Influence of Cytogenetics on the Outcome of Patients With High-Risk Myelodysplastic Syndrome Including Deletion 5q Treated With Azacitidine With or Without Lenalidomide
Funding: This work was supported by Nordic Cancer Union, Cancerfonden, Celgene.
ABSTRACT
In myelodysplastic syndromes (MDS), cytogenetic characteristics of the malignant bone marrow cells influence the clinical course. The aim of this study was to evaluate whether cytogenetics is useful to predict outcome and response in patients with del(5q) under azacitidine (AZA) ± lenalidomide (LEN) therapy. We therefore performed comprehensive cytogenetic analyses in MDS patients with del(5q) treated within the randomized phase II trial NMDSG10B. Seventy-two patients were enrolled in the study and 46 patients (64%) had sufficient cytogenetics at inclusion and response evaluation. Karyotyping was significantly more sensitive during follow-up to detect del(5q) compared to FISH, 34 patients (97%) versus 27 patients (77%) (p = 0.027). The overall response rate (ORR) did not differ between the 11 patients with < 3 aberrations (median 1 aberration) and the 59 patients with ≥ 3 aberrations (median 7 aberrations, range 3–16), while ≥ 3 aberrations were associated with shorter overall survival (OS), 9.9 months versus 25.2 months (p = 0.004). OS was significantly shorter in patients with unbalanced translocation of 5q than patients with del (5)(q14q34), 8.4 months versus 21.1 months (p = 0.004). Both complex karyotype and multi-hit TP53 alterations were more frequent in patients with unbalanced translocations of 5q versus del (5)(q14q34), 98% and 88% versus 67% and 47% (each p = < 0.001). Most patients with cytogenetic progression had multi-hit TP53 alterations at inclusion. Cytogenetic progression occurred at a similar frequency in the AZA arm and in the AZA + LEN arm. In summary, this study in homogenously treated MDS patients with different abnormalities of 5q demonstrates the influence of cytogenetics on treatment results.
Trial Registration: EudraCT number: 2011-001639-21; ClinicalTrials.gov identifier: NCT01556477.
1 Introduction
Patients with myelodysplastic syndrome (MDS) constitute a heterogeneous group with varying prognosis. Clonal chromosomal aberrations detected in approximately half of MDS patients [1] provide valuable diagnostic and prognostic information [2]. Lower-risk MDS with an isolated deletion of 5q (del(5q)) is known to have a favorable prognosis with a median survival of 80 months and a low risk of transformation into acute myeloid leukemia (AML) [3]. On the other hand, patients with an increased number of marrow blasts, particularly those with a complex karyotype, have a poor prognosis and a high risk of transformation into AML. A complex karyotype including del(5q) or t(5q) nearly always an unbalanced translocation is common in high-risk MDS and encompass approximately 15% of the high-risk population [4, 5]. If the complex karyotype contains del(5q), the prognosis is dismal with a median survival of approximately 7 months [5].
Among MDS patients with a complex karyotype, TP53 mutation is associated with highly complex karyotypes defined as more than four chromosome abnormalities, including del(5q) and other abnormalities of 5q [6-9]. Moreover, TP53 mutated patients usually show only few co-mutated genes [9]. TP53 mutations can affect one or both alleles. In MDS, about one third of the patients carry monoallelic mutations while two thirds of the patients carry multiple (multi-) hits consistent with biallelic targeting, monoallelic with 17p deletion or copy-neutral loss of heterozygosity (cnLOH). Established associations with complex karyotype, few co-occurring mutations, high-risk presentation, and poor outcome were specific to multi-hit patients only. There is also a correlation between multi-hit TP53 alterations and chromosomal aneuploidies and a higher number of deletions. In particular, del(5q) was detected in 85% of multi-hit patients compared to 34% of monoallelic patients [10]. In contrast, the outcome of patients with monoallelic TP53 mutations were more similar to wild-type (WT) TP53, reflecting one functioning TP53 allele.
In higher-risk MDS patients treatment with azacitidine (AZA), a hypomethylating agent, is first-line treatment and in Europe the only licensed therapy for this MDS category. In a randomized phase III study of higher-risk MDS patients, AZA prolongs survival with a median of 9 months compared to conventional care regimes [11].
Lenalidomide (LEN), an immunomodulatory drug, is useful in lower-risk MDS patients with del(5q) and induce complete cytogenetic remission in 45% of the patients [12]. LEN target an E3 ubiquitin ligase (cereblon) inducing ubiquitination and degradation of haploinsufficient CSNK1A1 gene located on chromosome 5 resulting in apoptosis of del(5q) MDS cells [13, 14]. This process is dependent on functional TP53 [15]. The direct molecular effect of LEN raises the question if it could give a synergistic effect to AZA in treatment of higher-risk MDS patients with del(5q). Therefore the Nordic MDS group initiated a randomized phase II study of AZA ± LEN in higher-risk MDS and AML with a del(5q) and showed an overall response rate (ORR) in 39% for AZA and 44% for AZA + LEN (p = 0.63) [16].
With this study the aim was to further investigate the influence of cytogenetics on the treatment response in a cohort of high-risk MDS patients with del(5q) treated with LEN and AZA. We also addressed the question if specific cytogenetic findings may be useful to predict outcome and could help to optimize treatment strategies in this group patients.
2 Material and Methods
2.1 Patient Cohort
All 72 patients were from the Nordic MDS group's prospective multicenter open randomized phase II trial of higher-risk MDS (International Prognostic Scoring System Intermediate risk [IPSS INT-2] and High) and AML with multilineage dysplasia and 20%–29% blasts with a karyotype including del(5q). The patients were treated with either standard dose of AZA 5-2-2 (75 mg/m2/day subcutaneously) [17] with a cycle length of 4 weeks or AZA 5-2-2 + LEN. The dose of oral LEN was 10 mg daily 21/28 days. If tolerated the dose was increased to 25 mg daily during cycles 4–6.
The primary endpoints encompassed response according to the 2006 International Working Group (IWG) criteria for MDS [18] and were assessed by two independent observers after six cycles of AZA ± LEN treatment or at the end of study if this occurred at an earlier time point.
The secondary endpoint was cytogenetic response after three cycles (using del(5q) fluorescence in situ hybridization [FISH] positive clone in the bone marrow using the LSI EGR1/D5S23, D5S721 FISH probe), after six cycles of AZA or AZA + LEN treatment, or at the end of study if this occurred at an earlier time point. Cytogenetic response was defined as complete cytogenetic response (CCyR), disappearance of the del(5q) or any other chromosome aberration, and partial cytogenetic response (PCyR) (at least 50% reduction of the del(5q) FISH positive clone). Other secondary endpoints were mutational status, relapse, and survival from the time of randomization to death.
