Evaluation of the lodging resistance and the selection of identification indexes of maize inbred lines
Yunxiao Zheng and Peng Hou contributed equally to this study.
Abstract
Lodging is one of the main problems affecting the maize production. In this study, 220 maize inbred lines were used for determining the lodging resistance. Analysis methods such as the correlation analysis, the principal component analysis, the cluster analysis, the stepwise discriminate analysis, and the ridge regression analysis were deployed for data interpretation. The results of the correlation analysis showed that 17 characters were correlated with varying degrees. Strong and positive correlations between TID and FID (r = 0.910), TIL and FIL (r = 0.898), NSVB and NLVB (r = 0.775), ASVB and ALVB (r = 0.746), and LC and HC (r = 0.656) were observed. The first six principal components explained 79.13% of the phenotypic variation of the 17 characters, with the contribution rates being 20.77%, 18.12%, 14.09%, 11.17%, 8.66%, and 6.32%, respectively. Five categories were clustered in the 220 inbred lines. The result of stepwise discriminate analysis showed that 211 inbred lines were correctly discriminated and the identification rate was 95.91%, and 9 inbred lines were incorrectly discriminated and the identification rate was 4.09%, which meant that the result of cluster analysis was accurate and reliable. The stalk bending strength, ear height, cellulose content, number of small vascular bundles, and cross-sectional area were selected, and the regression model of lodging resistance of inbred lines was established by using the ridge regression method. Thirty of the 220 inbred lines, including R1656, 4003, and LD61, showed the highest lodging resistance. The results provide a reference for the selection of lodging-resistant germplasm resources in breeding for the lodging resistance hybrids.
1 INTRODUCTION
Maize (Zea mays L.) is one of the most important cereal crops in the world (Xu et al., 2017). High yields of maize are typically obtained at high plant densities (Ittersum & Cassman, 2013). However, maize lodging rates also increase at high densities. High plant density increases competition for nutrients between individual plants, which results in thinner maize stems and a higher risk of lodging (Shah et al., 2017). Studies have shown that lodging is divided into two types: root fall and stem fall (folding), and stem breakage causes greater losses to the yield and quality of maize (Li et al., 2015). According to statistics, the annual yield loss of maize due to stalk lodging is from 5% to 25%, and every 1% increase in lodging will cause a 108 kg/ha yield reduction (Norboerg et al., 1988; Xu et al., 2017). Additionally, lodging also increases the difficulty of mechanical harvesting and reduces harvest efficiency (Wang et al., 2022). Therefore, great attention should be paid to the lodging of maize.
Most studies on maize stalk lodging focused on aspects of stalk mechanical characteristics, plant morphology, stalk chemical composition, and stalk anatomical structure (Xue et al., 2017). Studies on stalk mechanical characteristics have shown that the rind penetration strength (RPS), crushing strength (CS), and bending strength (three-point bending flexural tests) are all significantly negatively correlated with the stalk lodging rate (Robertson et al., 2014). Additionally, stalk pull is significantly negative associated with lodging resistance (Feng et al., 2010; Liang, 2014). The most important morphological feature associated with stalk lodging is plant height and ear height (EH). Previous studies have also reported that reducing plant height can improve the crop lodging resistance (Peng et al., 2014; Shekoofa & Emam, 2008). Besides, maize with high resistance to stalk lodging should have a lower ear position to decrease center of gravity (Shah et al., 2021). Basal internodes is another morphological feature associated with stalk lodging. Previous research has indicated that maize plants with short and thick basal internodes display greater stalk lodging resistance (Ma et al., 2014).
Studies have reported that stalk strength is significantly and positively related to the contents of chemical compositions, such as cellulose, hemicellulose, and lignin, and the accumulation of these three chemical components improved the thickening and flexibility of the culm wall, which is an important factor affecting plant lodging (Appenzeller et al., 2004; Chen et al., 2007; Fu et al., 2013; Tanaka et al., 2003). Anatomical characteristics of maize plants are essential and have a significant effect on the lodging resistance. Xu et al. (2017) found that stalk strength can be improved by increasing the number and area of vascular bundles in the effects of applying EDAH (a plant growth regulator containing 27% ethephon and 3% DA-6). Sclerenchyma cells around the vascular bundles in the stem are responsible for mechanical strength, and a reduction in sugar supplies can reduce the vigor of sclerenchyma cells, inducing stalk lodging (Novacek et al., 2013; Shah et al., 2021).
