Among- and within-population variability in tolerance to cadmium stress in natural populations of Daphnia magna: Implications for ecological risk assessment
Abstract
Previous attempts to test the hypothesis that laboratory selection of isogenetic populations can produce test organisms with a significantly increased mean tolerance to toxic substances have failed. One possible explanation for such failure is that the tolerance of laboratory populations is largely constrained by their origins (were the source populations composed of tolerant genotypes?). To address this question, among- and within-population variability in stress tolerance was assessed by calculating the variance in individual fitness and longevity across a cadmium gradient (0—10 μg/L). The study employed Daphnia magna clones from four geographically separate European populations. Results revealed significant differences in tolerance to lethal levels of toxic stress among populations. The distribution of tolerances within two of the studied populations showed high amounts of genetic variation in tolerance. Genetic relationships between tolerance traits and life history performance under nonstressful environments differed among the studied populations. One population showed significant but low costs associated with tolerance, whereas no costs were associated with tolerance in the other population. These results suggest that laboratory selection will favor individuals with high fitness or reproductive performance under optimal laboratory conditions resulting in laboratory populations with similar or lower tolerance to toxic stress than their original field populations. Given that populations can exhibit high levels of genetic variability in tolerance to toxic stress, minimizing genetic diversity in toxicity tests will increase the uncertainty attendant in extrapolating from the lab to the field.
INTRODUCTION
A major concern in ecotoxicology is that toxicity testing is done without clear understanding of how selecting laboratory populations with reduced genetic diversity can bias test results and how such biases can be corrected so that an adequate level of environmental protection can be achieved [1]. Indeed many standard toxicity tests employ populations of test organisms with little or no genetic diversity [2]. Thus, it is essential to study how tolerance to toxic stress in laboratory populations differs from that of their original field populations.
Laboratory population responses are directly related to the selection regime posed by laboratory culture conditions, which are wholly unrepresentative of natural conditions [3]. Thus, laboratory population responses may deviate from those of the field populations from which they are derived [4]. Baird [1] suggested that because of genetic bottlenecking, laboratory populations could exhibit a significantly increased mean tolerance to toxic substances since laboratory cultures are established by selecting individuals that are tolerant to laboratory conditions and possibly also to other types of stress. While supported by theory [5] and despite the existence of substantial amounts of variation in tolerance among laboratory populations [6, 7], previous attempts to test this hypothesis have been largely inconclusive [8]. A possible explanation for this is that tolerance of laboratory populations to toxic stress is related to the distribution of tolerances within their original source populations. This will include local adaptation [9], the amount of genetic variability present in the original field population, and the genetic relationships between tolerance traits and those phenotypic traits favored under laboratory conditions [10]. In ecotoxicology, populations of test organisms with high reproductive performance under optimal laboratory conditions are usually required in order to increase statistical power [5, 11]. From this, three outcomes are possible: By selecting out individuals who are intolerant to laboratory conditions and who are by inference less tolerant to other forms of stress [5], laboratory populations may also be more tolerant to environmental changes [1, 3, 5]; if fitness costs associated with tolerance to toxic stress exist and the laboratory environment favors genotypes with high fitness or reproductive performance, then laboratory populations sensitive to toxic stress will be selected; and, alternatively, if no fitness costs are associated with resistance to stress, then the tolerance of laboratory populations will be similar to those occurring in the field populations from which they are derived. Hence, differences in tolerance among and within field populations will ultimately determine the relative tolerance of laboratory populations. Thus, in order to quantify the uncertainty attendant in extrapolating from responses measured in laboratory to the field, it will be essential to assess the within- and among-population variability in tolerance to toxic stress and to evaluate the existence of costs associated with tolerance in field populations of existing test organisms.
Among existing test organisms, Daphnia magna Straus offers an ideal model system for studying the distribution of tolerances to toxic stress within and among field populations.
Daphnia magna reproduces asexually by ameiotic parthenogenesis. Thus, single genotypes can be tested across a range of toxic concentrations, and among- and within-population variability in life-history responses to a stress gradient (reaction norms sensu Falconer [12]) can be easily determined. Furthermore, in the wild, D. magna populations consist of two components: a free-living population and a seed bank of dormant eggs known as ephippia [13]. Therefore, it is possible to sample field populations by collecting ephippia from the sediment of water bodies. Ephippia are produced by sexual reproduction and can constitute the complete gene pool of the population at certain times of the year [14]. They can be sampled and hatched in the laboratory in a common environment and can be used to gauge population responses of outbreed field populations.
