Reconfigurable flexible thermoelectric generators based on all-inorganic MXene/Bi2Te3 composite films
Abstract
Flexible thermoelectric generators (FTEGs) represent an excellent solution for energizing wearable electronics, capitalizing on their ability to transform body heat into electrical energy. Nevertheless, their use in the wearable industry is limited by the insufficient thermoelectric (TE) efficiency of materials and the minimal temperature variation among the devices. In this study, we have developed a Lego-like reconfigurable FTEG by combining flexible TE chips, rheological liquid-metal electrical wiring, and a stretchable substrate in a mechanical plug-in configuration. The flexible TE chips are constructed from n-type all-inorganic MXene/Bi2Te3 composite films, which have their TE properties further enhanced through heat treatment. A demonstration of the FTEG illustrates its capability to convert heat into vertical temperature difference (ΔT), leading to a substantial ΔT at the cold end in contact with the environment, resulting in a power output of 7.1 μW with a ΔT of 45 K from only 5 TE chips. The reconfigurable FTEG presents significant potential for wearable devices to harness low-grade heat.
1 INTRODUCTION
With the continuous growth of the smart wearables market, there is a rising demand for whole-body power in wearable devices.1-3 Although chemical batteries with high energy density, such as lithium-ion pouch cells, have been incorporated into wearable systems,4, 5 their practical use in portable electronics faces significant challenges due to frequent charging requirements and potential leakage issues.6-8 Consequently, there is considerable interest in alternative and sustainable energy generation methods, such as solar cells,9 triboelectricity,10 and thermoelectricity.11 Flexible thermoelectric generators (FTEGs) offer a consistent electricity output by directly converting low-grade heat from the environment, rendering them well-suited for self-powered wearable electronics due to their immunity to external factors such as day-night cycles and intermittent mechanical movement.12-16 However, despite substantial progress in continuously converting various heat sources, the reported FTEGs still produce subpar output, restricting their capacity to continuously power microelectronics.17, 18 As a result, there is still a strong need for the advancement of high-efficiency FTEGs.
Two key strategies are essential for maximizing the performance of FTEGs. One strategy focuses on developing advanced TE materials, such as organic semiconductors and organic/inorganic composites.19-25 However, due to the coupling effect, these materials cannot simultaneously achieve a high Seebeck coefficient and excellent conductivity, resulting in a low power factor. TE materials with reduced dimensions can enhance the electron state density near the Fermi level by leveraging size confinement effects, thereby advancing TE efficiency.26, 27 We have previously reported mechanically stable all-inorganic films prepared using carbon nanomaterials and two-dimensional (2D) chalcogenide nanoplates.28 These films exhibit a high Seebeck coefficient and a conductivity of −180 μV K−1 and 292 S cm−1 at ambient temperature, respectively. Dun et al. demonstrated that metallic nanoparticles can selectively grow on chalcogenide nanoplates, presenting a 25% increase in the Seebeck coefficient and a 600% increase in conductivity.29, 30 In their study, Hou and colleagues described a thermoelectric film utilizing 1D Ag2Se nanowires that demonstrated a high power factor of 17 μW cm−1 K−2.31 These findings inspire the development of low-dimensional nanomaterials for constructing flexible and highly efficient TE materials.
Another strategy involves structural design aimed at maximizing power output by creating a larger temperature difference (ΔT). Current research mainly focuses on two aspects: (1) optimizing the ΔT along the thermal gradient of the heat surface, and (2) enhancing heat dissipation efficiency at the cold end. First, the ΔT between the environment and heat source is perpendicular to the skin; thus, three-dimensional (3D) structures provide a well-matched thermal impedance for TE materials by means of out-of-plane architecture.32 For example, Lv and colleagues introduced a spring-like FTEG design that can stand vertically on a heated surface, showcasing a high output power density of 416.22 nW cm2 33 Second, advancements in enhancing heat exchange capability can effectively lower the cold end temperature so as to produce a substantial ΔT across both ends of FTEGs. Zhou et al. designed a leaf-inspired FTEG that maximizes the ΔT using a vertical TE array as an environmental cooling fan, illustrating the benefit of an out-of-plane structure for improving TE output.34
To summarize, the development of a category of FTEGs that not only integrate low-dimensional nanomaterials but also facilitate efficient thermal impedance matching via cross-plane architectures is especially appealing, although limited research has been undertaken. Furthermore, existing TE materials remain securely affixed within the substrate or matrix,35, 36 and the challenge of being unable to add or remove TE couples as necessary complicates the management of intricate and variable application scenarios, including alterations in heat surface and power requirements. Therefore, assembly flexibility should also be considered during the design of FTEG structures.
