The effect of 17β-Estradiol on the gonopodial development and sexual activity of Gambusia holbrooki
Abstract
Many chemicals have recently been demonstrated to possess estrogenic activity and may potentially interfere with normal sexual development. In the present study, we quantified the effects of waterborne exposure to 17β-estradiol on the gonopodial development and sexual activity of male eastern mosquitofish (Gambusia holbrooki). Juvenile males were exposed during the period of sexual maturation to nominal concentrations of 20, 100, and 500 ng/L of 17β-estradiol and a solvent control (0.00003% w/v ethanol) for 84 d under continuous-flow conditions. Following exposure, significant differences were found among the test treatments with respect to gonopodial length and degree of gonopodial elongation of the fish. Sexual activity, measured as the number of approaches and copulatory attempts made by the exposed males to nonexposed females, also significantly decreased with increasing concentrations of 17β-estradiol. Because the degree of gonopodial maturation and frequency of sexual activity are important characteristics for the reproductive success of male mosquitofish, it is suggested that these traits provide sensitive and ecologically relevant endpoints for assessing estrogenic activity under both laboratoryand field-exposure conditions.
INTRODUCTION
Currently, widespread concern exists regarding the presence of pollutants in the environment that can interfere with normal endocrine function in animals, including humans. Particular attention has focused on chemicals that are capable of mimicking or modulating the effects of gonadal hormones, thereby potentially interfering with the reproductive morphology and behavior of an organism [1]. A number of reproductive abnormalities have been observed in wild populations of fish and reptiles that have been attributed to exposure to endocrine-modulating substances. Reported abnormalities have included reduced penis size and testicular abnormalities in alligators from Lake Apopka, USA [2]; delayed gonadal maturation in lake whitefish (Coregonus clupeaformis) near pulp mills in Jackfish Bay, Canada [3]; and a high incidence of intersex gonads and testicular deformities in roach (Rutilus rutilus) and flounder (Platichthys flesus) collected near sewage treatment plants in the United Kingdom [4-6].
Poeciliid fish, particularly mosquitofish (Gambusia sp.), may be valuable indicators of exposure to endocrine-modulating substances, because they exhibit hormone-dependent sexual dimorphism. Male mosquitofish are smaller than females and possess a modified anal fin, the gonopodium, which is used to transfer sperm during copulation (Fig. 1). Development of the gonopodium is under androgenic control and normally occurs in males as the testes begin to produce androgens at the time of sexual maturation [7, 8]. However, gonopodial development can also be induced in female mosquitofish on exposure to androgenic hormones, such as ethynyltestosterone [9-11] and 11-ketotestosterone [7]. Consequently, inducement of gonopodial development in female mosquitofish has been suggested as a potential indicator of exposure to androgenic substances [7], and female mosquitofish exhibiting gonopodium-like structures have been reported following both laboratory and in situ exposure to paper mill effluents [12].
In comparison to androgenic hormones, the effect of estrogens and estrogenic xenobiotics on gonopodial development in male mosquitofish has received very little attention. However, given the androgen-dependence of gonopodial development, it has been hypothesized that exposure of male mosquitofish to estrogens and estrogenic xenobiotics could potentially interfere with the normal development of this secondary sexual characteristic. Dreze et al. [13] recently observed partially developed gonopodia in male eastern mosquitofish (G. holbrooki) following laboratory exposure to 4-nonylphenol, the estrogenic activity of which has previously been demonstrated using both in vitro and in vivo techniques [14, 15]. In addition, Batty and Lim [16] reported shorter gonopodia in male eastern mosquitofish inhabiting sewage-contaminated streams in Sydney, Australia, compared to male fish from sites upstream of sewage treatment plants and streams where no sewage treatment plants are present. Effluent from sewage treatment plants has been identified as a significant contributor of estrogens and estrogenic xenobiotics to aquatic systems [e.g., 17–20], and this led Batty and Lim [16] to conclude that the shorter gonopodia they observed were the result of exposure to exogenous estrogens. Because male mosquitofish can only inseminate females when the gonopodium is fully developed [21], impairment of gonopodial development following exposure to exogenous estrogens may potentially affect the reproductive success of this species.
