Natural exposure of coastal river otters to mercury: Relation to age, diet, and survival
Abstract
We evaluated effects of location (i.e., Jackpot Bay, a naturally contaminated site, and Herring Bay, reference site), diet as determined by stable isotopes, and age on mercury concentrations in individual river otters (Lontra canadensis) from Prince William Sound, Alaska, USA. We also investigated the effects of mercury accumulation on survival of river otters from these two locations. Our results indicated that mercury concentrations in fishes from Jackpot Bay were significantly higher than those in fishes from Herring Bay and those in pelagic fishes. In addition, a predominant intertidal fish diet in both areas influenced the accumulation of mercury concentrations in otters. Concentrations of mercury in fur of river otters from Jackpot Bay were significantly higher than those of animals from Herring Bay. Nonetheless, we did not detect significant differences in survival between otters inhabiting the two areas, suggesting that this natural contamination was not high enough to impair survival. Our ability to investigate the effects of various factors such as location, diet composition, and age on mercury accumulation and subsequent survival of individuals offers an example for a link between individual-based captive studies and population-level field investigations.
INTRODUCTION
Bioaccumulation of mercury (in its methylated form) can have far-reaching consequences for individuals and populations. Numerous studies demonstrated the negative influence of mercury on survival of adults and juveniles in several species of mammals [1-5]. High concentrations of mercury in the diet also have adverse effects on reproduction of both males and females [2, 6]. In addition, nonlethal concentrations of mercury were reported to damage the central nervous system in humans as well as in domesticated and wild mammals [3, 7, 8]. Such damage may influence performance of wild, free-ranging animals and result in additional indirect mortality. Several studies indicated that mercury concentrations bioaccumulate over time, a phenomenon that may result in high mercury concentrations in older animals [8-10]. Reduced reproduction and high juvenile mortality likely result in low recruitment, and together with high adult mortality would likely lead to decline in populations [11]. Recent studies demonstrated that the effects of mercury contamination could be exacerbated because of synergistic effects of other environmental pollutants or stressors such as disease and extreme temperatures [4, 5, 12].
Anthropogenic sources for mercury pollution such as inputs from mining and smelting, environmental acidification, and increased atmospheric deposition due to burning of fossil fuels rapidly increase concentrations of mercury in the environment. This is particularly true in freshwater systems [13-16]. Although anthropogenic inputs rapidly change concentrations of mercury, natural geologic and hydrologic processes can create conditions of high mercury exposure similar to those reported for human-caused pollution [17]. Thus, risk of mercury pollution is highest for human and animal populations that rely heavily on aquatic food webs [17].
Piscivorous mammals such as river otters (Lontra canadensis), European otters (Lutra lutra), and American mink (Mustela vison), which represent top predators in different aquatic systems, have been used as model species for toxicologic studies on the effects of bioaccumulation of mercury [1, 3-5]. The potential relation between high concentrations of mercury in these mustelids and population declines in different locations in North America and Europe make these species a reliable indicator or sentinel species of environmental mercury pollution [10, 11, 15, 17-22].
Prince William Sound, Alaska, USA, was predominantly glaciated by the late Wisconsin period (about 15,000 years ago) [23], although nunataks (i.e., ice-free refugia) are hypothesized to have existed on the mountains of Montague, Hinchinbrook, and Knight islands [24]. Most of the area was freed from ice during the last major episode of ice retreat about 11,000 years ago [23] and forestation was evident about 3,000 to 4,000 years ago [25]. Nonetheless, glaciers dominate the landscape today. Although effects of glaciation on the distribution of river otters in the region are unknown, collection of river otter remains (dating from the Wisconsin period) from caves in southeastern Alaska [26] suggest that these mustelids inhabited the region during, or shortly after the receding of glaciers. Between 1897 and 1942 numerous gold, silver, and copper mining operations were established in Prince William Sound [27] and other sites, such as a quartz vein in Jackpot Bay [27; Fig. 1, S-91], were explored. Although no occurrences of mercury were reported and no records or signs of mineral production are known from Jackpot Bay [27], preliminary analysis of fishes in the area indicated high concentrations of mercury contamination. This unique occurrence of natural mercury contamination in an area with few anthropogenic inputs, well-established populations of river otter, and known time span of potential contamination (i.e., around 4,000 years) prompted us to investigate the influence of age and diet on bioaccumulation of mercury in coastal river otters in Prince William Sound.
