceramic matrix. It is known that aragonite forms in hydraulic mortars and cements as a secondary phase, although not from the carbonation of Ca(OH)2 (e.g., Secco et al., 2019). In either case, the presence of more soluble aragonite together with calcite indicates good overall preservation of plaster through time. This can be assessed using the method proposed by Toffolo, Regev, et al. (2019) for aragonite-calcite mixtures, based on the intensity of the ν4 infrared absorption at 713 cm−1, which is common to both calcite and aragonite. The same method allows the analysis of calcite, which instead might in part derive from the recrystallization of aragonite into calcite. Additionally, SEM-CL can identify pristine pyrogenic aragonite and calcite based on the emission of blue luminescence, whereas orange luminescence is indicative of Ca2+ substitution by Mn2+ and thus of diagenesis (Toffolo, Regev, et al., 2020; Toffolo, Ricci, Chapoulie, Caneve, & Kaplan-Ashiri, 2020; Toffolo, Ricci, et al., 2019; Wendler, Wendler, Rose, & Huber, 2012; Figure 6).
3.4 Absolute dating
The instability of aragonite under acidic conditions entails the loss of its original isotopic signature. This has far-reaching implications and becomes especially apparent in the case of U-series dating of speleothems and corals and 14C dating of shells, given the extremely small amount of contamination necessary to significantly affect measurements, ultimately leading to problematic chronologies.
3.4.1 U-series dating
The radioactive decay of uranium and thorium isotopes embedded in the CaCO3 lattice can be exploited to calculate the age of aragonitic speleothems and corals from the last ~500,000 years (Ivanovich & Harmon, 1992). These materials provide absolute ages for the archaeological sediments, fossils and artifacts with which they are associated (e.g., Dirks et al., 2017; Pickering et al., 2010). In addition, flowstones underlying and overlying parietal art can be used to constrain the age of prehistoric paintings, or at least provide a minimum/maximum age (e.g., Pons-Branchu et al., 2014). Corals may occur at open-air sites, where they were brought by humans for ritual purposes (e.g., Weisler, Hua, & Zhao, 2009), as tools (Burley, Weisler, & Zhao, 2012), or as architectural elements (Sharp, Kahn, Polito, & Kirch, 2010). In addition, corals are found at sites that were once submerged (e.g., Walter et al., 2000). Another major contribution of the U-series method lies in the dating of corals and speleothems used to build and improve the 14C calibration curve beyond 13,000 cal BP (Reimer et al., 2013).
The U-series method is accurate only if the materials selected for dating do not exchange U and/or Th with the environment after their formation, that is, they represent a closed system. This condition is not always satisfied in corals, which require removal of contaminated areas before dating. Under unfavorable conditions, primary aragonite recrystallizes into secondary aragonite or calcite and loses its original isotopic composition (e.g., Hendy et al., 2007; Pons-Branchu, Hillaire-Marcel, Deschamps, Ghaleb, & Sinclair, 2005). Therefore, calcite in corals should always be considered younger than aragonite. It has been shown that a calcite content under 1%, as determined by XRD, does not affect U-series ages (Durand et al., 2013 and references therein). For larger calcite contents, calcite and aragonite may be separated by centrifugation in lithium polytungstate based on their different specific gravity, following the method of Douka, Hedges, and Higham (2010) developed for 14C dating of mollusk shells. The purity of the separates should be checked with XRD. When secondary aragonite is present, it may be distinguished based on crystal habit (often acicular) and trace-element composition, and should not be selected for U-series dating (e.g., Bar-Matthews, Wasserburg, & Chen, 1993; Enmar et al., 2000; Perrin, 2004).
