Electroconductive hydrogels for bioelectronics: Challenges and opportunities
Abstract
Electroconductive hydrogels (ECHs) have been extensively explored as promising flexible materials for bioelectronics because of their tunable conductivity and tissue-like biological and mechanical properties. ECHs can interact intimately with biosystems, transmit physiological signals, and are expected to revolutionize the convergence between organisms and electronics. However, there are still some challenges in utilizing ECHs as flexible materials for bioelectronics, such as mismatched stretchability with tissues, a lack of environmental adaptability, susceptibility to mechanical damage, inferior interface compatibility, and vulnerability to bacterial contamination. This review categorizes these challenges encountered in the bioelectronic applications of ECHs and elaborates on the strategies and theories for improving their performance. Furthermore, we present an overview of the recent advancements in ECHs for bioelectronic applications, specifically focusing on their contributions to healthcare monitoring, treatment of diseases, and human–machine interfaces. The scope of future research on ECHs in bioelectronics is also proposed. Overall, this review offers a comprehensive exposition of difficult issues and potential opportunities for ECHs in bioelectronics, offering valuable insights for the design and fabrication of ECH-based bioelectronic devices.
1 INTRODUCTION
Bioelectronics is an interdisciplinary field that combines electronic engineering, biology, medicine, and material science, focusing on the development of devices for biomedical research and clinical healthcare.1-3 Monitoring physiological signals, including physical signals and electrochemical signals, by bioelectronic devices on/in the human body is of significance for health surveillance and clinical diagnosis. Furthermore, exploiting bioelectronic devices to realize bidirectional communication between artificial electronic systems and biological electrical systems will effectively facilitate the development of human–machine integration. In conventional bioelectronic devices, metal and silicon typically serve as conductors and semiconductors, while polymers serve as insulators.4 However, the mismatch between soft biological tissues and traditional rigid electronic devices has become a considerable obstacle when developing next-generation bioelectronic devices.5-7 Exploring novel materials that could seamlessly integrate bioelectronic devices onto/into human tissues as well as promote the restoration of indigenous properties of the human tissues are crucial issues to which special attention should be paid.
Hydrogels are cross-linked 3D network structures with high water content and have been extensively studied in biomedical applications owing to their excellent biomechanical properties, favorable biological characteristics, and remarkable biocompatibility.8, 9 The mechanical properties of hydrogels are similar to those of biological soft tissues and can be optimized for a variety of demands by modifying the chemical structure of hydrogels.10, 11 Similarly, by adjusting the composition, hydrogels can be endowed with excellent electrical properties, which are referred to as electroconductive hydrogels (ECHs).12 According to the principle of conduction, ECHs can be classified as ionically conductive hydrogels and electronically conductive hydrogels.13 Owing to the large amount of water in the matrix, hydrogels are inherently capable of obtaining conductivity by adding free ions. Ionically conductive hydrogels mainly introduce free ions such as acids, salts, polyelectrolytes, and ionic liquids into hydrogel networks to endow hydrogels with conductivity.14-17 Electronically conductive hydrogels are mainly prepared by compounding the hydrogel network with conductive fillers such as metal nanoparticles/nanowires (e.g., silver, copper),18-20 carbon-based materials (e.g., carbon nanotube [CNT],21-23 carbon nanofiber,24 graphene25-27) or conductive polymers (e.g., polyaniline,28, 29 polypyrrole30-32).
ECH is an ideal candidate for bioelectronic applications due to its uniquely superior electronic property and inherent biocompatibility. It has a substantial influence on medical devices that can be implanted or worn. In recent years, ECH-based devices have attracted much attention in bioelectronics and are generally used in wearable sensing, the treatment of diseases, and the realization of human–machine interfaces (HMIs).33-36 Nevertheless, the existing ECHs are still incapable of satisfactorily meeting the performance requirements for bioelectronic devices. For instance, ECHs should have excellent conductivity to reduce power consumption and noise of sensing electronics.37, 38 However, simply increasing the ratio of conductive components or electrolytes in hydrogels would cause phase separation of the hydrogel matrix or salting-out effect, thereby impairing the mechanical properties and biocompatibilities of the hydrogel materials.39-42 Moreover, the challenges ECH materials face in different service environments are diverse, resulting in wide variations in performance requirements such as stretchability, environmental adaptability, adhesion, self-repair, and antibacterial properties of ECHs for bioelectronic devices. By manipulating the hydrogel's chemical composition and physical structure, an ECH can fulfill specific functions according to desired requirements.
Till now, reviews focusing on ECHs have been reported in the view of their classification,13-17 materials,12, 43-45 fabrication techniques,46, 47 and applications.48 Additionally, several reviews have been compiled from the perspective of endowing ECHs with functionalities such as self-adhesion,49 self-healing,50 and injectability.51, 52 However, a comprehensive analysis and summation of the intricate factors hindering the expansive development of ECHs in bioelectronics remain conspicuously absent. This review focuses on the various constraints that currently impede the progressive development of ECHs and explores their potential applications in bioelectronic advancement (Figure 1). We first emphasize challenges faced by ECHs in bioelectronics and corresponding strategies proposed in recent works. The structure-properties relationships of ECHs are discussed here, which provide guidelines for the rational design of ECHs to address the problems of stretchability, environmental adaptability, physical damage, interface compatibility, and bacterial contamination. Subsequently, typical applications in the field of bioelectronics, including healthcare monitoring, treatment of diseases, and HMIs, are highlighted. Furthermore, we recapitulate some perspectives for the future development of ECHs in bioelectronics.

Schematic summary of challenges and applications of electroconductive hydrogels in bioelectronics.
2 CHALLENGES FACED BY ECHs IN BIOELECTRONICS
2.1 Stretchability
Stretching, twisting, and folding of muscle, organ, or skin tissues are essential for achieving the flexibility of the human body. However, the variations in the deformation ability of different human body parts cause extensive problems that the mechanical performance of materials in bioelectronics does not match the tissues, especially for joint skins with natural wrinkled structures that provide greater strain tolerance.53 There is a considerable demand to develop highly stretchable materials that can simultaneously enhance the durability of electronic devices and provide comfort when worn in bioelectronics. Achieving high stretchability of hydrogels has always been a big hurdle in application, although hydrogel-based materials have similar characteristics to human tissues.54 The low stretchability of conventional hydrogels is generally attributed to the inhomogeneous chemically crosslinked networks and the lack of an effective energy dissipation network. Furthermore, this drawback is more apparent for ECHs in which rigid nanometallic materials, conductive polymers, or carbon materials usually serve as conductive fillers.55-57 In recent years, many researchers have begun to focus on the design of highly stretchable ECHs in bioelectronics and three prevalent approaches have been exploited to improve the stretchability of ECHs.
First, the construction of double-network hydrogels, which consist of two interpenetrating polymer networks, is an effective approach. Generally, the brittle network in double-network hydrogels provides sacrificial bonds to dissipate substantial energy under large deformation, while the flexible network allows the hydrogels to remain intact.58, 59 The work by Yang et al. was implemented by developing double-network ECHs with a rigid and conductive network of chemical cross-linking poly(thiophene3-acetic acid) (PTAA) and a flexible and biocompatible network of photo-crosslinking methacrylated aminated gelatin (MAAG) (Figure 2A).60 Increasing the soft MAAG network content in ECHs from 0% to 50% clearly improved the flexibility of the hydrogels, as evidenced by an increase in fracture strain from 23% to 37% (Figure 2B). The primitive interpenetrating networks have effectively improved the stretchability of ECHs in this study, but it remains challenging to meet larger deformation demands in bioelectronics. Subsequently, more creative double-network ECHs were further designed for various applications. Liang et al. reported a tough and stretchable ECH with a physically cross-linked dual ionic network of poly (acrylamide-co-acrylic acid)-Fe3+ (P(AM-co-AA)-Fe3+) and chitosan- (CS-) via free radical polymerization and soaking processes (Figure 2C).61 Specifically, interpenetrating networks composed of P(AM-co-AA) and crosslinked chitosan (CS) chains are pre-synthesized by UV-initiated free radical polymerization. Subsequently, the hydrogels are submerged in Fe(NO3)3 and Na2SO4 aqueous solutions, resulting in the formation of superior dual ionic networks. CS chains are served as flexible networks to endow the ECH with high strength. This double ionic network ECH has achieved unexpected stretchability that fracture strain up to ∼1225% (Figure 2D). Moreover, other studies have also employed the introduction of ions between double networks as an approach to enhance the stretchability of ECHs.62, 63 Recently, our team also made a flexible ECH for electroluminodynamic therapy (ELDT) device, which could effectively kill drug-resistant bacteria without any traditional antibacterial agents.64 The ELDT hydrogel with polyacrylamide (PAM)/sodium alginate (SA) double networks was prepared through a two-step process of free radical polymerization and ionic physical cross-linking and could extend >300% of its original length.
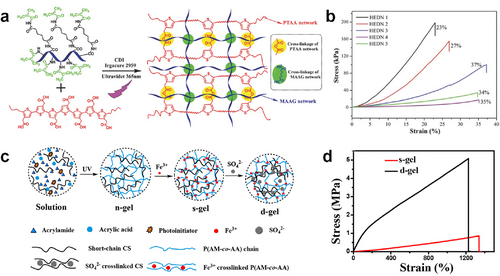
(A) Synthetic method and chemical structure of PTAA/MAAG-based double-network ECH. Reproduced with permission.60 Copyright 2015, Wiley-VCH. (B) Compressive stress–strain curves of PTAA/MAAG-based double-network ECHs. (C) Schematic illustration of the preparation procedure and network structure of the P(AM-co-AA)-Fe3+/CS- hydrogel. Reproduced with permission.61 Copyright 2020, American Chemical Society. (D) Stress−strain curves of P(AM-co-AA)-Fe3+/CS and P(AM-co-AA)-Fe3+/CS-. ECHs, electroconductive hydrogels; PTAA/MAAG, poly(thiophene3-acetic acid)/methacrylated aminated gelatin.
The introduction of reversible interactions in hydrogel networks is another practical approach to improving the stretchability of ECHs. These reversible interactions include dynamic covalent interactions such as Schiff bases, disulfide bonds, and borate ester bonds, as well as non-covalent interactions including but not limited to ionic bonds, hydrogen bonds, metal-coordination interactions, hydrophobic forces, and van der Waals forces. These diverse interactions that existed in ECHs could be sacrificed to dissipate energy effectively.54 Yang and co-workers synthesized a dual physically cross-linked ECH with excellent mechanical and electronic properties through micellar copolymerization of N-hydroxymethyl acrylamide (HMA) and stearyl methacrylate based on hydrogen bonds and hydrophobic associations (Figure 3A).65 Due to the dual physical cross-linking, poly(N-hydroxymethyl acrylamide) (PHMA) hydrophobic association ECH demonstrated a high tearing energy of 1509 J m−2 and a high tensile strength of 1.09 MPa (Figure 3B,C). Moreover, a notched PHMA gel could be elongated to 8.7 times its original size, which indicates it is notch-insensitive. This ECH can serve as a sensor of strain and pressure for monitoring human movements. Cui et al. recently reported a toughened alginate/polyvinyl alcohol (Alg/PVA) ECH reliant on the synergistic effect of ion-induced cross-linking and salting-out, achieving significant enhancements in fracture stress and fracture energy by up to 530- and 1100-folds without sacrificing the ductility (fracture strain of 750%) (Figure 3D).66 The authors attributed the enhancements to both the cross-linking and salting-out effects, which densify the network structure and facilitate the formation of inter-/intra-polymer interactions.
