Gonadal development and endocrine responses in Japanese medaka (Oryzias latipes) exposed to o,p′-DDT in water or through maternal transfer
Abstract
Various isomers and metabolites of DDT disrupt endocrine systems and gonadal development in fish and wildlife, and o,p′-DDT has been shown to be an relatively potent estrogen agonist. In this study, we exposed Japanese medaka (Oryzias latipes) to o,p′-DDT using two exposure protocols: direct exposure of early life stages to aqueous solutions from 1 to 100 d posthatch and exposure of female medaka to aqueous solutions, followed by mating with unexposed males to produce offspring that were exposed through mechanisms of maternal transfer. In treatments with direct aqueous exposures, an intersex condition of the gonad (testis-ova) was observed in male medaka exposed at early life stages to nominal o,p′-DDT concentrations of 50, 10, and 5 μg/L, indicating that this estrogen agonist can alter gonadal development when exposure occurs continuously over the period of gonadal differentiation. Comparisons with previously published data on the induction of testis–ova by exposure to nonylphenol (NP) and octylphenol (OP) indicated that the relative potencies for induction of intersex in medaka are o,p′-DDT > NP ≈︁ OP, which is not consistent with the relative estrogenic potencies of OP > NP > o,p′-DDT observed in the yeast estrogen screening (YES) assay. In the maternal transfer protocol, no testis-ova were observed in the offspring, although there was some delay in time to hatch of the offspring. Medaka exposed by maternal transfer showed no other toxicological responses during early life stages, but when treated fish reached sexual maturity, the females showed more advanced development of oocytes. In addition, when medaka exposed by maternal transfer were subsequently exposed at 10 months of age to 17β-estradiol (12 mg/L), there was a significantly greater induction of hepatic vitellogenin in DDT-exposed males in comparison to control males, indicating that exposure to estrogenic chemicals during early life stages may potentiate vitellogenin induction following exposure events later in the life of the fish.
INTRODUCTION
A broad range of environmental contaminants are endocrine-disrupting substances that have the potential to alter sexual development and reproductive success in fish. Impacts observed in fish populations that have been attributed to exposure to endocrine-modulating substances include altered serum steroid levels and delayed gonadal maturation in lake whitefish (Coregonus clupeaformis) near pulp mills [1], masculinization of mosquitofish (Gambusia affinis) in streams downstream of pulp mills [2], synthesis of egg-yolk protein, vitellogenin and reduced serum testosterone in male carp (Cyprinus carpio) captured near sewage treatment plants [3], and a high prevalences of intersex gonads in roach (Rutilus rutilus) collected near sewage treatment plants [4] and in flounder (Platichthys flesus) collected from contaminated estuaries [5]. We do not know the identity of the compounds inducing these responses, and we know little about the mechanisms of endocrine modulation and alterations to gonadal differentiation in teleosts.
Isomers of DDT and their metabolites have been identified as compounds with endocrine-modulating activity. The o,p′-DDT isomer has been identified as a relatively potent estrogen agonist in in vitro tests with estrogen-responsive tumor cells [6, 7] and in the yeast estrogen screening (YES) assay [7-9]. This isomer also induced gonadotropin release and enhanced ovarian growth in Atlantic croaker (Micropogonius undulatus) exposed to the test compound at dietary doses of 0.02 and 0.1 μg/g body weight per day for 3 and 7 weeks [10]. This compound also induced feminization of the gonads in male California gulls (Larus californicus) when eggs were injected with doses as low as 2 μg/g [11]. Finally, DDT isomers (including o,p′-DDT) and their metabolites have been implicated in reduced development of penises in male alligators from contaminated Lake Apopka, Florida, USA [12]. It is widely believed that alterations to the development of gonads and genitalia in fish and wildlife occur when exposure to DDT and other endocrine-modulating substances begins in ovo or in utero, during the period of differentiation of these tissues into the male and female phenotype [13]. It is reasonable to assume that exposure to lipid-soluble endocrine-modulating substances begins as a result of maternal transfer of these compounds to the young through deposition of egg yolk in oviparous animals or through transplacental or lactational exposure in mammals. One possible result of exposure of fish and wildlife to endocrine-modulatory substances during critical periods of development is modification to the organization of the reproductive and endocrine system, resulting in reduced reproductive success at sexual maturity [13].