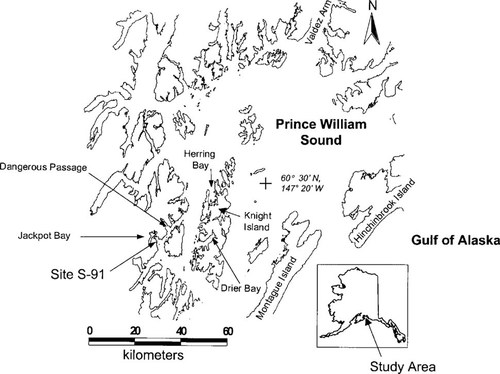
Location of study areas in Prince William Sound, Alaska, USA, where river otters were live-captured and fishes were collected in 1996 and 1997. Mineral exploration in Jackpot Bay occurred in 1909 (site S-91).
In this study, we describe diet of individual river otters using stable isotope analysis and evaluate the effects of location, diet, and age on mercury concentrations in these animals. Stable isotopes of carbon and nitrogen have been used as tracers in examining the origins of prey in several mammalian carnivores including coastal river otters [28-30]. The tendency of δ13C and δ15N to accumulate with trophic position allows for evaluation of the relation between diet and bioaccumulation of environmental pollutants [31]. We hypothesized that animals inhabiting locations closer to the natural source of mercury contamination would have higher concentrations of mercury than animals inhabiting other locations. We also hypothesized that older animals would have higher concentrations of mercury, as would animals feeding largely on freshwater fishes. Finally, we hypothesized that survival of individuals in areas with high mercury contamination would be lower than that of animals exposed to lower concentrations of mercury pollution.
MATERIALS AND METHODS
Study areas
Our study areas were located in Herring Bay and vicinity on Knight Island (Fig. 1; 60°30′N, 147°40′W) and Jackpot Bay and vicinity in Dangerous Passage (60°20′N, 148°10′W) in Prince William Sound. Both areas have a maritime climate; summers are cool and wet and winters are characterized by deep snow (2,200-mm annual precipitation). The snow-free period extends from early May to early November at lower elevations. Vegetation at higher elevations is typically alpine tundra, and at lower elevations is characterized by coastal, old-growth forest of Sitka spruce (Picea sitchensis) and western hemlock (Tsuga heterophylla) with a well-developed under-story (mainly Oplopanax horridus, Vaccinium spp., Menziesia ferruginea, and Rubus spp.). Alder (Alnus spp.) tends to occur on disturbed sites, and near the boundary of terrestrial vegetation and the intertidal zone. Herring Bay has only small freshwater streams that support a limited number of freshwater fishes. In comparison, Jackpot Bay has an extensive freshwater system including a fourth-order stream (Jackpot Creek) and a lake with numerous tributaries.
Between 1897 and 1942, numerous gold, silver, and copper mining operations were established in Prince William Sound [27]. Active copper mining occurred in Drier Bay on southern Knight Island (Fig. 1) until the late 1930s but no other mining operations were done in the vicinity of either Herring Bay or Jackpot Bay [27]. In 1909, a quartz vein containing arsenopyrite, galena, and sphalerite was reported in Jackpot Bay (S-91) [27]. Samples from the site yielded 42.6 g gold per ton and 88.0 g silver per ton as well as 0.2% copper, 0.5% zinc, and 0.05% lead [27]. No occurrences of mercury were reported and no records or signs of mineral production are known from the bay [27]. Nonetheless, mercury deposits in this marine environment usually occur in conjunction with the metals listed above [27].
In 1989, the super tanker Exxon Valdez ran aground on Bligh Reef just beyond Valdez Arm in Prince William Sound, spilling 39,000 metric tons of North Slope crude oil. That oil spread across 3,500 km of shoreline in western Prince William Sound. Herring Bay on Knight Island was one of the heavily oiled bays in the region. Our studies documented the deleterious consequences of the Exxon Valdez oil spill on river otters inhabiting oiled areas compared with nonoiled areas of Prince William Sound (R.T Bowyer et al., unpublished data), and led to the listing of river otters as an injured resource by the Exxon Valdez oil spill Trustees Council in 1993. By 1997 to 1998 differences in body mass, biomarkers, diet, home-range size, habitat selection, and indices of population size recorded in the earlier studies were no longer evident (R.T. Bowyer et al., unpublished data). These results indicated that river otters in Herring Bay have recovered from the effects of this spill and can be used as reference samples in this study.
