Modeling zinc toxicity for terrestrial invertebrates
Abstract
Acute and chronic ecotoxicity tests with zinc were performed with the earthworm Eisenia fetida, the potworm Enchytraeus albidus, and the springtail Folsomia candida. To assess the influence of the soil type on zinc toxicity for these soil invertebrates, these tests were carried out in a standard artificial soil, a sandy and a loamy field soil. Based on the results of this experimental work and data taken from literature, models were developed relating the ecotoxicity of zinc to the most important parameters controlling bioavailability: pH and cation exchange capacity. Models were developed for E. fetida and F. candida using the regression technique partial least squares projection to latent structures (PLS). Acute as well as chronic toxicity data of both organisms could be normalized on the basis of the pH and the cation exchange capacity of the test soils. For other terrestrial invertebrates, not enough data were available to develop reliable models.
INTRODUCTION
Risk assessments of metals are currently mainly based on laboratory toxicity data. These data are used to fit species sensitivity distributions from which a hazardous concentration for the ecosystem is derived [1]. Not only does terrestrial ecotoxicity vary between species, but the soil characteristics also greatly influence the effect concentration of metals by altering the bioavailability. Van Straalen and Denneman [2] and Van Straalen [3] tried to account for metal bioavailability by normalizing toxicity data to a reference value for a standard soil (25% clay and 10% organic matter) using linear equations developed for relating natural background concentrations of metals in soil to clay and organic matter content. However, the previously mentioned equations are not necessarily suited to predict the ecotoxicity of metals. Indeed, pH and cation exchange capacity (CEC) were recently reported to be the most important parameters affecting zinc toxicity to Enchytraeus albidus [4]. Normalization of toxicity data using these soil characteristics may considerably improve current risk assessment.
In this paper, toxicity data are presented for Eisenia fetida, E. albidus, and Folsomia candida exposed to zinc in standard artificial soil, a sandy and a loamy field soil. These data, together with toxicity data for terrestrial invertebrates obtained from literature, were used to develop statistical models relating zinc toxicity to the pH and the CEC of the test substrate.
MATERIALS AND METHODS
Test organisms
The culture of E. albidus Henle 1847 was kindly provided by J. Römbke. The culture substrate of E. albidus consists of artificial soil [5], and animals are fed once a week with ground rolled oats. The culture of F. candida Willem 1902 was obtained from Aquasense B.V. (Amsterdam, The Netherlands). Animals are cultured on a substrate of plaster of paris and pulverized chemical activated charcoal in a ratio of 8:1 (wt/wt). Granulated dry yeast is added weekly as a food source. The culture of E. fetida Savigny 1826 was obtained from a commercial earthworm breeding plant. In our laboratory, animals are cultured in Sphagnum peat adjusted to pH 6 to 7 with CaCO3, and animals are weekly fed with cow dung. All cultures have been maintained in our laboratory for at least five years at 20°C and in complete darkness.
Test substrates
The artificial soil was composed as prescribed by Organization for Economic Cooperation and Development (Paris, France) Guideline 207 [5]: 70% sand, 20% kaolin clay, and 10% finely ground Sphagnum peat, adjusted to pH 6 with CaCO3. The sandy field soil originated from Kalmthout (Antwerp, Belgium) and the loamy field soil from Termunck (Flemish Brabant, Belgium). The main characteristics of the soils are summarized in Table 1.
Toxicity assays
Toxicity tests with E. albidus were carried out according to draft OECD Guideline 220 [6]. Acute toxicity assays with E. fetida were performed following OECD Guideline 207 [5] and chronic toxicity tests as suggested by Van Gestel et al. [7]. Tests with the springtail F. candida were carried out according to the International Organization for Standardization [8]. In the acute toxicity tests, 10 adult worms with a fully developed clitellum or 10 synchronized springtails of 10 to 12 d were exposed per glass vessel. Per vessel, 20 g wet weight of soil were added for E. albidus, 30 g for F. candida, and 750 g for E. fetida. Four replicates were used per exposure concentration. Surviving animals were counted after 14 d of exposure. Mortality in the controls never exceeded 10%; in most cases no mortality occurred.