All in all, the stalk lodging resistance is a comprehensive indicator of mechanical characteristics, plant morphology, chemical composition, and anatomical structure. Therefore, accurate evaluation of stalk lodging resistance is very important. In this study, 220 maize inbred lines were used as test materials, and 17 traits closely related to the lodging resistance were investigated. Furthermore, a multivariate statistical analysis of the traits was conducted to establish a set of mathematical evaluation models and methods for accurately evaluating the lodging resistance of inbred lines and screening identification indicators. These results provide a reference for the identification and genetic improvement of maize lodging resistance germplasm resources and provide a reference for breeding lodging-resistant maize hybrids.
2 MATERIALS AND METHODS
2.1 Plant materials
A natural population containing 220 diverse maize inbred lines was used as research materials. The 220 inbred lines were classified into six subgroups based on population structure Q matrix. The detailed information on this natural population can be found in Table S1 (Yang, 2016). For all the 220 inbred lines, a randomized complete block design with three replications was used in this study. Each material was planted in a plot with two rows in 0.60 m inter-row spacing and 3.0 m long row using a population density of 75,000 plants per hectare at the Experimental Station of Hebei Agricultural University in Baoding (115.48°E, 38.85°N) and Experimental Station of Shijiazhuang (115.12°E, 37.54°N) in May 2019 and 2020. All lines followed standard local field management practices using local maize tillage methods throughout the whole growth period.
2.2 Measurements of the lodging resistance
The lodging resistance related traits measured in this study included four categories: morphological characteristics, mechanical characteristics, chemical composition, and anatomical characteristics of maize, with a total of 17 traits. The specific determination method was as follows:
2.2.1 Determination of mechanical properties of stems
One week after pollination of maize in the field, three plants were selected with the same growth trend in each inbred line except for the side plants. The thrust meter (DIK-7401) was used to apply force in the direction perpendicular to each stalk at the middle of the ear node until the whole plant angles to 45° from the ground. At this time, the value displayed by the thrust meter was defined as the stalk pushing resistance (SPR).
Under natural growing conditions in the field, the stalk bending strength (SBS) and rind penetrometer strength (RPS) were measured using a stalk strength tester (YYD-1, Zhejiang Top Instrument Co Ltd.) at the 10th day after anthesis, and two representative plants with uniform growth were selected in each row. The stalk strength tester was used to apply force in the direction perpendicular to each stalk at the ear node until the stalks broke or bent greater than 45°, and the peak force required to bend or break the stalk was defined as the SBS (Yang et al., 2020). In addition, the probe of the stalk strength tester was inserted into the stalk between the third and fourth internodes at a slow, uniform speed perpendicular to the stalk. The maximum value required to penetrate the stalk epidermis was read three times, and the average value was calculated as the RPS (Yang et al., 2020).
2.2.2 Determination of morphological character of stem
The length (TIL and FIL) and diameter (TID and FID) of the third and fourth internodes were measured from the base of the maize plant using a Vernier caliper and the central part shall prevail. Plant height of the main stem (PH) was measured from the ground to the tassel at physiological maturity. Ear height of the main stem (EH) was measured from the ground to the top of the effective female ear at physiological maturity.
2.2.3 Determination of chemical composition of stem
At the 10th day after anthesis, two representative plants with uniform growth in each plant row were selected, and the third and fourth stem nodes of the base were collected. The samples were inactivated at 105°C for half an hour and then transferred to a 65°C oven for drying. Dried samples of the same tissue were pooled, grounded, and screened through a combined 40-mesh sieve. The NIRS model of fiber quality established by Guan et al. (2018) was installed on the DA7200 near-infrared analyzer, and then the samples were placed in a 75 mm-diameter rotating sample cell, and the surface was scraped with a ruler. Each sample was loaded twice, and the scan was repeated twice. Finally, the physiological data of cellulose content (CC), hemicellulose content (HC) and lignin content (LC) were obtained; the unit was “%”.