The objective of the present study was to determine the effect of exposure to exogenous estrogens on the gonopodial development and sexual activity of male G. holbrooki. In particular, we were interested in quantifying the effects of 17β-estradiol (E2), because it is the principal estrogen in fish and is commonly found in sewage effluents [19, 20].
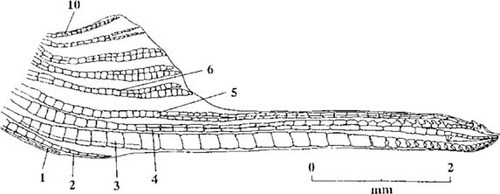
Camera lucida drawing of the modified anal fin of a sexually mature male mosquitofish. The gonopodium forms from the elongation of rays 3 to 5 of the anal fin and is used for sperm transfer during copulation.
MATERIALS AND METHODS
Collection of fish
Juvenile G. holbrooki (Girard, 1859) were collected in January (summer) 2001 using dip nets from a substantially sized population in South Creek, a tributary of the Hawkesbury River (Australia), away from any known sources of estrogens. The fish were returned live to the laboratory where they were anesthetized by immersion in 100 mg/L of benzocaine and sexed under a dissecting microscope. Juvenile males can be distinguished from females by the length of the first segment of the third anal fin ray, as has been demonstrated for G. affinis [22].
Exposure conditions
The dilution water was dechlorinated Sydney (NSW, Australia) mains water maintained at a temperature of 25 ± 2°C (mean ± SE). The conductivity of the test water ranged from 180 to 220 mmhos/cm, whereas pH was 7.5 ± 0.5. The dissolved oxygen concentration was maintained at greater than 80% saturation throughout the duration of exposure.
Exposures were conducted under a continuous-flow regime using three nominal concentrations of E2 (20, 100, and 500 ng/L) and a solvent control (0.00003% ethanol). Concentrated stock solutions of E2 (Sigma, Castle Hill, NSW, Australia) were prepared in absolute ethanol and stored at −20°C for a maximum of 40 d. Working stock solutions, used for dosing the experimental aquaria, were prepared every 3 to 4 d by diluting appropriate aliquots of concentrated stock solution with deionized water. Each working stock solution was delivered to a mixing tank using a peristaltic pump at a flow rate of 0.1 ml/min and mixed with the flow of dilution water (270 ml/min). After mixing, the test water was delivered from the mixing tank into three replicate glass exposure tanks at a flow rate of 50 ml/min. Using a test volume of 10 L, this flow rate corresponded to 7.2 volume additions every 24 h, or a true 99% replacement time of <15 h [23]. Diluent water and toxicant flow rates were checked three times a week to ensure delivery of appropriate volumes to the mixing tanks. The E2 concentrations in the test solutions were measured at six different time intervals during exposure using a commercially available enzyme-linked immunosorbent assay kit (ELISA Systems, Windsor, QLD, Australia) that has previously been validated [20]. Because only one mixing tank was used for each treatment, it was considered that E2 concentrations would not vary significantly between replicate tanks. Therefore, E2 concentrations were measured for only one replicate tank per treatment.
Ten juvenile male G. holbrooki (total lengths ranging from 14.4–22.0 mm, mean length of 18.5 mm) were randomly allocated to each tank and exposed for a total of 12 weeks (84 d). Fish were fed twice daily with commercial food flake and once a week with freshly hatched brine shrimp. A 16:8-h light: dark photoperiod was provided. Fecal material was siphoned from the bottom of the tanks weekly.
Assessment of sexual activity
Following exposure, each fish was assessed for frequency of sexual activity. Tests were conducted in a glass tank containing 20 L of diluent water. Two adult females, collected from the same site as the juveniles, were isolated in a 400-ml glass beaker containing diluent water and suspended in the tank toward one side. Five juvenile males, also isolated in a 400-ml beaker, were placed on the other side of the tank. Previous studies on the sexual activity of male G. holbrooki have not isolated the females, allowing actual contact between the genitals of the two sexes [21, 24]. However, females can prevent copulation by turning right angles to the approaching male [24], and males will usually attempt to copulate with females only if they are stationary or swimming very slowly [25]. We therefore isolated the females in a beaker to minimize their influence on the sexual behavior of the males.