In this study, life-history responses of clones from four different field populations of D. magna to cadmium stress were used to assess among- and within-population variability in tolerance. Lethal and sublethal (reproduction) toxicity tests were selected to assess tolerance to cadmium in D. magna, since these are among the few ecotoxicological tests that provide the basis for legislative decisions [11]. Cadmium was used as a stressor since previous studies conducted with D. magna have reported substantial levels of genetic variability in tolerance to lethal and nonlethal exposure levels among laboratory populations [8, 15]. Two questions were addressed: (1) Do populations from different origins and hence with a different prior selection history show similar levels of tolerance to cadmium, and (2) do the distribution of tolerances within populations and costs associated with tolerance vary among populations from different origins?
MATERIALS AND METHODS
Study populations and culture conditions
Clonal lineages were established from D. magna populations from four habitats located in northern Germany and southern Spain. German clones originated from two populations located near Plön: a small fishless eutrophic pond from Rixdorfer Pferdertränke [9] and a large eutrophic semipermanent pond from Lebrader Schleswig-Holstein used to rear fish [16]. Spanish clones originated from two small fishless eutrophic ponds: one located in Villafranco del Guadiana (Badajoz), which was exploited for Daphnia biomass production, and the other located in San Fernando (Cadiz, Spain). All the studied populations were located in relatively pristine environments with no known history of pollution, but their habitats differed in climate, predation regime, and husbandry. Therefore, these populations can be considered representative of unpolluted European habitats. All experimental clones originated from ephippia isolated from sediment samples collected at the start of the growing season for each habitat [17]. Ephippia collected at a given habitat were hatched in the laboratory at various temperatures and photoperiod regimes to ensure a nonselective regime [17]. All hatchlings were isolated to initiate clonal lineages, with a success rate of 95%, and each female was followed until it released the second or third brood. From each of these females, two neonates were isolated in separate vessels as replicates of the clonal line. After five generations, a total of 10 clonal lines from Lebrader and Cadiz and 40 clones from Rixdorfer and Badajoz populations were randomly chosen for inclusion in the experiments. It was assumed that each clone was genetically distinct as a result of sexual reproduction. Subsequent allozyme analysis by standard cellulose acetate allozyme electrophoresis [18] at five different polymorphic loci (malate dehydrogenase, glutamate-oxaloacetate transferase, phosphoglucose isomerase, manose phosphate isomerase, phosphoglucomutase) confirmed that 60 ± standard error 5% of the clones studied were electrophoretically different.
Single Daphnia females were maintained in 120 ml of American Society for Testing of Materials (ASTM) hard water [19] in 150-ml screw-top glass with the addition of a standard organic extract [20]. Animals were fed every other day with Chlorella vulgaris Beijerinck (5 × 105 cells/ml, corresponding to 1.8 μg C/ml [20]). Photoperiod was maintained to a 14:10-h light:dark cycle and temperature to 20°C.
Experimental design
Tolerance to sublethal and lethal cadmium stress was determined from life-history responses of D. magna individuals exposed to five cadmium concentrations: 0, 0.5, 1, 2, and 10 μg/L. Previous studies have showed that ecologically relevant life-history responses to cadmium are driven mainly by effects on reproduction and growth responses at low exposure levels (<2 μg/L) and by effects on lethality responses at high exposure levels (10 μg/L) [21]. Therefore, by exposing Daphnia individuals to low and high levels of cadmium, it was possible to determine simultaneously sublethal and lethal responses to toxic stress. Life-history responses to cadmium were assessed using a shortened version of the Daphnia reproduction test [8] in which animals were held individually from birth until first reproduction, at which point the experiment was terminated. During the assays, reproduction (age and size of first brood) and survival of experimental animals were assessed at 8-h intervals. Experiments were started with <8-h neonates, originated from second- or third-brood females previously acclimated to the laboratory environment for at least five generations. Test solutions and algae were changed every other day. To better control aqueous cadmium speciation and hence to minimize differences in toxicity across experiments, D. magna exposures were conducted in artificial hard ASTM water of known composition [19]. In a previous study, we showed that physicochemical parameters and cadmium speciation for ASTM hard water were controlled under our laboratory conditions [15].