Collection of samples
Thirty-two river otters were live-captured in Jackpot Bay and 31 were captured in Herring Bay in spring and summer of 1996 and 1997, with number 11 Sleepy Creek® leg-hold and Hancock traps (Sleepy Creek Traps, Manistique, MI, USA) [32]. Otters were anesthetized with Telazol (9 mg/kg; A.H. Robins, Richmond, VA, USA) administered using Telinject® darts and a blowgun. Blood and tissues, including samples of fur, were collected from each individual otter at that time and the gender was recorded. Blood and soft tissue samples were analyzed for biomarker responses to hydrocarbon exposure (R.T Bowyer et al., unpublished data). Otter age (juvenile = 1, young adult = 2, adult = 3, old adult = 4) was estimated from body size and tooth wear and staining. In 1997, the first upper molar was extracted from 22 individuals for aging by counting cementum annuli (Matson Laboratory, Milltown, MT, USA). Estimated age was correlated with tooth age (r = 0.66, p = 0.001 [30]). The juvenile age group included one-year-old animals, animals in the young adult group were estimated to be between two and three years old, those in the adult group were estimated to be between four and five years old, and old adults included animals six years old and older. For additional details on trapping and handling of otters see Blundell et al. [32].
After measurements and samples were collected, a subset of the captured river otters (n = 41), which were deemed to be in good health, were implanted with hermetically sealed radiotelemetry transmitters (IMP/400/L, Telonics®, Mesa, AZ, USA) into the peritoneal cavity [30]. All surgeries employed sterile techniques (for details see Blundell et al. [30]). The transmitter signaled a mortality mode if the otter remained motionless for 9 h. In 1996, only otters captured in Jackpot Bay were implanted, whereas in 1997, otters in both Herring and Jackpot bays were implanted with transmitters. Battery life for transmitters varied from 21 to 36 months.
Fish samples were obtained from companion studies by Ben-David et al. [29], A. Hirons (Institute of Marine Sciences, University of Alaska Fairbanks, Fairbanks, AK, USA), and T.C. Kline (Prince William Sound Science Center, Cordova, AK, USA). Freshwater and intertidal fishes were caught in baited minnow traps or through beach seining [28] in the Jackpot Bay freshwater system and the intertidal zone of Jackpot Bay and Herring Bay. Samples of pelagic fishes were collected in several nearshore locations in northwestern Prince William Sound (Fig. 1). Samples of pelagic fishes included adult pink salmon (Oncorhynchus gorbuscha), juvenile salmon (Oncorhynchus spp.), capelin (Mallotus villosus), Pacific herring (Clupea pallasii), and Pacific sand lance (Ammodytes hexapterus). Intertidal and demersal fishes included rockfish (Sebastes sp.), kelp greenling (Hexagrammos decagrammus), gadids (Gadus macrocephalus and Theragra chalcogramma), cresent gunnels (Pholis laeta), intertidal sculpins (Oligocottus aculosus and Icelinus borealis), and pricklebacks and ronquils (Anoplarchus purpurescens, Stichaeus punctatus, Bathymaster signatus, Lumpenus maculatus, Xiphister atropurpureus, and X. muscosus). Freshwater fishes sampled were coastrange sculpin (Cottus aleuticus), juvenile Dolly Varden (Salvelinus malma), and three-spined stickleback (Gasterosteus aculeatus). All samples of fish were dried at 60 to 70°C for 48 h before analysis.
Stable isotope analysis
Samples of fur collected from live-captured otters were washed with distilled water and dried at 60 to 70°C for 48 h. Subsequently, a subsample of 1 to 1.5 mg of fur was weighed into a miniature tin cup (4×6 mm) for combustion. We used a Europa 20/20 (PDZ Europa, Cheshire, UK) continuous flow isotope ratio mass spectrometer to obtain the stable isotope ratios. Each sample was analyzed in duplicate and results were accepted only if the variance between the duplicates did not exceed that of the peptone standard (δ13Cstd = —15.8, δ15Nstd = 7.0, coefficient of variation [CV] = 0.1). Stable isotope values for fish tissues collected in Prince William Sound were obtained using identical procedures. Dried fish tissues were ground finely with a Wig - L - Bug grinder (Crescent Dental, Chicago, IL, USA) before weighing of subsamples.