Each exposed male fish was tested separately. Male mosquitofish achieve copulation by gonopodial thrusting. In this process, the male approaches the female from behind, rotates the gonopodium forward, and forcibly introduces the gonopodial tip into the female's genital pore. Each fish was introduced into the center of the tank, and the number of approaches and copulatory attempts made toward the females was counted for 10 min, with observations starting 3 min after introduction into the tank. A female approach was defined as a lunge forward by the male in the direction of the females so that the snout of the male made contact with the glass beaker housing the females. A copulatory attempt was defined as a thrusting of the erect gonopodium forward in the direction of the females. Observations were also made of the amount of time spent in proximity to the females relative to the amount of time spent in proximity to the juvenile males.
Morphological measurements
After behavioral assessment, each fish was euthanized by immersion in 400 mg/L of benzocaine, weighed, and photographed with a Leica DC 100 digital microscope camera (Leica Microsystems, North Ryde, NSW, Australia) mounted on a stereomicroscope. Morphological measurements were made on the images using the Leica Qwin image processing and analysis system (Leica Microsystems).
Measurements of both total length and gonopodium length were made for each fish. Gonopodium length was defined as the length from the anterior base of the anal fin to the gonopodial tip [16]. Measurements were also taken of the length of the fourth and sixth rays of the anal fin. The fourth fin ray elongates as the gonopodium forms, whereas the sixth ray does not (Fig. 1). Therefore, the ratio of the lengths of rays 4 and 6 provides an index of elongation [7]. In addition, the thickness of rays 3 and 4 was also measured. New bone is added to ray 3 while the gonopodium is developing, causing it to become thicker than the other rays (Fig. 1). Therefore, the ratio of the thickness of ray 3 to that of ray 4 provides a measure of the degree of thickening [7]. The thickness measurements were taken at the sixth segment of each ray. Observations were also made of the presence or absence of hooks on the distal portion of rays 4 and 5 (Fig. 2). These hooks act as holdfast devices during copulation, and their presence on the gonopodial tip is an indication that the gonopodium has fully developed.
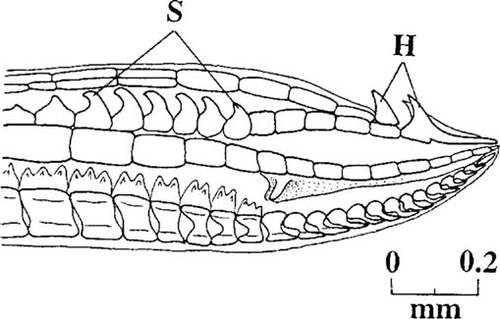
Camera lucida drawing of the distal tip of the gonopodium of a sexually mature male mosquitofish. The hooks (H) and serrae (S) act as holdfast devices during sperm transfer, and their presence indicates that the gonopodium is fully developed.
Statistical analyses
Data failed the Kolmogrov-Smirnov normality test irrespective of the transformation used. Therefore, the distribution- free Kruskal-Wallis test was performed using Statisticat for Windows (Statsoft, Tulsa, OK, USA) to determine significant differences among the test treatments with respect to the behavioral and morphological traits measured (except for the presence/absence of gonopodial hooks). When a significant difference was observed, the Mann-Whitney U test was used to determine which treatments differed significantly from each other. Significant differences in the frequency of fish exhibiting gonopodial hooks were determined using the chi-square analysis [26].
RESULTS
The mean concentrations of E2 measured in the test solutions during the exposure period are presented in Table 1. The E2 concentrations varied between each of the six measurements, with the greatest variation being observed in the 500-ng/L treatment. However, the overall mean of the measured concentrations was close to the nominal value, ranging from 86 to 128% of the desired concentrations. No E2 was detected in the control tanks for the duration of exposure.