Here, we propose a reconfigurable FTEG that optimizes thermal pathways through the utilization of flexible TE chips composed of all-inorganic composite films. For the fabrication of high-performance TE chips, we chose 2D MXene and Bi2Te3 nanoplates as the main components of the composite films, with the MXene serving as a conductive scaffold to bridge the Bi2Te3 nanoplates. Specifically, the TE chips can be integrated into the FTEG assembly by embedding them within liquid-metal electrodes enclosed in the silicone substrate, offering the advantage of Lego-like reconfiguration capability. With just 5 TE chips, the FTEG achieves an output power of 7.1 μW at a ΔT of 45 K. These design features give the reconfigurable FTEG strong durability and impressive TE efficiency, making it ideal for harvesting low-grade heat.
2 RESULTS AND DISCUSSION
Figure 1 depicts the schematic illustration of the fabrication of the reconfigurable FTEG, which comprises flexible TE chips as electrical output, liquid-metal as electrical connection, and elastic silicon as flexible substrate. The TE chips contribute to the entire power output of the FTEG by converting diverse heat; thus, the properties of integrated TE materials play a critical role. Theoretical and experimental evidence has confirmed that low-dimensional nanomaterials, such as Bi2Te3 nanoplate, exhibit exceptional TE properties due to the quantum confinement effect.27-29 Nevertheless, the multitude of interfaces between the assembled nanomaterials hinder charge carrier transport, leading to the low conductivity of macroscopic materials. To optimize TE performance, we incorporate highly conductive MXene flakes into 2D Bi2Te3 nanoplates to prepare all-inorganic composite films. Figure 1A illustrates the procedure for producing MXene/Bi2Te3 composite films. The MXene flakes were derived from exfoliating the MAX precursor using the Li-ion intercalation method, as detailed in our previous work.37-40 Subsequently, the composite films were prepared via vacuum filtration of a mixed solution containing MXene flakes and Bi2Te3 nanoplates, followed by heat treatment at 300°C to optimize the TE properties. Based on the above design strategy, we successfully produced all-inorganic composite films with excellent TE performance for incorporation into TE chips. Figure 1B depicts the structure of TE chip and its integrated architecture within the reconfigurable FTEG. Notably, line-shaped liquid-metal was pre-encapsulated in elastic silicone as electrical wiring, ensuring that it remains confined within the designated gaps of the TE chips due to the inherent surface tension. Following gap filling, the TE chips were electrically connected in series and thermally connected in parallel, enabling efficient heat energy harvesting through the out-of-plane structure, with heat transfer occurring along the in-plane direction of the TE chips. The inset in Figure 1B shows the optical images of the assembled FTEGs, highlighting their exceptional flexibility when subjected to bending.
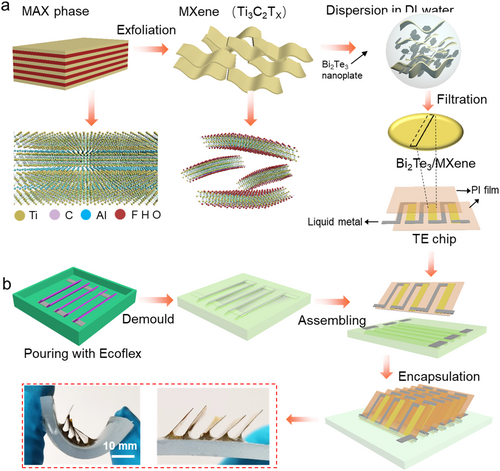
Design and fabrication process for the reconfigurable FTEG. (A) Illustration showing the steps involved in creating MXene/Bi2Te3 composite films. (B) Schematic diagram of the fabrication process for TE chips and reconfigurable FTEG. FTEG, flexible thermoelectric generator.