Chronic toxicity tests with E. albidus took six weeks to complete. Rolled oats were put on the soil surface weekly as a food source. After three weeks of exposure, the adults were removed, and after another three weeks the juveniles were counted. To facilitate counting, the substrate was fixed with ethanol, and a few drops of Bengal Red solution (Chroma-Gesellschaft, Köngen, Germany) were added. The next day the substrate was washed through a 300-μm sieve, and the juveniles were counted. The minimum number of 25 juveniles per vessel in the controls, as prescribed by the guideline, was always exceeded.
| OECD soila | Sandy field soil | Loamy field soil | |
|---|---|---|---|
| Sand (%) | 70 | 77 | 11 |
| Silt (%) | 0 | 99 | 72 |
| Clay (%) | 20 | 1 | 17 |
| PH | 6.0 | 4.4 | 6.3 |
| Organic matter (%) | 10 | 4.8 | 1.5 |
| CEC (cmol/kg)b | 15.0 | 10.1 | 11.5 |
| WHC (%)c | 110 | 45 | 55 |
- a OECD = Organization for Economic Cooperation and Development.
- b CEC = cation exchange capacity.
- c WHC = water holding capacity.
Reproduction tests with F. candida took four weeks to complete. Granulated dry yeast was added weekly on the soil surface as a food source. At the end of the test, juveniles were counted after flotation. The number of juveniles in the controls always exceeded the prescribed minimum of 100 instars per vessel.
In the chronic toxicity tests with E. fetida, adults were exposed for three weeks. At the start of the test, 2% (dry wt) finely ground cow dung was supplied in a shallow depression in the test soil. At the end of the test, the number of cocoons was determined after washing the soil through a 1-mm sieve.
During exposure, vessels were kept at 20 ± 1°C and a 16: 8-h light:dark cycle of at 400 to 800 lux or constant illumination in the case of E. fetida. Soil moisture content was adjusted twice a week by replenishing weight loss with the appropriate amount of deionized water. Zinc was added as aqueous solutions of chloride salt (ZnCl2, Vel, Leuven, Belgium, pro analysis). Zinc was spiked in logarithmic series with four concentrations per order of magnitude. Test organisms were exposed immediately after spiking.
Metal analysis
Soils were digested in hot acid (HC1:HNO3 1:5 v/v, microwave heating), and soil zinc concentrations were measured using flame atomic absorption spectrometry (Varian, SpectrAA-100, Victoria, Australia) with a deuterium background correction. A calcareous loamy soil (CRM 141 R, Community Bureau of Reference, Brussels, Belgium) was used as certified reference material. Nominal concentrations of the spiked soils and certified concentrations in the reference material never differed more than 10% from the measured concentrations.
Characterization of soil parameters
At the end of the exposures, pH (KCl) was measured with a pH meter (Consort, P407, Turnhout, Belgium) at a 1:2.5 soil: liquid ratio with 1 M KCl [9]. The CEC was determined with the AgTu method in 0.4 M ammoniumacetate [10]. Water holding capacity (WHC) was determined by measuring the water content of the soil substrate after leaving the substrate for 3 h under water and subsequently draining it for 2 h [11]. The water content was determined by weighing the sample, drying it to constant mass at 105°C, and reweighing it. For the field soils, the clay fraction and the percentage organic matter were also measured. The clay fraction (<2μm) was determined by sedimentation velocity, and the organic matter content was estimated from the determination of carbon (multiplying by a factor 1.7), which was done by oxidation with potassium dichromate in sulfuric acid medium [12].
Literature data
For the model development, additional toxicity data for terrestrial invertebrates exposed to zinc were obtained from literature. Only toxicity data were used of tests with soils into which zinc was homogeneously mixed. Studies in which animals were exposed via the food or via contaminated sludge that was put on top of uncontaminated soil were rejected from the data set. Several papers reported growth as well as reproduction as endpoints. As the later parameter was in most cases more sensitive, data for growth were not included.