Speleothems, too, are prone to dissolution and recrystallization (Hopley et al., 2009). Therefore, in areas predominantly characterized by aragonite, the presence of calcite should be regarded as possible evidence of diagenetic alteration. Secondary calcite can be identified using XRD and optical, electron and FTIR/Raman microscopy. More specifically, in layered structures pristine aragonite grows in bundles of acicular crystals, whereas secondary calcite occurs mainly as large equant crystals (sparitic calcite), rather than the columnar crystals typical of primary calcite (Frisia et al., 2002; Martín-García et al., 2009; Perrin et al., 2014). Recrystallization of aragonite into calcite leads to U loss that causes older apparent ages, assuming that Th is immobile (Th4+ is insoluble in most aqueous environments). This is due to the fact that the large CaO9 polyhedron of aragonite is better suited to trap large cations such as UO22+, compared with the smaller CaO6 octahedron of calcite (Kelly et al., 2003; Reeder, Nugent, Lamble, Tait, & Morris, 2000). Upon recrystallization, part of the uranyl is leached and U-series ages are thus overestimated (e.g., Bajo et al., 2016; Lachniet, Bernal, Asmerom, & Polyak, 2012; Ortega, Maire, Devès, & Quinif, 2005; Railsback et al., 2002), although in some cases secondary calcite might form in semi-closed geochemical conditions and exhibit similar U content as parent aragonite (Dominguez-Villar et al., 2017). This is a consequence of the limited diffusion capability of large chemical species containing uranyl within thin films of water, and the affinity of some calcite surfaces for the incorporation of U chemical species (Reeder et al., 2001). In addition, it should be kept in mind that aragonite exposed to dripping water, for example, at the tip of stalagmites, may be affected by U leaching due to partial dissolution (Martín-García et al., 2019). Cross-checking of nearby speleothems can help identify anomalies in the chronological sequence. Another way of identifying secondary calcite before dating is to measure U contents via microprobe analysis. Secondary calcite derived from the dissolution of aragonite shows higher U content compared to primary calcite (Ortega et al., 2005). When fragments of aragonitic speleothems are found embedded in cave sediments, for instance the so-called straw stalactites used as maximum age for the layer in which they are found, these should be carefully characterized to ascertain that no secondary phases such as calcite or calcium phosphate formed from the reaction between aragonite and groundwater.
Pons-Branchu et al. (2020) postulated that the recrystallization of aragonite into calcite at Nerja Cave (Spain), which is known to contain aragonite straw stalactites, is the cause of the inverse relation between U content and 230Th/234U ratios and wrong U-series ages obtained from speleothems covering Upper Paleolithic parietal art at the site. According to the authors, the same process might have caused the overestimation of U-series ages of speleothems covering presumed Middle Paleolithic paintings at three other caves in Spain, which have been attributed to Neanderthals (Hoffmann et al., 2018). The characterization of speleothems selected for U-series dating is therefore a crucial step to avoid open-system conditions where secondary minerals derive from the diagenesis of primary aragonite. µFTIR and µRaman on thin sections can provide spatially resolved information on the occurrence of secondary calcite, whereas SEM can identify diagenetic structures. As stated, secondary aragonite may form in speleothems. However, it is not known whether its U content is altered compared to primary aragonite (Perrin et al., 2014). Thus, further research on this aspect is highly desirable. In addition to optical microscopy, secondary aragonite crystals may be identified based on Raman peak broadening (Sibony-Nevo et al., 2019) and sampled by laser ablation (Lin et al., 2017).
3.4.2 14C dating
Aragonite contains carbon and, therefore, can be dated using 14C if the age of the sample falls within the range of the method, that is, ~50,000 years. Aragonitic speleothems, mollusk shells and fish otoliths have been extensively used to obtain absolute dates at archaeological sites across the globe. The main requirement for accurate dating is that the 14C content of the selected material changes over time only due to radioactive decay, and not because of external factors such as diagenesis (Bowman, 1990). In addition, the 14C content of fresh and sea water differs considerably from that of the atmosphere, and region-specific corrections for reservoir age effect should be applied (e.g., Brook et al., 2010; Reimer & McCormac, 2002).
