Plant-facilitated mobilization and translocation of weathered 2,2-bis(p-chlorophenyl)-1,1-dichloroethylene(p,p′-DDE) from an agricultural soil
Abstract
Field experiments were conducted to determine the uptake and translocation of highly weathered 2,2-bis(p-chlorophenyl)1,1-dichloroethylene (p,p′-DDE) from an agricultural soil. In soil containing known amounts of p,p′-DDE, experimental plots containing zucchini, pumpkin, or spinach were constructed. At destructive harvest, three soil fractions were collected that differed in the degree of influence exerted by the plant roots. The bulk soil was vegetation-free, the near-root zone was within the area encompassed by the roots, and the rhizosphere remained physically attached to the roots at harvest. For each crop, statistically significant decreases were found in the concentration of p,p′-DDE in either the near-root zone or the rhizosphere relative to the bulk soil, suggesting plant-facilitated mobilization and/or degradation of the residue. Plant tissues were analyzed to determine the extent of contaminant removal from the soil and the magnitude of translocation through the shoot system. The concentration of p,p′-DDE in the roots of both zucchini and pumpkin was more than an order of magnitude larger than the bulk soil concentration, followed by significant translocation through the plant tissues to the fruit. The data indicate that certain plants may effectively accumulate residues of persistent organic pollutants in their tissues, suggesting phytoremediation as a possible treatment strategy.
INTRODUCTION
Considerable scientific evidence has accumulated during the last decade that suggests the bioavailability of organic compounds in natural solids declines with residence time [1, 2]. This time-dependent decrease in availability has been observed with laboratory-contaminated soils as well as with field-weathered pollutants. The list of compounds that have been shown to undergo sequestration in soil includes pesticides such as 1,2-dibromoethane [3], simazine [4], DDT [5, 6], dieldrin [5], and heptachlor [5]; polychlorinated biphenyls [7]; and polycyclic aromatic hydrocarbons such as naphthalene, phenanthrene, anthracene, pyrene, and benzo[α]pyrene [8-10]. The assays employed to demonstrate this time-dependent loss in availability include mild solvent extraction [11], mineralization or biodegradation by bacteria [9], uptake by earthworms [11, 12], genotoxicity to bacteria [10], and lethality to insects [6]. This impressive set of data has been used to suggest that current analytical methods employed in the estimation of contaminant exposure are inappropriate, because they rely on total pollutant concentration and not on the bioavailable fraction. Consequently, arguments have been offered that the estimation of risk from contaminated soils and the remediation endpoints that result from such analyses have been overstated [1, 2, 12].
Persistent organic pollutants are a class of compounds of particular concern in the environment due to their recalcitrance in natural solids, global transport and distribution, and toxicity [13]. Included in this group of pollutants are polychlorinated biphenyls, DDT, chlordane, dieldrin, and dioxin. Although certain constituents of this group have been linked to carcinogenicity [14] and endocrine disruption [15], the risk to humans from highly weathered residues of these persistent compounds is unknown. Previous work from this institution has indicated that significant amounts of chlordane, weathered in soil for nearly 40 years, could be readily translocated into certain plant species and, on several instances, into the edible tissues of vegetable crops [16]. In fact, in several cases, the quantity of contaminant removed from the soil has led to speculation regarding plant-based clean-up or phytoremediation of such recalcitrant compounds from natural solids. Similarly, White [17] showed that 2,2-bis(p-chlorophenyl)1,1-dichloroethylene (p,p′-DDE) residues could be mobilized from soil under nonrigorous growing conditions by alfalfa, rye, and bean. Hulster et al. [18] as well as Campanella and Paul [19] have described similar observations concerning the availability of dioxins and furans to certain plant species. Given the highly weathered and sequestered nature of the pollutants (i.e., decades old), these findings contrast with the evidence indicating time-dependent reductions in bioavailability and the concomitant arguments for revised exposure values from allegedly overstated risk assessments.