Mercury analysis
Mercury analysis for fish samples was conducted with cold-vapor atomic florescence spectroscopy [33]. For the dried tissue, 0.5 to 1.0 g of the homogenized sample was weighed into a Teflon® vial. To the vial, 7.0 ml of a 7:3 (v/v) mixture of HNO3:H2SO4 was added. The sample was placed on a 125°C hotplate for 2 h after the onset of reflux or until all organic matter was dissolved. After cooling, the sample was diluted to 25 ml with a 10% solution of 0.2 N BrCl. Milli Q water (Milli-Q Corp, Bedford, MA, USA) (100 ml) and 0.3 ml of SnCl2 were added to an aliquot of the digested sample in a bubbler. After mercury ions were reduced to mercury with SnCl2, the mercury was purged onto gold-coated sanded traps as a means of preconcentration by using N2 and a bubbler. The mercury was released from the gold sand traps using heat and determined using a Tekran detector (Frontier Geosciences, Seattle, WA, USA).
Mercury analysis for fur of river otters was conducted at Northern Testing Laboratories (Fairbanks, AK, USA). A 0.2-g sample in 5 ml of distilled H2O was digested in 5 ml of acid solution (1:l HC1:HNO3, v/v) by heating the sample for 2 min at 95°C. After cooling to room temperature, 6 ml of a sodium chloride-hydroxylamine solution was added, followed by dilution with H2O and addition of 5 ml of SnCl2. After mercury was reduced to elemental state, absorbency was measured at 253.7 nm with a Perkin-Elmer 2380 atomic absorption spectrophotometer (Norwalk, CT, USA; EPA Method 7000; U.S. Environmental Protection Agency, Washington, DC).
Survival analysis
Otters on both study areas were protected from sport and subsistence harvest, precluding human-related mortality. In 1997 through 1998, all radiotracking of river otters was conducted with aerial telemetry. Aerial tracking occurred about every 4 d in mid-April to mid-June; thereafter, tracking was conducted weekly until September. Tracking was attempted every two to three weeks during winter, depending upon weather. Observers recorded the signal mode of each transmitter at each flight and determined mortality based on the signal. Animals were determined dead only if the mortality mode was detected in the same location in three consecutive flights. The time of death was denoted as the last flight when the animal was alive, representing days known alive.
Data analysis
To determine whether isotopic signatures of intertidal fishes, pelagic fishes, and freshwater fishes significantly differed from each other we employed the K-nearest randomization test [34]. A one-way analysis of variance (ANOVA) with multiple comparisons was used to determine whether mercury levels differed between locations and fish groups [35; SPSS for Windows, SPSS, Chicago, IL, USA].
We determined relative contribution of pelagic, intertidal, and freshwater fishes to the diet source for each otter based on the combined values of δ13C and δ15N using a dual-isotope multiple-source mixing model [28]. The mixing model assumes that each individual otter consumes all possible types of food. Therefore, this model will tend to overestimate the proportion of food items that are rarely consumed and underestimate the proportion of commonly used prey. Consequently, we used the model as an index of relative contribution of each food type rather than as actual proportions in the diet. We used fractionation values of 3‰ for carbon and 4‰ for nitrogen based on results from feeding experiments in captivity (M. Ben-David, unpublished data). Using results from the mixing model, we determined the cutoff points in the nontransformed δ13C data that represented relative contribution of pelagic, intertidal, and freshwater fish to the diet and used this information in subsequent analyses.
We compared the concentrations of mercury in otter fur between the two study areas using a two-sample t test assuming equal variance [35; SPSS for Windows], and further investigated the relations between concentrations of mercury, location, diet, and age using a three-way ANOVA model. In this model location (Jackpot or Herring bays), age group (juvenile, young adult, adult, and old adult), and diet type as represented by δ13C (pelagic, intertidal, and freshwater) were used as factors, and mercury concentration was the dependent variable [35; SPSS for Windows]. Similarly, we investigated differences in concentrations of mercury between males and females in our sample using a one-way ANOVA model. In this model gender was used as a factor, and location, age group, and diet were used as covariates [35].