Speleothems embedded in archaeological sediments may provide chronological pegs (flowstone) or a maximum age (straw stalactite) if aragonite is pristine. This can be verified with the same methods outlined above for U-series dating, for example, optical and electron microscopy, XRD, Raman, and FTIR. Only primary aragonite should be targeted for 14C dating. If speleothems are layered and well-preserved, a chronological sequence can be established for organic material found embedded in aragonite, for example, pollen, and for climatic proxies such as δ18O and δ13C and Mg/Ca ratios, provided that corrections for carbon fractionation and reservoir age effect are applied (Brook et al., 2010; Railsback, Brook, Chen, Kalin, & Fleisher, 1994).
Shells of live bivalves/gastropods brought to the site for food consumption (as opposed to shells collected post mortem) provide an age for the sedimentary matrix in which they are found and for the associated artifacts. XRD or FTIR should be performed to verify that no calcite is present due to diagenetic alteration (e.g., Barbieri et al., 2018; Bosch et al., 2015). Standard sample pretreatment often includes etching of the shell surface with diluted HCl aimed at removing calcite, although there is no control over the reaction and how it may affect the preserved aragonite (e.g., Chiu, Fairbanks, Mortlock, & Bloom, 2005). The method developed by Douka et al. (2010) allows separating the two polymorphs based on specific gravity, and the purity of the separates should be verified with XRD. Toffolo et al. (2017) improved the method by adding a sodium phosphate buffer that increases the pH of the initial acidic solution to 8, thus minimizing carbonate dissolution. As shown above (Figure 5), aragonite crystals in shells may recrystallize into secondary aragonite due to Ostwald ripening or to secondary biomineralization and geogenic processes, which entail isotopic exchange with the environment (Busschers et al., 2014; Webb et al., 2007). This phenomenon poses a major problem in the selection of suitable samples for 14C dating. Bosch et al. (2015) proposed to use the degree of intra-crystalline protein diagenesis based on amino acid racemization as an indicator of closed-system behavior in aragonite and thus support the selection of specific samples for dating. Given that polymorph separation by specific gravity is ineffective in such instances, the degree of preservation of crystals may be determined in cross section based on SEM imaging, assuming that the inner layers are less exposed to diagenesis. If the inner layers are indeed well-preserved, they may be isolated manually by abrasion. In addition, Raman peak broadening may differentiate aragonite crystals characterized by varying degrees of local structural order as a result of different formation paths (Sibony-Nevo et al., 2019). Secondary calcite in shells may also be a result of exposure to elevated temperatures. Lindauer, Milano, Steinhof, and Hinderer (2018) showed that this transition does not significantly affect the accuracy of 14C measurements, presumably because it does not involve a major isotopic exchange.
Secondary aragonite or calcite in otoliths has not yet been reported, however, this should be considered a likely scenario in view of the abovementioned studies on speleothems and mollusk shells. The degree of preservation of aragonite and the presence of calcite should be verified using XRD or FTIR before dating (Long et al., 2014, 2018). Calcite should be removed by specific gravity or abrasion rather than etching in acid.
Recently, also hackberry endocarps have been dated. Quade et al. (2014) obtained 14C ages from endocarps recovered at Neolithic Aşıklı Höyük (Turkey), which are systematically younger than associated charcoal by ∼130 14C years. This discrepancy has been ascribed to possible aragonite recrystallization into calcite; the latter has been identified with XRD in some specimens.
Since most organisms do not mineralize aragonite in equilibrium with the environment, a correction for carbon fractionation should be applied. In some cases, mollusks precipitate aragonite with a 14C content in equilibrium with that of water, thus allowing the identification of specific age reservoirs for different types of water and regions (e.g., Lev, Boaretto, Heller, Marco, & Stein, 2007). Alternatively, independent reservoir age corrections must be compiled, for both sea water and lakes (e.g., Reimer & McCormac, 2002). With regard to the fish otoliths dated at Lake Mungo (Australia), a reservoir age effect was excluded based on the analysis of modern freshwater shells (Long et al., 2014).