Until it was banned in 1970 by the U.S. Environmental Protection Agency, DDT was a widely used organochlorine pesticide, and it is currently designated as a persistent organic pollutant [13]. It can be converted microbiologically [20, 21] and abiotically [22] to one of two metabolites: DDE, and dichlorodiphenyldichloroethane (DDD). However, due to the bioaccumulative potential and long half-lives of the two metabolites, both DDE and DDD themselves are classified as persistent organic pollutants and are of environmental concern [13]. Previous work from this institution has shown that certain plants of the genus Cucurbita accumulate significant amounts of chlordane from soil [16]. The present study seeks to address the ability of two cucurbits, zucchini and pumpkin, as well as of spinach to accumulate highly weathered residues of p,p′-DDE from an agricultural soil. This type of information is necessary to accurately describe the fate, exposure, and ultimate risk posed by recalcitrant organic compounds in the environment.
MATERIALS AND METHODS
Experimental plots
Zucchini (Cucurbita pepo Raven), pumpkin (Cucurbita pepo Baby bear), and spinach (Spinacia oleracea Hector) were purchased from Johnny's Selected Seeds (Albion, ME, USA). Experimental plots were established at our Lockwood Farm (Hamden, CT, USA) in areas previously shown [17] to contain measurable levels of p,p′-DDE (50–500 ng/g). No DDT or DDD was detectable in the soil. Precise application records were not available, but this area of the farm received regular applications of DDT for insect control from the late 1940s to the early 1970s. The soil is designated as Cheshire fine sandy loam (56% sand, 36% silt, 8% clay; pH 6.7) and has an organic carbon content of 1.4%. Individual plots were 2 × 2 m. A total of eight plots were constructed: three of zucchini, four of pumpkin, and one of spinach. Zucchini and pumpkin seeds were planted in three separate mounds per plot (six to eight seeds per mound) on May 15, 2000. This resulted in four to five separate zucchini or pumpkin plants per plot. Spinach seeds (∼50 per row) were planted in two rows. The plots were weeded and watered as necessary. On August 5, 2000, the soil and vegetation (i.e., roots, stems, leaves, and fruit) were harvested and composited by tissue or soil compartment and plot number.
Soil extraction procedure
The extraction procedure utilized was that described by Mattina et al. [23]. Bulk soil samples were collected from the top 15 cm of individual plots (two to three feet from vegetation) at harvest. The near-root zone soil was operationally defined as the soil that fell off the plant at destructive harvest as well as the surrounding soil within the area encompassed by the plant roots. The soil from both these treatments was stored in 40-ml, amber glass vials and sealed with screw caps containing Teflon®-lined septa. The rhizosphere soil was operationally defined as the soil that did not fall off the roots at harvest. Root samples of all plants were harvested and placed on aluminum foil for approximately 1 to 2 h to allow the attached soil to air-dry. The rhizosphere soil was then removed with a fine-bristle toothbrush. All soil fractions were air-dried to ensure homogeneous moisture content and sieved to 0.5 mm to remove particulates and provide sample uniformity. A representative 3.0-g portion of each soil treatment was placed in an oven at 100°C for 24 h for moisture determination for every two to three soil samples that were extracted. The moisture content of the air-dried soil (all fractions) ranged from 4 to 7% (by weight). Portions (3.0 g) of the remaining soils from each plot were transferred to a Teflon PFA-lined digestion vessel from the CEM MES-1000 microwave solvent extraction system (CEM, Mathews, NC, USA). Fifty milliliters of 2:3 (v/v) hexane:acetone (Ultra-Resi-Analyzed; J.T. Baker, Phillipsburg, NJ, USA) was added to the vessel, followed by 500 ng of transnonachlor as an internal standard. The vessels were sealed and placed in the CEM MES-1000 oven and then extracted using 100% power, 7-min ramp to 120°C, and 20-min hold time. The liquid phase was decanted into Kuderna-Danish flasks fitted with 10-ml concentrator tubes containing a boiling chip. The remaining soil in the extraction vessels was rinsed twice with 15-ml portions of 2:3 (v/v) hexane:acetone, and the solvent was combined with the original extractant. A Snyder column was fitted to the flask, and the solvent was reduced to less than 1 ml in a 95°C water bath. Three milliliters of 2,2,4-trimethylpentane (Ultra-Resi-Analyzed; J.T. Baker) were added through the Snyder column, and the volume was again reduced to 1 ml. The flask was then removed and rinsed with an additional 3 ml of trimethylpentane. On cooling, the final volume of solvent was adjusted to 10 ml. Before analysis, the soil extract was passed through a glass microfiber filter (0.2 μm; Laboratory Science, Sparks, NV, USA) to ensure complete particulate removal. The adequacy of this procedure has been previously demonstrated [17, 23] utilizing an external standard calibration.