Because of the elevated number of population clonal toxicant replicate treatments involved (2,400), the study was repeated over five experiments where clones were randomly assigned to each experiment in time. In the first experiment, 10 clones from each of the four populations studied, hereafter designated as Rixdorfer, Labrader, Badajoz, and Cádiz, were exposed to cadmium simultaneously to assess differences in tolerance among populations. In the remaining four experiments, within-population (genetic) variability in tolerance and costs associated with tolerance were assessed in Rixdorfer and Badajoz populations. From each population, 40 different clones were exposed to cadmium, with 20 clones randomly assigned to each experiment in time. The number of replicates per clone varied with exposure concentration and experiment in order to maximize the number of clones and populations tested in each trial and to improve the accuracy of the responses measured. In the first experiment, two and four replicates per clone were used at low (<2 μg/L) and high (10 μg/L) cadmium exposure concentrations, respectively, whereas in the remaining experiments four and eight replicates were used at low and high exposure concentrations, respectively. Replicates were randomized, and their location was changed every other day after feeding.
Water chemistry
Stock solutions of cadmium (expressed as total ion concentrations) were prepared by adding analytical reagent-grade (BDH Anar grade, BDH, Poole, UK) salt (3CdSO4.8H2O) to deionized water (Milli-Q, Bedford, MA, USA; 18-MΩ/cm resistibility). Nominal test concentrations ranging from 0.5 to 10 g/L were subsequently prepared by adding aliquots of each metal stock solution to the synthetic ASTM hard water.
Duplicate (100 ml) water samples at 0 and 48 h were collected at the beginning and end of the tests to measure cadmium, pH, and oxygen levels. Effective ionic concentrations of cadmium were measured using an ATI Unicam Model 939 graphite furnace atomic absorption spectrophotometer (Unicam, Cambridge, UK) following the procedure described by Barata et al. [15]. Dissolved oxygen concentration was measured using a polarographic oxygen electrode coupled to a YSI model 57 dissolved-oxygen meter (Yellow Springs Instruments, Yellow Springs, OH, USA). The pH was measured using an epoxy-body combination electrode, coupled to a Chemix® pH meter (Chemix, Pittsfield, MA, USA) and calibrated with standard pH buffer solutions (BDH).
Data analysis
For D. magna, the criterion normally used to assess sublethal and lethal tolerance is a significant drop in mean fecundity and survival, relative to unstressed controls, respectively [11]. Here, among- and within-population variability in tolerance to sublethal and lethal stress levels was determined from the variance in performance of individual fitness and lethal responses of populations and clones across cadmium concentrations (0–10 μg/L). This is a standard method of calculating genotype or population sensitivity (hereafter referred to as tolerance) to stress [12] and has been previously employed in environmental risk assessment [22]. For each individual, fitness (here referred as individual fitness) was estimated as λ = er, and r was determined as loge (clutch size)/(time to first reproduction). It should be noted that this measure of λ only accounts for the first clutch. However, measures of λ calculated using more clutches are normally highly correlated with first-clutch values [23]. We use individual fitness, characterized as λ, instead of reproductive output for two reasons: λ can be used to measure the fitness of a genotype in clonal organisms [24], and tolerance to toxic stress and costs associated with tolerance are better assessed from fitness-related traits [10].
- (1).
Among-population variability in sublethal and lethal tolerance to cadmium was assessed from analysis of variance (ANOVA) performed on individual fitness and neonatal longevity responses considering population and cadmium as random and fixed factors, respectively, and only life-history data obtained in the first experiment. Within-population interclonal differences were not the main focus of interest in this analysis. Therefore, replicate values of the clones were averaged and entered as the lowest level of replication in the analysis, yielding a total of four populations-four cadmium concentrations-10 clonal means per population observations for individual fitness and four populations-10 clonal means per population observations in the case of longevity.
- (2).