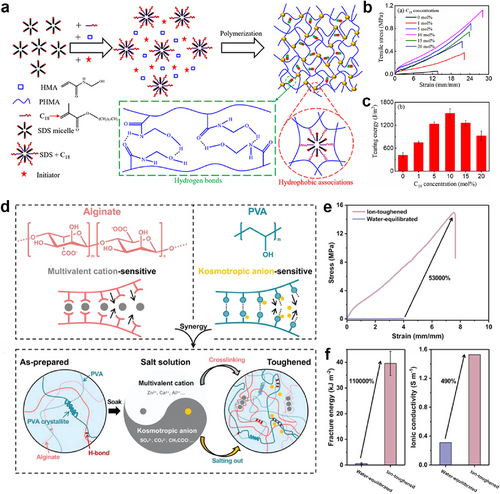
(A) Schematic illustration of the synthesis and structure of the PHMA hydrophobic association ECH. Reproduced with permission.65 Copyright 2019, American Chemical Society. (B) Tensile curves and (C) tearing energies of PHMA hydrophobic association ECHs. (D) Schematic illustration of synthesis and structure of tough Alg/PVA hydrogel. Reproduced with permission.66 Copyright 2022, Wiley-VCH. (E) Tensile curves of the water-equilibrated and ion-toughened Alg/PVA hydrogels. (F) Fracture energy and ionic conductivity of the water-equilibrated and ion-toughened Alg/PVA hydrogels. Alg/PVA, alginate/polyvinyl alcohol; ECH, electroconductive hydrogel; PHMA, poly(N-hydroxymethyl acrylamide).
In addition, nanomaterials like CNTs, graphene, and Fe3O4 filling in the hydrogels are also available to improve the stretchability of ECHs. The mechanism of this process is that the interactions between the polymer network and nanocomposites could dissipate energy and effectively reduce the polymer intermolecular force.67, 68 The study by Qin et al. presented a highly stretchable hydrogel in which CNTs were dispersed in a hydrophobically associated polyacrylamide (HAPAAm) network with the assistance of amphiphilic sodium dodecyl sulfate (Figure 4A).21 In addition to improving the conductivity of hydrogels, CNTs also served as physical crosslinkers via strong hydrophobic interactions, which could effectively dissipate the deformation energy. Specifically, the optimum CNTs/HAPAAm hydrogel containing 0.5 wt% CNTs showed ultrahigh stretchability, which is up to 3000%, and toughness of 3.42 MJ m−3 (Figure 4B,C). Likewise, introducing graphene oxide (GO) into the PVA/polyethylene cross-linked networks could improve the mechanical properties of hydrogels as well.70 Li and coworkers reported conductive poly (N-hydroxyethyl acrylamide)/Laponite/PEDOT:PSS hydrogels (PHEA/Laponite/PEDOT:PSS hydrogels) (Figure 4D).69 PHEA chains can be physically self-crosslinked by themselves and also crosslinked by laponite nanoplatelets. The PEDOT:PSS endows the hydrogels with good electronic conductivity. Furthermore, the PHEA/Laponite/PEDOT:PSS conductive gels exhibited a high tensile strength of 0.43 MPa (Figure 4E), a high toughness recovery of 82%, and a high conductivity of 0.09 S m−1 (Figure 4F). This hydrogel could be used as a strain sensor to monitor human movement for fingers and wrists, as well as minute respiration.
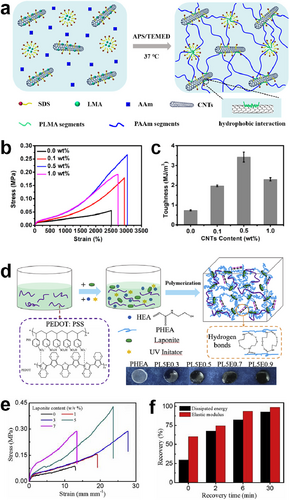
(A) Schematic illustration of the preparation process of the CNTs/HAPAAm hydrogel. Reproduced with permission.21 Copyright 2020, American Chemical Society. (B) Tensile stress−strain curves and (C) the corresponding toughness of the CNTs/HAPAAm hydrogels with various CNTs contents. (D) Schematic illustration of the preparation of PHEA/Laponite/PEDOT:PSS ECH. Reproduced with permission.69 Copyright 2019, Elsevier. (E) Tensile stress–strain curves and (F) recovery ratios of toughness and stiffness of PHEA/Laponite/PEDOT:PSS ECHs. CNTs/HAPAAm, carbon nanotubes/hydrophobically associated polyacrylamide; ECH, electroconductive hydrogel.
2.2 Environmental adaptability
Although hydrogel is one of the most suitable materials to mimic the natural human body and has remarkable biocompatibility with human tissues among various artificially synthesized materials, environmental adaptability still hinders its application. Just as organisms could not live in some extreme situations, hydrogel also faces crucial problems such as evaporation and freezing, which are essentially caused by the physical properties of water. Hydrogels are naturally prone to dehydration without protection, especially in an exposed environment, which could significantly affect the original mechanical performance and conductivity designed by researchers and even break the structural integrity of hydrogels. On the other hand, hydrogels with abundant water are easily frozen at low temperatures (below 0°C) and lose their basic performance. Therefore, research on maintaining the environmental adaptability of ECHs is of great importance for hydrogel bioelectronics.
2.2.1 Water retention
Various efforts have been made to avoid the rapid evaporation of water in the ECHs network and maintain the stable performance of ECHs so far. In general, hygroscopic salts dissolving in an aqueous state would ionize into cations and anions and further bring about the formation of hydrated ions. This process reduces the vapor pressure, which stabilizes the water molecules in the hydrogels.71 In particular, this approach is most convenient and efficient for ionically conductive hydrogels. For example, lithium chloride (LiCl)-based ionic conductive poly (sulfobetaine-co-acrylic acid) hydrogel exhibited extraordinary water retention capacity. The hydrogel retained nearly 100% of its water content after being exposed to a typical environment (25°C, 54% relative humidity) for 1 week (Figure 5A–C).72 The water retention capacity of hydrogels is determined by the degree of ionic hydration, the salt and its concentration. Water molecules will hardly evaporate from hydrogels if there is a strong bond between cation/anion-water molecule pairs, which means a higher water retention ability. Bai et al. proved that LiCl is the most efficient hygroscopic salt for water retention in dehydrated conditions, followed by potassium acetate (KAc) and magnesium chloride (MgCl2), and sodium chloride (NaCl) is the least effective salt among all salts (Figure 5D–F).73 Besides, dual-ionic (free Ca2+ and bonded ) ECHs have also been developed for anti-dehydration, which have achieved storage in the general environment for 8 months without apparent dehydration.76 However, hydrogels containing hygroscopic salts would not dry out in the air but swell and de-swell in response to fluctuations in the ambient humidity, and the corresponding fluctuations in water content would cause challenges in their electrical properties.
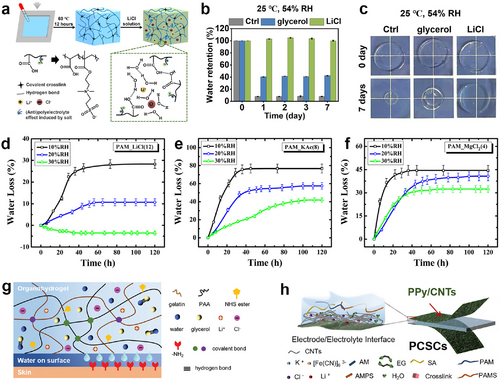
(A) Schematic depiction of preparation of LiCl-based poly (sulfobetaine-co-acrylic acid) ECHs. Reproduced with permission.72 Copyright 2021, Elsevier. (B) Water retention of control, Gly-based and LiCl-based hydrogels after storage at 25°C and 54% relative humidity from 0 to 7 days. (C) Images of control, Gly-based and LiCl-based poly (sulfobetaine-co-acrylic acid) hydrogels placed at 25°C and 54% relative humidity for 0 and 7 days. Evolutions of water loss over time for (D) LiCl-based; (E) KAc-based; and (F) MgCl2-based ECHs with high salt content under varying levels of relative humidity at 25°C. Reproduced with permission.73 Copyright 2014, AIP Publishing. (G) Structural schematic of an organo-hydrogel by introducing a Gly/water binary solvent system. Reproduced with permission.74 Copyright 2021, Wiley-VCH. (H) Schematic illustration of an organo-hydrogel by introducing an EG/water binary solvent system. Reproduced under terms of the CC-BY 4.0 license (https://creativecommons.org/licenses/by/4.0).75 Copyright 2022, The Authors, published by Wiley-VCH. ECHs, electroconductive hydrogels; KAc, potassium acetate; LiCl, lithium chloride; MgCl2, magnesium chloride.
Organo-hydrogel, which is filled with organic solvent and water in the network, is another solution for the long-term water retention of hydrogels.77, 78 Polyol molecules like ethylene glycol (EG) and glycerin (Gly) serve as humectants in preventing water evaporation of ECHs due to their exceptional hygroscopicity and non-toxic nature. Their excellent water retention capability can be attributed to their higher boiling points and the strong interaction (hydrogen bonds) between the polyol molecules and water molecules, thereby reducing the overall vapor pressure of the binary solvents.79 According to related research articles, Gly is the most frequently used agent compared to other organic molecules because of its higher boiling point and lower vapor pressure. The implementation of the Gly/water binary solvent strategy in ECH research has led to improved long-term water retention capacity in hydrogels. These improvements, with minimal weight loss, were observed for periods ranging from a few days to several months (Figure 5G).74, 80-82 However, it is important to note that the use of Gly may impede the free movement of conductive ions within ECHs, ultimately leading to decreased conductivity. Thus, EG as another solvent was incorporated into the hydrogels, allowing for ions to move freely in the ECHs (Figure 5H).75, 83
Inspired by organisms that counteract natural evaporation, protective layers have been developed on the surface of ECH, similar to the skin of a mammal or the leaf epidermis, to prevent dehydration of the hydrogel body. A strategy for the fabrication of skin-liked surface structure via assembling hydrogel-elastomer hybrids with robust interfaces was developed by Yuk et al. for bioelectronics application (Figure 6A).84 By covering the elastomer (Ecoflex), the hydrogel–Ecoflex hybrid exhibited a desirable anti-dehydration performance under the ambient condition (24°C and 50% relative humidity) that did not exhibit significant weight changes for 48 h. Previous research has identified oily substances on the skin and waxy substances on the leaf as crucial barriers that prevent water evaporation from the body. Consequently, further research has been conducted to develop hydrophobic coatings that can mitigate water evaporation in ECHs. The organogel–hydrogel hybrid is a newly developed composite structure that integrates hydrophilic hydrogel and hydrophobic organogel.85 By serving as the outer layer, the hydrophobic organogel enables this hybrid to greatly enhance its water retention capability. Additionally, double-hydrophobic coatings can serve as the external layer of ECHs to prevent water evaporation for the hydrogel network.88, 89 Figure 6B illustrates the manufacturing process of the double hydrophobic coating, which involves three straightforward steps. Initially, the bulk hydrogel is immersed in a solution containing hydrophobic monomer and initiator, and polymerization occurs, forming the hydrophobic polymer layer on the surface. An oil treatment is then performed to fill the hydrophobic polymer layer with oil while the oil-swollen surface is constructed. Finally, the robust, homogeneous structure on the surface is achieved by the quenching of the surface. This double-hydrophobic coating serves as a strong barrier against water transfer and considerably reduces drying. In this research, acrylate-based hydrophobic monomers with three different chain lengths, octyl acrylate (OA; short chain), dodecyl acrylate (DA; medium chain) and stearyl acrylate (SA; long chain) are viable options as alternative hydrophobic monomers. Furthermore, a hydrogel containing ZnO nanoparticles (ZnO NPs) with a dense structure could also serve as a protective layer for the dual-layer gel, effectively reducing excessive water evaporation and preserving the ECH stability as a skin patch (Figure 6C).86
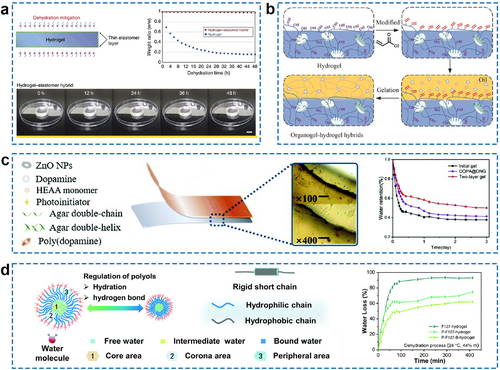
(A) Schematic illustration and anti-dehydration performance of the hydrogel–elastomer hybrid. Reproduced under terms of the CC-BY 4.0 license (https://creativecommons.org/licenses/by/4.0).84 Copyright 2016, The Authors, published by Springer Nature. (B) Process for the preparation of an organo-hydrogel hybrid. Reproduced with permission.85 Copyright 2018, Wiley-VCH. (C) Structural schematic of a two-layer gel in which hydrogel is doped with ZnO NPs serving as a protective layer for water retention. The water retention of three different gels stored at 28°C and 56% relative humidity for 0 and 3 days is measured. Reproduced with permission.86 Copyright 2021, Royal Society of Chemistry. (D) The regulation mechanism of polyols/water multi-solvent on micelle self-assembly during gelation in a superior water-anchored hydrogel and the water loss curves of three different hydrogels under natural conditions. Reproduced with permission.87 Copyright 2020, Royal Society of Chemistry. NPs, nanoparticles.