Vegetative extraction procedure
The method described by Pylypiw [24] was used to extract p,p′-DDE residues from various portions of the vegetation. Samples of roots, stems, leaves, and fruit were collected from the field. In preparation for analysis, the vegetation was thoroughly rinsed with tap water to remove attached soil particles, because residual soil particles on the surface of the root samples would confound results obtained from vegetative extracts of this plant tissue. Consequently, after rinsing, the root samples were added to 300 ml of distilled water and sonicated for 15 min (FS30; Fisher Scientific, Springfield, NJ, USA). All vegetative samples were finely chopped and either extracted immediately or stored in 500-ml, amber glass bottles with Teflon-lined caps in a freezer. Fifty-gram portions of the vegetation were then weighed into a one-quart blender container with 50 ml of 2-propanol (Ultra-Resi-Analyzed; J.T. Baker) and 3,000 ng of transnonachlor as an internal standard. The sample was blended at high speed for 30 s. A 100-ml volume of petroleum ether (Ultra-Resi-Analyzed; J.T. Baker) was added to each of the containers, and the sample was blended at 40% of full speed for 4 min. After blending, the sample was allowed to settle for 30 s. The extract was then decanted into a funnel packed with glass wool, and the extract was collected in a 500-ml glass separatory funnel with a Teflon stopcock. After complete draining of the solids (∼20 min), 200 ml of distilled water and 10 ml of saturated sodium sulfate solution were added separately to each funnel. The funnel was then capped, shaken gently for 1 min, and allowed to sit for 15 to 20 min for phase separation. Next, the water was drawn off, and the ether was rinsed twice with distilled water. The final extract (∼60–70 ml) was then amended with 15 g of anhydrous sodium sulfate and allowed to sit for 2 to 3 h before analysis.
Chemical analysis
The amount of p,p′-DDE in the trimethylpentane soil extracts and the petroleum ether vegetative extracts was determined on a Hewlett-Packard (Avondale, PA, USA) 5890 gas chromatograph (GC) with a 63Ni electron-capture detector (ECD). The column (30 m × 0.53 mm × 0.5 μm) contained a SPB-1 film (Supelco, Inc., Bellefonte, PA, USA), and the GC program was as follows: 175°C initial temperature, ramped at 1°C/min to 205°C, then ramped at 15°C/min to 250°C, with a hold time of 5 min. The total run time was 38 min. A 2-μl splitless injection was used, and the injection port was maintained at 250°C. The carrier gas was He, and the make-up gas was 5% CH4 in Ar at 20 ml/min. The ECD was maintained at 325°C.
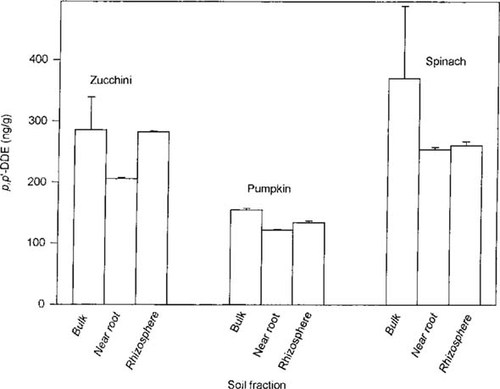
Concentration of 2,2-bis(p-chlorophenyl),1,1-dichloroethylene (DDE; ng/g dry wt) in the bulk, near-root, and rhizosphere soils of selected plots containing zucchini, pumpkin, and spinach. Data from zucchini and pumpkin are from one of three and from one of four plots, respectively. Error bars indicate standard deviation.
A stock standard of p,p′-DDE was purchased from Chem Service (West Chester, PA, USA). The stock was transferred to either petroleum ether (for vegetative extracts) or trimethylpentane (for soil extracts) and diluted to prepare a series of calibration standards of p,p′-DDE at 10, 25, 50, 100, 150, 250, and 500 ng/ml for each solvent. Each calibration level contained 100 ng/ml of transnonachlor. The retention times of transnonachlor and p,p′-DDE were 20.95 and 23.95 min, respectively.