We calculated Kaplan-Meier survival estimates using a staggered-entry model described by Pollock et al. [36] for both Herring Bay and Jackpot Bay. These estimates were then compared using a log-rank test [36]. In this analysis we excluded otters that traveled between study areas. We followed this analysis with a logistic regression model with fate (dead coded 0 and censored coded 1) as the dependent variable and mercury levels, age, and location as the independent ones [37] to determine whether mercury concentrations influenced survivorship in our study animals. Censored animals were defined as those animals for which the radio signal was not detected in three consecutive flights and that did not reappear in the study areas until the end of tracking. The cause of signal disappearance could have been due to one of four reasons: complete battery drain at the end of 21 to 36 months; transmitter failure; emigration from the study areas; and death underwater. Therefore, our censored data may be biased by including animals that perished together with surviving ones. To evaluate the degree of bias we calculated the number of days known alive for each animal and compared this variable between the two survival categories using a one-way ANOVA with number of days alive as the dependent category, fate as the independent category, and age and location as covariates [35; SPSS for Windows]. All animals in our study were either dead or censored by the end of the tracking period in January 1999.
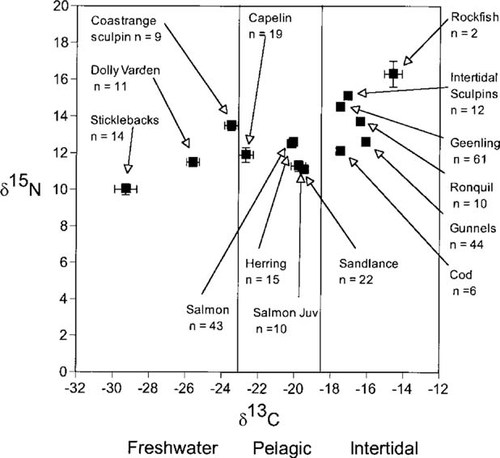
Stable isotope values for fish tissues collected in Prince William Sound, Alaska, USA, and obtained from companion studies [29; A. Hirons, Institute of Marine Sciences, University of Alaska Fairbanks, Fairbanks, AK, USA; T.C. Kline, Prince William Sound Science Center, Cordova, AK, USA]. When pooled by groups, intertidal, pelagic, and freshwater fishes had all significant isotopic signatures (p < 0.05; K-nearest randomization test). Adapted from Blundell et al. [30].
RESULTS
Of all the species of fish, only herring and adult salmon, and sand lance and juvenile salmon had overlapping isotopic ratios (K-nearest randomization test, p = 0.55 and 0.76, respectively; Fig. 2). When pooled by groups of intertidal fishes, pelagic fishes, and freshwater fishes, clusters had significantly different isotopic values (K-nearest randomization test, p < 0.05; Fig. 2). Although values for these groups were significantly different in bivariate space, the difference was most pronounced in values of δ13C (Fig. 2).
Sensitivity of the cold-vapor atomic fluorescence spectroscopy allowed for detection of mercury in a subsample of the fishes (Fig. 3). Values of mercury in fishes ranged from 0.02 to 0.38 mg/kg dry weight (or 0.02–0.38 ppm) and were significantly different between fish groups and locations (Fig. 3; ANOVA, p < 0.001). Multiple comparisons indicated that concentrations of mercury in freshwater and intertidal fishes in Jackpot Bay were not significantly different from each other (p = 0.2), although the mean values of freshwater fishes were elevated. In contrast, those values were significantly higher than those of intertidal fishes in Herring Bay as well as pelagic fishes in northwestern Prince William Sound (Fig. 3; p = 0.015).
Mercury was detected in the fur of river otters and ranged from 2.2 to 18.8 mg/kg dry weight (or 2.2 to 18.8 ppm; Fig. 4). Mercury concentrations in the fur of river otters from Jackpot Bay and Dangerous Pass were significantly higher from those inhabiting Herring Bay and vicinity (Fig. 4; two-sample t test p = 0.045, one-tailed test). Subsequent analysis indicated that location (Jackpot Bay vs Herring Bay), diet as represented by δ13C, and age had significant effect on mercury concentrations in fur of river otters (Fig. 5; three-way ANOVA, overall model p = 0.039). Although effects of location and diet were significant for the entire data set (p = 0.008 and 0.021, respectively), age was not (p = 0.69). However, within each location, the influence of age and diet varied. In Herring Bay, older animals exhibited higher values of mercury, although this trend was marginally nonsignificant (Fig. 5; p = 0.07). No such effect was detected in Jackpot Bay (p = 0.71). Also, in each location diet affected mercury concentrations within each age group (Fig. 5). The ANOVA model indicated that location had a significant influence on diet (p = 0.015), with animals in Herring Bay exhibiting more enriched δ13C values representing a predominantly intertidal fish diet (Fig. 5). In contrast, age had no influence on diet composition (p = 0.58). Gender had no influence on mercury concentrations in our sample (ANOVA, p = 0.3) when location, age, and diet were introduced as covariates to the model.