Statistics
Ten percent effective concentrations (EC10s) and 50% effective concentrations (EC50s) were calculated using the probit method, 50% lethal concentrations (LC50s) were calculated using the moving average method, and if less than two partial mortalities occurred, the binomial method was used [13]. In the latter case, no confidence intervals could be calculated. No-observed-effect concentrations were calculated by Kruskal-Wallis analysis of variance followed by post hoc multiple comparisons [14].
For the frequently used test organisms, zinc toxicity was related to the most important environmental variables influencing bioavailability using partial least squares projection to latent structures (PLS) models [15]. The PLS is a generalization of regressions based on latent variables to find a linear or polynomial relationship between a set of prediction variables X (NobservationsṁKvariables) and a set of response variables Y (NobservationsṁMvariables) or one single response variable y. The R2 of the model is a measure for the variance explained by the model, while Q2 is measure for the variance of the variables that can be predicted by the model. The Q2 for a significant model has to be greater than a critical value (Q2 limit = 0.097, which corresponds with p < 0.05 [15]). Partial least squares regression coefficients, which are related to the centered and scaled data, express the relationship between the response variable Y and the prediction variables X. The PLS has some important advantages if compared with multiple linear regression: PLS allows the number of prediction variables to be greater than the number of observations, PLS can work with high numbers of correlated X variables (multiple collinear data), and PLS tolerates a certain amount of missing data. Multiple linear regressions do not work adequately under these conditions. Because pH and CEC are the most important parameters influencing zinc bioavailability to E. albidus [4], PLS models were developed on the basis of these parameters. As the CEC was reported only for the Budel soil (11 cmol/kg) and Lufa soils (Lufa, Speyer, Germany; 6.1 cmol/kg) [16], estimations had to be used for the other soils. The CEC of artificial soils was estimated according to the equation CEC (cmol/kg) = 1.41 OM (% dry wt peat) + 0.93 [4]. Cation exchange capacity of field soils was estimated according to the equation CEC (cmol/kg) = 2 OM (% dry wt) + 0.5 clay (% dry wt). The CEC and total zinc concentration of the soils were log transformed prior to PLS analyses; pH was not transformed because it is already based on a log scale.
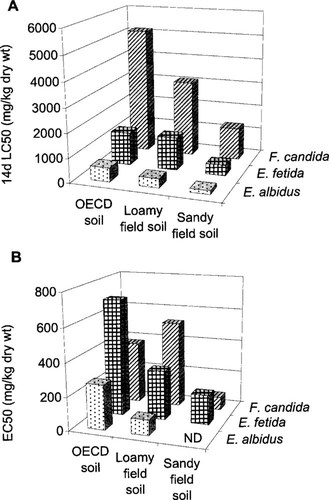
Acute (A) (14-d LC50s) and chronic (B) toxicity of zinc toward Folsomia candida, Enchytraeus albidus, and Eisenia fetida (28-d EC50s number of juveniles, 42-d EC50s number of juveniles, and 21-d EC50s number of cocoons, respectively). ND = not determined.
RESULTS
Ecotoxicity tests
Acute toxicity tests with the three invertebrates used in this study revealed that E. albidus was most sensitive to zinc followed by E. fetida and F. candida (Fig. 1A and Table 2). The interspecies difference in chronic toxicity was less clear and depended on the used soil type (Fig. 1B and Table 2). Ecotoxicity was always highest in the acid sandy soil, intermediate in loamy soil with a relatively high pH and high clay content but a relatively low organic matter content, and toxicity was lowest in the standard artificial OECD soil with a high pH, a high clay content, and the highest organic matter content. With F. candida, however, the chronic toxicity in the loamy soil was somewhat less than in OECD soil. Chronic toxicity toward E. albidus could not be assessed in the sandy soil because this species did not reproduce in this substrate.
Modeling zinc toxicity
To develop models relating the toxicity of zinc to the most important parameters controlling bioavailability, the experimental results in this study were combined with data obtained from literature. The PLS models based on pH and the CEC resulted in reasonably good models predicting acute or chronic zinc toxicity to E. fetida and F. candida. For other organisms, insufficient data were available to develop reliable models.