The distribution of tolerances within populations and costs associated with tolerance in the Rixdorfer and Badajoz populations were analyzed from ANOVA performed on individual fitness and neonatal longevity responses, considering clones and cadmium as random and fixed factors, respectively, and life-history data obtained in four experiments (2–5). As significant variation was observed between experiments within populations, separate tests for each experiment were performed. Genetic variability in sublethal and lethal tolerance to cadmium was assessed by determining values of heritability. Heritabilities for sublethal tolerance were determined as the proportion of variance explained by the genotype by environmental component of variance (Vge) and the total variance (Vp = Vg + Ve + Vge + Vr) of individual fitness responses across cadmium concentrations, where Vg, Ve, and Vr are the genetic, environmental, and residual components of variance, respectively. Components of variance were obtained from two-way ANOVA with clone and cadmium as random and fixed factors, respectively. Heritabilities for lethal tolerances of neonates exposed at 10 μg/L of cadmium were estimated from one-way ANOVA as the proportion of variance explained by the genetic (Vg, among clonal) and the total phenotype (Vp = Vg + Vr) variance. Variance components were estimated using the maximum-likelihood method (SPSS, SYSTAT, Chicago, IL, USA). Since clonal differences in fitness responses across cadmium concentrations existed, the distribution of sublethal tolerances within each population was further characterized determining the tolerances of the different genotypes and changes in heritability values of fitness responses within cadmium treatments. The environmental tolerance of a genotype was defined as the slope of the linear reaction norm of its fitness values plotted against the environmental values determined as the grand mean of individual fitness responses of all clones in a given cadmium concentration. This approach replaces a physical measurement, such as chemical concentration, by a difference in the trait measured. A genotype with a slope > 1 can be considered less tolerant than average, whereas a genotype with a slope < 1 can be considered more tolerant than average [12]. Heritability of individual fitness responses within cadmium concentrations was estimated from one-way ANOVA following the same procedure described for lethality responses. Costs associated with tolerance and genetic relationships between tolerance to lethal and sublethal stress levels were determined from product-moment correlations among clonal means of three traits: individual fitness under nonstress conditions (λ at 0 μg/L cadmium), the slopes of the reaction norms of λ across cadmium concentrations (0–2 μg/L), and mean longevity of neonates at 10 μg/L. Confidence intervals were obtained for correlations using the z transformation [25].
RESULTS
Chemical analysis
Actual concentrations of 0- and 48-h cadmium test solutions were within 10% of nominal concentrations (Table 1). Mean measured background concentrations of cadmium (0.025 μg/L) were very low, indicating that contamination was minimal. Oxygen levels in old test solutions were within 91% of saturation levels. Mean pH values were 8.3, and differences between initial and final values never exceeded 0.2 pH units.
Among-population variation in tolerance
Fitness and lethal responses of the studied four populations across cadmium concentrations are depicted in Figure 1A and B. Despite significant effects of cadmium on fitness responses, nonsignificant interaction terms denoted similar levels of sublethal tolerance among the studied populations (Interaction. Table 2). Alternatively, significant population effects on neonate longevity responses at 10 μg/L of cadmium denoted differences in lethal tolerance among the studied populations (Table 2).
| Measured cadmium | |||
|---|---|---|---|
| Nominal cadmium | n | t = 0 h mean (SD) | t = 48 h mean (SD) |
| 0 | 10 | 0.02 (0.01) | 0.02 (0.01) |
| 0.5 | 10 | 0.55 (0.08) | 0.51 (0.05) |
| 1 | 10 | 1.25 (0.06) | 1.12 (0.08) |
| 2 | 10 | 2.27 (0.12) | 2.12 (0.12) |
| 10 | 10 | 11.13 (1.10) | 10.46 (1.31) |
Within-population variation in tolerance
Interclonal variation in sublethal and lethal tolerances to cadmium within Rixdorfer and Badajoz populations in each of the four experiments performed are depicted in Figure 2A to D. In all four experiments, significant interaction terms denoted differences in sublethal tolerance among clones. Moreover, heritability levels of tolerance within Rixdorfer and Badajoz populations were similar (Vge, Table 3). The existence of interclonal differences in sublethal tolerance to cadmium was also assessed by comparing the slopes of individual clone reaction norms for fitness responses (Fig. 2A and B). Regression analysis indicated that clonal reaction norms of fitness responses were linear: 75 out of 80 regression equations obtained were significant (p < 0.05) and had a coefficient of determination (r2) greater than 0.6. Analysis of covariance indicated that slopes of the studied clonal reaction norms differed significantly (p < 0.001) in the four experiments (F40,265 = 4.94; F40,271 =3.92, F40,253 =5.2; F40,238 =5.4 for trials 2–5, respectively). A further characterization of the fitness responses of the D. magna clones within each of the studied cadmium concentrations denoted population differences in the amount and expression of heritable variance across cadmium concentrations. In particular, heritabilities (H) for fitness responses in the Rixdorfer population were low at low cadmium exposure levels and increased with exposure concentrations. On the contrary, heritabilities (H) for fitness responses in the Badajoz population always showed high levels of heritable variation for fitness responses.