A subgroup of 46 patients with sufficient cytogenetics, defined as available karyotype and/or FISH at inclusion, after three cycles of treatment and/or at final assessment was studied in more detail.
The study was approved by National Ethical committees in Sweden, Denmark, Norway, and Finland and carried out in accordance with the Declaration of Helsinki/Tokyo/Venice [19]. The patients provided written informed consent. The study was financed by the Nordic MDS group, and Celgene contributed with a research grant and study drug (LEN).
2.2 Fluorescence R-Banding
Karyotyping was performed at baseline and at final assessment at week 25. Unstimulated short-term cultures (24–48 h) were set up from bone marrow aspirates. Chromosome preparation and fluorescence R-banding were performed as previously described [20]. Twenty metaphases were analyzed when possible and the karyotypes were described according to the International System for Chromosome Nomenclature (ISCN, 2016).
Clonal evolution was defined as the acquisition of new cytogenetic aberrations in aberrant clones. Independent clones were cells carrying aberrations not detected in another clone. A complex karyotype was defined as three or more aberrations. Patients with less than 10 metaphases and no clonal aberrations were excluded from this study.
2.3 Cytogenetic Response
A CCyR was defined as disappearance of the del(5q) abnormality and any other chromosomal aberrations present at baseline. A PCyR was defined as a 50% reduction (vs. baseline) in the number of aberrant cells. Persistent clones or reduction < 50% defined a lack of cytogenetic response.
Cytogenetic relapse was defined as the reappearance of aberrations or ≥ 50% increase in abnormal metaphases after achievement of a PCyR. Cytogenetic progression was defined as the appearance of previously undetected aberrations in the sense of clonal evolution or development of new independent clones.
2.4 FISH Analysis
FISH analyses on interphase nuclei were performed for every patient at baseline, week 13, and as final assessment at week 25 using a dual color probe for the locus 5q31 (Vysis EGR1/5p14 FISH Probe Kit—Abbott, Wiesbaden, Germany). At inclusion and final assessment, FISH was performed on fixed cells prepared for classical banding analysis. FISH at week 13 was performed on bone marrow slides. For each sample 200 interphase nuclei were analyzed. We evaluated the cutoff level for this probe by analyzing 1000 interphase nuclei from 10 healthy donors. The cutoff level for fixed cells was set at 8% and for bone marrow slides 6% (data not shown).
2.5 Multicolor FISH (mFISH)
In patients with uncertain cytogenetic aberrations mFISH was performed in order to identify cryptic aberrations. For mFISH, metaphase preparations from heparinized bone marrow aspirates taken from every patient were prepared according to standard procedure. The analysis was carried out using a 24XCyte Multicolor FISH probe (mFISH) (Metasystems, Altlussheim, Germany). mFISH analysis was performed according to the manufacturer's instructions. The ISIS software was used for analysis (Metasystems, Altlussheim, Germany). Whenever possible, five metaphases were analyzed for each sample.
2.6 Telomere/Centromere Fluorescence In Situ Hybridization (T/C-FISH)
For T/C-FISH, metaphase preparations from heparinized bone marrow aspirates were prepared according to standard procedure. Whenever possible, 10 metaphases were examined from each sample after combined fluorescence R-banding and T/C-FISH analysis. We used the Telomere PNA FISH Kit (Dako, Glostrup, Denmark) for analysis. T/C-FISH was performed as described in the protocol of the manufacturer. The kit contains a ready to use telomere PNA probe. The centromere probe of chromosome 2 was developed by DAKO (supplied on request). For an area of 22 × 22 mm we used 9 μL telomere probe and 1 μL centromere probe [21]. The ISIS-Telomere module (Metasystems, Altlussheim, Germany) was used for analysis as described [22]. The software calculates a T/C value for each individual chromosome arm and the mean value of a metaphase was calculated [21].
We set up a cohort of five healthy individuals (adapt) (age-matched) for each patient as control for telomere length measurement (data not shown). The relative values of T/C-FISH (T/C value) can be expressed in absolute kb through the validation of the method by Southern blot in our lab (T/C-FISH: kb = 0.12 × T/C value + 7.83) [21].
2.7 Next Generation Sequencing (NGS)
Mononuclear cells or CD34+ cells from bone marrow and peripheral blood were isolated by using Lymphoprep and genomic DNA was separated from these cells by using GeneElute DNA extraction kit (Sigma Aldrich). Forty-two different genes were analyzed at screening phase (ASXL1, ATRX, BCL10, BCOR, BCORL1, BRAF, CALR, CDKN2A, CDKN2C, CEBPA, CSF1R, CSNK1A1, CTCF, DDX41, DDX54, DNMT3A, EGFR, EP300, EZH2, FLT3, GATA2, GNB1, IDH1, IDH2, JAK2, KMT2C, KRAS, LUC7L2, NF1, NRAS, PHIP, PPM1D, PTPN11, RAD50, ROBO1, RUNX1, SFRB1, SH2B3, SRSF2, TET2, TP53, and U2AF1). Gene mutations, at screening phase, as well as TP53 mutational status were analyzed after the end of study by deep targeted sequencing [10]. Variant allele frequency (VAF) was determined in all samples with a sensitivity of ≥ 2%. Following failure of initial sequencing six patients were analyzed by TruSight Myeloid Sequencing panel. TruSight analyzed 54 different genes (ABL1, ASXL1, ATRX, BCOR, BCORL1, BRAF, CALR, CBL, CBLB, CDKN2A, CEBPA, CBLC, CSF3R, CUX1, DNMT3A, ETV6, EZH2, FBXW7, FLT3, GATA1, GATA2, GNAS, HRAS, IDH1, IDH2, IKZF1, JAK2, JAK3, KDM6A, KIT, KMT2A, KRAS, MPL, MYD88, NPM1, NRAS, NOTCH1, PRGFRA, PTEN, PTPN11, PHF6, RAD21, RUNX1, SMC1A, SETBP1, SF3B1, SMC3, SRSF2, STAG2, TET2, TP53, WT1, U2AF1, and ZRSR2).
To evaluate the effect of treatment cryopreserved separated MNC or CD34+ cells were analyzed by TruSight, to analyze the presence of residual mutations. VAF was determined in all samples with a VAF ≥ 5%.
2.8 Statistical Methods
The randomization process was not stratified by any patient characteristics.
Continuous data were described by mean and median (range) values depending on the distribution of data. χ2- or Fisher's exact tests were used to measure the difference between responders and nonresponders.
Continuous variables regarding mutational status and clone size of FISH analyses used univariate analysis by a Mann–Whitney U test or t-test independent analysis.