In addition, it is possible to adjust the framework materials of a hydrogel to modify its water retention property. A water anchoring strategy that suggests water retention through the design of a hydrogel network skeleton has been proposed (Figure 6D).87 The hydrophilic chain from the micelle shell provides a strong hydrogen bonding effect, while the rigid hydrophobic chain provides a repelling effect. This accommodation reduces the kinetic activation energy of water molecules in the hydrogel, enabling more water to anchor in the hydrogel network. The appropriate strategy to adopt depends on the specific use of hydrogels. In vitro application scenarios require higher water retention compared to in vivo scenarios. Typically, the ECH should retain more than 80% of its weight in its working environment during its service period to ensure its conductivity and flexibility.
2.2.2 Freeze resistance
Water provides excellent biocompatibility for hydrogels, but it also limits their performance at low temperatures. The water existing in the hydrogel network at low temperatures could be categorized into three types: “free water,” “loosely bound water” and “unfrozen bound water.”90 Free water in the hydrogel network has no interaction with the other components and moves as freely as bulk water. Meanwhile, the physical properties of free water are similar to those of bulk water; freezing caused by phase transition would occur when the ambient temperature drops below the freezing point. Loosely bound water is in an intermediate state (also called “intermediate water”), which has little interaction with the surrounding hydrophilic groups, and whose movement is also affected to some extent. Therefore, loosely bound water can maintain a non-frozen state at subzero temperatures. Unfrozen bound water has strong interactions with other components, such as polymer networks or conductive functional materials, and there are basically multiple non-covalent interactions between a single water molecule and other components. Thus, unfrozen bound water is able to remain liquid at ambient temperatures below freezing. Generally, the content of free water in the hydrogel network is much higher than that of loosely bound water and unfrozen bound water, which makes ordinary hydrogels susceptible to freezing at low temperatures. Thus, reducing the amount of free water in the network is a feasible approach to improving the freeze resistance of ECHs.
Salts can be employed as anti-freezing agents in ECHs to lower freezing points while acting as conductive electrolytes. LiCl is the most commonly used inorganic salt to improve the freeze resistance of ECHs by inhibiting water crystallization and preventing water evaporation.91, 92 The ECHs containing LiCl could even endure ultralow temperatures (−80°C) over 30 days without freezing (Figure 7A,B).91 The introduction of other chlorides, such as NaCl, zinc chloride (ZnCl2), or calcium chloride (CaCl2) into ECHs also endowed the hydrogels with outstanding anti-freezing and ionic conductive properties.96-98 Some studies also tried to utilize iron(III) sulfate (Fe2(SO4)3) or organic acids like tannic acid and phytic acid as electrolytes for improving the low-temperature tolerance ability of ECHs and produced satisfactory results.99-101 Recently, researchers have developed advanced anti-freezing ECH systems with additional features. By complexing PAM (PAAm) with polyacrylic acid (PAA) chelated with Fe3+ ions and adding NaCl salts, Li et al. created a novel PAAm/PAA–Fe3+/NaCl hydrogel (Figure 7C).93 The addition of NaCl not only significantly lowered the freezing point of water trapped inside the PAAm/PAA–Fe3+/NaCl hydrogels to −24.7°C, but also endowed the hydrogels with high ionic conductivity (∼0.72 S m−1) (Figure 7D). These enhanced hydrogels could function as ionic skins, suitable for monitoring the health and movements of the human body when integrated into wearable devices. The addition of salt to ECHs could enhance their electrical conductivity, yet it may also impact the stability of ECHs.
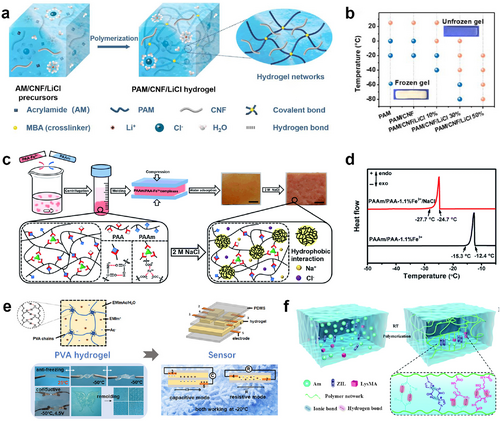
(A) Schematic illustration of the anti-freezing ECH synthesized by a one-step AM polymerization in the presence of CNF and LiCl. Reproduced with permission.91 Copyright 2021, Elsevier. (B) Experimental phase diagram (frozen/non-frozen state) of PAM, PAM/CNF, and PAM/CNF/LiCl hydrogels. The background color is absent when a hydrogel is frozen. (C) Schematic illustration of the preparation process of the anti-freezing PAAm/PAA–x%Fe3+/NaCl hydrogels. Reproduced with permission.93 Copyright 2020, Royal Society of Chemistry. (D) DSC curves of the PAAm/PAA–1.1%Fe3+ and PAAm/PAA–1.1%Fe3+/NaCl hydrogels. (E) Schematic representation of an anti-freezing ECH prepared using ILs and its antifreeze properties. Reproduced with permission.94 Copyright 2021, American Chemical Society. (F) Schematic illustration of the structure and the intermolecular noncovalent interactions of an ECH system using ZIL. Reproduced with permission.95 Copyright 2022, Royal Society of Chemistry. AM, acrylamide; CNF, cellulose nanofibrils; DSC, differential scanning calorimetry; ECH, electroconductive hydrogel; LiCl, lithium chloride; NaCl, sodium chloride; PAM, polyacrylamide; ZIL, zwitterionic liquid.
In recent years, ionic liquid (IL)-based gels (ionogels) have attracted attention due to their low-temperature tolerance, high electrochemical stability, and excellent conductivity.102 These materials utilize ILs as solvents and consist of cation–anion pairings of organic ions and inorganic counterions. They can flow like water but do not freeze at subzero temperatures, making them highly tolerant of extreme temperatures.103 Liu et al. synthesized a physically crosslinked hydrogel by dissolving PVA into an IL/water binary solvent, in which 1-ethyl-3-methylimidazolium acetate (EMImAc) was employed as IL (Figure 7E).94 The PVA/EMImAc/H2O hydrogel remains flexible and conductive at temperatures as low as −50°C and presents extraordinary water-retention properties, owing to the introduction of IL. Another study by Zhang et al. demonstrated that the conductive zwitterionic nanochannels formed by the zwitterionic liquid (ZIL) and methacrylate lysine (LysMA) also endowed the hydrogel with excellent conductivity and low-temperature tolerance (Figure 7F).95 They fabricated a novel hydrogel consisting of acrylamide, ZIL, and LysMA using a one-pot method. The hydrogel exhibited outstanding flexibility and conductivity even under −20°C. The authors believed that ZIL in this system decreased the freezing point by disrupting the hydrogen bond between water molecules. In general, this strategy still utilizes the interaction between ions and water molecules to reduce the content of free water in the hydrogel to achieve better anti-freezing performance.
Furthermore, organo-hydrogel has a lower freezing temperature because of the existing of organic solvent, which has a much lower freezing point than water. Liu and coworkers prepared an anti-freezing organo-hydrogel by introducing an EG/water binary solvent into PVA and conductive PEDOT:PSS networks (Figure 8A).104 The hydrogen bonds and crystalline regions formed with PVA chains and solvent molecules provide the conductive organo-hydrogel with reliable mechanical strength and self-healing properties. This organo-hydrogel can maintain stable flexibility and strain sensitivity at temperatures ranging from −55.0°C to 44.6°C (Figure 8B). Jian et al. synthesized anti-freezing and stretchable organo-hydrogels through the solvent-displacement method. The prepared hydrogels were immersed in Gly or a mixed solution of Gly and water (Figure 8C).105 After introducing potassium iodide (KI), the Gly-based PAAm organogel could be used as a conductive gel sensor with a working temperature range of −30°C to 60°C. Dimethyl sulfoxide (DMSO) has a higher dielectric constant than other molecules and has been used to prepare antifreeze hydrogels in recent years.107 In the presence of a DMSO/H2O organic solvent system, the ion-conducting hydrogel remains flexible and conductive (0.014 S cm−1) at −70°C.

(A) Schematic illustration of the preparation and structural characterization of the anti-freezing organo-hydrogel. Reproduced with permission.104 Copyright 2017, Wiley-VCH. (B) The mechanical properties of anti-freezing conductive organo-hydrogels and conductive hydrogels at low temperatures. (C) Schematic illustration of the solvent-displacement method for synthesizing organo-hydrogels and digital photos of organo-hydrogel blocks with different solvent components at 20°C and −10°C. Reproduced under terms of the CC-BY 4.0 license (https://creativecommons.org/licenses/by/4.0).105 Copyright 2019, The Authors, published by the American Association for the Advancement of Science. (D) The fraction of unfrozen water as a function of internanodomain spacing was determined using small angle neutron scattering. Reproduced with permission.106 Copyright 2019, American Chemical Society.
Doping hydrogel networks with anti-freezing agents maximally enhances their low-temperature resistance. However, the anti-freezing agents (solutes or solvents) are easily released from the material, which affects its basic properties and are potentially biotoxic. Fixing the freeze-resistant group on the polymer chain proves to be an ideal solution to overcome this issue. The presence of grafted residues and bound water can disturb the water structure, hindering ice nucleation and leading to a sub-zero freezing temperature of the hydrogel if a specific polymer chain group has a strong attraction with water molecules (energetically favored over water-water interactions). Wang et al. reported that supramolecular hydrogels consisting of statistical copolymers of 2-hydroxyethyl acrylate and 2-(Nethylperfluorooctane sulfonamido)ethyl methacrylate effectively suppressed ice formation within a synthetic material using biomimicry, resulting in an unprecedented level of suppression (Figure 8D).106 Another recent study designed a strategy to fabricate a family of double-network hydrogels with intrinsic, built-in anti-freezing and mechanical properties, without any anti-freezing additives.108 However, a drawback of this approach is that ECHs still require the inclusion of salts to provide conductive ions for achieving optimal conductivity. This method could overcome the disadvantage of the leakage of anti-freezing components and realize the long-term stable application of ECHs. However, the freeze resistance of this method is usually not very strong.