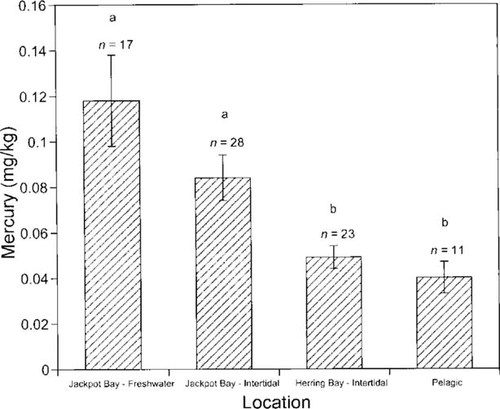
Mean concentrations (± standard error) of mercury (mg/kg dry weight) in tissues of fish collected from Jackpot Bay and Herring Bay, Prince William Sound, Alaska, USA. Sample sizes are provided above error bars. Concentrations of mercury were significantly different between fish types and locations (analysis of variance, p < 0.001). Letters represent significant differences at the α level of 0.05 as determined from Scheffé multiple comparisons.
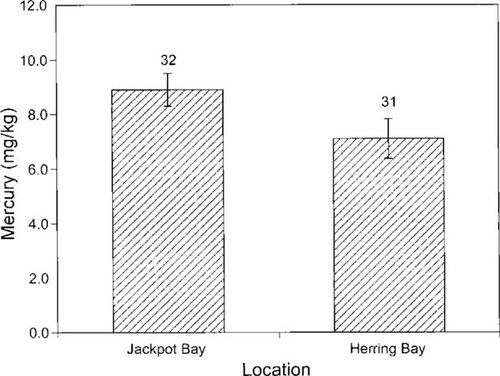
Mean concentrations (± standard error) of mercury (mg/kg dry weight) in fur of river otters live captured in Jackpot Bay and Herring Bay, Prince William Sound, Alaska, USA, in 1996 and 1997. Sample sizes are provided above error bars. Concentrations of mercury in river otters from Jackpot Bay were significantly higher than those in otters inhabiting Herring Bay (two-sample t test, p = 0.045, one-tailed test).
Survivorship of river otters, based on radiotelemetry at one half-month intervals from late June 1997 to early January 1999 did not differ significantly between Herring Bay and Jackpot Bay (Fig. 6; p > 0.2). Overall survivorship in both areas was comparatively high (>0.8 over 20 months). Number of days alive for censored animals (i.e., those animals for which the radio signal was lost, n = 33) was significantly higher than for those that died (n = 8) during the study (458.8 ± 40.6 [mean ± standard error] and 225.9 ± 78.9, respectively; ANOVA, p = 0.016), suggesting that the bias introduced to the censored category of fate by animals perishing underwater was relatively low. Age and location did not significantly influence number of days alive (p = 0.72 and 0.7, respectively) when included as covariates. Logistic regression indicated that levels of mercury, age, and location had no influence on fate of river otters in our study (logistic regression, overall model p = 0.65; mercury p = 0.73, age p = 0.61, location p = 0.19).
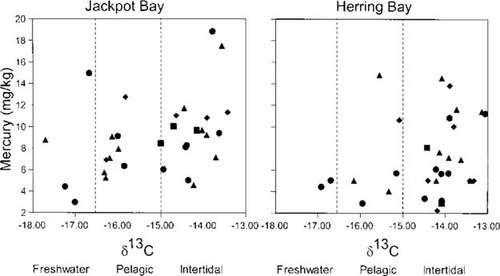
Influence of age group and diet on concentration of mercury (mg/kg dry weight) in fur of river otters live-captured in Jackpot Bay and Herring Bay, Prince William Sound, Alaska, USA, in 1996 and 1997. Otter age was estimated as juvenile (▪), young adult (•), adult (▴), and old adult (⋄). Although the effects of location and diet were significant for the entire data set (p = 0.008 and 0.021, respectively), age was not (p = 0.69). Nonetheless, in Herring Bay, older animals exhibited higher values for mercury, although this trend was only marginally significant (p = 0.07). No such effect was detected in Jackpot Bay (p = 0.71).