| Factors | df | F | Factors | df | F |
|---|---|---|---|---|---|
| Individual fitness (λ) | Longevity | ||||
| Population | 3,76 | 0.91 | Population | 3,28 | 7.59* |
| Cadmium | 3,9 | 28.23* | |||
| Interaction | 9,76 | 0.90 | |||
- * p < 0.05.
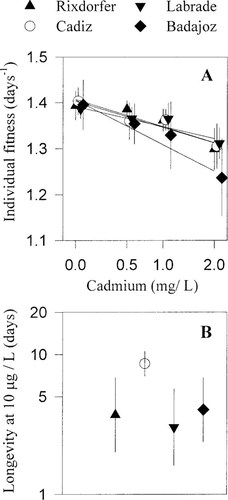
Individual fitness, λ (A) and longevity (B), responses of the four Daphnia magna populations across cadmium concentrations. Error bars are standard deviations. Cadmium concentrations and longevity values are depicted in loge scale.
In all four experiments, significant heritability levels for longevity responses of neonates exposed to 10 μg/L of cadmium (H, Table 3) indicated differences in lethal tolerance among clones. Furthermore, heritability levels for tolerance within Rixdorfer and Badajoz populations were similar (Table 3).
Genetic relationships (product-moment correlations) among three traits in D. magna related to fitness under non-stress conditions and tolerance at sublethal and lethal exposure levels of cadmium are depicted in Figure 3. Because genetic correlations among life-history traits were expected to be low and to be particularly sensitive to small sample size [12], within each population, data from both experiments were pooled to achieve a sample size of n = 40. Furthermore, clones with slopes >1 are considered less tolerant than those with slopes <1; thus, the sign of all relationships involving slopes of the reaction norms of λ across cadmium concentration were reversed (which would be the coefficient times -1) to reflect fitness correlations rather than purely numerical relationships [12, 24]. Correlation analysis among the three traits studied showed differences between Rixdorfer and Badajoz populations. Within the Rixdorfer population, no significant relationships were found between tolerance to sublethal and lethal stress levels and fitness performance under nonstress conditions, but a positive relationship between lethal and sublethal tolerance to cadmium existed. In contrast, within the Badajoz population, significant negative relationships between fitness performance under nonstress conditions and tolerance to sublethal and lethal stress levels denoted fitness costs associated with tolerance. However, tolerance to sublethal and lethal stress levels was not correlated.
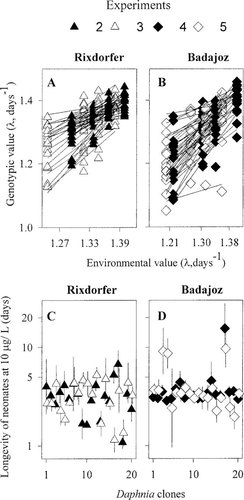
Interclonal variation in sublethal (A, B) and lethal tolerances (C, D) to cadmium within Rixdorfer and Badajoz populations in each of the four experiments performed. Sublethal tolerance is depicted as the slope of each genotype regression of individual fitness responses (λ) on the environmental value. An environmental value is calculated as the mean trait response of all genotypes in that environment. The steeper the slope, the smaller a genotype's relative tolerance to its environment. Lethal tolerances are expressed as longevity of neonates (loge transformed) exposed to 10 μg/L of cadmium. Each symbol represents the mean value for each genotype. Error bars in graphs C and D correspond to standard deviation.