The exceptional freeze resistance of ECHs can be achieved by various strategies, including incorporating salts as antifreeze agents, using IL-based gels, introducing organic solvents, and grafting specific polymer chain groups to prevent ice nucleation, thus extending the functionality of the material even at extremely low temperatures. The aforementioned methods are disparate operationally, yet collectively seek to diminish the quantity of free water present within the hydrogel, thereby enabling it to withstand freezing temperatures while preserving its essential properties. The ECH should demonstrate consistent electrical and mechanical performance at low temperature, ideally below −20°C. Furthermore, the ECH should ideally show reversible changes in its properties when transitioning between freezing and thawing cycles, without significant degradation. The design and preparation of anti-freeze ECHs are still relatively simple, and further research is needed to elucidate the mechanism and develop new synthesis strategies for anti-freeze ECHs.
2.3 Physical damage
Bioelectronic devices are susceptible to physical damage after prolonged bending and stretching, which can lead to reduced reliability and durability. Self-healing materials are inspired by self-repair mechanisms found in nature, such as the scab healing process of human surface wounds, and have the potential to improve the longevity and reliability of bioelectronic devices. Self-healing ECHs, which emulate human skin, have exhibited great potential in emerging bioelectronic fields. Additionally, electroconductive self-healing materials are alluring for the production of futuristic soft robots or cyborganics. However, developing ECHs with optimal mechanical behavior and self-healing properties is still a significant challenge. The rheological feature of self-healing in ECHs refers to the capability of the polymer matrix to autonomously recover its original state after sustaining damage. At present, self-healing ECHs can be designed and constructed by employing reversible non-covalent interactions or dynamic covalent interactions. Self-healing mechanisms in hydrogels can be categorized, depending on the cause, into two types: those that involve the autonomous behavior of the materials themselves, such as dynamic chemical bonds, noncovalent interactions and those that respond to external stimuli, such as heat, light or pH. The self-healing mechanisms, healing requirements, and healing efficiencies of some representative stimulus-responsive self-healing ECHs are summarized in Table 1.
| Stimulation types | Self-healing mechanisms | Healing requirements | Healing efficiencies | Refs. |
|---|---|---|---|---|
| Heat | Hydrogen bonds and metal coordination bonds | 70°C for 2 h | Mechanical property: 41.7% | 109, 110 |
| Heat | Hydrogen bonds | 90°C for 30 min | Electrical property: 95.2% | 111 |
| Heat | Diels-Alder reactions | 125°C for 30 min and 60°C for 24 h | Mechanical property: 77.6% | 112 |
| Light | Borate bonds | Near-infrared (NIR) light irradiation for 1 min | Mechanical property: 93% | 113 |
| Light | Borate bonds and fluidity of macromolecular chains | NIR light irradiation for 100 s | Mechanical property: 97.1% | 114 |
| Light/pH | Hydrogen bonds | Acidic or basic condition with NIR light irradiation for 3 min | Mechanical property: ≈90% | 110 |
| Electrical property: ≈100% | ||||
| Electricity/light | Ag–S bonds and hydrogen bonds | Electrical stimulus for 5 min or NIR light irradiation for 5 s | Mechanical property: 73% (electric field), 101% (NIR light) | 115 |
| Microwave/pH | π–π interactions, disulfide bonds, and hydrogen bonds | Microwave irradiation for 15 s or acidic condition for 24 h | Mechanical property: 84% (microwave), 50% (acid) | 116 |
| Electrical property: 74% (microwave), 78% (acid) |
Generally, self-healing hydrogels are made from reversible non-covalent interactions, including host-guest recognition, hydrogen bonds, hydrophobic interactions, and ionic bonds. These sacrificial combinations in self-healing ECHs act as predetermined breaking points that absorb stress and can then reform, allowing the hydrogel to repair itself after minor damage. Among the above-mentioned non-covalent interactions, hydrogen bonds are the most common interactions in nature and significantly contribute to the mechanical properties and conformation of biomacromolecules. They have also been widely used as reversible links in the production of self-healing hydrogel matrices.117, 118 Hydrogen bonds have lower binding energy than covalent bonds or ionic bonds, typically no more than 15 kcal mol−1. However, the benefits of rapid bonding and wide distribution make them critical components in the self-healing process of hydrogel materials. Recently, Yu et al. prepared soft and rapid self-healable ECHs by incorporating polyaniline nanoparticles (PANI-NPs) into the poly(poly(ethylene glycol) methacrylate-co-acrylic acid) (P(PEG-co-AA)) scaffolds. The novel conductive composite hydrogels exhibit high mechanical performance and excellent anti-fatigue performance (Figure 9A).119 Most importantly, the fracture strain and maximum stress of the ECHs in this study were able to recover to 93.3% of their initial state after mechanical damage, showing excellent self-healing ability. Furthermore, self-healing ECHs reliant on multiple hydrogen bonds have been extensively reported for the preparation of wearable bioelectronic devices.123, 124 Ionic bonds and metal-coordination interactions are also used to fabricate self-healing materials because they are easy to control and synthesize. Fe3+ can coordinate with different anionic groups, such as carboxyl and sulfonic acid groups, and has been adopted in many studies to prepare self-healing hydrogels.120, 125-128 As shown in Figure 9B, Fe3+ is introduced into the hydrogel network, allowing for reversible interactions with anionic groups. This reversible interaction enables damage, such as cuts and scratches, to heal quickly and repeatedly.120 Ionic interactions between ammonium groups and catechol groups in poly(γ-glutamic acid) (γ-PGA) were also reported preparing self-healing ECHs based on their excellent biocompatibilities (Figure 9C).121 In this study, cation–π interaction is induced into the hydrogel matrix which could provide extra links during the healing process. Other non-covalent interactions, while significant in the preparation of self-healing hydrogels, are typically used in conjunction with hydrogen bonds or ionic bonds for self-healing purposes. For instance, Ren et al. prepared a self-healing ECH based on O-carboxymethyl chitosan (O-CMCS) and PVA. Due to the presence of the host-guest inclusion complexing of poly(β-cyclodextrin) with adamantane groups, borate bond, Schiff base, and hydrogen bond, the CS-based ECH exhibited rapid self-healing ability with healing efficiency as high as 97%–103% (in 15 s) (Figure 9D).122
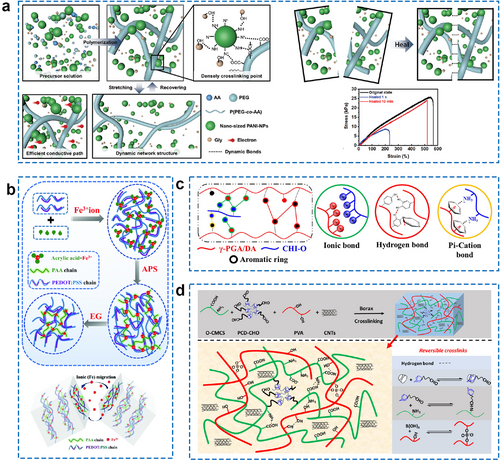
(A) The schematic structure of the soft and rapid self-healable P(PEG-co-AA) hybrid ECHs and tensile curves of the three hydrogels in the original and healed states with different healing times. Reproduced with permission.119 Copyright 2022, Wiley-VCH. (B) Schematic illustration of the fabrication process of PEDOT:PSS/PAA–Fe3+ hydrogel and the self-healing mechanism. Reproduced with permission.120 Copyright 2022, Royal Society of Chemistry. (C) The gelation mechanism of the self-healable γ-PGA-based hydrogel and the non-covalent interactions in this hydrogel. Reproduced with permission.121 Copyright 2021, Elsevier. (D) Synthesis route and cross-linking mechanism of a rapid self-healing CS-based hydrogel by host-guest interaction and dynamic covalent bond. Reproduced with permission.122 Copyright 2021, Elsevier. CS, chitosan; ECHs, electroconductive hydrogels; P(PEG-co-AA), poly(poly(ethylene glycol) methacrylate-co-acrylic acid); γ-PGA, poly(γ-glutamic acid).
Dynamic covalent interactions could also facilitate the healing of hydrogels through the reconstruction of covalent bonds such as Schiff base, boronate ester, disulfide, and Diels-Alder reactions. The chemical bonds formed by dynamic covalent interactions are more stable than the non-covalent interactions described above. However, the formation of dynamic covalent bonds often requires a longer reaction time or a special reaction condition. Wang et al. recently developed self-healing ECHs through a Schiff base reaction between dialdehyde-functionalized PEG and acrylamide, resulting in self-healing hydrogels (Figure 10A).129 The authors investigated the self-healing mechanism of the system using proton nuclear magnetic resonance spectroscopy (1H NMR) and Fourier-transform infrared spectroscopy and demonstrated that the cooperative effect of dynamic covalent (Schiff base reaction) and non-covalent interactions (hydrogen bond) leads to the self-healing ability of the gel. The other work designed a self-healing ECH with an island-bridge by merging PVA/CNTs hydrogel and PVA/graphene hydrogel (Figure 10B).130 Dynamic borate–dol ester bonds between the borate ion and the –OH group in the hydrogel matrix allow the ECH to heal without any external stimulation (Figure 10C). The PVA/CNTs/graphene hydrogel presents a new concept for the advancement of wearable electronics, showcasing the potential of the upcoming generation of such technology.
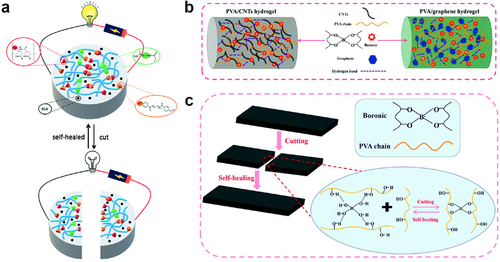
(A) A self-healing ECH made from dynamic covalent (Schiff base reaction) and non-covalent interactions (hydrogen bond). Reproduced under terms of the CC-BY-NC 3.0 license (https://creativecommons.org/licenses/by-nc/3.0/).129 Copyright 2021, The Authors, published by Royal Society of Chemistry. (B) Cross-linking reactions and multiple complexes inside the PVA/CNTs hydrogel and PVA/graphene hydrogel. Reproduced with permission.130 Copyright 2021, Royal Society of Chemistry. (C) Schematic diagram of the self-healing mechanism of the PVA/CNTs hydrogel and PVA/graphene hydrogel. ECH, electroconductive hydrogel; PVA/CNTs, polyvinyl alcohol/carbon nanotubes.