Initially, the morphologies and compositions of the composite films were investigated first, as shown in Figure 2. The TEM image (Figure 2A) revealed a regular hexagonal morphology, while the EDS mapping confirmed the successful synthesis of Bi2Te3 nanoplates by illustrating the distribution of corresponding elements. The crystal plane spacing of the sample, approximately ∼0.22 nm (Figure 2B), closely matches the (110) facets of hexagonal Bi2Te3 nanoplate.41, 42 The hexagonal spot pattern observed in the selected area electron diffraction (SAED) image serves as evidence for the monocrystalline structure of Bi2Te3 nanosheets, as depicted in the inset of Figure 2B. Additionally, the TEM image displayed in Figure 2C confirms the successful exfoliation of layered MXene, revealing the presence of large-area MXene flakes.
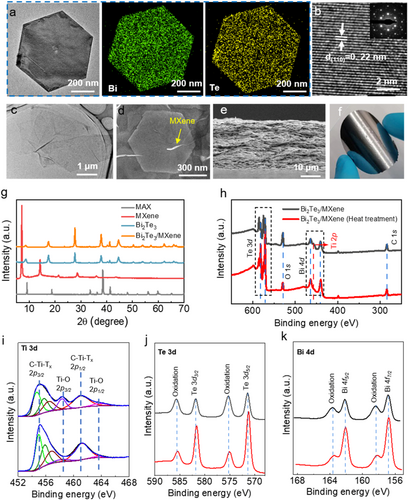
Morphologies and compositions of Bi2Te3 nanoplates, MXene flakes, and the composite films. (A) TEM and EDS mapping images of Bi2Te3 nanosheets. (B) High resolution TEM image reveals a nanosheet of Bi2Te3, with an inset image displaying the corresponding selected area electron diffraction (SAED) pattern. (C) TEM image of pure MXene flakes. (D) SEM image of the surface of Bi2Te3/MXene composite film. (E) Cross-sectional SEM image of the Bi2Te3/MXene composite film. (F) Photograph of the free-standing Bi2Te3/MXene composite film. (G) X-ray diffraction (XRD) patterns of MAX, MXene, Bi2Te3 and Bi2Te3/MXene composite films. (H) XPS spectra of the Bi2Te3/MXene composite before (upper panel) and after (lower panel) heat treatment at 300°C. (I–K) High resolution XPS spectra of Ti, Te and Bi peaks before (upper panel) and after (lower panel) heat treatment at 300°C.
The remarkably high conductivity of these MXene flakes was investigated in our previous work,43, 44 demonstrating the advantage of acting as carrier bridges between Bi2Te3 nanoplates to enhance the electrical performance of MXene/Bi2Te3 composite films. Figure 2D displays the surface morphology of MXene/Bi2Te3 composite films, highlighting the role of the MXene layer as a bridge connecting nanoplates to enhance mechanical properties and TE performance through direct contact with the surface of the Bi2Te3 nanoplates. Utilizing the vacuum assembly process, the nanoplates can be stacked along the in-plane direction (Figure 2E), thereby creating favorable conditions for the realization of high-performance MXene conductive pathways. Consequently, the freestanding MXene/Bi2Te3 composite film exhibits a noticeable metallic luster and excellent flexibility, allowing it to be curled without breakage (Figure 2F).