McNemar's test was used to compare binary data of the detection of del(5q) with classical banding analysis versus FISH.
Survival estimates were calculated by the Kaplan–Meier method, and tests for differences in survival were done using log-rank tests. Survival was calculated from date of randomization to death; censoring date was the date patient was last known to be alive.
3 Results
3.1 Patients’ Characteristics
Seventy-two patients were enrolled into the NMDSG10B study between March 2012 and January 2017: 54 patients (75%) were diagnosed with MDS and 18 patients (25%) with AML. The median age was 72 (range 35–84). In 54 patients (75%) the cytogenetic risk group, as per IPSS-R, was categorized as very poor.
Twenty-six patients (36%) terminated the study early without a final cytogenetic assessment. The reasons for this were disease progression in 9 patients (35%), adverse events in 13 patients (50%), and subject request in 4 patients (19%). Fifteen patients (58%) with early termination died before the end of the study (Figure S1).
Forty-six patients (64%) with sufficient cytogenetics at inclusion, after three cycles of treatment (week 13) and/or at final assessment were analyzed in detail in this study. The baseline characteristics were similar in the cohort of 46 patients with sufficient cytogenetics and the cohort of 26 patients without follow-up cytogenetics except of patients without sufficient cytogenetics who terminated the study before reaching the endpoint had more unfavorable baseline parameters like bone marrow fibrosis, prior treatment with induction chemotherapy, low platelet counts, transfusion dependency, and TP53 mutations than patients with sufficient cytogenetics (Table 1).
| All included patients | Patients with or without cytogenetics at end | |||
|---|---|---|---|---|
| Total | Patients with cytogenetics at start and end | Patients without follow-up cytogenetics | Significant vs. nonsignificant | |
| Factor | n = 72 | n = 46 | n = 26 | p |
| Age (years), median | 71.5 (35–84) | 71.5 (35–84) | 72 (52–83) | NS |
| Female | 30 (42) | 18 (39) | 12 (46) | NS |
| RCMD | 12 (17) | 9 (20) | 3 (12) | NS |
| RCMD-RS | 2 (3) | 2 (4) | 0 | NS |
| RAEB-1 | 16 (22) | 10 (22) | 6 (23) | NS |
| RAEB-2 | 24 (33) | 16 (35) | 8 (31) | NS |
| tMDS | 15 (21) | 8 (17) | 7 (27) | NS |
| AML_de novo | 0 | 0 | 0 | NS |
| AML de novo relaps | 1 (1) | 0 | 1 (4) | NS |
| AML-MDS | 17 (24) | 9 (20) | 8 (31) | NS |
| MDS/AML diagnosis, days before randomization, median | 61.5 (0–5191) | 63.5 (0–5191) | 61.0 (8–3298) | NS |
| Marrow blasts, MDS | 7.0 (0–19) | 6 (0–18) | 8 (4–19) | NS |
| Marrow blasts, AML | 21.5 (1–30) | 22.5 (1–30) | 21.5 (4–27) | NS |
| Marrow cellularity, MDS | 75 (25–100) | 70 (25–100) | 80 (45–100) | 0.04 |
| Marrow cellularity, AML | 70 (10–100) | 70 (30–100) | 73 (10–90) | NS |
| Marrow fibrosis, grade 2 or 3 | 18 (25) | 8 (17) | 10 (38) | 0.046 |
| TP53 mutation present | 53 (74) | 31 (67) | 22 (85) | NS |
| TP53 mutation monoallelic | 4 (8) | 2 (6) | 2 (9) | NS |
| TP53 mutations multi-hit | 49 (92) | 29 (94) | 20 (91) | NS |
| TP53 1 mutation + del | 15 (31) | 9 (31) | 6 (30) | NS |
| TP53 1 mutation + cnloh | 16 (33) | 10 (34) | 6 (30) | NS |
| TP53 > 1 mutations | 18 (37) | 10 (34) | 8 (40) | NS |
| TP53, WT | 13 (18) | 12 (26) | 1 (4) | 0.024 |
| Number of mutations, median | 2 (0-6) | 2 (0-5) | 2 (1-6) | NS |
| Comorbidity solid tumor, past | 17 (24) | 11 (24) | 6 (24) | NS |
| Hematological malignancies, past | 10 (14) | 6 (13) | 4 (15) | NS |
| Hematological malignancies, current | 2 (3) | 0 | 2 (8) | NS |
| Prior radiation | 6 (8) | 2 (4) | 4 (16) | NS |
| Prior transplant | 2 (3) | 0 | 2 (8) | NS |
| Prior treatment | ||||
| No previous treatment | 43 (60) | 29 (63) | 14 (54) | NS |
| Prior chemo | 19 (26) | 10 (22) | 9 (35) | NS |
| Induction chemotherapy | 5 (7) | 1 (2) | 4 (16) | 0.039 |
| ESA | 11 (15) | 10 (22) | 1 (4) | NS |
| One course of Azacitidine | 11 (15) | 6 (13) | 5 (19) | NS |
| Cytogenetics IPSS-R Good | 11 (15) | 8 (17) | 3 (12) | NS |
| Cytogenetics IPSS-R Intermediate | 1 (1) | 1 (2) | 0 | NS |
| Cytogenetics IPSS-R Poor | 6 (8) | 5 (11) | 1 (4) | NS |
| Cytogenetics IPSS-R Very poor | 54 (75) | 32 (70) | 22 (85) | NS |
| IPSS-R Low + Intermediate | 6 (8) | 5 (11) | 1 (4) | NS |
| IPSS-R High | 14 (19) | 10 (22) | 4 (16) | NS |
| IPSS-R Very high | 34 (47) | 22 (48) | 12 (46) | NS |
| WPSS High | 23 (32) | 18 (39) | 5 (19) | NS |
| WPSS Very High | 31 (43) | 19 (41) | 12 (46) | NS |
| HgB (g/dL) | 9.2 (6.2–12.0) | 9.3 (6.2–12.0) | 9.1 (7.8–10.2) | NS |
| ANC (×109/L) | 1.00 (0.10–20.60) | 1.0 (0.10–12.8) | 0.99 (0.10–20.60) | NS |
| Platelets (×109/L) | 43 (4–252) | 59 (9–222) | 30 (4–252) | 0.023 |
| Transfusion dependent at inclusion | 62 (86) | 37 (80) | 25 (96) | 0.022 |
- Note: Continuous variables (min–max) and categorical variables (%) are reported. Bold indicates statistically significant values.