| H at a given cadmium concentration | ||||||
|---|---|---|---|---|---|---|
| Trait | 0 | 0.5 | 1 | 2 | 10 | Vge |
| Experiment 2 | ||||||
| R-λ | 18.8 | 20.9 | 29.7* | 63.1* | 28.4* | |
| R-L | 70.0* | |||||
| Experiment 3 | ||||||
| R-λ | 23.6 | 65.6* | 70.2* | 60.1* | 16.5* | |
| R-L | 65.0* | |||||
| Experiment 4 | ||||||
| B-λ | 57.2* | 63.3* | 75.3* | 68.2* | 20.1* | |
| B-L | 75.8* | |||||
| Experiment 5 | ||||||
| B-λ | 84.0* | 88.3* | 53.2* | 69.9* | 22.2* | |
| B-L | 64.0* | |||||
DISCUSSION
A number of hypotheses have been forwarded to explain observed differences in the degree of genetic variation in response to different chemicals or within single chemicals across toxic stress levels. For example, it has been postulated that in the case of heavy metal stress, because of differences in selection intensity, genetic variability in tolerance should converge with decreasing levels of toxicity and from essential to nonessential metals [7, 15]. Previous attempts to test these hypotheses have been inconclusive. A possible explanation for this is that tests have been conducted with laboratory clones derived from geographically distinct populations [2], and thus tolerance measured in the laboratory clones was related to the distribution of tolerances of their original field populations. Here we have shown that the degree of variation among clones across nonlethal and lethal cadmium exposure levels varies within populations, that lethal responses vary among populations, and that the relationship between lethal and sublethal responses varies between populations. This means that laboratory clones derived from different populations may show variable response patterns, including variable clone-to-clone differences in tolerance between low and high sublethal exposure levels of single toxicants, different degrees of genetic variation in lethal responses across chemicals, and different degrees of concordance between lethal and sublethal responses. As discussed in the following, our results provide experimental evidence to supporting the view that strong potential exists to evolve resistance to toxic stress within natural populations, and as a result, genetic variability in susceptibility to toxic exposure should be included in ecological risk assessment [10, 26].
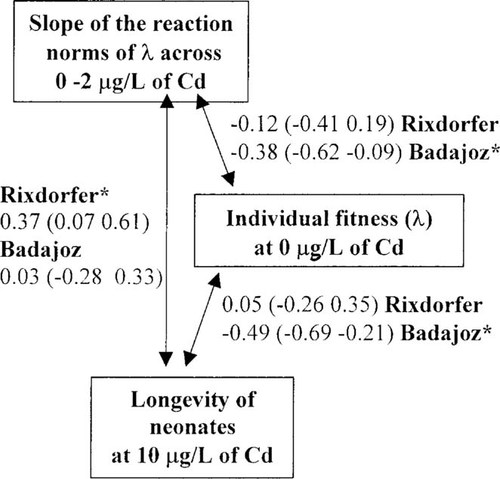
Genetic relationships (product-moment correlations) among three traits in Daphnia magna related to fitness in unstressed conditions (λ at 0 μg/L cadmium) and tolerance at sublethal and lethal exposure levels of cadmium. Individual fitness (λ) at 0 μg/L cadmium was selected as a fitness-related trait in unstressed conditions, the slopes of the reaction norms of λ across cadmium concentrations (0–2 μg/L) were selected as measures of sublethal tolerance to cadmium, and longevity of neonates exposed to 10 μg/L cadmium concentration was selected as a measure of tolerance to lethal exposure levels of cadmium. Asterisks indicate whether correlations are significant (p < 0.05). 95% confidence intervals associated to correlation coefficients are in brackets. Because clones with slopes >1 are considered less tolerant than those with slopes <1, the sign of all relationships involving slopes of the reaction norms of λ across cadmium concentration has been changed (which would be the coefficient times -1).
A major concern in ecotoxicology is that toxicity testing is done without clear understanding of how selecting laboratory populations with reduced genetic diversity can bias test results and how such biases can be corrected so that an adequate level of environmental protection can be achieved [1]. The observed life-history responses among and within field populations of D. magna exposed to cadmium showed only significant differences in tolerance to lethal levels of toxic stress among populations, substantial levels of genetic variation in tolerance within populations, and low or no costs associated with tolerance. These results support our second and third hypotheses that selection of monoclonal populations with high fitness or reproductive performance under optimal laboratory conditions will lead to populations with lower or similar tolerance to toxic stress than their original field populations. In the latter case, differences in tolerance among and within field populations will ultimately determine the relative tolerance of laboratory populations. Hence, tolerance of laboratory populations will be highly variable. Two sets of results support these assumptions. Previous studies conducted in the past 10 years have always reported substantial levels of genetic variability in tolerance among geographically distinct laboratory clones of D. magna [6, 7]. Furthermore, studies conducted with tests organisms such as D. magna or Chironumus riparius have reported that tolerance of laboratory-derived populations to toxic chemicals was similar or significantly lower than that of field populations [4, 8].