2.2.4 Determination of the structure of stem vascular bundle
First, a phloroglucinol solution with a concentration of 5% was prepared, placed, and allowed to stand for one day. Then, the plant for stem thrust measurement was used as the research material, the central part of the male axis was cut for freehand sectioning, with a thickness of about 0.2–0.4 mm, and stained with a preconfigured phloroglucinol solution and concentrated hydrochloric acid (Huang et al., 2016). The stained slices were imaged by the Zeiss Axioskop 40 microscope. Zen (blue edition) 2012 image processing software was used to collect data of vascular bundle-related traits. Generally speaking, small vascular bundles with relatively close arrangement are located in the 1–2 layer of the tissue edge, and large vascular bundles with loose arrangement are located in the organization. The area of the large and small vascular bundles and the cross-sectional area (CSA) of the stem were calculated as “S = πab/4” (a is the long axis of the ellipse and b is the short axis of the ellipse) (Yang, 2016). Finally, the physiological data of number of small vascular bundle (NSVB), number of large vascular bundle (NLVB), average area of single small vascular bundle (ASVB), average area of single large vascular bundle (ALVB), CSA were obtained.
2.3 Data and statistical analysis
2.3.1 The value of membership function for each comprehensive character of maize inbred line
In the formula, Xj represents the jth comprehensive trait, U (Xj) represents the membership function value of the j comprehensive trait, Xmin and Xmax represent the minimum and maximum values of the jth comprehensive trait, respectively (Xue et al., 2013).
2.3.2 The weight of each comprehensive trait
In the formula, Wj represents the importance of the jth comprehensive trait in all comprehensive traits, that is, the weight; Pj represents the contribution rate of the jth comprehensive trait of each maize inbred line obtained by principal component analysis (PCA).
2.3.3 The lodging resistance of each maize inbred line
In the formula, D is the comprehensive evaluation value of the lodging resistance of each maize inbred line, and the Euclidean distance and deviation square sum method (ward. D2) were applied at the same time (Dai et al., 2014; Danojevi et al., 2016).
In order to eliminate the deviation caused by environmental impact as much as possible, the best linear unbiased prediction (BLUP) was performed on the two-year two-point data using the “lmer” function in R's “lme4” software package (R Core Team, 2015). In order to eliminate the influence of different dimensions, the BLUP value was first standardized by the Z-score method (Prolla et al., 2012), then the “corrplot” package of R platform was used for correlation analysis, the “hclust” function of R platform was used for cluster analysis, and SPSS 21.0 software was used for PCA of each trait, stepwise discriminant analysis, and ridge regression analysis.
3 RESULTS
3.1 Variability in lodging resistance traits
The phenotypic characteristics for the 17 lodging-related traits across four environments are shown in Table 1 of this study. As shown in Table 1, the coefficients of variation across four environments ranged 8.69–31.31, 12.21–28.63, 12.01–40.30, and 11.84–32.13 (%), respectively. In addition, the means varied widely among the RPS, SBS, and FIL in different environment (Table 1). The means of phenotypic values in Shijiazhuang are higher than that in Baoding, such as FIL, TID, FID, LC, and NSVB (Table 1). And the means of phenotypic values in 2020 are higher than that in 2019, such as PH, EH, ASVB, and CSA (Table 1).