- Abbreviations: AML, acute myeloid leukemia; ANC, absolute neutrophil count; cnloh, copy-neutral loss of heterozygosity; del, deletion; ESA, erythropoiesis stimulating agent; HgB, hemoglobin; IPSS, International Prognostic Scoring System; IPSS-R, Revised-IPSS; MDS, myelodysplastic syndrome; RAEB, refractory anemia with excess blasts; RCMD, refractory cytopenia with multilineage dysplasia; RS, ring sideroblasts; t, treated; WT, wild type; WPSS, World Health Organization Prognostic Scoring System.
In the patients with sufficient cytogenetics (n = 46) who were analyzed in detail in this study, baseline data were similar between the two treatment arms except of bone marrow fibrosis, grade 2 or 3, that were more frequent in the AZA + LEN arm (32%), versus (4%) in the AZA arm (p = 0.047) (Table S1).
3.2 Cytogenetics
3.2.1 Cytogenetic Characteristics at Inclusion in the Study
Classical banding analysis was performed at inclusion and at final assessment in all patients (Figure 1A; Table S2). Sufficient data were obtained in 70 patients (97%) at inclusion, and in 41 patients (95%) at final assessment. However, in two patients (3%) no metaphases were possible to analyze due to a low number of cells in the bone marrow aspirate.
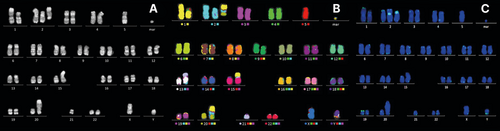
To identify cryptic aberrations, mFISH was performed in 43 patients with uncertain aberrations at inclusion (Figure 1B). In 40 of these patients (93%) several structural aberrations were characterized more clearly which have not been identified by karyotyping. Thus, mFISH was an integrated part of the cytogenetic analysis.
Fifty-nine out of 70 karyotyped patients (84%) had a complex karyotype at inclusion, among them 12 patients (20%) with three to four aberrations and 47 patients (80%) with five or more aberrations. Eleven patients (16%) had less than three aberrations. Seventeen patients (24%) had a karyotype including 17p aberration (Figure 2).
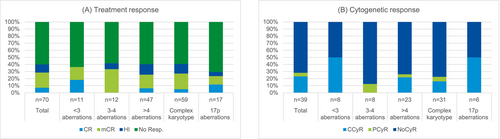
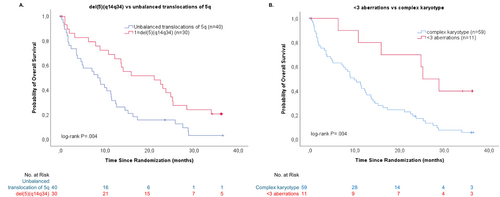
The ORR was 36% among patients with less than three aberrations (median 1) at inclusion and 41% in patients with a complex karyotype (median 7, range 3–16) (p = 1.0) (Figure 2). The number of aberrations at inclusion was associated with survival but not with response to treatment. The median survival was 11.4 months for the entire study population, 9.9 months in patients with less than three aberrations, and 25.2 months in patients with a complex karyotype (p = 0.004) (survival, Figure 3B).
Forty-seven patients (67%) showed interstitial deletions of 5q. Twenty-three patients (33%) all with complex karyotypes showed other aberrations of chromosome 5 which included dicentric chromosomes, for example, dic(1;5), dic(5;7), and dic(5;17), and unbalanced translocations leading to loss of parts of 5q. Recurrent gains were trisomy 8 and trisomy 21; recurrent losses, except 5q, involved particularly 7q, as well as chromosomes 11, 12, 16, 18, and 20. In total, 27 patients (39%) showed other chromosome abnormalities such as dicentric chromosomes (dic), and isodicentric chromosomes (ider) that involve breakpoints in the vicinity of centromeres. Except for three patients with three and four aberrations respectively, all patients with dic or ider carried complex karyotypes with five or more aberrations. Five patients showed double minutes (dmin), resulting from gene amplifications (Table S2). The ORR was 30% in patients with a dic or an ider involving breakpoints in the vicinity of centromeres versus 47% in patients with no dic or ider (p = 0.16).
3.2.2 Identification of the del(5q) Clone by FISH
To detect aberrations of the locus 5q31 (EGR1) FISH was performed on interphases at inclusion, after three cycles of treatment (week 13) and at final assessment. For FISH fixed cells prepared for classical banding analysis as well as on bone marrow slides were used. At inclusion, del(5q) was detected by karyotyping and FISH in all cases. At final assessment, the classical banding analysis was significantly more sensitive to detect the deletion in smaller clones compared to FISH (p = 0.027). Notably, the aberrant clone was identified by karyotyping, but not by FISH in eight patients (23%), versus in only one patient (3%) the aberrant clone was detected by FISH, but not by karyotyping (Table 2).