The Japanese medaka (Oryzias latipes) is an ideal test organism for studies of the effects of endocrine-modulating substances in fish [14]. Several endpoints that impact on reproductive success in fish have been studied with medaka, including embryolarval toxicity, developmental abnormalities, differentiation of the gonad, development of secondary sexual characteristics, sex reversals, vitellogenin induction, fecundity, and mating behavior [14, 15]. An intersex condition of the gonad called testis–ova is induced in male medaka when exposures to aqueous solutions of estrogenic chemicals begin during differentiation of the testis [16, 17]. Techniques have also been developed for measuring the vitellogenic response in medaka exposed to estrogenic chemicals [18].
One of the goals of this project was to determine the effects of a known estrogen agonist, o,p′-DDT, on the development of the gonad in male medaka. Our previous studies have shown that alkylphenol xenoestrogens that have a relatively low potential for bioaccumulation in fish induce testis–ova in male medaka at concentrations in the 50- to 100-μg/L range [16, 17]. We wanted to determine whether an estrogen agonist with high potential for bioaccumulation in fish could induce this intersex condition when medaka are exposed to low aqueous concentrations. Early life stages of medaka were exposed to aqueous concentrations of o,p′-DDT between 1 and 50 μg/L, beginning at 1 d posthatch during differentiation of the gonad to a testis. The potency of this in vivo response (testis–ova induction) was compared to the estrogenic potency of o,p′-DDT in the in vitro YES assay. Exposure of populations of fish to DDT and other lipid-soluble contaminants during critical stages of development are most likely to begin in ovo. Therefore, a second goal of this study was to determine whether alterations to gonadal development could occur in medaka exposed to o,p′-DDT through maternal transfer of this compound from females to eggs. Finally, we assessed whether exposure to o,p′-DDT by maternal transfer can modify endocrine responses in medaka after they reach sexual maturity and, specifically, whether the vitellogenic response in mature medaka is affected by prior exposure at early life stages. This research goal is important in determining whether early life stage exposures induce organizational effects that are expressed later in the life of the organism.
MATERIALS AND METHODS
Direct aqueous exposures
Eggs were collected from female medaka from a breeding stock of adult fish as described previously [16]. Eggs from several females were pooled in embryo-rearing medium in a petri plate and checked for fertilization. Chronic exposures of medaka to o,p′-DDT (Supelco, Toronto, ON, Canada) were initiated at 1 d posthatch in a static-renewal system. Exposures took place in glass containers of progressively larger sizes (1, 2, and 10 L) as the medaka grew, filled with dechlorinated tap water at 20 to 22°C. The aqueous solutions were renewed every 48 h and exposures terminated at 100 d posthatch.
Preliminary toxicity tests with early life stages of medaka conducted as described previously [16] indicated that o,p′-DDT was embryotoxic to medaka at aqueous concentrations of about 100 μg/L. Based on these data, sublethal treatments were selected at nominal o,p′-DDT concentrations of 50, 10, 5, 1, and 0 (control) μg/L. The test solutions were prepared by adding volumes (<10 μl) from a DDT stock solution in acetone to the water in test containers. In the control treatment, acetone alone was added in the same volumes as the experimental treatments. No control treatments without solvent were included in this experiment. There were 60 fish in each treatment at the start of the experiment. The fish were maintained in a 16:8 LD (light:dark) cycle and were fed a diet of newly hatched brine shrimp twice daily for the duration of the experiment as described previously [16].
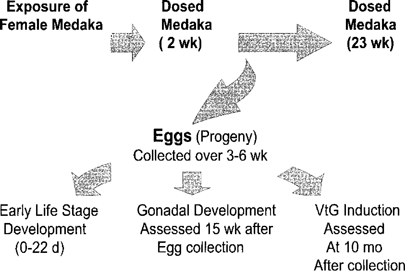
Flow diagram of the maternal transfer experiment showing the timing of exposure of female medaka to o,p′-DDT and experimentation with all life stages of the progeny of the dosed medaka.