In addition, we conducted analysis of the crystal structure and chemical composition of the MXene, Bi2Te3 nanoplates, and MXene/Bi2Te3 composite films using X-ray diffraction (XRD) patterns and XPS. As presented in Figure 2G, the sharp diffraction peaks observed in the XRD patterns verify the well-crystallized Bi2Te3 nanoplates. There are no XRD peaks assigned to MXenes occurred, which is attributed to their low content (1 wt%) in the composite, lower than the detective level of XRD.45-48 XPS was conducted to verify the presence of MXene and analyze the chemical bonding changes in the composite films before and after heat treatment, as depicted in Figure 2H–K. The presence of MXene in composite films is confirmed by the peak at 455.1 eV in the spectrum of the MXene/Bi2Te3 composite film, corresponding to Ti 2p3/2 binding energies (Figure 2I). Bi2Te3 nanoplates are topological insulators with metallic surfaces and insulating bulk, making them susceptible to surface oxidation. To address this issue, we reduce the oxidation degree through heat treatment and then analyze the changes in binding energies using XPS. We compared changes in peak intensities of the elements to assess the impact of heat treatment on composite films. Figure 2I–K reveal the level of surface oxidation of the Bi2Te3 nanoplates and MXenes based on the peaks of Bi, Te, and Ti elements, with a noticeable decrease in intensity of the oxidation peaks following heat treatment. These pieces of evidence sufficiently demonstrate that heat treatment effectively reduces the oxidation content in MXene and Bi2Te3 nanoplates, which facilitates charge carrier transport in these materials. The dense composition and altered oxidation of the materials, as revealed by SEM and XPS findings, are likely to positively influence the TE performance of the composite films.
We assessed the impact of heat treatment on the TE performance of the composite films by evaluating them before and after the treatment. The experiments were conducted on the thin films within the in-plane at room temperature. Notably, the Seebeck coefficient and conductivity of the composite films without heat treatment are significantly impaired due to their extremely poor electrical properties, which are attributed to the surface oxidation of Bi2Te3 nanoplates in the presence of a low amount of MXene. The TE properties of the composite films are consistently detected through 3 repeated tests after heat treatment at 300°C in an argon atmosphere. Heat treatment effectively provides the electrical characteristics and thermoelectric performance of the composite films. In addition, the MXene content also plays a crucial role in TE performance by enhancing electrical properties, as exhibited in Figure 3A. As MXene content increases, the conductivity increases monotonically, demonstrating the advantage of optimizing electrical performance. Increasing the MXene content to 4 wt% yields a composite film with an outstanding electrical conductivity of up to 256 S cm−1, markedly exceeding the 8 S cm−1 conductivity of bare Bi2Te3 nanoplates. However, the Seebeck coefficient presents an inverse trend compared to the change in conductivity, that is, it decreases with increasing MXene content from −112 to −20 μV K−1 (Figure 3B). It is worth nothing that the negative Seebeck coefficient corresponds to n-type semiconductors, indicating that the conducting carriers are electrons. The power factor of the n-type MXene/Bi2Te3 composite film was calculated using the provided conductivity and Seebeck coefficient data (Figure 3C). Thus, the power factor exhibits an initial increase followed by a gradual decrease, reaching its peak at 66.2 μW m−1 K−2 with 1 wt% MXene content, which is a value 6 times greater than that of the bare Bi2Te3 film.
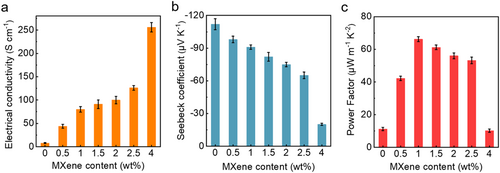
TE performance of the MXene/Bi2Te3 composite films. (A) The Seebeck coefficient, (B) electrical conductivity, and (C) power factor with increasing the MXene content (wt%). The error bars are the results of three tests.