| Inclusion | Final assessment | |||||||
|---|---|---|---|---|---|---|---|---|
| Patient number | % metaphases with del(5q) | Detection via karyotyping | % interphases with del(5q) | Detection via FISH | % metaphases with del(5q) | Detection via karyotyping | % interphases with del(5q) | Detection via FISH |
| 1 | 100 | Yes | 89 | Yes | 0 | Yes | 100 | Yes |
| 2 | 92 | Yes | 64 | Yes | n.d. | n.d. | n.d. | n.d. |
| 3 | 100 | Yes | 83 | Yes | n.d. | n.d. | n.d. | n.d. |
| 4 | 33 | Yes | 76 | Yes | 0 | No | 30 | Yes |
| 5 | 90 | Yes | 65 | Yes | 38 | Yes | 10 | Yes |
| 6 | 73 | Yes | 49 | Yes | 0 | No | 0 | No |
| 7 | 100 | Yes | 38 | Yes | n.d. | n.d. | n.d. | n.d. |
| 8 | 45 | Yes | 18 | Yes | 15 | Yes | 0 | No |
| 9 | 91 | Yes | 34 | Yes | 0 | No | 0 | No |
| 10 | 52 | Yes | 23 | Yes | 0 | No | 0 | No |
| 11 | 100 | Yes | 79 | Yes | n.d. | n.d. | n.d. | n.d. |
| 12 | 100 | Yes | 21 | Yes | n.d. | n.d. | n.d. | n.d. |
| 13 | 83 | Yes | 83 | Yes | n.d. | n.d. | n.d. | n.d. |
| 14 | 95 | Yes | 95 | Yes | n.d. | n.d. | n.d. | n.d. |
| 15 | 100 | Yes | 94 | Yes | n.d. | n.d. | n.d. | n.d. |
| 16 | 100 | Yes | 96 | Yes | 0 | No | 0 | No |
| 17 | 75 | Yes | 83 | Yes | 20 | Yes | 0 | No |
| 18 | 90 | Yes | 76 | Yes | 55 | Yes | 47 | Yes |
| 19 | 86 | Yes | 60 | Yes | 20 | Yes | 8 | Yes |
| 20 | 90 | Yes | 84 | Yes | 86 | Yes | 24 | Yes |
| 21 | 96 | Yes | 85 | Yes | 100 | Yes | 85 | Yes |
| 22 | 100 | Yes | 93 | Yes | n.d. | n.d. | n.d. | n.d. |
| 23 | 75 | Yes | 60 | Yes | 0 | No | 0 | No |
| 24 | 80 | Yes | 85 | Yes | n.d. | n.d. | n.d. | n.d. |
| 25 | 100 | Yes | 89 | Yes | 47 | Yes | 22 | Yes |
| 26 | 100 | Yes | 96 | Yes | 100 | Yes | n.d. | n.d. |
| 27 | 75 | Yes | 53 | Yes | n.d. | n.d. | n.d. | n.d. |
| 28 | 100 | Yes | 90 | Yes | n.d. | n.d. | n.d. | n.d. |
| 29 | 100 | Yes | 91 | Yes | 0 | No | 0 | No |
| 30 | 100 | Yes | 92 | Yes | 100 | Yes | 83 | Yes |
| 31 | 100 | Yes | 66 | Yes | n.d. | n.d. | n.d. | n.d. |
| 32 | 95 | Yes | 63 | Yes | n.d. | n.d. | n.d. | n.d. |
| 33 | 100 | Yes | 94 | Yes | 0 | No | 0 | No |
| 34 | 100 | Yes | 78 | Yes | 50 | Yes | 60 | Yes |
| 35 | 93 | Yes | 63 | Yes | 100 | Yes | 0 | No |
| 36 | 67 | Yes | 61 | Yes | 7 | Yes | 18 | Yes |
| 37 | 100 | Yes | n.a. | n.a. | n.d. | n.d. | n.d. | n.d. |
| 38 | 73 | Yes | 76 | Yes | 40 | Yes | 0 | No |
| 39 | 100 | Yes | 89 | Yes | 25 | Yes | 0 | No |
| 40 | 100 | Yes | 98 | Yes | n.d. | n.d. | n.d. | n.d. |
| 41 | 17 | Yes | 10 | Yes | n.d. | n.d. | n.d. | n.d. |
| 42 | n.a. | Yes | 87 | Yes | 0 | No | 0 | No |
| 43 | 100 | Yes | 65 | Yes | n.d. | n.d. | n.d. | n.d. |
| 44 | 100 | Yes | 91 | Yes | 53 | Yes | 28 | Yes |
| 45 | 73 | Yes | 33 | Yes | 28 | Yes | n.a. | n.d. |
| 46 | 27 | Yes | 24 | Yes | 70 | Yes | 89 | Yes |
| 47 | 100 | Yes | 84 | Yes | n.d. | n.d. | n.d. | n.d. |
| 48 | 100 | Yes | 93 | Yes | n.d. | n.d. | n.d. | n.d. |
| 49 | 100 | Yes | 85 | Yes | n.d. | n.d. | n.d. | n.d. |
| 50 | 100 | Yes | 97 | Yes | n.d. | n.d. | n.d. | n.d. |
| 51 | 100 | Yes | 93 | Yes | n.d. | n.d. | n.d. | n.d. |
| 52 | 47 | Yes | 19 | Yes | 53 | Yes | n.a. | n.d. |
| 53 | 62 | Yes | 45 | Yes | 10 | Yes | 0 | No |
| 54 | 100 | Yes | 88 | Yes | n.d. | n.d. | n.d. | n.d. |
| 55 | 0 | Yes | 68 | Yes | 0 | No | 0 | No |
| 56 | 69 | Yes | n.a. | n.a. | 100 | Yes | 19 | n.a. |
| 57 | 28 | Yes | 79 | Yes | n.d. | n.d. | n.d. | n.d. |
| 58 | 94 | Yes | 79 | Yes | n.d. | n.d. | n.d. | n.d. |
| 59 | 67 | Yes | 71 | Yes | n.d. | n.d. | n.d. | n.d. |
| 60 | 90 | Yes | 35 | Yes | 25 | Yes | 0 | No |
| 61 | 88 | Yes | 89 | Yes | 0 | No | 0 | No |
| 62 | 100 | Yes | 62 | Yes | 95 | Yes | n.d. | n.d. |
| 63 | 67 | Yes | 28 | Yes | n.d. | n.d. | n.d. | n.d. |
| 64 | 53 | Yes | 78 | Yes | 80 | Yes | 71 | Yes |
| 65 | 40 | Yes | 21 | Yes | n.d. | n.d. | n.d. | n.d. |
| 66 | 87 | Yes | 43 | Yes | n.d. | n.d. | n.d. | n.d. |
| 67 | 72 | Yes | 67 | Yes | 0 | No | 0 | No |
| 68 | 100 | Yes | 74 | Yes | n.a. | n.a. | n.d. | n.d. |
| 69 | 100 | Yes | n.d. | Yes | 13 | Yes | 0 | No |
| 70 | 100 | Yes | 88 | Yes | 0 | No | n.d. | n.d. |
| 71 | 100 | Yes | 86 | Yes | n.d. | n.d. | n.d. | n.d. |
| 72 | n.a. | n.a. | n.d. | n.a. | 0 | No | 0 | No |
- Note: McNemar's test, OR 9000, 95% CI: 1.247–394.479, p-value: 0.027.
- Abbreviations: del(5q), deletion in 5q; FISH, fluorescence in situ hybridization; n.a., not analyzable; n.d., not done/no material.
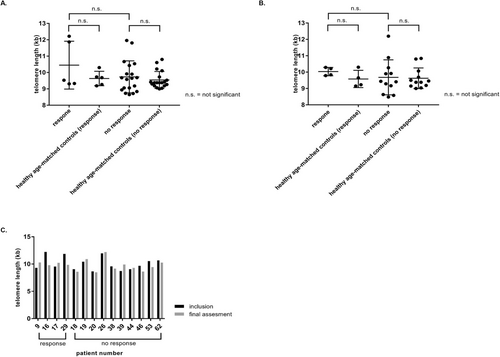
3.2.3 Telomere Length Measurement
Telomere length measurement was performed in 24 patients at inclusion and 16 patients at final assessment (Figure 1C). In parallel, age-matched controls were analyzed. No significant difference in telomere lengths could be detected at inclusion or at final assessment among the five patients (21%) with a CCyR and a complete remission (CR) to treatment versus the 19 patients without a response (Figure 4).