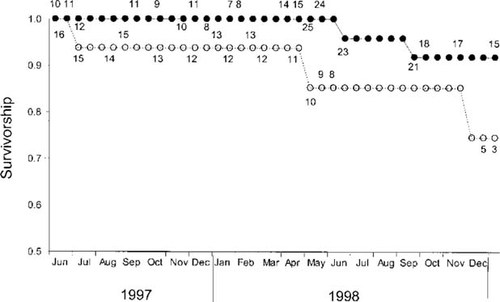
Survivorship of river otters with radiotransmitters inhabiting Jackpot Bay (○) and Herring Bay (•), Prince William Sound, Alaska, USA, by one-half-month intervals from late June 1997 to early January 1999. Numbers adjacent to circles represent changes in the otters at risk used for calculating survivorship from the Kaplan-Meier, staggered-entry model [36]. The log-rank test indicated no significant difference (p > 0.20) in survivorship of river otters inhabiting Jackpot Bay and Herring Bay. Adapted from R.T Bowyer (unpublished data).
DISCUSSION
Although no mercury was recorded in samples collected at site S-91 (Fig. 1) [27], the occurrence of arsenic, gold, silver, copper, and other metals in the formation indicates high likelihood of mercury deposits as well. The observation that mercury concentrations did not differ between the two fish groups (freshwater and intertidal) suggests that the input of mercury to Jackpot Bay can not be attributed solely to hydrologic action of Jackpot Creek (Fig. 1). Perhaps high surface and subsurface runoff from adjacent slopes due to high annual precipitation in conjunction with deterioration of rocks from freezing and thawing events may add to the action of the stream. Significantly lower mercury concentrations in pelagic fishes that acquire most of their resources in the Gulf of Alaska [30], as well as the lower mercury levels in intertidal fishes from Herring Bay (Fig. 3), supports the hypothesis of a point source of mercury contamination in Jackpot Bay.
Mercury concentrations in freshwater and intertidal fish in Jackpot Bay were lower than values recorded in other studies where anthropogenic inputs were the main source of contamination (0.88 ± 0.08 mg/kg wet weight [mean ± standard deviation] in omnivorous fishes, and 1.72 ± 0.53 mg/kg wet weight in carnivorous fishes [11]; range 0.17–0.43 ppm wet weight [38]; 0.051–1.544 mg/kg wet weight [13]; range of means 0.28–2.4 mg/kg [15]). However, consumption of fishes from Jackpot Bay would result in an average uptake of 3.65 mg/kg body weight per year (based on consumption of 1 kg of fishes per day and average body weight of 10 kg), which is higher than the World Health Organization (Paris, France) recommended 0.15 mg/kg per year for humans [11].
Mercury concentrations in otter fur in our study areas generally were lower than those reported by studies conducted in areas with anthropogenic contamination [11, 18, 22, 39]. The positive relation between total mercury in hair and that of methylmercury in liver is inconsistent between studies [11, 18, 22] preventing us from comparing concentrations of mercury in our animals with other studies that determined mercury concentrations in liver, muscle, kidney, and brain tissues [10, 11, 15, 18-22]. When compared with concentrations of mercury in fur of American mink, mercury concentrations in our study animals were lower than those reported for animals in contaminated areas and similar to those of animals from reference sites [22, 40]. Therefore, despite the significantly higher values of mercury in animals from Jackpot Bay compared with Herring Bay (Fig. 4), the level of natural contamination in that area was likely lower than that observed in areas contaminated with anthropogenic inputs. In light of these results, it is not surprising that we did not detect significant differences in survival between otters inhabiting Jackpot and Herring bays (Fig. 6) or that mercury concentrations did not significantly influence the fate of otters (i.e., dead and censored) in both areas. Kruuk et al. [10] determined that mercury accumulation in European otters in Scotland was likely too low to affect survival of individuals despite the significant negative relation between body condition and concentrations of mercury in their sample. In addition, Kruuk et al. [10] postulated that free-ranging European otters exposed to relatively low concentrations of mercury were able to excrete some of this temporally bioaccumulated contaminant. Thus, the concentrations of mercury found in the fur of river otters in Jackpot Bay suggest that the level of exposure is not likely to pose a significant risk to their subsequent survival.