2.4 Interface compatibility
Bioelectronic devices are expected to make intimate contact with tissues. Otherwise, mismatches in mechanical or electrical properties may cause low signal intensity, poor signal-to-noise ratios (SNRs), mis-action, or even device invalidation. Therefore, it is crucial to strengthen the interface compatibility of ECHs in bioelectronics to achieve satisfactory performance. Traditional bioelectronic devices typically require the use of adhesive tapes, bandages, or sutures to attach to the skin, heart, organs, and other human tissue. Unfortunately, this practice may result in additional injury to users and invalidation of the devices in long-term practical applications. A more effective strategy for addressing this issue involves modifying the internal characteristics of ECHs. Specifically, equipping ECHs with self-adhesive properties would enhance the stability and fidelity of the bioelectronics in signal detection. The adhesion of ECHs to biological tissues relies on the chemical bonding and intermolecular interactions at their interfaces. The functional groups abundant in biological tissues, including carboxylic acids, thiols, amino groups, and hydroxyl groups, provide adequate anchor points for hydrogel adhesion. By modifying the chemical components and functional groups of ECHs, an adhesive interface can be established to match biological tissues effectively. The core of this strategy is to create stable anchoring of the material by forming covalent or dynamic bonds between ECHs and tissue interfaces.131
High selectivity, specificity, and efficiency in chemical reactions are required for ECHs-tissue interfacial bonding. For example, Michael addition and Schiff base reactions are commonly used to establish covalent bonds at the interface. Gu et al. prepared a hydrogel sensor for monitoring human and organ motions based on O-CMCS and PVA.122 The carboxyl group in O-CMCS has strong hydrophilicity and can establish hydrogen bonds with hydrophilic groups present on the skin surface. The supramolecular cross-linker that existed in the ECH provides aldehyde groups that could react with the amino groups of proteins on the skin surface through Schiff base reactions. Therefore, the fabricated hydrogel exhibited a good self-adhesive property (Figure 11A). In another work, the prepared double-network hydrogel strain sensor also achieved tight adhesion to skin tissue, which could be explained by the Schiff base interaction between the aldehyde groups in hydrogel and the amino groups in skin proteins and the strong hydrogen bonding between skin protein molecules and the carboxyl/amino groups in the hydrogel.134
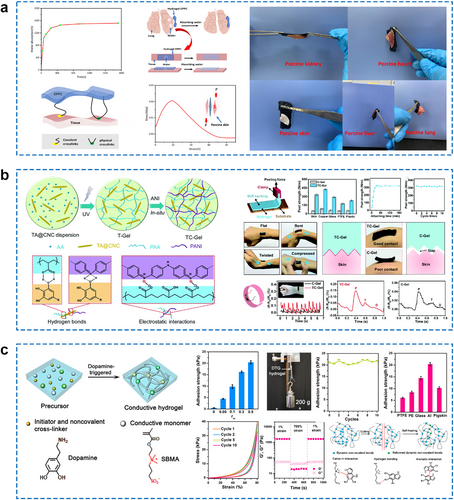
(A) Water absorption capacity, adhesion mode, and adhesion stress–strain curve of the self-adhesive CS-based hydrogels and photographs of the hydrogels adhering to the tissue surface of various organs. Reproduced with permission.122 Copyright 2021, Elsevier. (B) The schematic preparation process of a dynamically self-adhesive ECH and the self-adhesive ECH for enhanced conformal contact with human skin. Reproduced with permission.132 Copyright 2019, Royal Society of Chemistry. (C) Schematic illustration of the preparation of DTG hydrogel and the adhesion, elasticity, and self-healing properties of DTG hydrogels. Reproduced with permission.133 Copyright 2021, American Chemical Society. CS, chitosan; DTG, dopamine-triggered gelation; ECH, electroconductive hydrogel.
In addition, hydrogen bonds and electrostatic interactions are commonly introduced at the interface between the hydrogel and the tissue to enhance interfacial compatibility, resulting in favorable adhesion.135, 136 Shao and co-workers have reported a dynamic self-adhesive ECH that combines both chemical cross-links and physical cross-links mediated by hydrogen bonding and electrostatic interactions.132 The ECH integrates the interpenetrating conductive PANI network and the covalently cross-linked PAA network involving reversible non-covalent interactions using tannic acid-coated cellulose nanocrystals (TA@CNCs) as the reinforcing physical cross-linkers. As a result, the ECH simultaneously achieves superior mechanical and electrical performances (Figure 11B). The dynamic hydrogen bonds and electrostatic interactions in hydrogel networks could be easily broken and re-formed at room temperature, exhibiting excellent self-healing capability. Specifically, the resulting hydrogel provides a mechanically stable interface with good conformal contact to human skin, effectively improving sensing performance for large, intense human movements, small muscle movements, and other physiological signals. Cao and colleagues reported a boron nitride/PEDOT:PSS/poly(N-isopropylacrylamide) ECH with exceptional stretchability, toughness, and multiple functionalities, including self-healing, adhesive, conductive, and photothermal properties.137 The abundant hydrogen-bonding crosslinks and stiffness of the 2D functionalization of boron-nitrogen nanosheets (f-BNNS) enhanced the self-healing and adhesion of ECH materials. Other physical interactions between the hydrogel and the substrate, such as π–π stacking, cation–π interaction, and metal coordination, might also contribute to the adhesiveness of hydrogel materials. Particularly, hydrophobic interaction can overcome the effect of the interfacial hydration layer and realize the effective adhesion of the ECH in water. In Table 2, representative works of these non-covalent interactions in facilitating the interface compatibility of ECH with other substrates are summarized.
| Adhesion mechanisms | Hydrogel components | Adhesion strengths | Applications | Refs. |
|---|---|---|---|---|
| π–π stacking and hydrogen bonding | PDA@CNT/polyacrylamide (PAM) | Shear strengths to polyvinylidene difluoride (PVDF), Al plate, paper, acrylic plate, and glass are 0.8, 25.6, 70.3, 5.6, and 4.3 kPa | Strain sensor | 138 |
| π–π stacking and other non-covalent interactions | Terpyridine derivative/oxidized polyethyleneimine/poly(acrylic acid) (PAA) | Adhesion strengths to wood, copper, iron, skin, and plastic surfaces are 63, 54, 55, 57, and 2 kPa | Strain sensor | 139 |
| π–π/cation–π interaction, hydrogen bonding, and hydrophobic interaction | Poly(acrylamide-hydrophobic/cationic micelles) | Adhesion strength to porcine skin is 30 kPa | Strain sensor | 140 |
| Cation–π interaction and hydrogen bonding | Polymerization of acrylic acid-dopamine, acrylic acid, methacryloxyethyltrimethyl ammonium chloride, tannic acid, methacrylated gelatin, and gelatin | Adhesion strengths to polyethylene terephthalate (PET), muscle, and iron sheet are 5.5, 9.51, and 16.6 kPa | Tissue repair | 141 |
| Metal coordination and hydrogen bonding | Polymerization of dialdehyde carboxymethyl cellulose (DCMC), CS, PAA, and aluminum ions (Al3+) | Adhesion strength to porcine skin is 24.1 kPa | Strain sensor | 142 |
| Metal coordination and other non-covalent interactions | Tannic acid-coated cellulose nanofibrils (TA@CNFs)/PAM/ferric ions (Fe3+) | Adhesion strengths to wood and steel are 9.3 and 4.7 kPa | Skin sensor | 143 |
| Hydrophobic interaction and hydrogen bonding | Hydrophobic alkyl monomers/adhesive catechol derivatives/PAM or PAA | Adhesion strength to tissue is 22.39 ± 1.03 kPa in NaCl solution | Electrocardiogram (ECG) monitoring | 144 |
| Hydrophobic interaction and hydrogen bonding | CNTs/Tara tannin/gelatin/PVA | Adhesion strengths to pigskin, nitrile gloves, glass, and wood are 14, 47, 57, and 63 kPa in water | Strain sensor | 145 |
| Host–guest interaction and hydrogen bonding | Monoacrylamide cucurbit[7]uril/poly(acrylamide-co-methylenebisacrylamide)/and PVA | Adhesion strength to porcine skin is 3.63 kPa | Human–machine interface | 146 |
| Van der Waals' force and other non-covalent interactions | Poly([2-(methacryloyloxy) ethyl] dimethyl-(3-sulfopropyl)-co-acrylamide) | Adhesion strength to paper is 39.1 kPa | Sensor | 147 |
Moreover, great efforts have been made in developing adhesive hydrogels driven by biomolecules such as dopamine molecules, nucleobases, and proteins. Polydopamine (PDA) exhibited a superior binding adhesive ability to a wide range of material surfaces due to its structural similarity to the mussel-adhesive proteins with catechol and amine groups.148-150 Several PDA-based materials are explored as components of enhanced adhesive ECHs. For example, Zhang and co-workers reported a dopamine-triggered gelation (DTG) strategy for fabricating mussel-inspired and transparent ECHs (Figure 11C).133 The researchers evaluated the DTG hydrogel's microscopic adhesion properties using a shear adhesion test. They discovered that increasing the dopamine dosage from 5 wt% to 50 wt% resulted in an increase in the adhesion strengths of the DTG hydrogels from 4.4 to 20.4 kPa. Such a significantly improved effect stems from the elevated levels of dopamine within DTG hydrogels, leading to a substantial boost in adhesion sites. Yu et al. prepared a highly self-adhesive and conductive PVA-based hydrogel for wearable sensors by incorporating PDA as a crosslinking agent.151 The maximum adhesion strength of the PVA-COOH/PDA hydrogel was achieved at a carboxyl group content of 12.01 wt%, owing to the increased number of active groups and interaction sites on its surface. PDA-based materials are investigated as building blocks for modified adhesive hydrogels. Nucleobase-driven adhesive ECHs were also prepared for the fabrication of wearable strain sensors.152 The adhesive layer of this bilayer ECH is prepared by a PAM hydrogel with adenine and thymine. The nucleobase-driven adhesive ECHs could easily adhere to various substrates such as steel, wood, ceramic, and polymer based on the robust nucleobase recognition interaction. Qian's team designed and synthesized a series of ECHs with adhesive, electroconductive, and mechanically robust properties using resilin-like proteins.153 Gly in the hydrogel network promotes the self-assembly of proteins, exposing a large number of amino acid motifs on the hydrogel surface and endowing the hydrogels with excellent adhesion properties. Among the various levels tested, the addition of 20% Gly showed the greatest increase in hydrogel adhesive strength, achieving a significantly high level of 23.8 ± 2.3 kPa.
2.5 Bacterial contamination
Bioelectronics that are composed of ECHs exhibit superior biocompatibility compared to traditional electronics that are composed of metals or semiconductors when used internally for implantation. Nevertheless, hydrogel bioelectronics are inevitably contaminated by pathogenic microorganisms, which can cause serious infection and inflammation. These issues can adversely affect the performance of ECHs and cause vascular and tissue damage during implantation, thus further leading to the misfunction of bioelectronic devices. Therefore, it is necessary to prevent bacterial contamination of ECHs during implantable/wearable applications by designing materials rationally.
Generally, strategies for fabricating antimicrobial hydrogels are summarized into two types. The comparatively straight-forward as well as predominantly used method is to dope the hydrogel network with substances having antibacterial properties by physical mixing. This generic strategy has been widely implemented in the design of ECHs as well. These substances with antimicrobial activity could be antibiotics (e.g., ciprofloxacin, gentamicin, vancomycin), biological extracts (e.g., curcumin, CS), synthetic antibacterial drugs (e.g., nitroimidazoles, sulfa drugs), or antimicrobial inorganic materials (e.g., metal nanoparticles, metal oxide nanoparticles). Notably, some components that serve as conductive fillers for ECHs, such as graphene or Mxene, also exhibit inherent antibacterial properties. These two-dimensional conductive substances have sharp edges that allow them to work as “nano-knives,” which can physically damage bacterial membranes and thereby inactivate the bacteria. However, this effect is not sufficient for ECHs to have reliable antibacterial performance. Additional antibacterial components shall be introduced into the ECH to achieve synergistic or additive effects. In Fan's work, a composite hydrogel with multifunctional segments was rationally designed and mainly fabricated from four raw materials, including biocompatible PVA, self-adhesive PDA, antibacterial activity Ag0 nanoparticles, and conductive graphene (denoted as G) nanosheet.154 The PVA−G−PDA−AgNPs hydrogel exhibits outstanding antibacterial activity to typical pathogenic microbes such as Gram-negative Escherichia coli and Gram-positive Staphylococcus aureus, which effectively prevents bacterial infections that harm human health. Wang et al. reported an antibacterial ECH by incorporating silver nanowires (AgNWs) with a highly crosslinked poly(N-isopropylacrylamide) (PNIPAM) hydrogel.155 In this work, AgNWs in the matrix could improve the conductivity of hydrogel as well as endow the hydrogel with excellent antibacterial performance (Figure 12A). The ECH with the highest AgNWs content exhibited >99.99% killing ratio for both Gram-positive S. aureus and Gram-negative E. coli, which demonstrated strong broad-spectrum antibacterial activity.