3.2.4 Cytogenetic Response
Forty-six patients had sufficient cytogenetics at inclusion, after three cycles of treatment and/or at final assessment. Forty-one of the 46 patients (89%) had sufficient cytogenetics at final assessment (Figure S2). A CCyR (karyotype) was achieved in 11 patients (27%), a PCyR was achieved in 2 patients (5%), and 28 patients (68%) had no CyR at final assessment (Table 3). Twelve patients (43%) showed a cytogenetic progression at final assessment, six patients (38%) in the AZA arm, and six patients (50%) in the AZA + LEN arm (p = 0.60). A clonal evolution was detected in eight patients (67%), five patients (63%) in the AZA arm and three patients (37%) in the AZA + LEN arm (p = 0.70). Four patients (33%) developed a new independent clone, one patient (25%) in the AZA arm and three patients (75%) in the AZA + LEN arm (p = 0.29) (Figure S2). Six patients (75%) with a clonal evolution and two patients (50%) with a new independent clone, at final assessment, had multi-hit TP53 alterations at inclusion (Figure S2).
| Patients, N (%) | Total (n = 46) | ORR treated cohort | Complete remission | Marrow CR | HI |
|---|---|---|---|---|---|
| Cytogenetics, 3 cycles and/or final assessment | 30 (65) | 6 (13) | 16 (35) | 8 (17) | |
| Cytogenetics, final assessment | 41 (89) | 29 (71) | 6 (15) | 16 (39) | 7 (17) |
| Cytogenetic CR final assessment | 11 (27) | 11 (100) | 5 (45) | 6 (55) | 0 |
| Cytogenetic PR final assessment | 2 (5) | 2 (100) | 1 (50) | 1 (50) | 0 |
| No cytogenetic response | 28 (68) | 16 (57) | 0 | 9 (32) | 7 (25) |
| Cytogenetics, 3 cycles (FISH) | 43 (93) | 28 (65) | 6 (14) | 16 (37) | 6 (14) |
| Cytogenetic response, CR, 3 cycles (FISH) | 25 (58) | 19 (76) | 6 (24) | 11 (44) | 2 (8) |
| Cytogenetic response, PR, 3 cycles (FISH) | 6 (14) | 3 (50) | 0 | 1 (17) | 2 (33) |
| No cytogenetic response, 3 cycles (FISH) | 12 (28) | 6 (50) | 0 | 4 (33) | 2 (17) |
- Abbreviations: CR, complete remission; FISH, fluorescence in situ hybridization; HI, hematologic improvement; ORR, overall response rate; PR, partial remission.
Forty-three of 46 patients (93%) had sufficient cytogenetics after three cycles of treatment. A CCyR (FISH) was achieved in 25 patients (58%), a PCyR was achieved in 6 patients (14%), and no CyR in 12 patients (28%) (Table 3). Eleven patients (44%) lost their CCyR after three cycles of treatment, seven patients (64%) due to clonal progression, involving five patients (45%) with a clonal evolution and two patients (18%) with a new independent clone, one patient (4%) showed a PCyR at final assessment and two patient (8%) stopped the study due to adverse events (AE). One patient (4%) was transplanted after three cycles and cytogenetics from week 13 was used as final assessment (Figure S2).
Patients without a complex karyotype reached CCyR more frequently, but not significantly, than patients with a complex karyotype or patients with an aberration of 17p (4 of 11 [36%] vs. 5 of 59 [8%] [p = 0.065] and 3 of 17 [8%], respectively).
Patients were analyzed in different subgroups, for example, < 3, ≥ 3, < 5, or ≥ 5 aberrations and del(5q) or unbalanced translocations of 5q to see if the clone size at inclusion, detected by RPS14 or EGR1, differed between subgroups. The clone size, identified by EGR1, did not affect the response to treatment, except in patients with a marrow CR where the clone size was larger, that is, 88.5% (range 60–91) in patients with del(5q) versus 51% (range 0–94) in patients with unbalanced translocations of 5q (p = 0.031). In patients analyzed with a RPS14 FISH probe at inclusion, the clone size differed significantly in patients with a CCyR. The clone size was smaller in patients with < 3, < 5 aberrations, or with del(5q) 64% (range 45–77) versus 91% (range 91–95) in patients with ≥ 3, ≥ 5 aberrations, or unbalanced translocations of 5q (p = 0.016). Patients without a CyR had a larger RPS14 clone in patients with del(5q) 76% (range 33–96) versus 65% (range 8–86) in patients with unbalanced translocations of 5q (p = 0.044) (Tables 4a–4c).
| Total | < 3 aberrations | ≥ 3 aberrations | ||
|---|---|---|---|---|
| N | (n = 46) | (n = 7) | (n = 39) | p |
| EGR1 (%), inclusion | 76 (0–98) | 61 (22–93) | 79.5 (0–98) | 0.15 |
| ORR | 76 (0–96) | 69 (60–70) | 78 (0–96) | 0.38 |
| Complete remission | 80 (70–89) | 70 (70) | 80 (80) | na |
| Marrow CR | 72.5 (0–94) | 64.5 (60–69) | 82 (0–94) | 0.50 |
| CCyR | 70 (0–94) | 69 (60–70) | 80 (0–94) | 0.40 |
| PCyR | 89 | 0 | 89 (89) | na |
| NoCyR | 75 (17–96) | 61 (34–93) | 76 (17–96) | 0.41 |
| HI | 76 (60–96) | 0 | 76 (60–96) | na |
| Total | < 3 aberrations | ≥ 3 aberrations | ||
|---|---|---|---|---|
| N | (n = 48) | (n = 7) | (n = 41) | p |
| RPS14 (%), inclusion | 74 (8–97) | 47 (19–90) | 79 (8–97) | 0.077 |
| ORR | 79.5 (20–96) | 64 (45–77) | 84 (20–96) | 0.15 |
| Complete remission | 87 (77–95) | 77 (77) | 95 (95) | n.a. |
| Marrow CR | 73 (20–91) | 54.5 (45–64) | 81.5 (20–91) | 0.21 |
| CCyR | 87 (45–95) | 64 (45–77) | 91 (91–95) | 0.016 |
| PCyR | 87 | 0 | 87 (87) | n.a. |
| NoCyR | 67 (8–96) | 47 (33–90) | 68 (8–96) | 0.32 |
| HI | 82 (66–96) | 0 | 82 (66–96) | n.a. |
- Note: Bold indicates statistically significant values.