Mortalities were observed in all test treatments during the 12-week duration of exposure, with the greatest mortality occurring in the control tanks (Table 2). Most of the dead fish had damaged or missing tail fins, suggesting possible mortality through aggressive behavior. No significant difference was observed in the percentage of mortality among the different test treatments (p = 0.51). For surviving fish, no significant differences were observed in total length (p = 0.30) or wet weight (p = 0.61) among the test treatments (Table 2).
| Measured concentration (ng/L) | ||
|---|---|---|
| Nominal concentration | Range | Mean |
| Solvent control | 0 | 0 ± 0 |
| 20 ng/L | 14.2–41.4 | 25.6 ± 4.3 |
| 100 ng/L | 91.9–116.8 | 101.6 ± 3.6 |
| 500 ng/L | 228.7–691.6 | 429.0 ± 71.4 |
| Treatment | Mortality (%) | Total length (mm) | Wet weight (g) |
|---|---|---|---|
| Solvent control | 23 | 23.6 ± 0.5 | 0.12 ± 0.01 |
| 20 ng/L | 10 | 23.6 ± 0.5 | 0.12 ± 0.01 |
| 100 ng/L | 20 | 22.2 ± 0.7 | 0.11 ± 0.01 |
| 500 ng/L | 17 | 22.7 ± 0.6 | 0.11 ± 0.01 |
Gonopodial development
Significant differences were observed in total gonopodium length and degree of anal fin elongation (ray 4 to ray 6 length ratios) among the test treatments (p < 0.001) (Table 3). The Mann-Whitney U test revealed fish exposed to 100 and 500 ng/L of E2 had significantly reduced gonopodia and lower ray 4 to ray 6 length ratios compared with those of the control fish (p < 0.05). The lowest ray 4 to ray 6 length ratio was 1.00, which was observed for several fish exposed to 500 ng/ L of E2 and indicated that no elongation of the anal fin had occurred.
A significant difference was observed among the test treatments with respect to the degree of thickening of ray 3 (p < 0.05) (Table 3). However, only the 100-ng/L treatment differed significantly from the control group for this trait (p < 0.01).
The percentage of fish with hooks on the gonopodial tip decreased in a dose-dependent manner (Fig. 3). Whereas 52% of the control fish exhibited gonopodial hooks, indicating complete gonopodial development, none of the fish exposed to 500 ng/L of E2 had any hooks on rays 4 or 5 of the anal fin. The chi-square analysis revealed significant differences in the frequency of fish with gonopodial hooks among the control fish and those exposed to 100 and 500 ng/L of E2 (p < 0.001).
Sexual activity
Significant differences were observed among the test treatments in the number of approaches and copulatory attempts made toward the females (p < 0.001). However, it was clear during the behavioral assessments that not all fish had undergone sufficient elongation of the gonopodium to physically enable a copulatory attempt. This was particularly so for fish exposed to the highest E2 concentrations, as evidenced by the ray 4 to ray 6 length ratios (Table 3). Sexually mature male mosquitofish usually have a ray 4 to ray 6 length ratio > 2.0 [7]. Therefore, we removed from the dataset fish with a ray 4 to ray 6 length ratio < 2.0, because we considered these fish to be physically unable to copulate.
When these fish were removed from the dataset, significant differences remained among the test treatments in the number of approaches and copulatory attempts made by the males toward the females (p < 0.001) (Table 4). Fish exposed to 500 ng/L of E2 made significantly fewer approaches and copulatory attempts compared to the controls (p < 0.001). Of all the males exposed to 500 ng/L of E2, only a single copulatory attempt was made toward the females (Table 4). Fish exposed to 100 ng/L of E2 made significantly fewer female approaches than the control fish (p < 0.05), but no significant difference was observed in the number of copulatory attempts made (p = 0.08). Also, no significant difference was observed among the test treatments with regard to the time spent in proximity to the females (p = 0.06).
| Treatment | n | GP length (mm) | Ray 4 to ray 6 length ratio | Ray 3 to ray 4 width ratio |
|---|---|---|---|---|
| Solvent control | 23 | 6.36 ± 0.28 | 2.45 ± 0.12 | 1.88 ± 0.08 |
| 20 ng/L | 27 | 6.06 ± 0.27 | 2.51 ± 0.13 | 1.87 ± 0.07 |
| 100 ng/L | 24 | 4.90 ± 0.30* | 1.91 ± 0.11* | 1.48 ± 0.07** |
| 500 ng/L | 25 | 5.29 ± 0.24* | 1.88 ± 0.14* | 1.66 ± 0.07 |
- * Significant differences from the solvent control values at p < 0.05.