| Lodging resistance traitsa | 2019BD | 2019SJZ | 2020BD | 2020SJZ | ||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | CV (%) | Mean ± SD | CV (%) | Mean ± SD | CV (%) | Mean ± SD | CV (%) | |
| SPR (N/mm2) | 3.86 ± 1.21 | 31.31 | 3.86 ± 0.78 | 20.08 | 6.31 ± 2.54 | 40.30 | 9.41 ± 3.02 | 32.13 |
| RPS (N/mm2) | 50.31 ± 12.92 | 25.67 | 115.41 ± 16.87 | 14.61 | 60.16 ± 17.41 | 28.95 | 37.21 ± 9.55 | 25.67 |
| SBS (N/mm2) | 404.69 ± 117.42 | 29.01 | 685.11 ± 145.47 | 21.23 | 421.22 ± 124.84 | 29.64 | 366.88 ± 115.91 | 31.59 |
| TIL (mm) | 88.15 ± 15.92 | 18.06 | 84.68 ± 15.94 | 18.82 | 82.60 ± 18.70 | 22.64 | 93.59 ± 16.81 | 17.96 |
| FIL (mm) | 105.84 ± 19.73 | 18.64 | 108.70 ± 18.14 | 16.69 | 87.71 ± 18.88 | 21.52 | 112.23 ± 18.56 | 16.54 |
| TID (mm) | 16.28 ± 1.98 | 12.19 | 19.39 ± 2.50 | 12.88 | 17.26 ± 2.75 | 15.95 | 17.75 ± 2.16 | 12.17 |
| FID (mm) | 16.13 ± 1.92 | 11.93 | 18.53 ± 2.26 | 12.21 | 16.52 ± 1.98 | 12.01 | 17.44 ± 2.07 | 11.84 |
| PH (cm) | 185.18 ± 23.33 | 12.60 | 179.52 ± 24.55 | 13.68 | 188.77 ± 25.01 | 13.25 | 189.52 ± 24.13 | 12.73 |
| EH (cm) | 70.22 ± 14.76 | 21.02 | 64.83 ± 14.33 | 22.11 | 70.75 ± 17.86 | 25.24 | 73.98 ± 15.53 | 20.99 |
| CC (%) | 31.41 ± 2.73 | 8.69 | 33.42 ± 3.98 | 11.90 | 27.81 ± 3.58 | 12.87 | 26.67 ± 2.94 | 11.01 |
| HC (%) | 22.37 ± 2.71 | 12.10 | 21.89 ± 3.81 | 17.39 | 18.35 ± 2.75 | 15.01 | 19.56 ± 2.92 | 14.95 |
| LC (%) | 26.14 ± 3.13 | 11.96 | 30.60 ± 3.95 | 12.92 | 28.43 ± 4.14 | 14.56 | 28.53 ± 4.62 | 16.20 |
| NSVB | 57.24 ± 10.03 | 17.53 | 61.87 ± 10.72 | 17.33 | 57.89 ± 10.81 | 18.67 | 61.70 ± 10.23 | 16.59 |
| NLVB | 60.39 ± 15.67 | 25.94 | 66.07 ± 18.92 | 28.63 | 62.14 ± 16.29 | 26.21 | 60.99 ± 16.40 | 26.89 |
| ASVB (mm2) | 0.019 ± 0.005 | 23.47 | 0.018 ± 0.005 | 24.62 | 0.021 ± 0.006 | 26.37 | 0.021 ± 0.005 | 22.94 |
| ALVB (mm2) | 0.031 ± 0.009 | 28.96 | 0.024 ± 0.006 | 26.62 | 0.033 ± 0.010 | 29.25 | 0.029 ± 0.007 | 23.86 |
| CSA (mm2) | 15.42 ± 4.24 | 27.52 | 16.12 ± 4.43 | 27.48 | 19.37 ± 5.24 | 27.06 | 17.35 ± 4.38 | 25.22 |
- a SPR, RPS, SBS, TIL, FIL, TID, FID, PH, EH, CC, HC, LC, NSVB, NLVB, ASVB, ALVB, and CSA stand for stalk pushing resistance, rind penetrometer strength, stalk bending strength, the length of the third internodes, the length of the fourth internodes, the diameter of the third internodes, the diameter of the fourth internodes, plant height, ear height, cellulose content, hemicellulose content, lignin content, number of small vascular bundle, number of large vascular bundle, average area of single small vascular bundle, average area of single large vascular bundle, and cross-sectional area, respectively.