- Abbreviations: CCyR; complete cytogenetic response; CR, complete remission; HI, hematologic improvement; n.a., not analyzable; NoCyR, no cytogenetic responseORR, overall response rate; PCyR, partial cytogenetic response.
| < 5 aberrations | ≥ 5 aberrations | ||
|---|---|---|---|
| N | (n = 19) | (n = 19) | p |
| EGR1 (%), inclusion | 74.5 (0–94) | 79 (0–98) | 0.26 |
| ORR | 73 (60–89) | 76 (0–96) | 0.26 |
| Complete remission | 70 (70) | 0 | n.a. |
| Marrow CR | 78.5 (60–89) | 57.5 (0–94) | 0.10 |
| CCyR | 69 (60–70) | 80 (0–94) | 0.40 |
| PCyR | 89 (89) | 0 | n.a. |
| NoCyR | 63 (24–93) | 76 (17–96) | 0.31 |
| HI | 76 (76) | 80 (60–96) | n.a. |
| < 5 aberrations | ≥ 5 aberrations | ||
|---|---|---|---|
| N | (n = 27) | (n = 29) | p |
| RPS14 (%), inclusion | 67 (8–90) | 82 (10–97) | 0.062 |
| ORR | 70.5 (45–90) | 86 (20–96) | 0.32 |
| Complete remission | 77 (77) | 95 (95) | n.a. |
| Marrow CR | 67 (45–90) | 83.5 (20–91) | 0.41 |
| CCyR | 64 (45–77) | 91 (91–95) | 0.016 |
| PCyR | 87 (87) | 0 | n.a. |
| NoCyR | 64 (8–90) | 68 (20–96) | 0.20 |
| HI | 71 (71) | 84 (66–96) | n.a. |
- Note: Bold indicates statistically significant values.
- Abbreviations: CCyR; complete cytogenetic response; CR, complete remission; HI, hematologic improvement; n.a., not analyzable; NoCyR, no cytogenetic response; ORR, overall response rate; PCyR, partial cytogenetic response.
| Total | del(5q) | Unbalanced translocations of 5q | ||
|---|---|---|---|---|
| N | (n = 45) | (n = 21) | (n = 24) | p |
| EGR1 (%), inclusion | 76 (0–98) | 85 (22–98) | 76 (0–98) | 0.077 |
| ORR (n = 21) | 76 (0–96) | 81 (60–96) | 76 (0–95) | 0.090 |
| Complete remission (n = 2) | 75 (70–80) | 70 (70) | 80 (80) | n.a. |
| Marrow CR (n = 12) | 72.5 (0–94) | 88.5 (60–91) | 51 (0–94) | 0.031 |
| CCyR (n = 6) | 69.5 (0–94) | 69 (60–70) | 80 (0–94) | 0.40 |
| PCyR (n = 1) | 89 (89) | 89 (89) | 0 | n.a. |
| NoCyR (n = 22) | 75 (17–96) | 81 (34–96) | 69.5 (17–85) | 0.074 |
| HI (n = 7) | 76 (60–96) | 74 (60–96) | 80 (76–95) | 0.29 |
| Total | del(5q) | Unbalanced translocations of 5q | ||
|---|---|---|---|---|
| N | (n = 47) | (n = 22) | (n = 25) | p |
| RPS14 (%), inclusion | 72 (8–97) | 79.5 (19–96) | 71 (8–97) | 0.13 |
| ORR (n = 21) | 77 (20–96) | 79.5 (45–96) | 76 (20–95) | 0.27 |
| Complete remission (n = 2) | 86 (77–95) | 77 (77) | 95 (95) | n.a. |
| Marrow CR (n = 12) | 73 (20–91) | 78.5 (45–91) | 70 (20–91) | 0.21 |
| CCyR (n = 6) | 84 (45–95) | 64 (45–77) | 91 (91–95) | 0.016 |
| PCyR (n = 1) | 87 (87) | 87 (87) | 0 | n.a. |
| NoCyR (n = 22) | 67 (8–96) | 76 (33–96) | 65 (8–86) | 0.044 |
| HI (n = 7) | 82 (66–96) | 82 (66–96) | 78.5 (66–91) | 0.40 |
- Note: Bold indicates statistically significant values.
- Abbreviations: CCyR; complete cytogenetic response; CR, complete remission; HI, hematologic improvement; n.a., not analyzable; NoCyR, no cytogenetic response; ORR, overall response rate; PCyR, partial cytogenetic response.
3.3 Del (5)(q14q34) versus 5q Deletion due to Unbalanced Translocations of 5q
Seventy patients were analyzed with karyotype and FISH at inclusion. Thirty patients (43%) had a del (5)(q14q34) and 40 patients (57%) had a del(5q) due to other abnormalities, nearly always due to unbalanced translocations leading to partial loss of 5q (Table S3).
Complex karyotype was more frequent in patients with unbalanced translocations of 5q compared to del (5)(q14q34), 39 patients (98%) versus 20 patients (67%) (p < 0.001). Notably, a complex karyotype with more than four aberrations was more frequent among patients with unbalanced translocations of 5q compared to patients with del (5)(q14q34), 33 patients (83%) versus 13 patients (43%) (p < 0.001). Therapy-related MDS or AML were more common in patients with unbalanced translocations of 5q compared to del (5)(q14q34), 14 patients (35%) versus 3 patients (10%) (p = 0.019). Five patients were classified as IPSS-R low or intermediate, all of those had a del (5)(q14q34) (p = 0.006) (Table S3).
The ORR was 38% among patients with unbalanced translocations of 5q and 43% for patients with del (5)(q14q34) (p = 0.62) and there were no other significant differences in responses to treatment between the two groups. However, patients with del (5)(q14q34) versus unbalanced translocations of 5q showed a longer overall survival (OS), 21.1 months versus 8.4 months (p = 0.004) (survival, Figure 3A).
Thirty-six patients (90%) with unbalanced translocations of 5q carried aTP53 mutation versus 16 patients (53%) with del(5q) (p < 0.001). Multi-hit TP53 alterations (p < 0.001) and among those biallelic TP53 mutations (p = 0.041) were more common in the group with unbalanced translocations of 5q versus del (5)(q14q34). Ten patients (33%) with del (5)(q14q34) were TP53, WT versus two patients (5%) among patients with unbalanced translocations of 5q (p = 0.002) (Table S3).
The median number of oncogenic gene mutations was 1 (range, 0–5) in patients with del (5)(q14q34) and 1 (range, 0–4) in patients with unbalanced translocations of 5q (p = 0.002). TP53 mutations with another driver mutation (TET2, SF3B1, ASXL1, RUNX1, BCOR, and/or CBL) were more common among patients with del (5)(q14q34), 13 patients (45%) versus 6 patients (15%) in patients with unbalanced translocations of 5q (p = 0.007). Eight patients (29%) with del (5)(q14q34) had SF3B1 mutations compared to one patient with unbalanced translocations of 5q (3%) (p = 0.008) (Table S3).