A PLS model based on the 21 LC50s for E. fetida (Table 2) could predict the acute toxicity of zinc quite well (R2 = 0.70, (Q2 = 0.62) (Fig. 2A). Similarly, the PLS model based on the 20 EC50s for E. fetida and Eisenia andrei cocoon production (Table 2) predicted the chronic toxicity of zinc quite well (R2 = 0.69, (Q2 = 0.62) (Fig. 2B). The PLS model based on the 16 LC50s for F. candida (Table 2) could predict the acute toxicity of zinc very well (R2 = 0.87, (Q2 = 0.85) (Fig. 3A), although it should be noted that not much variation existed in the soil types used. The PLS model based on 26 EC50s for F. candida juvenile production did not predict the chronic toxicity of zinc as well as the acute toxicity (R2 = 0.38, (Q2 = 0.36) (Fig. 3B). The toxicity data of the soils that were equilibrated for one month and longer before testing [17, 18] were removed for the development of the latter model. In all models, pH and CEC were both important variables with a similar contribution to the explanation of the variance (Table 3). Addition of quadratic factors (pH2 and CEC2) or interaction terms (pH·CEC) slightly increase the explained variance (R2) but considerably reduced the predictive value (Q2) of the models for E. fetida, while the models for F. candida were only marginally improved by the inclusion of the interaction coefficients.
DISCUSSION
The chronic toxicity test with E. albidus could not be performed in the Kalmthout field soil because the enchytraeids did not reproduce in this soil. This was probably due to its low pH of 4.4. Dirven-Van Breemen et al. [19] already reported that the reproduction in artificial soils was inversely related to the pH of the soil with reproduction almost ceasing at pH 4. These findings indicate that reproduction tests with E. albidus may not be suited for toxicity assessments of all types of field soils.
The presented literature data clearly indicate that an increasing number of reliable terrestrial ecotoxicity data are becoming available. However, the number of test species used is still limited, and therefore it is not possible to calculate reliable hazardous concentrations (e.g., hazardous concentration for 5% of the species [HC5] [1]) for soil ecosystems. Furthermore, hazardous concentrations based on the type of toxicity data as presented in this study for terrestrial invertebrates exposed to zinc (Table 2) would considerably overlap with background concentrations of zinc. This is due to the fact that both toxicity data and background concentrations are expressed in total and not in bioavailable concentrations. Indeed, not accounting for changes in bioavailability can have serious consequences for environmental risk assessment. This is especially true when it is considered that zinc is an essential element, which means that not only toxicity but also deficiency should be considered. In addition, the toxicity varies not only between species but also between soil types. Consequently, at the same total zinc concentration in the soil, deficiency may occur in a soil with a high CEC and a high pH, whereas toxicity may occur in a soil with low values for these parameters.
In their review on the influence of soils characteristics on the toxicity of metals to soil invertebrates, Van Gestel et al. [20] concluded that pH, cation exchange capacity, and clay and soil organic matter content are probably the most important soil parameters affecting metal toxicity. Spurgeon and Hopkin [21] found that the 21-d LC50 and the 21-d EC50 (cocoon production) of the earthworm E. fetida exposed to zinc decreased with increasing pH or increasing organic matter content of the artificial soil. Using a fractional factorial design, Lock et al. [4] found that CEC and pH were the most important parameters affecting zinc toxicity to E. albidus in spiked artificial soils. The subsequent use of a central composite design indicated that acute zinc toxicity to E. albidus could be modeled in function of pH and CEC, and this model was validated with spiked field soils. Van Straalen and Denneman [2] tried to account for metal bioavailability by normalizing toxicity data to a reference value for a standard soil (25% clay and 10% organic matter) using linear equations developed for relating natural background concentrations of metals in soil to clay and organic matter content. However, the previously mentioned equations are not necessarily suited to predict the ecotoxicity of metals. By varying only the type of clay or organic matter of standard artificial soil [5], Lock and Janssen [22] found that acute zinc toxicity to E. albidus varied over more than an order of magnitude, depending on the type of clay or organic matter, while zinc toxicity in these experiments could be accurately predicted with the equations on the basis of pH and CEC as developed by Lock et al. [4].