After 100 d of exposure, all individuals were sacrificed with an overdose of tricaine methanesulfonate (MS-222). The body weight (g) and length (cm) were recorded and the fish placed in tissue capsules and fixed in Calex fixative (Fisher, Toronto, ON, Canada). The fixed medaka were prepared for histological examination by embedding whole in paraffin wax and sectioning (5–10 μm) with a microtome. On average, five step-sectioned slides were prepared per fish to ensure sectioning of the gonad. The sections were stained using hematoxylin and eosin, mounted in DPX, and examined under a light microscope. The gonad was examined to determine the phenotypic sex of the fish or the presence of the intersex condition, testis–ova. Sex ratios were calculated to the nearest two significant digits.
To determine the persistence of o,p′-DDT in test containers under the conditions used in chronic tests, stock solutions in acetone were added to 5 L of water in 10-L glass aquaria at nominal test concentrations of 0,1,5, and 10 μg/L as described previously. There were 40 fish (0.05–0.10 g) present in each aquarium for this study. All tests were conducted in triplicate. Water samples of 250 ml were collected from each test container at 0, 24, and 48 h postaddition.
Maternal transfer
Figure 1 illustrates the protocols used in the maternal transfer experiment, including the timing of exposures of female medaka to o,p′-DDT and the various treatments with the progeny of these dosed medaka. Female medaka from the brood stock (six months old; 0.2–0.3 g) were divided into control and experimental treatments (n = 20/treatment) and placed in separate all-glass containers filled with 20 L of dechlorinated tap water at 20 ± 2°C. Water in the experimental group was spiked with a stock solution of o,p′-DDT in acetone to a nominal concentration of 2.5 μg/L. This concentration was chosen because it is within the range of concentrations (1–50 μg/L) that induced sublethal responses in a previous study with the aquarium fish Poecilia latipinna [19]. The control treatment received a 50-μl volume of acetone only. The water was changed every 48 h in both the experimental and the control treatment throughout the uptake phase of the experiment (2 weeks). Fish were fed a diet of brine shrimp twice daily for the duration of the exposure phase. Medaka (n = 3) were removed for analysis of DDT residues at 0, 1, and 2 weeks of the exposure phase.
At the end of the 2-week exposure phase (Fig. 1), dosed female fish were removed from the experimental and control containers and placed in clean water at 26°C with unexposed male medaka from the brood stock. The photoperiod was adjusted, and the fish were fed newly hatched brine shrimp to induce spawning as described previously [16]. Within 2 weeks, the females began to produce eggs, which were collected daily from spawning females over a 27-d period, pooled according to the day of collection, and placed in rearing solution. No attempt was made to determine which individual females from the exposed and control treatments were spawning. A pooled sample of approximately 40 newly fertilized eggs (approx. 0.1 g) was collected at the beginning and end of the spawning period (3 and 6 weeks postexposure, respectively) for analysis of DDT concentrations. Dosed female medaka were removed for analysis of DDT residues at 5 weeks (n = 3), 9 weeks (n = 1), and 23 weeks (n = 3) after exposures began.
Progeny of females from experimental and control treatments (pooled according to day of collection) were monitored for development during early life stages (Fig. 1). Eggs were placed in plastic petri plates in rearing solution in an incubator at 26°C and monitored for 22 d from fertilization to the beginning of exogenous feeding (swim-up) to determine time to hatch and cumulative mortalities. Fish that hatched and reached swim-up were transferred to 1-L glass containers filled with dechlorinated tap water at 20 to 22°C and fed newly hatched brine shrimp twice daily. Fish were transferred to 2-L glass containers and 10-L glass aquaria as they grew, and at 15 weeks after collection of the eggs, subsamples of the fish (n = 140 exposed; n = 80 control) were taken at random from aquaria for histological analysis of gonadal development (Fig. 1) as described previously. The gonad was examined to determine the phenotypic sex of the fish or the presence of the intersex condition, testis–ova. At the end of this part of the experiment (21 weeks postexposure, 15 weeks after collection of eggs), several medaka from the experimental and control treatments (n = 3 per treatment) were removed for analysis of DDT residues.