Statistical analysis
All reported concentration values of p,p′-DDE are the average of duplicate injections expressed on a dry-weight basis of either soil or vegetation. The variation between duplicate injections was less than 3%. At harvest, the separate tissue compartments (i.e., root, stem, leaves, and fruit) of the four to six zucchini or pumpkin plants per plot were composited, and subsamples were subsequently extracted. The statistical significance of differences in p,p′-DDE concentrations in soil or vegetation was assessed utilizing an unpaired Student's t test, and the corresponding p values are reported in the text. Recovery of the internal standard was 102% (±9.5%).
RESULTS AND DISCUSSION
Soil compartments
Bulk, near-root, and rhizosphere soil fractions were collected from separate plots of zucchini, pumpkin, and spinach at destructive harvest. The data from randomly selected zucchini and pumpkin plots and from the spinach plot are shown in Figure 1. For each of the crops investigated, the concentration of p,p′-DDE in the near-root zone or rhizosphere (or both) was significantly reduced (p < 0.05) relative to the amount in the bulk soil. The average bulk soil concentrations in the three separate zucchini plots were 397 (±61.3), 286 (±53.2), and 225 (+75.5) ng/g (three replicates each). In the four pumpkin plots, the bulk soil values were 397 (±23.9), 340 (±132), 179 (±43.3), and 155 (±2.61) ng/g (three replicates each). To express all soil data collectively for a given crop was difficult because of the variability between different plots of the same crop. Therefore, the data within each individual plot were normalized to the average bulk soil concentration for that specific plot. Thus, the data from all plots of a given crop could be averaged and expressed collectively as a percentage of the bulk soil concentrations (i.e., as a fraction of 1.0). On this basis, the near-root zone concentrations of p,p′-DDE for all zucchini, pumpkin, and spinach plots were 0.65, 0.82, and 0.69, respectively (all significantly different from a bulk soil value of 1.0 at p < 0.05). The values in the rhizosphere of all zucchini and pumpkin plots were slightly reduced (0.88 and 0.96, respectively) as compared to that in the bulk soil, but these differences were not statistically significant. The value in the rhizosphere of spinach was 0.72, but due to large variability in the bulk soil concentration, this reduction is only significant at p < 0.10.
It is hypothesized that the observed trends in p,p′-DDE distribution within the different soil compartments are the result of plant-facilitated mobilization of the residue. This plant-mediated increase in availability is viewed as a dynamic process, and as the contaminant is released from its sequestered state in the bulk soil, the residue may temporarily resorb as it is drawn through the near-root zone and rhizosphere toward the root. Thus, differential trends in the concentrations of p,p′-DDE may be evident with different crops, or even with separate individuals of the same species. Regardless, the visualization of this plant-mediated mobilization through the various operationally defined soil compartments is evident. White [17] observed similar gradients of p,p′-DDE in the near-root zone and rhizosphere of alfalfa, rye, and bean, with the precise distribution of the contaminant being related to the total amount absorbed by the roots. Similarly, White et al. (unpublished data) in greenhouse experiments described the plant-facilitated mobilization of highly weathered chlordane residues within soil in which zucchini plants were grown. Those authors hypothesized that the release of exudates from the zucchini roots served to solubilize the sequestered contaminant, thereby promoting its mobility into the aqueous phase and its ultimate availability to the plant. Schnoor et al. [25] have estimated that nearly 20% of the products of photosynthesis are transported directly to the roots, where they are summarily released to the rhizosphere. These exudates are a complex mixture of simple organic acids, carbohydrates, proteins, and lipids that, collectively, have enzymatic activity and stimulate the growth of enhanced microbial communities within the rhizosphere. Both Hulster et al. [18] and Campanella and Paul [19] have speculated regarding the presence of several molecules in the root exudates and leaf tissues of zucchini and melon that appear to increase the aqueous solubility of weathered dioxin residues in soil. Huang et al. [26] have shown that certain aromatic acids that are common to plant root exudates promote desorption and plant uptake of weathered uranium from soil. The precise mechanism by which the highly sequestered residues of organic contaminants are mobilized remains unknown, however, and is the topic of ongoing research.