| Organism | Medium | pH | OM | Clay | E | D | NOECa | LOECb | EC10 (95% C.L.c) | EC50 (95% C.L.) | LC50 (95% C.L.) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caenorhabditis elegans | Cecil | 6.2 | 1.7 | 16 | 1 d | 1 | 360 (340–379) | [25] | ||||
| Caenorhabditis elegans | Worksham | 5.1 | 3 | 16 | 1 d | 1 | 255 (242–268) | [25] | ||||
| Caenorhabditis elegans | Davidson | 6.1 | 3.4 | 20 | 1 d | 1 | 392 (235–588) | [25] | ||||
| Caenorhabditis elegans | Dyke | 6.2 | 2.2 | 39 | 1 d | 1 | 549 (523–575) | [25] | ||||
| Aporrectodea caliginosa | Al-Qanater | 7.1 | 21.6 | 0 | 56 | 568 (217–1,480) | 826 (540–1,270) | 3,610 (3,510–3,710) | [26] | |||
| Aporrectodea caliginosa | Rockalls | 6.4 | 12.4 | 9.7 | 7 d | 42d | 297 | 400 | 206 (133–268) | 442 (370–522) | 1,695 (1,621–1,775) | [27] |
| Aporrectodea rosea | OECDe | 6 | 10 | 20 | 3 d | 14 | 561 (499–633) | [28] | ||||
| Eisenia andrei | OECD | 6 | 10 | 20 | 0 | 21 | 320 | 560 | 659 (552–786) | [29] | ||
| Eisenia andrei | OECD | 6 | 10 | 20 | 0 | 56 | 320f | 560f | 512f | [29] | ||
| Eisenia fetida | OECD | 6 | 10 | 20 | 0 | 14 | 662 (574–674) | [30] | ||||
| Eisenia fetida | OECD | 6.3 | 10 | 20 | 0 | 14 | 1,010 (780–1,370) | [31] | ||||
| Eisenia fetida | OECD | 6.3 | 10 | 20 | 0 | 56 | 100 | 400 | 276(202–375) | 745 (591–957) | [31] | |
| Eisenia fetida | OECD | 6.1 | 10 | 20 | 3 d | 21 | 100 | 400 | 357 | 1,078 (789–1,449) | [24] | |
| Eisenia fetida | OECD | 6 | 10 | 20 | 3 d | 21d | 350 | 620 | 623 | 1,106 (1,010–1,212) | [28] | |
| Eisenia fetida | OECD | 6 | 5 | 20 | 0 | 21 | <100 | 136 | 620 (510–679) | [21] | ||
| Eisenia fetida | OECD | 5 | 5 | 20 | 0 | 21 | <100 | 199 | 591 | [21] | ||
| Eisenia fetida | OECD | 4 | 5 | 20 | 0 | 21 | 100 | 350 | 142 | 451 (411–493) | [21] | |
| Eisenia fetida | OECD | 6 | 10 | 20 | 0 | 21 | 350 | 620 | 462 | 791 | [21] | |
| Eisenia fetida | OECD | 5 | 10 | 20 | 0 | 21 | 100 | 350 | 343 | 601 (528–704) | [21] | |
| Eisenia fetida | OECD | 4 | 10 | 20 | 0 | 21 | 100 | 350 | 189 | 617 (545–730) | [21] | |
| Eisenia fetida | OECD | 6 | 15 | 20 | 0 | 21 | 350 | 620 | 592 | 1,613 (1,515–1,794) | [21] | |
| Eisenia fetida | OECD | 5 | 15 | 20 | 0 | 21 | 350 | 620 | 548 | 992 (913–1,106) | [21] | |
| Eisenia fetida | OECD | 4 | 15 | 20 | 0 | 21 | 100 | 350 | 230 | 474 (440–539) | [21] | |
| Eisenia fetida | OECD | 6 | 10 | 20 | 0 | 21d | 350 | 620 | 382 | 1,598 (1,460–1,760) | [32] | |