3.2 Correlation analysis of various traits
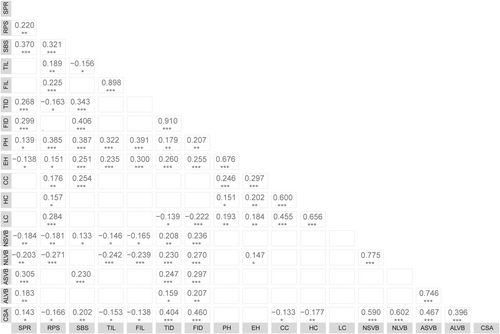
It was found through the correlation analysis of the 17 traits (Figure 1) that there was a correlation between the lodging resistance traits. Pearson's correlation analysis showed that 45 of these coefficients is extremely significantly correlated at p < 0.001, 16 of these coefficients is extremely significantly correlated at p < 0.01, and 18 of these coefficients is significantly correlated at p < 0.05. Among them, it was observed that stronger correlations between TID and FID (r = 0.910), TIL and FIL (r = 0.898), NSVB and NLVB (r = 0.775), ASVB and ALVB (r = 0.746), and LC and HC (r = 0.656). In the anti-lodging mechanism, the roles of different traits are not the same, but the traits showed significant or extremely significant correlation, so that the functions they produced partially overlapped and then had an impact on lodging resistance evaluation. In order to make up for the shortcomings of using a few traits to evaluate the lodging resistance, it is necessary to make a comprehensive evaluation of maize inbred lines using PCA, cluster analysis, and ridge regression analysis.
3.3 Principal component analysis
The PCA on 17 lodging resistance-related traits was carried out to calculate the feature vector and contribution rate of each principal component (PC). The number of PCs was determined by examining the feature values greater than 1 (Patto et al., 2009). It can be seen from Table 2 that the cumulative contribution rate of the first six PCs had reached 79.134%, so these six PCs can be used to comprehensively evaluate the lodging resistance.
| Traita | Principal component | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| SPR | 0.225 | 0.334 | −0.371 | −0.430 | −0.265 | 0.143 |
| RPS | −0.286 | 0.530 | −0.072 | −0.186 | −0.066 | 0.577 |
| SBS | 0.390 | 0.543 | 0.097 | −0.264 | −0.267 | 0.383 |
| TIL | −0.322 | 0.430 | −0.413 | 0.575 | 0.276 | −0.061 |
| FIL | −0.295 | 0.517 | −0.394 | 0.551 | 0.241 | −0.035 |
| TID | 0.722 | 0.314 | −0.022 | 0.032 | −0.326 | −0.414 |
| FID | 0.776 | 0.326 | −0.074 | 0.057 | −0.319 | −0.299 |
| PH | −0.051 | 0.788 | 0.066 | 0.261 | −0.217 | 0.121 |
| EH | 0.079 | 0.654 | 0.297 | 0.407 | −0.056 | −0.111 |
| CC | −0.173 | 0.451 | 0.582 | −0.230 | 0.192 | −0.071 |
| HC | −0.272 | 0.420 | 0.491 | −0.365 | 0.252 | −0.364 |
| LC | −0.325 | 0.426 | 0.397 | −0.401 | 0.318 | −0.109 |
| NAVB | 0.573 | −0.119 | 0.496 | 0.320 | 0.288 | 0.270 |
| NLVB | 0.601 | −0.178 | 0.545 | 0.299 | 0.211 | 0.174 |
| ASVB | 0.441 | 0.243 | −0.539 | −0.363 | 0.397 | −0.058 |
| ALVB | 0.393 | 0.153 | −0.497 | −0.257 | 0.562 | −0.027 |
| CSA | 0.821 | 0.003 | 0.016 | 0.045 | 0.362 | 0.158 |
| Eigen value | 3.531 | 3.081 | 2.396 | 1.899 | 1.471 | 1.075 |
| Contribution rate (%) | 20.768 | 18.124 | 14.093 | 11.173 | 8.655 | 6.321 |
| Cumulative contribution rate (%) | 20.768 | 38.892 | 52.985 | 64.158 | 72.813 | 79.134 |
- a SPR, RPS, SBS, TIL, FIL, TID, FID, PH, EH, CC, HC, LC, NSVB, NLVB, ASVB, ALVB, and CSA stand for stalk pushing resistance, rind penetrometer strength, stalk bending strength, the length of the third internodes, the length of the fourth internodes, the diameter of the third internodes, the diameter of the fourth internodes, plant height, ear height, cellulose content, hemicellulose content, lignin content, number of small vascular bundle, number of large vascular bundle, average area of single small vascular bundle, average area of single large vascular bundle, and cross-sectional area, respectively.