According to Hoffmann and Parsons [10, 27], differences in tolerance to toxic stress among populations within a species are directly related to selective pressures experienced in their local habitats leading to local adaptation, whereas genetic variability in tolerance within populations is inversely related with the level of stress. This means that populations derived from distinct habitats with no known history of pollution should show similar levels of tolerance to toxicants and substantial amounts of genetic variance in tolerance. Our results have demonstrated that tolerance to sublethal toxic levels of cadmium seems to vary significantly among D. magna clones only within populations, whereas tolerance to lethal toxic levels varies both within and among populations. In particular, all populations studied, with the exception of the Cadiz population, showed similar lethal responses to cadmium. According to a previous study [28], specific responses, such as survival responses to high toxic stress levels, are likely to be regulated by few genes of large effect, and thus differences in lethal tolerance among populations from different habitats are likely to occur because of the spread of resistant genotypes during sporadic episodes of selection during periods of high stress exposure. In contrast, sublethal responses, such as individual fitness impairment at moderate toxic stress levels, are likely to be regulated by many genes of small effect; thus, negative pleiotropic effects among these genes (trade-offs between traits and environments) will prevent selection of genotypes and hence of populations tolerant to moderate exposure levels of toxic stress [10, 27, 29]. This means that the observed higher levels of tolerance in the population from Cadiz may be related to sporadic episodes of metal contamination. These results support the concerns of Baird and Barata [6] and Forbes [26] that application factors for prediction of no-effect concentrations from test data should be maintained or even increased to account for the observed differences in tolerance within and among populations.
Two potential effects of toxicant exposure on genetic variability of natural populations exist. First, exposure to toxicants may cause reductions in the amount of genetic variation leading to a loss of future adaptability, hence increasing the probability of population extinctions [30]. Second, that exposure to toxicants may select for resistant genotypes, and as a result of costs associated with tolerance, such genotypes will have reduced fitness relative to nonresistant genotypes in the absence of exposure [31]. Clearly, either of these effects will reduce the ability of organisms within populations or of populations within species to adapt to chemical pollutants and to respond to subsequent environmental perturbations [32]. Here, studying the distribution of tolerances of genotypes within two geographically distinct populations of D. magna, we have showed that heritable variation of fitness-related traits increased or remained unchanged across toxic stress levels. These results support the view of Hoffmann and Hercus [33] that moderately stressful conditions may influence evolutionary rates by generating and maintaining genetic variability. The study of genetic relationships among traits showed that genetic correlations between tolerance traits and fitness responses under optimal laboratory conditions varied among the two populations studied, with negative and nonsignificant correlation values, respectively. Nevertheless, significant negative correlations between traits were < - 1 and hence, according to Via and Lande [34], did not guarantee costs associated with tolerance [31]. This means that resistant genotypes from the two studied populations showed low or no costs associated with tolerance to cadmium. Our results agree with the hypothesis that the mechanisms of resistance to heavy metals varied among species and among populations within species [35, 36] and that resistance to toxic chemicals does not necessary imply fitness costs [31]. Indeed costs associated with tolerance to cadmium have been reported only in those cases where resistance involved major genes with strong pleiotropic effects on fitness traits [37]. More specifically, fitness costs associated with tolerance to cadmium were related with changes in mineral metabolism that caused an increased need for essential metals such as zinc [38].
Overall, our results and those reported by Hairston et al. [39] indicated that a strong potential exists to evolve resistance to toxic chemicals within natural populations of Daphnia. As a result, differences in susceptibility to toxic chemicals within and among Daphnia populations are likely to be high. Although our results were restricted to one species and a single chemical, the occurrence of genetic adaptation as well as genetic variability to toxic stress have been reported for many other populations species and chemicals [29, 35, 36], thus suggesting that genetic differences in susceptibility to toxicant exposure should be more explicitly considered in the ecological risk assessment process [6].
Acknowledgements
Carlos Barata was supported by a Portuguese Fundação para a Ciência e Tecnologia grant PRAXIS XXI/1629/98 while carrying out this research.