| Eisenia fetida | OECD | 6 | 10 | 20 | 0 | 21d | 350 | 620 | 308 | 1,235 (811–2,855) | [32] | |
| Eisenia fetida | OECD | 6 | 10 | 20 | 0 | 21d | <190 | 234 | 1,131 | [32] | ||
| Eisenia fetida | Rockalls | 6.4 | 12.4 | 9.7 | 7 d | 42d | 838 | 1,650 | 246 (212–311) | 1,898 (953–2,174) | 3,172 (3,150–3,215) | [27] |
| Eisenia fetida | OECD | 6 | 10 | 20 | 0 | 21 | 560 | 1,000 | 438 (389–480) | 704 (658–754) | 1,340 | P.S.g |
| Eisenia fetida | Termunck | 6.3 | 1.5 | 17 | 0 | 21 | 180 | 320 | 127 (100–150) | 294 (264–323) | 1,340 | P.S. |
| Lumbricus rubellus | OECD | 6 | 10 | 20 | 3 d | 14 | <190 | 348 | 728 (627–845) | [29] | ||
| Lumbricus rubellus | Rockalls | 6.4 | 12.4 | 9.7 | 7 d | 42d | 638 | 724 | 88 (52–404) | 599 (483–757) | 1,734 (1,651–1,791) | [24] |
| Lumbricus terrestris | Rockalls | 6.4 | 12.4 | 9.7 | 7 d | 42d | 1,767 | 2,217 | 115 (62–706) | 1,029 (567–1,426) | 2,378 (2,132–2,636) | [24] |
| Enchytraeus albidus | OECD | 6 | 10 | 20 | 0 | 42d | 180 | 320 | 132 (86–168) | 267 (220–327) | 566 (530–600) | P.S. |
| Enchytraeus albidus | Termunck | 6.3 | 1.5 | 17 | 0 | 42d | 100 | 180 | 35.7 (28.7–41.9) | 92.4 (83.2–103) | 375 (328–433) | P.S. |
| Enchytraeus albidus | Kalmthout | 4.5 | 4.8 | 1 | 0 | 42d | 147 (131–163) | P.S. | ||||
| Enchytraeus crypticus | OECD | 6 | 10 | 20 | 7 d | 28 | 188 (149–237) | [33] | ||||
| Folsomia candida | Budel | 6 | 3 | 1.4 | 0 | 28 | 185(158–216) | [16] | ||||
| Folsomia candida | LUFA | 6 | 3.3 | 3.3 | 0 | 28 | 348 (307–393) | [16] | ||||
| Folsomia candida | Budel | 6 | 3 | 1.4 | 0 | 42 | 210 (167–264) | [16] | ||||
| Folsomia candida | LUFA | 6 | 3.3 | 3.3 | 0 | 42 | 363 (289–455) | [16] | ||||
| Folsomia candida | OECD | 6 | 10 | 20 | 2 d | 28 | 620 | 1,200 | 900 | [34] | ||
| Folsomia candida | OECD | 5 | 10 | 20 | 2 d | 28 | 300 | 1,000 | 600 | [34] | ||
| Folsomia candida | OECD | 4.5 | 10 | 20 | 2 d | 28 | 300 | 1,000 | 590 | [34] | ||
| Folsomia candida | Panheel | 4.7 | 2.4 | 1.9 | 0 | 28 | 178 (140–228) | 266(229–310) | 727 (648–816) | [35] | ||
| Folsomia candida | Panheel | 4.7 | 2.4 | 1.9 | 0 | 28 | 239 (138–416) | 306 (234–400) | 796 (714–888) | [35] | ||
| Folsomia candida | Panheel | 4.7 | 2.4 | 1.9 | 0 | 42 | 864 (777–961) | [35] | ||||
| Folsomia candida | Panheel | 4.7 | 2.4 | 1.9 | 0 | 42 | 821 (740–911) | [35] | ||||
| Folsomia candida | Panheel | 4.7 | 2.4 | 1.9 | 0 | 42 | 116 (79.6–168) | 244 (206–289) | 741 (690–795) | [35] | ||