The first PC (PC1) contributed the most to variability (20.768%), followed by PC2 (18.124%), PC3 (14.093%), PC4 (11.173%), PC5 (8.655%), and PC6 (6.321%), as shown in Table 2. In addition, PC1 was mostly contributed by traits CSA, FID, and TID which showed considerable positive factor loadings. PC2 was mostly contributed by traits PH, EH, and SBS which showed considerable positive factor loadings. Traits that determined the size of PC3 were CC and NLVB with positive loadings. For PC4, trait TIL showed considerable positive factor loadings. The trait that determined the size of PC5 was ALVB with positive loadings. For PC6, trait RPS showed positive factor loadings.
3.4 Cluster analysis
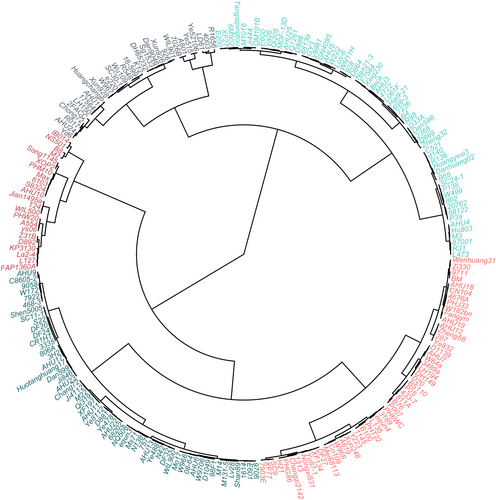
Two hundred and twenty inbred lines were clustered into five categories (Figure 2). There were 30 maize inbred lines in group I, including R1656, 4003, and LD61, which had the best lodging resistance; 60 maize inbred lines in group II, including L473, R31, and 87,001, which had a strong lodging resistance; and 55 maize inbred lines in group III, including AHU9, C8605-2, and 9058, which had a general lodging resistance; 52 maize inbred lines in group IV, including 7903E, XF77, and SC9, which had a weak lodging resistance; and 23 in group V, including IB014, NS501, and B8, which had the weakest lodging resistance (Table S2).
3.5 Discriminant analysis
The results of cluster analysis were verified by multi-class stepwise discriminant analysis. Based on the scores and clustering results of the six PCs of inbred lines, the Fisher Discriminant Function (Si) coefficients were calculated to establish the discriminant function (Kiani & Jafari, 2012).
Based on the discriminant rule: if the value of Si (i = 1, 2, 3, 4, 5) is the largest, then the inbred lines are classified into category i, and 220 inbred lines are reclassified. On the whole, compared with the cluster analysis results, 211 inbred lines were correctly identified with a probability of 95.91%; 9 inbred lines were misjudged with a misjudgment rate of 4.09%, indicating that the results of cluster analysis were accurate and reliable.
3.6 Regression model establishment and verification
In order to screen the traits to measure the comprehensive ability of lodging resistance, a mathematical evaluation model for accurately evaluating the lodging resistance of inbred lines was established, and the comprehensive evaluation value (D value) of the lodging resistance was used as the dependent variable. Each trait was used as an independent variable for ridge regression analysis, and regression equations were established. The final regression equation established was D = 0.477 + 0.014SBS + 0.027EH + 0.012CC + 0.031NSVB + 0.023CSA, and the equation determination coefficient R2 = 0.932, F = 597.516, p < 0.001 after adjustment. This equation reached a very significant level, indicating that the SBS, EH, CC, NSVB, and CSA were extremely associated with the comprehensive evaluation value (D value) of the lodging resistance relationship. The regression value was got after the standardized data of five characters were substitute into the equation, and the root mean square error (RMSE) between the original D value and the regression value was calculated to be 0.020, indicating that the equation has high accuracy and can be used to determine the lodging resistance of maize inbred lines in actual production.