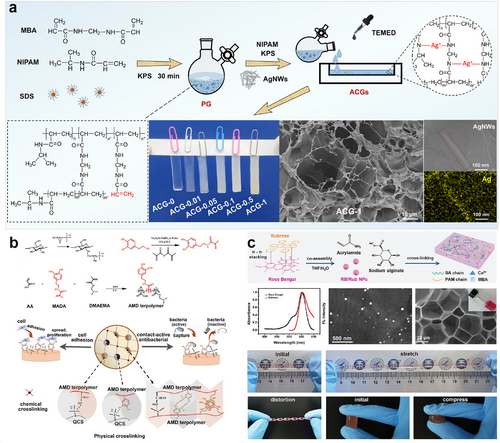
(A) Synthesis and characterization of an antibacterial ECH using AgNWs. Reproduced with permission.155 Copyright 2022, American Chemical Society. (B) The synthetic scheme of the mussel-inspired contact-active antibacterial ECH. Reproduced with permission.156 Copyright 2018, Wiley-VCH. (C) Synthesis process of an antibacterial ECH with EL molecules. Reproduced with permission.64 Copyright 2022, Wiley-VCH. AgNWs, silver nanowires; ECH, electroconductive hydrogel; EL, electroluminescent.
Another strategy for the fabrication of antimicrobial ECHs is to prepare antimicrobial polymer backbones by chemical grafting or copolymerization. The most relevant examples of inherently antimicrobial materials employed to fabricate hydrogels include antimicrobial peptides, CS, or synthetic polymers with antimicrobial functional groups (quaternary ammonium, N-halamines, etc.).157, 158 Du et al. developed a novel hydrogel patch composed of poly(ethylene glycol) diacrylate/quaternized chitosan/tannic acid (PEGDA/QCS/TA) based on mussel-inspired chemistry.159 This hydrogel patch possessed a compact microstructure, a low swelling ratio and tough mechanical properties, good antibacterial activities against S. aureus and E. coli, and excellent dry/wet adhesive ability to a wide range of substrates. Besides, a contact-active antibacterial hydrogel is proposed by copolymerization of methacrylamide dopamine and 2-(dimethylamino)ethyl methacrylate and forming an interpenetrated network with QCS inspired by the mussel adhesion chemistry (Figure 12B).156 This mussel-inspired hydrogel with inherent long-term and effective antibacterial ability not only can avoid many problems associated with using antibiotics, including high cost, short half-life, bacterial resistance, and burst release, but also provides a new solution to balance the cell compatibility and antibacterial activity of biomaterials.
To avoid antimicrobial components in hydrogels negatively affecting the biocompatibility of the materials themselves, many works have been carried out recently to explore responsive antimicrobial strategies. These works generally involve the introduction of photothermal or photodynamic antimicrobial components into the matrix of the hydrogel to achieve the killing of bacteria under light irritation.160-162 In addition, some other works have utilized gas transmitters to release small molecules of gases such as nitric oxide to achieve bacterial elimination under external stimulus and prepared responsive ECHs on this basis.163, 164 Our group designed a hydrogel-based flexible wound dressing by coordinating electroluminescent (EL) material with photosensitizer together, which can generate reactive oxygen species (ROS) in situ to inhibit drug-resistant bacteria under an electric field without an external light source (Figure 12C).64 The emitted fluorescence from the EL molecules activates the photosensitizer, leading to the production of singlet oxygen (1O2) which is instrumental in causing oxidative damage to pathogens. The flexible device based on ELDT demonstrates strong ROS-induced antibacterial properties, particularly against drug-resistant bacteria, with an effectiveness of over 99.9%. This capability significantly helps in preventing surface-level infections and aids in the acceleration of wound healing.
3 BIOELECTRONIC APPLICATIONS OF ECHs
3.1 Healthcare monitoring
In the realm of health surveillance, ECH could monitor biological, physical, and chemical signals from the human body, resulting in numerous applications across various fields. Depending on their intended functions and application scenarios, ECH-based sensors can be categorized as biosensors, physical sensors, or chemical sensors. Biosensors are composed of a sensing element and a transducer. The sensing element can be made up of antibodies, enzymes, nucleic acids, cells, or other materials with identifiable properties. The transducer converts the detection of the chemical or biological target molecule through the sensing element into separate signals, such as optical and electrical signals. Due to their mechanical similarity to human skin and tissues, ECHs have favorable properties such as flexibility, biocompatibility, and effective binding to target biomolecules. As a result, they are deemed an ideal material for use in biosensors. Physical sensors predominantly consist of strain, pressure, and temperature sensors. Among them, strain sensors are capable of imitating the pliability and sensory characteristics of human skin by translating a range of external stimuli into electrical signals. When external forces, such as joint movement, muscle contraction, and breathing, cause deformation, the resistance or capacitance of the ECH changes, allowing for strain monitoring. Additionally, the ECH's temperature-sensitive properties enable accurate detection of temperature changes, making it useful as a temperature sensor for monitoring temperature fluctuations in the body. ECHs are also utilized in chemical sensors for signal transduction. This is achieved by adding multiple active elements (carbon-based, metal-based, and conductive polymers). These elements convert chemical signals into either electrical or optical signals for output.165 The hydrogels are sufficiently sensitive and selective to precisely detect particular chemicals like blood glucose, blood oxygen, and lactic acid. Owing to their excellent biocompatibility and plasticity, ECHs can be fabricated into diverse forms and sizes to cater to varied monitoring requirements and application contexts. Therefore, ECH holds the potential to serve as a valuable material for personal health monitoring and medical diagnostics in the future.
Bao and Gurtner et al. created a bioelectronic system by utilizing PEDOT:PSS.166 The device continuously monitors wound impedance and temperature, while also providing electrical stimulation (ES) to enhance wound healing. In a study with mice that received ES for wounds, the healing process showed a 25% acceleration, and dermal remodeling saw a 50% improvement compared to the control group. The result can be attributed to the activation of pro-regenerative genes in both the monocyte and macrophage populations, which may stimulate the regeneration of tissue, neovascularization, and dermal repair. Recently, Roy and colleagues developed a self-reporting biosensor for cancer detection. They developed a mineralized ECH, named M-Hydrogel, by introducing disulfide-functionalized alginate-loaded carbonized polydopamine (cPDA) polymer dots (PD@cPDA) as redox and conductive agents, along with calcium carbonate (CaCO3) as crosslinkers, within a hydrogel matrix (Figure 13A).167 High levels of glutathione promote the cleavage of disulfide bonds, releasing cPDA in the hydrogel matrix. This alteration in the conductivity of M-hydrogel enables self-reporting of cancer (Figure 13B). Furthermore, the hydrogel exhibits outstanding tensile strength, adhesive strength, and biocompatibility, particularly at lower viscosities. This hydrogel-based biosensor presents an efficient and convenient strategy for cancer diagnosis.
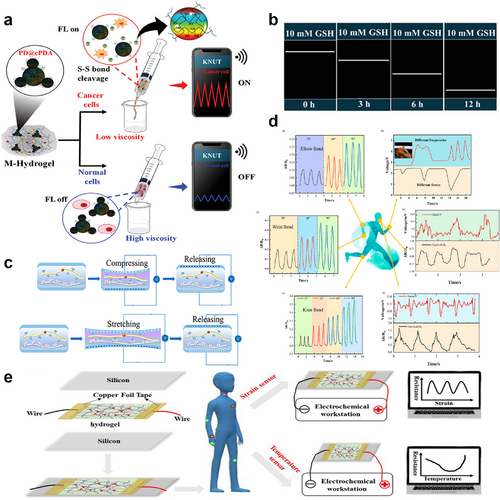
(A) Schematic illustration of cancer self-reporting in mineralized ECH. Reproduced with permission.167 Copyright 2023, Wiley-VCH. (B) Strain response, and wireless strain sensing. (C) Mechanism diagram of dipolar interaction of the hydrogel during tension and compression. Reproduced with permission.168 Copyright 2022, American Chemical Society. (D) Detection of human motion and subtle movements by flexible ECH sensors. (E) Schematic diagram of the ECH used as a strain or temperature sensor to detect human movement or abnormal hyperthermia of the users. Reproduced with permission.169 Copyright 2022, American Chemical Society. ECH, electroconductive hydrogel.
Tao et al. reported a tactile hydrogel sensor (THS) based on micro-pyramid-patterned double-network ionic organo-hydrogels to detect subtle pressure changes by measuring the variations of the triboelectric output signal without an external power supply.170 The self-powered THS shows the advantages of remarkable flexibility, good transparency (≈85%), and excellent sensing performance, including extraordinary sensitivity (45.97 mV Pa−1), fast response (≈20 ms), a very low limit of detection (50 Pa) as well as good stability (36 000 cycles). Hu and co-workers developed a composite ECH for smart wearable strain sensors.168 The ECH was synthesized by using cross-linked chitosan quaternary ammonium salt as the hydrogel matrix, PEDOT:PSS as the conductive filler, and poly(vinylidene difluoride-co-trifluoroethylene) as the piezoelectric filler (Figure 13C). The synthesis was carried out using a one-pot thermoforming and solution exchange method. These ECHs have both piezoresistive and piezoelectric sensing capabilities, offering the advantages of very high sensitivity (gauge factor: 19.3), fast response (response time: 63.2 ms), and wide frequency range (response frequency: 5–25 Hz), while maintaining excellent mechanical properties (elongation at break up to 293%). They have a wide range of applications, including monitoring human motion, detecting subtle movements, recognizing object contours, and serving as hydrogel-based array sensors (Figure 13D). Pang and colleagues prepared a dual lattice temperature-sensitive hybrid ECH by introducing polyvinylpyrrolidone/TA/Fe3+.169 Based on the experimental results, the hydrogel exhibits good stretchability and conductivity, making it a promising material for efficient and sensitive strain sensors. Additionally, by incorporating PNIPAAm with a low critical solution temperature, it can monitor ambient temperature through temperature-conductivity sensitivity. This characteristic renders it suitable for applications such as temperature sensors for detecting human body heat or for tissue thermotherapy (Figure 13E).
Ye's team has developed a multifunctional ECH paper patch capable of simultaneously monitoring ECG signals and biochemical signals (glucose levels) in sweat during exercise in real-time (Figure 14A).171 As a flexible and highly integrated wearable device, it can be used to detect electrophysiological and biochemical changes during exercise in a synergistic manner (Figure 14B). The highly conductive PEDOT:PSS hydrogel was self-assembled on paper fibers to form an ECH/paper composite. On-body epidermal tests were performed under actual fitness conditions. The electrophysiological signals, including heart rate and ECG, showed stable test curves and were not affected by glucose (Figure 14C). As a disposable and low-cost ECH for monitoring chemical-electrophysiological signals, it holds great promise for the future of medical health monitoring. Fu and colleagues have developed a chemical sensor capable of real-time detection of various ions present in human sweat (Figure 14D).172 The sensor was prepared by electrochemical polymerization of EDOT monomers in a pre-existing PAA hydrogel network. This resulted in the formation of an interpenetrating PAA–PEDOT hydrogel, which introduced a dense and highly conductive PEDOT network into the PAA matrix. The hydrogel has the potential to be converted into an electrochemical sensor electrode due to its interpenetrating properties. This can be used to detect various ions in solution, including significant human sweat chemical biomarkers such as glucose and lactate (Figure 14E). Additionally, it can be used to assess an array of electrophysiological signals, including electroencephalogram, ECG, and electromyogram, as well as in vivo neural signal recording and stimulation.