Pyrogenic aragonite may provide accurate 14C dates as well. As stated, upon carbonation Ca(OH)2 reacts with atmospheric CO2. The latter bears the 14C signature of the atmosphere and the resulting CaCO3 may be used to obtain age determinations. Indeed, pyrogenic calcite has been targeted since the 1960s to date lime mortars (Labeyrie & Delibrias, 1964). However, the accuracy of the method is significantly limited by several carbonate contaminants, such as secondary calcite, to the point that presently a single approach to date any type of mortar is not available (Hajdas et al., 2017). Similar problems affect calcite in wood ash (e.g., Koumouzelis et al., 2001), although promising results have been obtained from well-preserved crystals (Regev, Eckmeier, Mintz, Weiner, & Boaretto, 2011). Since aragonite is more soluble than calcite and does not precipitate out of bicarbonate, one could safely assume that if the pyrogenic form is found in sediments, then it is likely pristine (Boaretto, 2015; Toffolo & Boaretto, 2014). In a recent study, Toffolo et al. (2017) succeeded in extracting pyrogenic aragonite from ash found in an Iron Age destruction layer at Megiddo (Israel; Figure 7). Acicular aragonite crystals were separated from other phases, and especially calcite, based on its high specific gravity using centrifugation in sodium polytungstate buffered at pH 8. Carbon recovery was performed via thermal decomposition in vacuum of the purified fraction. The pMC values of aragonite crystals matched the values of charred seeds collected from the same destruction layer, thus confirming that pyrogenic aragonite may be considered a closed carbon system. This method can be used to date ash layers within the range of 14C in the absence of organics, provided that no secondary aragonite crystals are selected. The latter have not yet been reported. Given that calcite is usually the main component of wood ash, the application of the specific gravity method to high-temperature calcite produced through a CaO step would raise considerable interest.
4 CONCLUSIONS
The occurrence of aragonite in materials commonly found at archaeological sites, such as mollusk shells, corals, otoliths, speleothems, ash, and lime plaster, offers the possibility of extracting microscopic embedded information of crucial importance for the study of the archaeological record. This information includes postdepositional processes that determine the diagenesis of biogenic, geogenic, and pyrogenic aragonite, with far-reaching implications for the reconstruction of paleoenvironments and the establishment of absolute chronological sequences, and pyrotechnological processes linked to human use of fire. The elemental and isotopic composition of different types of aragonite is reliable only if crystals are pristine and reflect environmental conditions at the time of nucleation. This review has shown that a simple mineralogical assessment of the presence of aragonite is not sufficient to exclude contamination from secondary phases, and this becomes especially apparent in the case of paleoenvironmental reconstructions and absolute dating. The nucleation of secondary aragonite is particularly troublesome in this sense and requires the application of methods that address the basic structural and chemical properties of crystals and can identify secondary phases produced by Ostwald ripening. In particular, peak broadening in transmission FTIR has provided significant insights into the degree of local structural order of different types of aragonite, and allowed distinguishing pyrogenic aragonite from natural forms. Peak broadening in µRaman has produced promising results in the study of structural order in single biogenic aragonite crystals and may be used to retrieve spatially resolved information from speleothems and pyrogenic materials. In addition, further research is necessary to better characterize the elemental composition of secondary aragonite.
ACKNOWLEDGMENTS
This study was funded by a grant that I received from IdEx Bordeaux (grant n. ANR-10-IDEX-03-02), and is built on previous research that I conducted with the support of the Alexander von Humboldt Foundation and the Kimmel Center for Archaeological Science (Weizmann Institute of Science), which I gratefully acknowledge. I wish to thank Maïlys Richard for help in preparing Figure 1, Yannick Lefrais for help with SEM imaging, Daniel Master for access to the Iron Age shell during fieldwork at Ashkelon and Steve Weiner for providing Lisan and kettle aragonite samples. Finally, I would like to thank the Reviewers and Review Editor for their insightful comments, which improved the manuscript.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