A plot-by-plot analysis of the soil data from the zucchini and pumpkin plots reveals an inverse relationship between the bulk soil concentration and the extent of decreases in contaminant level in the other soil fractions (Table 1). One can observe that almost without exception, the lower the initial bulk soil value, the greater the percentage reduction in the contaminant concentration in both the near-root and rhizosphere soil compartments. The regression coefficients of the lines through normalized concentrations of near-root and rhizosphere soils versus bulk soil concentration are 0.62 and 0.82, respectively, for plots containing zucchini and 0.73 and 0.72, respectively, for plots containing pumpkin. These findings suggest that a concentration-dependent, mass-transfer process may ultimately control the degree of contaminant solubilization and the ultimate extent of plant uptake. For example, a constant amount of exudates being released from plant roots may yield more extensive contaminant solubilization in soils with lower pollutant levels as compared to that in soils with higher concentrations. Contrary to expectations concerning the bioavailability of highly weathered organic pollutants, these findings suggest that at the soil concentrations examined in this study, contaminant desorption (i.e., bioavailability) may not be the rate-limiting step in a phytoremediation effort. Therefore, a greater concentration of root exudates (perhaps achievable by higher plant density) may yield significantly greater contaminant removal from the soil.
| Normalized concentration (%) | ||||
|---|---|---|---|---|
| Plant | Plot | Bulk soil concentration (ng/g) | Near-rootab | Rhizospherea |
| Zucchini | 1 | 397b (61.3)c | 64.2–81.3 | 115–120 |
| 2 | 286 (53.2) | 71.5–72.0 | 99.0–99.5 | |
| 3 | 225 (75.5) | 44.3–54.6 | 43.9–55.5 | |
| Pumpkin | 1 | 397 (23.9) | 88.6–89.3 | 108–120 |
| 2 | 340 (132) | 81.5–81.6 | 91.4–94.8 | |
| 3 | 179 (43.3) | 76.3–81.9 | 88.3–91.1 | |
| 4 | 155 (2.61) | 78.4–79.4 | 85.4–88.1 | |
- a Values are expressed as a percentage of the bulk soil concentration of each plot.
- b Range of duplicates.
- c Values in parentheses are the standard deviation of four replicates; each replicate is the average of duplicate injections.
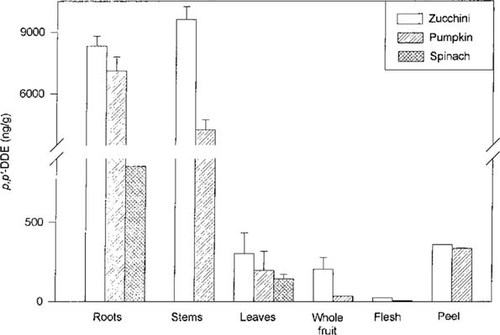
Concentration of 2,2-bis(p-chlorophenyl)1,1-dichloroethylene (DDE; ng/g oven-dry wt) in the vegetative tissues of zucchini, pumpkin, and spinach. Tissue samples from zucchini and pumpkin are a composite and subsample of four to six plants harvested from one of three and from one of four plots, respectively. Tissue samples from spinach are a composite and subsample of the vegetation from one plot. Error bars indicate standard deviation.
| Plant type | Root (n)b | Stem (n) | Leaf (n) | Whole fruit (n) | Peel (n) | Flesh (n) |
|---|---|---|---|---|---|---|
| Zucchini | 1.0 Acd | 1.4 B | 0.08 C | 0.03 D | 0.08 De | 0.01 E |
| (7) | (13) | (11) | (7) | (4) | (4) | |
| Pumpkin | 1.0 A | 0.66 B | 0.05 C | 0.02 Cf D | 0.06 C | 0.01 E |
| (11) | (11) | (12) | (6) | (7) | (7) | |
| Spinach | 1.0 A | NAg | 0.17 B | NA | NA | NA |
| (1) | (3) |
- a For each crop type, all values have been normalized to the average root concentration in each plot.
- b n = Number of replicates; each replicate is a composite and subsample of the tissues from four to five plants per plot (for zucchini and pumpkin) or a composite of all harvested tissues (for spinach).
- c Values are normalized to root concentrations within individual plots; three separate plots for zucchini, four for pumpkin, and one for spinach.
- d Within rows, values followed by different uppercase letters are significantly different at p < 0.05.
- e Significantly different from whole fruit at p < 0.10.
- f Significantly different from leaves at p < 0.10.
- g NA = not applicable.