- ** Significant differences from the solvent control values at p < 0.01.
DISCUSSION
Exposure to E2 during the period of sexual maturation had significant effects on the reproductive morphology of male G. holbrooki, with males exposed to E2 having smaller and incompletely developed gonopodia compared to those of control fish. Significant effects on gonopodial development, as assessed by the degree of gonopodial elongation and the presence of hooks on the gonopodial tip, were observed at concentrations of and exceeding 100 ng/L. At a concentration of 500 ng/L, the highest concentration tested, no fish had developed gonopodial hooks, and several fish had not undergone gonopodial elongation (i.e., ray 4 to ray 6 length ratio of 1.00). Dreze et al. [13] reported similar results for male G. holbrooki exposed to the estrogenic chemical 4-nonylphenol under semistatic conditions. They observed partially developed gonopodia in males following exposure to 4-nonylphenol at a concentration of 5 μg/L, whereas at 50 μg/L, no males had initiated gonopodial development.
The exact mechanisms by which exogenous estrogens affect gonopodial development are currently unknown. However, gonopodial growth in mosquitofish is paralleled by the progressive maturation of the testes, with the stage of gonopodial development indicating the degree of gonadal maturation [21]. Therefore, the presence of fish with partially developed gonopodia indicates the testes have not completely matured, which suggests a possible direct effect of E2 on testicular development. Dreze et al. [13] reported male mosquitofish with partially developed gonopodia following exposure to 4-nonylphenol also had symptoms of underdeveloped gonads. Several other studies have also found an inhibition of testicular development in male fish following exposure to exogenous estrogens [e.g., 27,28]. In addition, exposure of fish to E2 via implants or oral administration has been demonstrated to significantly reduce serum levels of testosterone and 11-ketotestosterone [29, 30]. It is probable, therefore, that E2 has a direct effect on testicular development and/or androgen levels, which has subsequent effects on gonopodial development.
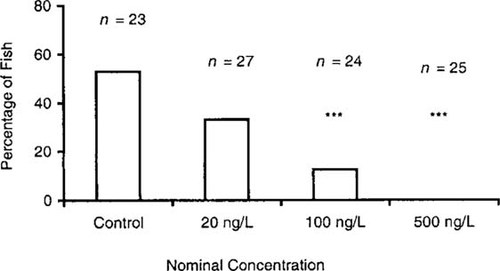
Percentage of fish exhibiting hooks on the distal tip of the gonopodium following exposure to 17β-estradiol. Significant differences from the solvent control values at p < 0.001.
With the exception of males in the 100-ng/L treatment, no significant difference was observed in the degree of thickening of the third anal fin ray of fish exposed to E2. Normally, new bone is added to ray 3 during gonopodial development, causing it to become much thicker than the other rays, but this thickening does not occur in female mosquitofish [7]. Thus, it was hypothesized that the thickness of ray 3 in males could potentially be affected by exposure to exogenous estrogens. If so, this could have significant effects on reproductive success, because it would limit the ability of males to swing the gonopodium forward for copulation. However, thickening of the third anal fin ray takes place relatively early during the process of gonopodial development and can be readily observed in immature male fish. The male fish used in the present study were at a late differentiation stage before exposure to E2 and had already undergone some thickening of the third anal fin ray. It is possible, therefore, that exposure of male mosquitofish to estrogens at an earlier stage of the life cycle could affect the thickening process, but further study would be required to determine this.
A significant reduction was observed in the sexual activity of male G. holbrooki following exposure to E2, with the number of approaches and copulatory attempts made by males toward females decreasing in a dose-dependent manner. Negative effects on normal reproductive behavior following exposure to E2 have been reported for other fish species, although at much higher concentrations than those used in the present study. For example, Bjerselius et al. [31] observed a reduction in the sexual activity of male goldfish (Carassius auratus) following exposure to E2 at a concentration of 1 μg/L, whereas Bayley et al. [32] noted a significant decline in the mating behavior of male guppies (Poecilia reticulata) following exposure to E2 at a concentration of 10 μg/L. Negative effects on sexual behavior have also been reported for other chemicals with estrogenic activity. For example, exposure to 4-tert-octylphenol has been observed to decrease the sexual activity of male mosquitofish (L. Gray, personal communication) and Japanese medaka (Oryzias latipes) [33]. The decreased sexual activity of male Japanese medaka following exposure to 4-tert-octylphenol resulted in decreased fertilization rates [33].