The main difference in bioaccumulation of mercury in river otters inhabiting Jackpot and Herring bays was the effects of diet and age (Fig. 5). In Herring Bay, animals feeding mainly on intertidal fishes exhibited higher mercury concentrations (Fig. 5), although this relation could have been influenced by age. Few older animals in Herring Bay consumed significant amounts of freshwater or pelagic fishes. In Jackpot Bay, the highest values of mercury occurred in animals feeding on intertidal and freshwater fishes regardless of age, although concentrations of mercury were higher in animals feeding on intertidal fishes within each age group (Fig. 5). That animals consuming relatively large amounts of pelagic fishes had high mercury concentrations in their fur was unexpected given the low levels of mercury in these fishes (Fig. 3). Nonetheless, these animals must have consumed large amounts of intertidal fishes during periods when pelagic fish were not available in Prince William Sound (i.e., November to May) [30]. Thus, a predominant diet of intertidal fishes for otters in both areas likely influenced the accumulation of mercury.
Older animals exhibited higher values of mercury in Herring Bay, although this relation was only marginally significant. In contrast, no such effect of age was detected in Jackpot Bay. A parallel observation in which a significant age difference occurred in a noncontaminated site but no difference occurred in a contaminated site was reported by Stevens et al. [40] for muskrats (Ondatra zibethicus). However, whether mercury deposition in fur is related to the rate of temporal bioaccumulation in soft tissues (which will be higher in contaminated areas) is unclear and merits further investigation. Unfortunately, we were unable to determine mercury concentrations in soft tissues for the river otters in this study because all samples were utilized in examination of biomarker responses to hydrocarbon exposure (R.T Bowyer et al., unpublished data). In addition, because of logistical constraints related to the location of our study areas, we were unable to collect carcasses of the dead otters before they decomposed, which prevented us from investigating the levels of methyl-mercury in their soft tissues.
Our study demonstrates the importance of individual-based analysis in pollution studies. Our ability to investigate the effects of various factors such as location, diet composition, and age on mercury accumulation and subsequent survival of individuals offers a link between traditional individual-based captive studies and standard population-level field investigations. Our ability to conduct this analysis relied largely on the advances made in radiotelemetry as well as the development of stable isotope analysis in contaminant studies [31]. Although bioaccumulation of naturally occurring mercury in coastal river otters in Prince William Sound may have little influence on animal survival, such accumulation may have larger consequences for the marine-terrestrial interface in that area. Coastal river otters are part of an assemblage of species that bridge the marine and terrestrial systems. These nexus species deposit marine-derived nutrients in the riparian and beach-fringe forests; nutrients that are incorporated into terrestrial vegetation and primary consumers [29, 41]. Thus, through their excretions, coastal river otters may biomagnify mercury levels available for incorporation in terrestrial vegetation and primary consumers in the beach-fringe forest. A study investigating the relation between mercury accumulation in livers of river otters, the diversion of mercury into fur or alternative excretion in feces, and the subsequent biomagni- fication of mercury in the terrestrial system will provide a link between individual animal physiology and ecosystem processes.
Acknowledgements
We thank L. Faro, P. Berry, S. Andersson, and C. Durham for assistance in the field, and J. Decreeft our radiotelemetry pilot. M.K. Hecker, N. Haubenstock, and C. Restrepo assisted with chemical analyses. This work was funded in part by the Institute of Arctic Biology, the Alaska Cooperative Fish and Wildlife Research Unit at University of Alaska, Fairbanks, the Cooperative Institute for Arctic Research, a National Oceanographic and Atmospheric Administration–sponsored program, and the Exxon Valdez oil spill Trustee Council. We thank H. Golden and D. Rosenburg of the Alaska Department of Fish and Game for logistical support. We are indebted to the people of Chenega Village and the Chenega Native Corporation for permission to conduct research on their lands. All methods used in this research were approved by the Institutional Animal Care and Use Committee at Univesity of Alaska, Fairbanks; trapping permits were issued by Alaska Department of Fish and Game; and all procedures adhere to the guidelines for animal care and use adopted by the American Society of Mammalogists (Animal Care and Use Committee 1998).