| Folsomia candida | Panheel | 4.7 | 2.4 | 1.9 | 0 | 35 | 159 (122–207) | 276 (246–308) | 650 (573–738) | [35] | ||
| Folsomia candida | Panheel | 4.7 | 2.4 | 1.9 | 0 | 28 | 136 (113–163) | 261 (240–283) | 699 (663–738) | [35] | ||
| Folsomia candida | Panheel | 4.7 | 2.4 | 1.9 | 0 | 23 | 247 (214–285) | 341 (313–371) | 580 (547–615) | [35] | ||
| Folsomia candida | Panheel | 5.7 | 2 | 2.9 | 1 m | 56 | 979 (892–1,074) | [17] | ||||
| Folsomia candida | Panheel | 6.7 | 2 | 2.9 | 14 m | 35 | 1,491 (1,143–1,947) | [17] | ||||
| Folsomia candida | Panheel | 6.7 | 2 | 2.9 | 26 m | 35 | 1,749 (886–3,450) | [17] | ||||
| Folsomia candida | OECD | 6 | 10 | 20 | 0 | 28 | 626 (526–744) | [36] | ||||
| Folsomia candida | OECD | 6 | 10 | 20 | 0 | 42 | 683 (547–855) | [36] | ||||
| Folsomia candida | OECD | 6 | 10 | 20 | 0 | 28 | 620 | 1,200 | 900 | [37] | ||
| Folsomia candida | OECD | 6 | 10 | 20 | 0 | 42 | 300 | 1,000 | 590 | [37] | ||
| Folsomia candida | OECD | 6 | 10 | 20 | 0 | 28 | 368 | 269 (181–400) | 487 (407–582) | [18] | ||
| Folsomia candida | Panheel | 4.7 | 2.4 | 1.9 | 0 | 28 | 335 | 355 (288–438) | 572 (507–646) | [18] | ||
| Folsomia candida | Panheel | 6.5 | 2.4 | 1.9 | 20 m | 28 | 709 | 1,059 (655–1,713) | 2,178 (1,527–3,106) | [18] | ||
| Folsomia candida | Panheel | 4.7 | 2.4 | 1.9 | 0 | 28 | 298 | 136 (113–163) | 261 (240–283) | 719 (681–759) | [24] | |
| Folsomia candida | Panheel | 4.7 | 2.4 | 1.9 | 0 | 28 | 160 | 98.6 (56.8–171) | 184 (148–228) | 625 (425–918) | [38] | |
| Folsomia candida | Panheel | 5 | 2.4 | 1.9 | 0 | 28 | 256 | 369 (187–726) | 389 (290–521) | 476 (410–554) | [38] | |
| Folsomia candida | Panheel | 4.7 | 2.4 | 1.9 | 0 | 28 | 256 | 165(113–240) | 258 (216–309) | 670 (483–927) | [38] | |
| Folsomia candida | Panheel | 4.7 | 2.4 | 1.9 | 0 | 28 | 410 | 149 (33.7–663) | 291 (145–582) | 1,085 (332–3,540) | [38] | |
| Folsomia candida | OECD | 6 | 10 | 20 | 0 | 28d | 100 | 180 | 123 (89.8–155) | 375 (321–437) | 5,150 (4,360–6,180) | P.S. |
| Folsomia candida | Termunck | 6.3 | 1.5 | 17 | 0 | 28d | 320 | 560 | 316(225–381) | 522 (444–615) | 3,100 (2,680–3,580) | P.S. |
| Folsomia candida | Kalmthout | 4.5 | 4.8 | 1 | 0 | 28d | 18 | 32 | 14.6 (10.4–19) | 77.7 (65.7–92.1) | 1,310 (1,190–1,430) | P.S. |
- a NOEC = no-observed-effect concentration.
- b LOEC = lowest-observed-effect concentration.
- c C.L. = confidence limit.
- d Duration of acute test is 14 d.