Utilizing the optimized MXene/Bi2Te3 composite films, a proof-of-concept FTEG with an out-of-plane structure (typically consisting of 5 TE chips) was fabricated for performance evaluation (Figure 4A), where each TE chip consists of 3 composite films connected by liquid-metal electrodes electrically in series. The distinctive advantage of this concept is that the FTEG enables Lego-like reconfiguration of TE chips based on reserved gaps and rheological liquid-metal electrodes, where the TE chips can be inserted directly into or removed from the elastic silicone substrate with encapsulated liquid-metal wiring. Importantly, this behavior does not compromise the overall conductivity of the device. The FTEG's reconfiguration capability allows it to be customized for specific thermal conditions and desired electrical output. In the case of the out-of-plane structure, the performance in terms of ΔT under heat sources is a critical criterion for assessing the heat capture capability. In Figure 4B, the infrared camera recorded the temperature distribution of the FTEG, showing its temperature profile. It clearly indicates that the cold end temperature remains significantly lower than the hot end even after 1 hour of stabilization at ambient temperature, highlighting the FTEG's ability to maintain distinct ΔT at both ends. This feature can be primarily attributed to the TE chips functioning as cooling fins, and the enhancement effect is further supported by the temperature data at a higher heat source temperature in Figure 4C. In order to ensure the material's strength, we conducted a test involving repeated bending, using a radius of curvature of 5 mm. As presented in Figure 4D, the normalized resistance change remains constant, indicating that repeated bending does not cause deteriorating resistance signs. This is crucial for the practical reliability of FTEG, particularly in scenarios involving frequent bends. The inset in Figure 4D shows an enlarged view of the localized normalized resistance of the device, where the periodic fluctuations primarily result from the deformation of the liquid metal electrode with the FTEGs, suggesting that there is still a very small resistance change during bending. Based on our measurements and calculations, the contact resistance between the Bi2Te3/MXene composite and the liquid metal electrode remains consistently at 0.05 Ω.
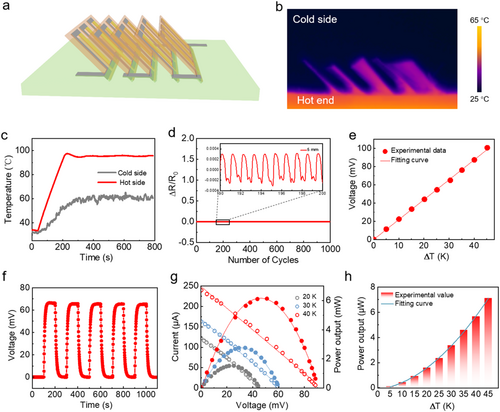
Mechanical stability and energy harvesting performance of reconfigurable FTEG. (A) Schematic representation of the integrated device structure. (B) Infrared imaging photograph illustrating the significant ΔT across the device following a 1 h stability test. (C) Temperature of the top and bottom ends of reconfigurable FTEG. (D) The measurement involved in repeating the normalized resistance change (ΔR/R0) 1000 times using a bending radius of 5 mm, with a closer view provided in the inset. (E) Graph showing the variation of open-circuit voltage with increasing ΔT. (F) Cycling test of open-circuit voltage with ΔT = 30 K applied at regular intervals. (G) Current-voltage and power output-voltage characteristics at ∆T = 20, 30, and 40 K. (H) Maximum output power as a function of ΔT. FTEG, flexible thermoelectric generator.