3.7 Comparison of characteristics between lodging-resistant maize inbred lines
Five clusters of inbred lines divided by cluster analysis and regression results were compared, and the characteristics of five important traits among different lodging resistance inbred lines were compared to further evaluate the lodging resistance of maize inbred lines (Table 3).
| Group | Average of primary value | ||||
|---|---|---|---|---|---|
| SBS (N/mm2) | EH (cm) | CC (%) | NSVB | CSA (mm2) | |
| I | 512.127 | 81.934 | 30.217 | 70.909 | 20.426 |
| II | 489.858 | 73.828 | 29.961 | 64.052 | 18.857 |
| III | 451.992 | 70.3783 | 29.864 | 59.047 | 17.105 |
| IV | 452.566 | 62.449 | 29.767 | 55.134 | 15.441 |
| V | 424.979 | 58.710 | 29.260 | 47.329 | 12.822 |
- Note: SBS, EH, CC, NSVB, and CSA stand for stalk bending strength, ear height, cellulose content, number of small vascular bundle, and cross-sectional area, respectively.
Group I (the type of high lodging resistance) included 30 inbred lines, which was 13.64% of the total, and the values of SBS, EH, CC, NSVB, and CSA in inbred lines in this group were higher than other groups. Group II (the lodging resistance type) included 60 inbred lines, which was 27.28% of the total, and the values of SBS, EH, CC, NSVB, and CSA in inbred lines in this group were all higher than those of groups III, IV, and V, and the value of LC was lower than that of group III, and the length of the third internode was lower than that in group V. Group III (the middle-down type) included 55 inbred lines, which was 25.00% of the total, and the values of EH, CC, NSVB, and CSA in inbred lines in this group were higher than those in group IV and V, and the value of SBS was lower than that of group IV. Group IV (the reversible type) included 52 inbred lines, which was 23.63% of the total, and the values of EH, CC, NSVB, and CSA in inbred lines in this group were higher than those of group V, and the value of SBS was higher than the group III. Group V (the highly reversible type) included 23 inbred lines, which was 10.45% of the total, and the values of SBS, EH, CC, NSVB, and CSA in inbred lines in this group were lower than other groups.
4 DISCUSSION
4.1 Multivariate statistical analysis of the evaluation index
The lodging resistance of crops is a complex and comprehensive trait affected by multiple factors, which is easily affected by the differences of external environment and its own genotype and leads to unreliable results. Therefore, the evaluation of lodging resistance of crops should be more effective by the comprehensive analysis of the studied traits. Many scholars have used the comprehensive evaluation methods to study and apply the disease resistance, cold tolerance, and drought tolerance of different crops. Saeed et al. (2014) used correlation, PCs, and cluster analysis to evaluate for cotton leaf curl virus disease tolerance, fiber quality, and some yield-related traits. PCA showed first five PCs having eigen value >1 explaining 71.3% of the total variation with plant height, sympodia per plant, and seed cotton yield being the most important characters in PC1. Cluster analysis classified 100 accessions into four divergent groups and in cluster 3 and 4 included genotypes with higher tolerance of cotton leaf curl virus disease and better fiber quality along with higher yield potential were included. Zarei et al. (2013) used several multivariate analyses, including simple correlation, path coefficient analysis, stepwise regression, factor analysis, and cluster analysis, to evaluate the relationships between the durum grain yield and the related traits under drought conditions. Their field screening techniques suggested diversity-dependent strategy based on plant height, the number of tillers per plant, the number of spikes per plant, above ground biomass, and harvest index for breeding durum wheat under drought stress condition. However, using multivariate statistical analysis to evaluate crop lodging resistance is still rare.