The remaining fish were raised for an additional six months in glass aquaria (10 months after initial collection of eggs), and at the end of this period, male and female fish from the control and experimental groups were removed at random from aquaria for experiments to determine the degree of estradiol-induced vitellogenin induction (Fig. 1). All groups were exposed for 4 d in static exposures to 17β-estradiol at a concentration of 12 mg/L, as confirmed by enzyme-linked immunoassays of the solutions using immunoassay reagents provided by C. Munro, University of California at Davis, California, USA. Immediately after this 4-d exposure, male and female medaka were removed from exposure vessels and sacrificed for analysis of vitellogenin in liver samples.
YES assay
The in vitro YES assay was conducted essentially as described by Routledge and Sumpter [20] using a yeast strain provided by J. Sumpter of Brunel University, Middlesex, United Kingdom. The assay system utilizes a recombinant strain of yeast in which DNA sequences of the human estrogen receptor are integrated into the yeast genome and human estrogen-responsive elements are integrated into plasmids. Binding of chemicals to the human estrogen receptor expressed in the yeast is followed by binding of the ligand-receptor complex to estrogen-responsive elements, which signals transcription of the reporter gene for β-galactosidase that induces a colorimetric response.
Before the assay, stock solutions were prepared in methanol of o,p′-DDT (Supelco, Toronto, ON, Canada) and 4-nonylphenol (4-NP) and 4-tert-octylphenol (4-tert-OP) purchased from Aldrich, Toronto, Ontario, Canada. Dilutions of 17β-estradiol (Sigma, Toronto, ON, Canada) varying between 0.001 and 2.5 ng/ml were tested as a positive control. Blanks consisted of methanol only in the test wells. In a laminar flow hood, stock solutions of test compounds were serially diluted (1:2) and 10-μl aliquots of each dilution transferred to a 96-well microtiter plate. The methanol solvent was allowed to evaporate to dryness in the plate, and then 200-μl aliquots of assay medium with a chromogenic substrate, red-β-D-galactopyranoside, were dispensed into each well. The plates were sealed with autoclave tape and incubated at 32°C. After 3 d incubation, a plate reader (BioRad, Hercules, CA, USA) was used to measure color development of the medium at 540 nm, with corrections for turbidity measured at 630 nm. All test compounds were tested in replicates of n = 3 and data presented as the mean response ± the standard deviation.
Analysis of vitellogenin
Because plasma is difficult to obtain from medaka, individual livers from adult medaka exposed to estradiol were removed and homogenized using molecular grinding resin (Genotech, St. Louis, MO, USA) in 50 μl of buffer (0.1 M Tris-HCl, 0.15 M KCl, 1 mM ethylenediaminetetraacetic acid) with aprotinin (Sigma, St. Louis, MO, USA). After homogenizing, the tissue was centrifuged at 10,000 g for 10 min and the supernatent removed. A subsample was used to determine protein concentration (BioRad). For western blot analysis, 10 (xg of protein from each sample was run on a 7.5% sodium dodecyl sulfate–polyacrylamide gel and transferred to a nitrocellulose membrane. For slot blot analysis, 5 μg of protein from each sample were also directly applied to a nitrocellulose membrane. Two samples did not contain sufficient amounts of protein to be analyzed by both western and slot blot analysis, so the remainder of these two samples (<5 μg) was included in the slot blot procedure but excluded from quantitative analysis. Using a slot blot, each sample is confined to a single slot with the same dimensions, allowing the optical density of the slots to be compared directly. However, quantitative analysis of western blots is not practical because it requires integration of optical density across multiple bands.
All nitrocellulose membranes were developed by the same procedure. Briefly, the membranes were incubated for 1 h at room temperature in a 2% bovine serum albumin solution, followed by incubation overnight at 4°C with a 1:500 dilution of a mouse antivitellogenin antibody obtained from N. Denslow, University of Florida, Gainesville, Florida, USA. The membranes were washed, incubated for 1.5 h in a secondary alkaline phosphatase antimouse IgG, washed, and developed using 5-bromo-4-chloro-3-indolyl phosphate and p-nitroblue tetrazolium chloride, both obtained from Sigma.