Output performance is another critical factor in evaluating the practical utility of FTEG. Figure 4E shows the experimental output voltage at different steady-ΔT, where increasing the ΔT can lead to a higher voltage, as demonstrated by the linear correlation. An open-circuit voltage of 100 mV was reached at a ΔT of 45 K. This consistent linear trend confirms the reliable performance of the FTEG at varying temperature. For thermal reliability, the constant output voltage remains constant at approximately 66 mV during five test cycles at ΔT = 30 K (Figure 4F), indicating that the thermal conversion of this FTEG is in a steady state. Figure 4G illustrates the correlation between the loop current and output power based on the output voltage, which was captured using a data acquisition device. The relationship between loop current and output power is demonstrated by the experimental results, which show a negative linear and parabolic trend with increasing output voltage at applied ΔT of 20, 30, and 40 K. An output power of 1.54 μW is generated by the FTEG when a ΔT of 20 K exists across each individual leg, with corresponding load voltage and loop current values of 25.5 mV and 60.3 μA, respectively. When the ΔT is increased to 30 K, the maximum output power reaches 2.76 μW, accompanied by a load voltage of 34.2 mV and a loop current of 80.8 μA. Increasing ΔT to 40 K leads to a peak output power reaching 6.13 μW, achieved at 51 mV and 120 μA. These results indicate that the highest power output occurs when the external resistance is set to 425 Ω under varying ΔT conditions, which aligns with the value determined using the optimal load equation in our previous research.28 After connecting the ideal load, we examined the voltage and current of the load to match them with ΔT, and subsequently determined the maximum power output. Figure 4H shows a robust agreement between the experimental data and the quadratic fitting curve, supporting the increase in maximum output power with rising ΔT. This allows for evaluation of output power based on specific ΔT requirements, with a notable output power of 7.12 μW at ΔT = 45 K. These findings demonstrate the mechanical durability of the FTEG and its ability to efficiently convert low-grade heat into electricity, making it a promising technology for wearable applications.
3 CONCLUSIONS
In summary, our proposal involves creating a reconfigurable FTEG that efficiently harvests low-grade heat through vertically oriented TE chips. This work primarily emphasizes two aspects: the development of high-performance all-inorganic composite films based on highly conductive MXene flakes and n-type Bi2Te3 nanoplates, and the design of a reconfigurable structure that facilitates the transition of heat transfer modes from in-plane to out-of-plane. To improve the thermoelectric performance of inorganic composite films, we employed heat treatment to decrease the oxidation level of MXene and Bi2Te3 nanoplates. This results in an enhanced power factor of approximately 66.2 μW m−1 K−2 with a Seebeck coefficient of −91 μV K−1. Furthermore, the planar TE chips, which utilize all-inorganic composite films, can be easily inserted into or removed from the elastic substrate that encloses the liquid-metal electrodes while maintaining both thermal parallel and electrical series connections. By employing these approaches, our FTEG, consisting of just 5 TE chips, can generate 7.1 μW of power when exposed at a ΔT of 45 K. This renders it a practical choice for harnessing waste heat in the environment.
4 MATERIALS AND METHODS
4.1 Materials
Aladdin Industries Corp provided high purity Bi(NO3)2·5H2O (≥99%), Na2TeO3 (99.99%), LiCl (99.99%), and polyvinylpyrrolidone (PVP, MW = 40,000), while Jilin 11 Technology Co supplied Ti3AlC2 powder. Sinopharm Chemical Reagent Co provided ethylene glycol (≥99%), NaOH (96%), HF (14 M), and HCl (9 M). Shenyang Jiabei Co., Ltd supplied liquid-metal made of gallium (75%) and indium (25%). Ecoflex silicone solution was bought from Smooth-On (USA). Shanghai Fuzhi Information Technology Co., Ltd. provided the target of polylactic acid. All experiments utilized deionized water with a resistance of 18.2 Ω.
4.2 Exfoliation of MXene (Ti3C2Tx) flakes
1.0 g of Ti3AlC2 powder was added gradually to a solution for etching, which consisted of 6 mL of DI water, 12 mL of HCl, and 2 mL of HF. The mixture was stirred constantly during a 24-h etching reaction at 35°C. Following the reaction, the acidic mixture underwent purification using deionized water through centrifugation at 3500 revolutions per minute for 5 min, until the pH level approached 6. After that, 70 mL of deionized water was mixed with the sediment, then 1 g of lithium chloride was added and the mixture was shaken for 1 h. The suspension was rinsed with deionized water until the pH was approximately 6. To obtain high-quality MXene flakes, we also ultrasonically exfoliate the products in 100 mL DI water for 2 h while bubbling using N2 gas. Following centrifugation at 3500 revolutions per minute for 30 min, the upper layer is gathered and placed in a refrigerator at 4 degrees Celsius.