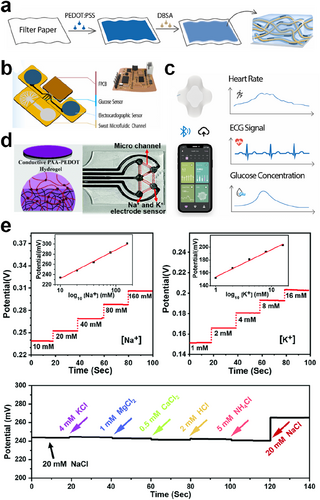
(A) Schematic of the fabrication process of the PEDOT:PSS ECH. Reproduced with permission.171 Copyright 2023, Elsevier. (B) Schematic diagram of the various components of the ECH-paper patch. (C) Schematic diagram of the wireless transmission of the paper-based hybrid device and the monitoring curves of different physiological signals. (D) Schematic of the interpenetrating PAA-PEDOT hydrogel and the image of the ECH-based sweat sensor. Reproduced with permission.172 Copyright 2021, Royal Society of Chemistry. (E) The properties of PAA-PEDOT hydrogel-based electrochemical sensors for detecting Na+ and K+. ECH, electroconductive hydrogel.
3.2 Treatment of diseases
Non-conductive hydrogels have become commonplace in disease management as they can serve as dressings for isolating wounds from the external environment, or as drug delivery carriers due to their excellent biocompatibility and similarity to human tissue. The exceptional electrical properties of ECHs provide an avenue for more innovative biomedical applications in disease management. ES is a non-pharmacological therapy that is essential in the management of a wide range of conditions, including neurological, cardiac, and autoimmune diseases. Implementing ES can encourage immune cell differentiation, fibroblast proliferation, or regulate neuronal cell growth. Thus, bioelectronic devices could make a significant contribution to the treatment of numerous diseases. With exceptional conductivity and biocompatibility, ECHs are excellent mediators for establishing effective interactions between organisms and electronic systems. Currently, multiple studies have exploited ECHs to prepare bioelectronic devices and achieved desirable therapeutic effects.
3.2.1 Wound healing
It has been proven that ES can be an adjunctive therapeutic approach to accelerate wound healing.173 The electrical field exerted on the wound could guide the proliferation and migration of cells, such as epithelial cells and fibroblasts, which involves the activation of ion channels and downstream signal transduction. Recently, Wang et al. reported a flexible electrical patch (ePatch) made with printed ECH electrodes for driving the electrical field in wound management (Figure 15A).174 The printable ECH was synthesized using AgNWs and methacrylated alginate. As shown in Figure 15B, the biological activities of the ePatch during the ES process mainly contain acceleration of fibroblast migration and proliferation, suppression of bacterial growth, promotion of angiogenesis, down-regulation of immune cell activities, and skin tissue remodeling. By assessing the metabolism of three cell growth factors, the authors demonstrated that pulsed electric fields could affect cell signaling pathways to promote NIH 3T3 fibroblast proliferation in this study. An in vivo study demonstrated that the ePatch with ES could significantly decrease the wound healing time of rats and increase angiogenesis, while preventing gram-positive and negative bacterial infections. Diabetic ulcers are the most common chronic wounds, and their treatment remains challenging, despite a great deal of effort that has been put into them. Zhao and colleagues fabricated an ECH namely PDA@AgNPs/CPH from a supramolecular assembly of PDA-decorated AgNPs, PANI, and PVA.177 The resultant hydrogel exhibits broad antibacterial activity against both Gram-negative and Gram-positive bacteria. Remarkably, it has a significant therapeutic effect on diabetic foot wounds by promoting angiogenesis, accelerating collagen deposition, inhibiting bacterial growth, and preventing wound infection. In addition, it could also monitor large-scale movements of the human body in real-time. Chronic wounds in diabetic individuals are hampered due to the excessive presence of ROS, proinflammatory and anti-inflammatory cells/factors that are not balanced in the microenvironment. As an effective antioxidant and electroactive wound dressing, ECHs can scavenge free radicals and support the wound healing process. A wound dressing with immunomodulating and electroconductive properties has been developed.17 To obtain ECH strips, [2-(acryloyloxy)ethyl] trimethylammonium chloride and gelatin methacrylate (GelMA) were 3D printed onto a doxycycline hydrochloride (DOXH)-loaded and ROS-degradable polyurethane nanofibrous membrane and then subjected to UV irradiation. DOXH is released more rapidly in a high ROS environment. The dressing facilitates endothelial cell migration and macrophage polarization to the anti-inflammatory phenotype (M2) in vitro. In a wound healing test on diabetic rats, the combination of conductivity and DOXH was found to be most effective in accelerating wound healing, collagen deposition, revascularization, and re-epithelialization by downregulating ROS and inflammatory factor levels, as well as by upregulating the M2 macrophage ratio.
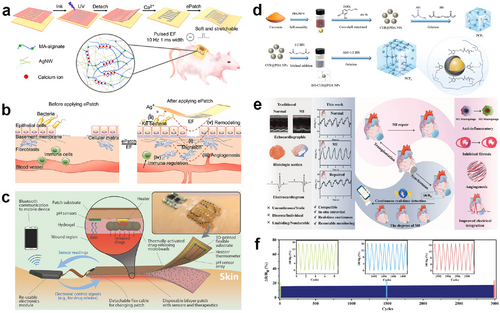
(A) Schematic of the flexible ePatch preparation and structural schematic of the double-crosslinked network of hydrogel ink. Reproduced with permission.174 Copyright 2022, Elsevier. (B) Mechanisms of the ePatch for promoting wound healing. (C) Structural schematic of an integrated smart bandage for chronic wound management and treatment. Reproduced with permission.175 Copyright 2018, Wiley-VCH. (D) Synthetic routes of the multifunctional nanocomposite-reinforced ion-conductive hydrogels. Reproduced with permission.176 Copyright 2023, Wiley-VCH. (E) Schematic of the multifunctional ECH-integrated MI detection and therapy. (F) Cyclic stability curve of the multifunctional ECH bioelectronic patch. ECH, electroconductive hydrogel; MI, myocardial infarction.
3.2.2 Drug delivery
Hydrogel delivery systems can enhance the therapeutic benefits of drug delivery and are already used in practice. Hydrogels allow for precise spatial and temporal control over the release of different therapeutic agents, such as small-molecule drugs, macromolecular drugs, and cells. Owing to their customizable physical properties, regulated degradability, and ability to protect unstable drugs from degradation, hydrogels provide an effective platform for various physicochemical interactions with the encapsulated drugs, controlling drug release. Gan et al. developed a tough ECH with excellent conductivity, superior mechanical properties, and good biocompatibility.178 The hydrogel can be utilized for programmable drug release through accurately controlled electrostimulation. This was demonstrated by the electrically stimulated release of a model drug from the hydrogel. Mostafalu et al. have developed a smart and automated flexible wound dressing with temperature and pH sensors integrated into flexible bandages for monitoring wound status in real-time (Figure 15C).175 A flexible electrochemical pH sensor with a linear response and sensitivity of −50 mV pH−1 was designed and fabricated on a flexible polyethylene terephthalate substrate. The dressing is fitted with a microcontroller that processes the data measured by the sensors and programs the drug release protocol for personalized treatment. This flexible, smart wound dressing has the potential to significantly impact the treatment of chronic wounds. Besides, Lin et al. have developed a composite hydrogel dressing with stepwise delivery of diclofenac sodium (DS) and basic fibroblast growth factor (bFGF) in the inflammation stage and new tissue formation stage for wound repair, respectively.179 The in vitro thermosensitive cargo delivery of this hydrogel showed that 92% of DS was sustainably delivered at 37°C within the early 3 days mimicking the inflammation stage, while 80% of bFGF was gradually released at 25°C within the later 8 days mimicking the new tissue formation stage. Zhu et al. have prepared a multifunctional hydrogel that shows stimulus responsiveness, good conductivity, and self-adhesive properties.180 This hydrogel is capable of releasing drugs on demand in response to changes in temperature or NIR light irradiation. In order to study the controlled drug release behavior of stimulus-responsive hydrogels or smart hydrogels under an applied electric field, Paradee and Sirivat studied the release behavior and the diffusion coefficient for the effect of cross-linking ratio, PEDOT particle size, and electric field strength.181 This study confirms that the drug release mechanism of benzoic acid in ECH is Fickian diffusion. The diffusion coefficient increased when the cross-linking ratio decreased, the electric field strength increased, or the PEDOT particles were smaller.
3.2.3 Cardiac repair
Electrical signals play a critical role in regulating the normal rhythm of the heart tissue, controlling the heart's contraction and relaxation.182 The electrophysiological properties of cardiomyocytes, including auto-rhythmicity, excitability, and conductivity, are determined by the bioelectric activity of the cardiomyocyte membrane.183 Myocardial infarction (MI) is a fatal coronary artery disease that can lead to heart weakness and failure. The use of ECHs is a suitable approach to achieving successful cardiac tissue repair due to their remarkable electrical conductivity, favorable cellular response, and facilitation of cellular signal transduction between cells. Several studies have developed injectable ECHs to replace damaged cardiac tissues. This can help protect cardiac function, reduce susceptibility to arrhythmias, and decrease the resistance of cardiac scar tissue.184-186 In addition to using prefabricated cardiac patches, researchers have also attempted to create injectable ECHs that can conform to the shape of the lesion, allowing for better adherence to cardiac tissue and reducing the risk of implantation.187-189 ECHs can not only conduct physiological electrical signals of the heart, but can also be employed to conduct externally exerted electrical signals for ES therapy. Li et al. prepared hybrid hydrogel scaffolds with extraordinary conductivity and mechanical property by dropping hydrophilic polydopamine-reduced graphene oxide (PDA-rGO) into GelMA hydrogels.190 The cell experimental results demonstrate that the cardiomyocytes seeded on GelMA−PDA−rGO hybrid hydrogel scaffolds exhibit favorable cell functionalities, including adhesion, retention, and beating behavior, along with a significant increase in the expression of two cardiac-related proteins and faster Ca2+ handling kinetics. This study confirms that ECHs have great potential for engineering a more functional and mature layer of myocardium. Shen et al. synthesized an ionically ECH reinforced by core-shell-structured nanocomposites for simultaneous heart monitoring and tissue repairing (Figure 15D).176 The prepared ECHs exhibited excellent bioactivity and ultrahigh mechanoelectrical sensitivity, which made them competent as implants to boost and monitor the repair process of MI (Figure 15E). The in vivo experimental results show that the nano-reinforced ECHs could prevent fibrosis from further deteriorating in the MI region and enhance both the systolic and diastolic functions of the left ventricle. The implantable hydrogel sensor is capable of detecting differences in mechanical deformation of the heart, which could potentially distinguish the severity of MI. The ECHs can also diminish the pacing threshold and improve the effectiveness of cardiac pacemakers. Figure 15F demonstrates that the strain sensor's relative resistance change (ΔR/R0) maintains exceptional stability and consistency through 3000 cycles of continuous stretching at a constant strain of 70%, showcasing its notable durability. An et al. have developed a highly conductive biomaterial known as poly-3-amino-4-methoxybenzoic acid-gelatin (PAMB-G) hydrogel.191 Injecting PAMB-G onto the electrode-tissue surface reduces the cardiac pacing threshold voltage while maintaining superior electrophysiological performance compared to direct electrode or non-conductive biomaterial pacing.
3.2.4 Neural tissue repair
Combining conductivity and biocompatibility, ECH can provide a suitable dynamic extracellular microenvironment for neuronal cells and is the most promising next-generation scaffold material for neural tissue engineering. In the process of neural tissue repair, neuronal survival, differentiation, and functional expression rely heavily on the stimulation and transmission of electrical signals. Besides, the porous structure of ECHs allows for efficient transport of nutrients and oxygen, which are critical for cell metabolism and function. Extensive research indicates that utilizing ECH as a neural tissue scaffold can proficiently stimulate neuronal cell growth and differentiation, boost neural tissue regeneration, and facilitate the restoration of impaired neural tissues.192-195 The spinal cord is a critical component of the human central nervous system, serving as a pathway for information transmission between the brain and peripheral nerves. By mimicking the high conductivity of the spinal cord, ECH can modulate the microenvironment of the spinal cord to restore the signaling pathway of damaged nerves, thereby facilitating the repair of spinal cord tissue.196-198 Incorporating live neural cells into ECH scaffolds is a more promising therapeutic strategy for neural tissue engineering, as it can also maintain cell viability and promote the formation of neural cell networks. Tringides et al. developed an adaptable biomaterial ECH scaffold composed of CNT and alginate and demonstrated its effectiveness in facilitating the growth and network development of a range of cell types, including mesenchymal stem cells, neural cells, and neural progenitor cells, when integrated into viscoelastic ECH structures.199 This scaffold has been shown to support neural cells for over 12 weeks and has sufficient conductivity to be used as implantable neural electrodes.