Vegetative compartments
The roots, stems, leaves, and whole as well as sectioned fruit of zucchini and pumpkin plants were analyzed for p,p′-DDE content. Only roots and leaves were available for spinach. The concentrations of p,p′-DDE in the plant tissues from a randomly selected plot are shown in Figure 2. Significant amounts of the contaminant were found in the roots of all three species, but in zucchini and pumpkin, the concentrations were more than an order of magnitude greater than those in the bulk soil. The variation in bulk soil concentrations of p,p′-DDE among different plots of the same crop led to similar variations in the plant tissues associated with those plots. For example, for zucchini in plot 1, the root concentration of p,p′-DDE 1 (soil concentration, 397 ng/g) was approximately 7,600 ng/g (oven-dry wt), but in plot 3 (soil concentration, 225 ng/g), this concentration was only 2,700 ng/g. Similarly, for pumpkin in plot 1 (soil concentration, 397 ng/g), the root concentration of p,p′-DDE was 7,100 ng/g, but in plot 4 (soil concentration, 155 ng/g), this concentration was only 1,700 ng/g. Consequently, all tissue concentrations for a given plant species were normalized to the contaminant concentration in the roots of each individual plot, and all normalized data are expressed as a percentage of that root value in Table 2. The pumpkin stems contained 35% less contaminant than the roots, but in zucchini, the opposite was true, with the stems containing approximately 40% more p,p′-DDE than the roots. In all cases, the concentrations of p,p′-DDE in the leaves and fruit were nearly an order of magnitude lower than those in the roots. The amount of p,p′-DDE in zucchini and pumpkin fruit peels was six- to eightfold greater than the amount of p,p′-DDE in the fruit flesh. Interestingly, that trend was not completely evident in the first set of zucchini that were analyzed due to an artifact in the methodology. Initially, the fruit were cut in half across the middle, and the top and bottom were analyzed randomly as whole fruit or sectioned into peel and flesh. The data from those fruit were mixed, with the whole-fruit value being greater than the peel value in certain cases. Subsequently, fruit were cut in half along the long axis, and the left and right halves were analyzed as either whole or sectioned fruit (i.e., peel and flesh). These are the data presented in Table 2 and Figure 2. In every case, the peel concentration was significantly higher than the whole-fruit concentration. On further analysis, the p,p′-DDE concentration was found to vary along the length of the fruit, from the stem end (i.e., top) to the blossom end (i.e., bottom). Three separate zucchini fruit from each of the plots were sectioned longitudinally, and the peel and flesh from the top and bottom halves of the fruit were extracted and analyzed separately. The amount of p,p′-DDE in the top sections of peel and flesh was 3- and 20-fold greater, respectively, than the amount in the bottom sections of the same fruit. The mechanism responsible for this gradient along the length of the fruit remains unknown.
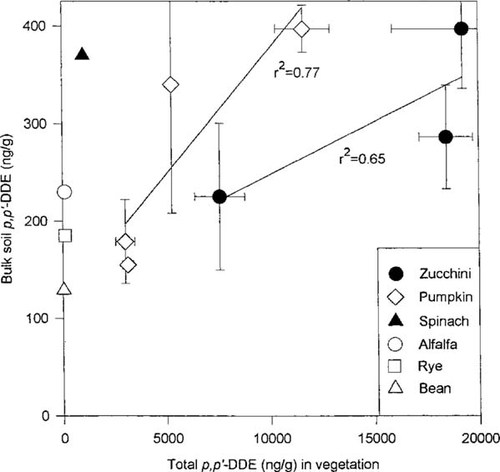
Correlation of bulk soil concentration of 2,2-bis(p-chlorophenyl)1,1-dichloroethylene (DDE) and total amount of contaminant in plant tissues. This total was calculated as the sum of the average concentration (ng/g oven-dry wt) of p,p′-DDE in the roots, stems, leaves, and whole fruit for zucchini and pumpkin or as the sum of the roots and leaves for spinach. Alfalfa, rye, and bean values are from White [17]. Error bars along the y-axis represent the standard deviation. Error bars along the x-axis represent the sum of the standard deviations for the averaged concentrations in roots, stems, leaves, and whole fruit.