| Treatment | n | Female approaches | Copulatory attempts | % Time in female half of tank |
|---|---|---|---|---|
| Solvent control | 19 | 33.0 ± 5.3 | 9.3 ± 2.3 | 67.5 ± 3.9 |
| 20 ng/L | 20 | 25.7 ± 6.3 | 7.7 ± 2.7 | 65.3 ± 4.4 |
| 100 ng/L | 8 | 17.5 ± 8.0* | 3.3 ± 1.8 | 69.0 ± 6.7 |
| 500 ng/L | 12 | 1.9 ± 0.9*** | 0.1 ± 0.1*** | 50.4 ± 5.0 |
- * Significant differences from the solvent control values at p < 0.05.
- *** Significant differences from the solvent control values at p < 0.001.
Unfortunately, we cannot determine the significance of the decrease in sexual activity of male G. holbrooki in terms of reproductive success from the present study, because we prevented contact between the genitals of the two sexes. However, previous studies have shown that males usually make copulatory attempts at a very high rate, with only a small proportion of attempts being successful in attaining sperm transfer [21]. It is probable, therefore, that any decrease in the sexual activity of male G. holbrooki would result in decreased insemination rates and, ultimately, lower reproductive success. Impairment of gonopodial development following exposure to estrogens is also likely to affect reproductive success. One of the most obvious effects of E2 on gonopodial development was a decrease in the percentage of fish with gonopodial hooks. These hooks act as holdfast devices during sperm transfer, and insemination can only occur after these hooks have formed [21]. Moreover, the partially developed gonopodia observed in males exposed to E2 is indicative of immature testes, which may not yet be capable of producing viable sperm.
The results presented herein support the hypothesis that estrogenic chemicals are affecting the reproductive morphology of mosquitofish populations inhabiting sewage-contaminated waters in Sydney [16]. Although no comprehensive surveys of aquatic systems have been performed in Australia with respect to endocrine disruption, reproductive abnormalities have been observed in other fish species inhabiting sewagecontaminated waters. For example, Smith and Suthers [34] reported abnormalities in the density and size of eggs in gravid female hulafish (Trachinops taeniatus) near sewage outfalls off the coast of Sydney, whereas elevated levels of vitellogenin, an egg-yolk precursor molecule usually only found in the plasma of females, have been detected in male toadfish (Tetractenos glaber) (D. Booth, personal communication). Of particular concern in Australia is that many waterways receiving discharged sewage effluent often have low-dilution capacities because of an erratic rainfall pattern. This is particularly so during periods of prolonged drought. Therefore, further research on the potential impacts of sewage effluent, and of endocrine disrupters in general, on Australia's aquatic systems is urgently needed.
Mosquitofish have been introduced from Central and South America into many regions of the world for mosquito control. In addition, mosquitofish have a ubiquitous distribution, and because of their tolerance of low oxygen concentrations, they can be found in severely degraded habitats as well as in pristine areas [16]. We have demonstrated that the normal gonopodial development and sexual activity of male mosquitofish are sensitive to disruption by exposure to exogenous estrogens. Thus, the gonopodial development and sexual activity of male mosquitofish are valuable endpoints for assessing estrogenic exposure. In particular, given the cosmopolitan and ubiquitous distribution of mosquitofish and the relative ease with which gonopodial development and sexual activity can be measured, these endpoints may provide a cost-effective screening tool for assessing estrogenic exposure under field conditions.
Acknowledgements
This research was funded by a University of Technology, Sydney, internal research grant (1213r) awarded to R.P. Lim. The animal use, care, and procedures described in this study were conducted under the guidelines of the Royal North Shore Hospital/ University of Technology, Sydney Animal Care and Ethics Committee (RNSH-UTS Protocol 0002–011A). All fish were collected under a permit issued by New South Wales Fisheries. The authors thank Lorraine Gray (Napier University, Edinburgh, UK) for providing valuable comments on earlier drafts of this manuscript.