3.3 Human–machine interfaces
ECH is an attractive material for building HMIs because of its conductivity, flexibility, and human tissue-like properties, enabling it to bridge the gap between the characterization of electronic devices and biological neural tissues. Extensively, HMI refers to the interaction and communication between humans and various mechanical or electronic devices to achieve effective manipulation of machines. HMI enables the combination of machines, software, and humans to create more efficient and convenient systems. In the field of bioelectronics, machines for HMI generally are highly integrated and miniature medical electronic devices such as cochlear implants, visual prostheses, and neurostimulators. Despite the multidisciplinary efforts that have contributed to the rapid development of HMIs, the HMIs intended to facilitate communication and interaction between humans and machines remain primitive and unreliable. Foreign body reaction, inflammation, long-term biocompatibility, and the mechanical heterogeneity between electrodes/probes and active neural tissue in the dynamic environment are crucial factors limiting the electronic device-human interaction. Optimal HMI materials must be able to couple tightly with soft neural tissue to enhance signal transmission with stable conductivity to enhance signal acquisition and excellent biocompatibility to minimize inflammation and immune responses. The use of soft materials, such as functionalized elastomers or gels, is the last resort to address these challenges as well as transform HMI into medical hope. A lot of work focusing on using ECH in neural tissue engineering and HMIs has been reported gradually, which promotes the development of HMI applications from the perspective of bioelectronics.
Ensuring the safety of ECH materials during use is a prerequisite for the development of ECH-based HMI. A peptide is an attractive option for ECH network construction due to its ability to control assembly structures and properties at the nanoscale through modifications in structure and sequence. Nam et al. developed a biocompatible, conductive, and biostable neural interface using a supramolecular β-peptide-based hydrogel that promotes signal amplification through close neural/hydrogel contact without causing neuroinflammation (Figure 16A).200 The strategic combination of nonbiodegradable β-peptide with conductive nanomaterials within the hydrogel results in the formation of a three-dimensional electrical network that can enhance signal transmission. Meanwhile, the viscoelasticity of this ECH ensures its smooth integration with adjacent neural tissues. Due to its moldability, the supramolecular β-peptide-based ECH has been successfully used in both acute and chronic intercortical and epidural neural signal recording, demonstrating enhanced neural signal amplification with minimal signal loss (Figure 16B).
(A) Chemical structure of a β-peptide and a diagram showing the supramolecular assembly process of betaVhex/CNTs complexes. Reproduced with permission.200 Copyright 2020, American Chemical Society. (B) The supramolecular β-peptide-based ECH for recording neural signals and the schematic of the 3D electrical network in the ECH. (C) Schematic illustration of fabrication of the graphene nanocomposite-based e-bioadhesive interface. Reproduced with permission.201 Copyright 2020, Wiley-VCH. (D) In vivo sciatic nerve stimulation via the e-bioadhesive interface. (E) Schematic illustration of in vivo polymerization of ECHs induced by endogenous metabolites. Reproduced with permission.202 Copyright 2023, The American Association for the Advancement of Science. (F) In vivo polymerization of the metabolite-induced ECH around the nervous system of medicinal leeches. CNTs, carbon nanotubes; ECH, electroconductive hydrogel.
To ensure the long-term effectiveness of neural stimulation and signal recording, it is crucial to establish conformal and stable contact between the electrode and the nervous system. Deng and colleagues developed an innovative electrical bioadhesive (e-bioadhesive) interface that is made by a thin layer of a graphene nanocomposite to achieve quick adhesion (forming in <5 s), robust (with an interfacial toughness exceeding 400 J m−2), and easily reversible integration (Figure 16C).201 This e-bioadhesive interface has been applied in conjunction with flexible electrodes, 50-μm-thick gold-coated polyimide, for direct in vivo ES of the sciatic nerve. In this study, the electrodes equipped with the e-bioadhesive interface successfully adhered to the rat's sciatic nerve (Figure 16D). Periodic and stable movements of the ankle joint were noted during the ES experiments, proving the efficacy of this ECH interface in providing stable ES to the sciatic nerve.
Strakosas and colleagues developed a versatile method for creating self-organizing, highly effective electrode structures in both the peripheral nervous system and the central nervous system.202 The process involves injecting cocktail gels, composed of enzymes and electroactive monomers, into biological tissues, using endogenous metabolites to trigger polymerization and gelation across different tissue types, resulting in conductive polymer gels with extensive conductivity (Figure 16E). The authors demonstrated the in situ formation of in vivo electrodes in zebrafish and leech models and were able to stimulate leech nerves (Figure 16F). This bioelectronic integration technology is expected to enable seamless integration of the ECH with the nervous system and drive a paradigm shift in the development of HMIs. Yang and colleagues devised a novel approach to creating hydrogel neural interfaces that are conductive, bioadhesive, photopatternable, antifouling, soft, and elastic. This multifunctional hydrogel is synthesized through the self-assembly of PEDOT:PSS in the presence of polyzwitterion. The resultant multifunctional hydrogel interface is characterized by its ability to adhere rapidly and establish strong electrical connections with wet tissues. It has shown great efficacy in facilitating electrical communication, enabling stable recording and stimulation of sciatic nerves in a rat model. The application of this multifunctional hydrogel as an electrical bioadhesive coating layer is anticipated to significantly enhance the long-term neuromodulation capabilities and reliability of bioelectronic devices.
To address the issue of performance degradation in ECH due to swelling upon tissue contact, Han et al. developed a novel poly(sulfobetaine vinylimidazolium)-graphene hydrogel, referred to as SG hydrogel.135 The SG hydrogel is non-swelling and retains 98% of its original volume even after 14 days immersed in water. The ECH is subsequently implanted into the sciatic nerve of a rat, and neuromodulation is verified by low-current ES. Lei et al. conducted a study where they engineered a fully biodisintegratable bio-hybrid neural interface (BHNI), leveraging extracellular matrix (ECM) technology to enhance nerve injury treatment.203 This novel BHNI features microelectrode array conductive paths crafted from an ECM-based ECH (Figure 17A,B). These ECH systems are distinct for their strong tissue-like chemical and physical characteristics, electrical conductivity, and compatibility with neural progenitor stem cells. The device's design allows it to wrap seamlessly around peripheral nerve fibers, enabling continuous neural signal tracking for assessing wound conditions. Notably, it combines controlled ES with cell transplantation to expedite motor function recovery. The biodegradable nature of this BHNI, which disintegrates within a month, eliminates the necessity for surgical removal. This feature ensures stable, ongoing interaction and integration with host tissues, showcasing an exemplary fusion of biological and electronic systems in an implant. In addition, Li et al. have pioneered a stretchable soft mesh electrode system (Figure 17C), showcasing its promise for prolonged electrophysiological interfacing with human cerebral organoids (hCOs).204 The key advantage of this system lies in its seamless integration with the 3D structure of organoids. This integration allows for repeated experiments and measurements on the same hCO at various stages of its development. The inherent stretchability of the materials used in the mesh electrode enables it to adjust and grow along with the 3D culture. Consequently, this system is capable of delivering consistent chronic stimulations to the organoids over extended periods, demonstrating its potential for long-term studies (Figure 17D).
(A) Synthesis route of ECM-based ECHs. Reproduced with permission.203 Copyright 2023, Wiley-VCH. (B) Photographs of the ECH scaffold MEA containing 7-channel 150 and 300 μm diameter microelectrodes integrated with the FPC. (C) Schematic of the mesh microelectronics and its fabrication process. Reproduced with permission.204 Copyright 2022, Elsevier. (D) Electrical interfacing between the mesh and organoid and the ES performance throughout the hCO. ECHs, electroconductive hydrogels; ECM, extracellular matrix; FPC, flexible printed circuit; MEA, microelectrode array.
4 CONCLUSIONS AND PERSPECTIVES
Although there have been significant advancements in ECHs over the last few decades, they are still in a very early stage of development. To better realize their potential in bioelectronics, several challenges need to be addressed. This review summarizes the challenges faced by ECHs in bioelectronic applications, such as stretchability, environmental adaptability, physical damage, interfacial compatibility, and bacterial contamination. It also systematically describes how these challenges can be addressed rationally through material design. In some cases, specific applications may require ECHs with additional functionalities to meet environmental and practical challenges. Therefore, our goal is to develop customized ECHs that meet various bioelectronic application needs. An ECH is a promising option in flexible bioelectronics, providing a dynamic platform for creating innovative medical devices, sensors, and treatment methods. Current investigations and breakthroughs in this field are poised to bring about transformative changes in the fields of healthcare and biotechnology.
To further enhance the applicability of ECH in bioelectronics, the following future perspectives are proposed. Firstly, the biocompatibility and biodegradability of ECHs are critical when used on/in the human body. Although the acute biosafety of ECH has been verified, its long-term stability in the biological environment, particularly those containing conductive nanomaterials, still requires exploration.205, 206 The development of fully degradable ECHs, or those that can fuse with tissues, is a promising research field. Additionally, the transmission of signals in the human body is a complex and variable process.207, 208 To effectively transmit complex signals, it is necessary to optimize the performance of ECH. Future research should prioritize enhancing and sustaining the conductivity of ECHs, as well as ensuring their robust integration into bioelectronic devices. In addition, optimizing the transmission system is important for ECH applications to improve the flux of signal transmission. Furthermore, designing effective hydrogel bioelectronics requires a deeper understanding of the biological interactions between ECHs and tissues.209 ECHs as non-living materials can be transformed into living materials that promote cell growth and differentiation, making them a viable option for bioelectronic applications.210 Finally, hydrogels differ significantly from traditional bulk metal or polymer materials. Injection molding or 3D printing has been extensively reported for their use.46, 47, 51 However, the processing and molding of hydrogels are still difficult due to their soft nature. The subsequent explorations of the more available fabrication and integration techniques for ECHs are necessary.
AUTHOR CONTRIBUTIONS
Nian Liu: Writing—original draft; conceptualization. Huifang Ma: Writing—original draft. Maorui Li: Writing—original draft. Rongrong Qin: Writing—review & editing. Peng Li: Writing—review & editing; supervision; funding acquisition; conceptualization.
ACKNOWLEDGMENTS
This work was financially supported by the National Natural Science Foundation of China (52473265 and 62288102), the Shaanxi Provincial Science Fund for Distinguished Young Scholars (2023-JC-JQ-32), the Innovation Foundation for Doctor Dissertation of Northwestern Polytechnical University (CX2023101), and the China National Postdoctoral Program for Innovative Talents (BX20230494).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interests.
Biographies

Nian Liu is a Ph.D. candidate at Northwestern Polytechnical University in China, under the supervision of Prof. Peng Li. He received his B.S. degree from Qingdao University of Science and Technology in 2017 and his M.S. degree from Nanjing Tech University in 2020. His research focuses on the development of wearable biomedical optoelectronic materials and devices.

Peng Li is a Professor at Northwestern Polytechnical University in China. He received his B.S. degree from Tianjin University in 2006 and his Ph.D. degree from Nanyang Technological University in 2013. In 2018, he joined the Institute of Flexible Electronics (IFE) at Northwestern Polytechnical University. His research team focuses on the development of innovative, flexible materials and devices for biomedical applications.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.