4.3 Synthesis of Bi2Te3 nanoplates
Initially, 1.94 g of Bi(NO3)2·5H2O, 1.328 g of Na2TeO3, and 1 g of PVP were individually placed into 70 mL of ethylene glycol. Then, 2 g of NaOH were added to regulate the pH. The blend was agitated with a magnet at a temperature of 35°C for a period of 5 h until a clear mixture was produced. The mixture was subsequently heated to a temperature of 180°C in sealed autoclaves for a duration of 10 h. Following the cooling process to ambient temperature, the resulting materials were cleansed through multiple rounds of centrifugation using deionized water, acetone, and dry ethanol, then dried under vacuum at 60°C for 12 h.
4.4 Preparation of MXene/Bi2Te3 composite film
Various quantities of Bi2Te3 nanoplates were mixed with varying amounts of MXene dispersion and then sonicated for 30 min using an ultrasonic disperser (Branson 3510, 350 W, Emerson). Composite films were created by suction filtering a mixed dispersion of varying concentrations. Then, the resulting films were subjected to heat treatment at 300°C for 120 min with a heating rating of 5°C, with circulating argon gas at 100 mL min−1 as a protective atmosphere. The composite films were obtained from the tube reactor after cooling to room temperature in an atmosphere of Ar.
4.5 Fabrication of TE chip
The MXene/Bi2Te3 composite films were precisely cut into uniform rectangular ribbons and arrayed on single-side adhesive polyimide (PI) tape. Subsequently, a liquid metal was utilized to establish electrical connections in series and thermal connections in parallel among the composite films, following the mask pattern illustrated in Figure 1A. Then, the PI tape was used to encapsulate TE array, resulting in the formation of a flexible TE chip avoiding bending damage.
4.6 Fabrication of FTEGs
The substrate mold was fabricated via 3D printing (n2plus, Raise3D), and subsequently cured for 8 h at room temperature after pouring the silicone solution into the mold. Liquid-metal was printed onto the reserved pattern as electrical wiring and then encapsulated by applying another layer of spin-coated silicone solution, with the reserved area for TE chips intentionally avoided during the spin-coating process. After curing, the TE chips can be inserted into the substrate, resulting in the fabrication of the reconfigurable FTEG.
4.7 Characterization and measurements
The microscopic structures of Bi2Te3 nanoplates and MXene flakes were investigated using a field emission transmission electron microscope (FETEM, Talos F200S) equipped with energy-dispersive (EDS), X-ray spectroscopy (XPS) and SAED. The morphology of the composite films was characterized using a field emission scanning electron microscope (FESEM, Hitachi SU800000). XRD analysis was performed using a MAX-2550 X-ray diffractometer from Rigaku in Japan, employing Cu Kα radiation with a wavelength of 1.5406 Å at 300 mA and 40 kV. Additionally, binding energies were analyzed using X-ray photoelectron spectroscopy (XPS, Escalab 250Xi). The data acquisition instrument (Keithley 2700) was used to measure the voltage, current, temperature, and resistance of FTEG. Surface temperatures were also confirmed using an infrared thermal camera (FLIR A310). Bending experiments were conducted using a home-built mechanical collider. The four-point probe instrument (MCP-T360, Mitsubishi Chemical) was utilized to measure conductivity. The Seebeck coefficient was acquired using a Thermoelectric Meter (PTM-3, JouleYacht).
AUTHOR CONTRIBUTIONS
Yunhe Xu: Writing – original draft; data curation; methodology; formal analysis; writing – review & editing. Bo Wu: Writing – review & editing; writing – original draft. Chengyi Hou: Resources; project administration; writing – review & editing; supervision. Yaogang Li: Resources. Hongzhi Wang: Resources. Qinghong Zhang: Resources; writing – review & editing; supervision; data curation.
ACKNOWLEDGMENTS
Bo Wu and Yunhe Xu contributed equally to this work. Financial support for this work was provided by the National Natural Science Foundation of China (No. 51873033) and the Fundamental Research Funds for the Central Universities (2232024G-07).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