- e OECD = Organization for Economic Cooperation and Development.
- f Juveniles instead of cocoons.
- g P.S. = present study.
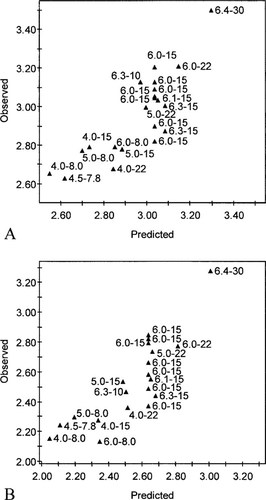
Partial least square showing observed acute (A) and chronic (B) log-transformed toxicity data (LC50s and EC50s, respectively) for Eisenia fetida and Eisenia andrei exposed to zinc versus the toxicity data predicted based on the pH and the log-transformed cation exchange capacity (CEC) with indication of the pH and the CEC (cmol/kg) of the used soils.
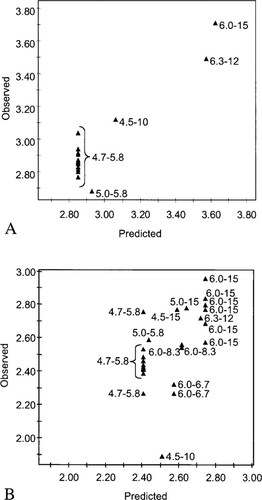
Partial least square showing observed acute (A) and chronic (B) log-transformed toxicity data (LC50s and EC50s, respectively) for Folsomia candida exposed to zinc versus the toxicity data predicted on the basis of the pH and the log-transformed cation exchange capacity (CEC) with indication of the pH and the CEC (cmol/kg) of the used soils.
| Species | Endpoint | pH | log (CEC) |
|---|---|---|---|
| Eisenia fetida | Acute | 0.58 | 0.48 |
| Chronic | 0.44 | 0.61 | |
| Folsomia candida | Acute | 0.46 | 0.52 |
| Chronic | 0.29 | 0.39 |
In the added risk approach [23], maximum permissible concentrations are derived by adding the HC5 to the natural background concentration. In this approach, the bioavailability of the background concentration is set to zero, while all adverse effects are assumed to be exclusively dependent on the anthropogenically added fraction. In this way, the maximum permissible concentration is artificially elevated without taking the process of aging into account: With time, the bioavailability of the added fraction will also decrease. As soil contamination is in general a gradual process, the bioavailability in contaminated field soils will rarely be as high as in spiked soils.
Most ecotoxicity tests use spiked soils that have not been aged long enough to allow the metal to reach equilibrium. A few studies compared the toxicity of zinc in standard artificial soil with the toxicity in field soils around a zinc smelter [16, 24]. Zinc toxicity in the latter soils was much lower in comparison with the spiked soils, but it remains unclear whether this is due to the soil parameters influencing bioavailability or to the effect of aging. In two other studies, Smit et al. [17] and Smit and Van Gestel [18] compared the toxicity immediately after spiking and after aging for up to 26 months in the field or in the laboratory. Zinc toxicity decreased considerably after aging, but a parallel increase in pH of at least one unit was observed. Therefore, it remains unclear which part of this decrease in zinc bioavailability is due to the increased pH and which part is caused by the effect of aging.
In the present study, acute and chronic zinc toxicity was assessed for E. fetida, E. albidus, and F. candida in three different soil types. These data, together with the existing literature data, allowed us to develop models, based on the partial least squares to latent structures regression technique, predicting zinc toxicity as a function of the pH and the CEC of the soil. This type of model allows the normalization of ecotoxicity data and is therefore a considerable step forward in the incorporation of metal bioavailability in risk assessments. However, most available toxicity data are based on experiments conducted in soils spiked with metals, which results in effect concentrations that are not representative for field situations. Therefore, studies assessing the effect of aging are urgently needed to allow an effect-based risk assessment of metal contaminated field soils.
Acknowledgements
This research was supported by the Public Waste Agency of Flanders. We would like to thank Tom Van Wichelen for the metal analyses.