4 Discussion
The prognostic and predictive power of cytogenetics depends on the specific treatment. For example, in chronic lymphocytic leukemia, after introduction of novel chemoimmunotherapeutic regimes, poor prognostic cytogenetic markers like del(11q) did not affect outcome anymore [23]. Therefore, homogenously treated patient populations are necessary to determine the influence of cytogenetics on the treatment results. Here, we report the cytogenetic findings of high-risk MDS and AML patients with a karyotype including 5q(del) enrolled in the randomized phase II NMDSG10B study of AZA or AZA + LEN.
A strength of this study was the comprehensive cytogenetic analysis including karyotyping, FISH, mFISH, and telomere measurement. Here, we did not find any significant differences in telomere lengths among the patient population compared to age-matched controls, in line with a previous report that telomere lengths in patients with MDS with del(5q) who do not progress is similar to healthy age- and gender-matched controls. However, the del(5q) clone in patients who later showed cytogenetic progression had significantly shorter telomeres than the del(5q) clone in patients without progression [24].
Notably, we found that the classical banding analysis was significantly more sensitive to detect del(5q) compared to FISH confirming data from the MDS-004 study [25]. This may be due to the fact that small del(5q) clones beyond the detection limit of FISH may have a proliferative advantage over normal cells.
Multicolor FISH was a helpful tool to detect cryptic aberrations. Particularly by comparing the findings with those of karyotyping and vice versa, mFISH identified a number of structural aberrations and resolved the degree of cytogenetic complexity.
The study cohort consisted of 46 high-risk myeloid patients that had sufficient cytogenetic results, that is, karyotype and/or FISH at inclusion, after three cycles of treatment and/or at final assessment after six cycles of AZA or AZA + LEN treatment. Twenty-six patients enrolled in the NMDSG10B study did not have follow-up cytogenetic analyses at final assessment or week 13, either due to disease progression or adverse events. The majority of patients had complex karyotypes, which are associated with a dismal prognosis [4]. This may have influenced the possibility to receive a follow-up cytogenetics, since this patient group showed significantly higher rates of fibrosis grade 2–3, therapy-related MDS or AML, lower platelet counts, and higher grade of transfusion burden which are known to be predictors of a poor outcome [26, 27].
Patients without a complex karyotype reached a CCyR more frequently, but not significantly, than patients with a complex karyotype or patients with an aberration of 17p. As reported, patients with del(5q) as the sole aberration and patients with < 3 aberrations had a significantly better outcome than patients with complex karyotypes [1].
As shown in this study, the poor prognosis of patients with complex karyotypes including del(5q) cannot be overcome with AZA + LEN treatment. It is well known that complex karyotypes, particularly those with biallelic inactivation of TP53, are resistant to all treatment regimes. Moreover, the relapse risk after allogeneic stem cell transplantation is very high [28].
Interestingly, a high proportion of patients (n = 11, 44%) reached CCyR after three cycles of treatment but lost their response at six cycles of treatment. This data support a more frequent monitoring, after each cycle of treatment, to enable an early transplantation regime in those patients with a short-lasting response.
Complex karyotypes with high genomic complexity, that is, ≥ 5 aberrations, were more common in the patient cohort with dicentric chromosomes or unbalanced translocations of 5q compared to del (5)(q14q34). This is in line with a report by La Starza et al. that deletions of the NPM1 locus on terminal band 5q35 occurred in > 40% of high-risk MDS/AML with a complex karyotype [29]. Highly complex chromosome rearrangements per se result in resistance against LEN and AZA treatment [30]. Moreover, TP53 mutations are associated with heavily rearranged chromosomes resembling chromotrypsis and increased chromosomal instability [31], loss of 17p [32], and with progression [15, 33].
TP53 mutations and high complexity > 4 abnormalities, both associated with a poor prognosis, are enriched for del(5q) but usually have only few additional somatic mutations. In a recent study [9], TP53 multi-hit alterations were more common in the patient cohort with unbalanced translocations of 5q, compared to del (5)(q14q34). On the other hand, del (5)(q14q34) showed a higher degree of TP53, WT, higher number of oncogenic gene mutations, and monoallelic TP53 mutations with another driver mutation, especially SF3B1 mutation [10]. The differences may have led to a shorter OS among patients with unbalanced translocations of 5q. Moreover, patients with unbalanced translocations of 5q frequently had therapy-related MDS/AML.
Most of the study population were patients with highly complex karyotypes including del(5q) with TP53 mutation. Stem and progenitor cells with del(5q) acquiring pathogenic TP53 mutations become resistant against LEN treatment and repopulate the bone marrow if the del(5q) had disappeared [34]. Albeit there are case reports that LEN can induce sustained hematological and cytogenetic remissions in patients with del(5q) and a complex karyotype [35] and the current data from a randomized multicenter clinical trial show that even if cytogenetic remissions were achieved they did not last long [16]. The German MDS study group saw no influence of TP53 WT and TP53 mutations on the achievement of cytogenetic remission. However, TP53 was the most powerful predictor of survival in MDS patients with del(5q) treated with LEN [36].
5 Conclusion
This study in homogenously treated high-risk MDS patients with different abnormalities of 5q demonstrates the influence of cytogenetics on treatment results. The poor outcome of patients with high-risk MDS, complex karyotypes including del(5q) and TP53 mutations cannot be improved with AZA + LEN treatment as it was the case for combinations with other drugs [36]. New concepts including timing of treatment, follow-up, and transplantation are needed to overcome the primary resistance of clones with complex karyotypes, particularly those with biallelic inactivation of TP53 and abnormalities of 5q other than the typical interstitial deletions.
Author Contributions
B.R., G.G., and B.S. collected and analyzed data and wrote the manuscript. B.R., F.E., A.M.O., J.M.N., L.M., and E.H.-L. designed the clinical trial. L.N., M.T., M.J., H.G., I.D., K.G., E.E., F.L., M.F., C.W.M., F.E., A.M.O., and J.M.N. provided clinical care to patients and provided critical input on the paper. L.C. and L.S. provided laboratory resources and provided critical input on the paper.
Acknowledgments
This work was supported by Swedish Cancer Society, Scientific Research Council Sweden, and Swedish Wallenberg Foundation. The Nordic MDS Group (NMDSG) received an unrestricted grant and study drug (lenalidomide) from Celgene for this academic study.
Ethics Statement
The study was approved by National Ethical committees in Sweden, Denmark, Norway, and Finland, carried out in accordance with the Declaration of Helsinki/Tokyo/Venice.
Consent
The patients provided written informed consent.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