These findings are similar to those of Lichtenstein [27] and of Lichtenstein et al. [28], who reported plant uptake of several chlorinated hydrocarbon insecticides from soil. More recently, Mattina et al. [16] detected chlordane in the tissues of numerous plant species, including zucchini, pumpkin, and spinach, grown in soil containing 40-year-old residues of the contaminant. Hulster et al. [18] as well as Campanella and Paul [19] similarly reported significant uptake of weathered dioxins and furans from soil by both zucchini and melon. In addition, White et al. (unpublished data) observed concentrations of chlordane in zucchini roots that were an order of magnitude greater than the level of the weathered residue in the bulk soil. Conversely, White [17] found relatively low levels of weathered p,p′-DDE in the roots of bean, alfalfa, and rye (25–100 ng/g), and Mattina et al. [16] observed considerable crop variability in the amount of weathered chlordane in the roots of 11 different crops (>25-fold differences). These data suggest that species-specific characteristics related to plant metabolism and physiology determine the extent of pollutant mobilization and the ultimate magnitude of uptake associated with a particular crop.
Figure 3 shows the average concentration of p,p′-DDE in the plant tissues of zucchini, pumpkin, and spinach as a function of the initial bulk soil concentration. The amount of contaminant in the plant tissue was obtained by summing the average concentration of p,p′-DDE (ng/g) in each plant compartment (i.e., root, stem, leaf, and whole fruit for zucchini and pumpkin; root and leaf for spinach). All plots of zucchini and pumpkin were treated individually and are represented as separate symbols, with a line regressed through that data. In addition, the soil and vegetative values from White [17] for alfalfa, rye, and bean were added to the figure. From the perspective of phytoremediation, plant species that cluster on the left-hand portion of Figure 3 (i.e., rye, alfalfa, bean, and spinach) are not desirable because of the relatively low concentrations of pollutant in the plant tissues relative to soil levels. Conversely, crops clustering on the center or right-hand portion of the figure indicate significant accumulation of contaminant within the plant tissues relative to soil concentrations. In addition, for plants in which several bulk soil concentrations were tested (i.e., zucchini and pumpkin), the slope of the linear regression through the data is instructive for phytoremediation efforts: The lower the slope of the regressed line through the data, the greater the contaminant accumulation in the tissues that is achieved with relatively small increases in soil concentration. Thus, assuming approximately equal weight and distribution of biomass among individual plant compartments, zucchini may be a better choice than pumpkin for phytoremediation of p,p′-DDE in soil. However, quantitative mass-balance studies, in which the weight of each soil and plant compartment is known, ultimately are necessary to determine and to compare both the efficiency of contaminant uptake and the extent of pollutant translocation by different plants. Such experiments are currently being designed.
The findings of this study are of particular interest in that they indicate considerable bioavailability and potential for bio-accumulation of highly weathered p,p′-DDE residues in soil. These data potentially contradict the considerable body of evidence in the scientific literature that indicates declining pollutant availability with time (i.e., sequestration) [1, 2]. The data herein represent availability only at one point in time, however, and the time course for the decline in p,p′-DDE bioavailability in this soil is unknown. Even so, the presence of pollutant concentrations 10- to 12-fold greater than those observed in the soil is a finding of particular interest. Although the list of contaminants that undergo time-dependent reductions in bioavailability and the assays utilized to demonstrate this process is incomplete, considerable support exists for considering such phenomena in the establishment of remediation endpoints and exposure estimates at contaminated sites in the field [2, 12, 29]. The data in this manuscript do not provide a mechanistic basis for the large bioavailability of weathered p,p′-DDE residues to cucurbits relative to that of other plants [17], but they do suggest caution regarding the current assumption of overly conservative risk determinations. In a soil containing a contaminant at residual levels that do not raise regulatory concerns (<400 ng/g dry wt), even when the total p,p′-DDE concentration is considered, 12 of 12 harvested zucchini fruit (whole-fruit concentration) exceeded action levels for p,p′-DDE in produce as regulated by the Department of Consumer Protection in the state of Connecticut (action level, 100 ng/g dry wt) [30]. Studies are currently underway to establish the likelihood of plant-based or phytoremediation strategies for soils contaminated with persistent organic pollutants such as p,p′-DDE.
Acknowledgements
I thank Lydia Wagner for technical assistance. I also thank Washington Braida and MaryJane Incorvia Mattina for manuscript review and MaryJane Incorvia Mattina for use of the Department of Analytical Chemistry's equipment and intellectual resources.