In this study, 17 traits caused maize stalk lodging, covering all aspects of plant mechanical properties, chemical composition, morphological structure, and vascular bundle structure, to recognize and analyze the lodging resistance mechanism, with more comprehensive data, which is conducive to scientific judgment of the lodging resistance performance of maize. Through PCA, 17 individual traits associated with lodging resistance were converted into six comprehensive traits independent of each other and the comprehensive value of different maize lodging resistance D value. Through cluster analysis, 220 maize inbred lines were divided into five types, including highly lodging-resistant, lodging-resistant, medium-falling, lodging, and weak lodging. A reliable regression model of maize inbred lodging resistance was established by gradual regression and selected five individual traits that significantly affected maize lodging resistance, including SBS, EH, CC, NSVB, and CSA. Under the same field conditions, five identification indexes of other maize inbred lines can be determined, and the evaluation model can be used to predict the lodging resistance of target inbred lines so that the identification and utilization of the lodging resistance is more predictable and can also provide a basis for lodging resistance breeding and resource identification and screening.
4.2 Evaluation of the lodging resistance and genetic improvement of maize
The predecessors have rated and evaluated the lodging resistance of maize. Wang et al. (2013) classified maize inbred lines into six types: highly lodging-resistant, general lodging-resistant, medium-falling, relatively easy-falling, easy-falling, and extremely easy-falling by different lodging rate. However, these studies are only based on one or two of the traits; thus, they cannot fully reflect the lodging resistance of maize. With an extensive review of previous and overseas relevant researches, this study studied 17 traits that caused maize stalk lodging, covering all aspects of plant mechanical properties, chemical composition, morphological structure, and vascular bundle structure, to recognize and analyze the lodging resistance mechanism, with more comprehensive data, which is conducive to scientific judgment of the lodging resistance performance of maize. Moreover, the performance of lodging resistance in maize inbred lines will vary with environmental factors, and the comprehensive evaluation of using BLUP values in different years and locations will be more convincing in this study.
At present, with the improvement of people's living standards, improving and breeding high-yield, high-quality maize has become the main problem in maize breeding, which can promote the development of the maize industry (Zheng et al., 2021). Maize is a model crop that uses heterosis, so breeding excellent inbred lines to match hybrids is the main task of maize breeding. Lodging is one of the main reasons for maize yield reduction (Li et al., 2014). Therefore, establishing mathematical models to conduct more accurate evaluation of maize inbred lines for lodging is of great significance to cultivating new maize varieties with high yield and lodging resistance. Therefore, in the genetic improvement of maize and breeding of varieties, emphasis should be given to its lodging resistance (Duvick, 2005; Ma et al., 2014). In this study, 17 traits closely related to lodging resistance were selected for identification. It was found that SBS, EH, CC, NSVB, and CSA were the factors that affect the stalk resistance. Therefore, lodging resistance should be taken into keen consideration for purposes of genetic improvement and maize variety selection and breeding. Among the 220 maize inbred lines, 30 highly resistant inbred lines, mainly R1656, 4003, and LD61, were selected, and their cultivation in the field should be strengthened. It at the same time provides a theoretical basis for the combination of lodging-resistant hybrids. Combining the results of the group structure analysis of Liu et al. (2014) and Liu et al. (2012), 30% of the 30 highly resistant inbred lines were from the Lvda Red Cob group, and the rest scattered in the Reid group, the P group, the Tangsipingtou group, and the mixed group, so attention should be paid to the utilization of germplasm resources of the Lvda Red Cob heterosis group in lodging resistance breeding. This paper focuses on the study of the lodging resistance of stalks of maize germplasm resources and provides references for the combination of maize hybrids.
5 CONCLUSION
This study divided 220 maize inbred lines into five categories: high lodging resistance, lodging resistance, middle-down, reversible, and highly reversible by multivariate statistical analysis. And the SBS, EH, CC, NSVB, and CSA were selected to establish the mathematical model of lodging resistance of maize inbred lines. The result obtained from this study could be useful for selecting lodging resistance inbred lines.
ACKNOWLEDGEMENTS
This work is financially supported by the Science and Technology Innovation Team of Maize Modern Seed Industry in Hebei (21326319D), State Key Laboratory of North China Crop Improvement and Regulation (NCCIR2021ZZ-10), and National Key Research and Development Program of China (2018YFD0300501).
CONFLICT OF INTEREST STATEMENT
The authors have declared no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding authors.