The amount of vitellogenin in each sample was compared by measuring the optical density of the enzyme-linked antibody staining in slots generated with the slot blot. The nitrocellulose membrane was scanned into a computer and opened in Scion Image (Scion, Frederick, MD, USA). In this program, a rectangle is drawn around one slot and the optical density measured; then the same rectangle is moved to the next slot and its optical density measured.
| Treatment | Length (cm) | Weight (g) | No. females | No. males | Sex ratio (M:F) | No. males with T-O |
|---|---|---|---|---|---|---|
| Control o,p′-DDT (μg/L) | 1.69 ± 0.47 | 0.052 ± 0.042 | 10 | 13 | 1.3:1 | 0 |
| 1 | 1.51 ± 0.23 | 0.030 ± 0.030 | 15 | 26 | 1.7:1 | 0 |
| 5 | 1.66 ± 0.46 | 0.050 ± 0.052 | 25 | 19 | 0.8:1 | 3 |
| 10 | 1.50 ± 0.26 | 0.031 ± 0.016 | 24 | 12 | 0.5:1a | 10 |
| 50b | − | − | 5 | 1 | 0.2:1 | 1 |
- a Sex ratio significantly different from controls.
- b No statistical analysis because of small sample number.
Analysis of o,p′-DDT
The DDT was extracted from water samples by liquid–liquid partitioning into distilled-in-glass dichloromethane (DCM). The 250-ml water sample was placed in a 1-L separatory funnel and extracted sequentially with 25, 20, and 15 ml DCM, each with 5 min stirring. The pooled solvent layer was dehydrated by passing through a 2-cm layer of anhydrous sodium sulfate (prewet with DCM). The eluate from sodium sulfate was concentrated on a rotary evaporator to a volume of approx. 2 ml and then under a stream of nitrogen to 0.5 ml. The samples were made up to 1 ml with hexane to yield a sample in hexane:DCM (1:1), which was subjected to further cleanup (see the following discussion) prior to analysis.
Medaka tissues and eggs were prepared for analysis of o,p′-DDT as described by Metcalfe and Metcalfe [21]. Briefly, samples of 0.1 to 0.5 g were Soxhlet extracted into hexane: DCM (1:1) and lipids separated from analytes in the extract by gel permeation chromatography with Biobeads® SX-3 (Merck, Darmstadt, Germany). The lipid fraction from the gel permeation chromatography column was collected and evaporated to dryness for gravimetric determination of lipid content. The gel permeation chromatography fraction containing analytes was concentrated to approx. 2 ml by rotary evaporation and then to 1 ml under a stream of nitrogen prior to further cleanup.
Extracts from water and medaka were further cleaned up on a 4-ml glass microcolumn packed with 2 cm of activated silica gel (60 × 200 mesh; Supelco) and topped with 1 cm of anhydrous sodium sulfate. The column was eluted with 15 ml of hexane:DCM(1:1). The eluate was concentrated on a rotary evaporator and then under a stream of nitrogen, transferred to iso-octane solvent, and placed in an autosampler vial for analysis of o,p′-DDT by high-resolution gas chromatography using a Varian model 3500 gas chromatography (Varian, Walnut Creek, CA, USA) with a 60-m DB-5 fused silica column (0.25-mm ID, 0.25-μm film thickness), splitless injection, 63Ni electron capture detector, and 8200 CX autosampler. The gas chromatography conditions were as described by Metcalfe and Metcalfe [21]. The analyte was quantified with limits of detection of 0.5 ng/ml by comparison to an external standard of o,p′-DDT purchased from Supelco. No other DDT isomers or metabolites were analyzed in extracts prepared from samples of water or medaka.
Statistical analysis
In tests with aqueous exposures of medaka to different concentrations of o,p′-DDT, data on lengths and weights of medaka were tested for differences between treatments by analysis of variance (p < 0.05). Data on the incidence of testis–ova in male medaka and the relative numbers of males and females were tested for between treatment differences by chi-square analysis (p < 0.05).
For the experiment with medaka exposed to o,p′-DDT through maternal transfer, the median time to hatch of progeny in the exposed and control treatments was calculated by probit analysis using SoftTox® software (WindowChem, Los Angeles, CA, USA). Data collected on mortalities and time to hatch of progeny were tested for differences between the exposed and control treatments with Student's t test. Among mature fish from these treatments, the numbers of female fish with oocytes in the vitellogenic stage and previtellogenic stage were compared by chi-square analysis (p < 0.05). Slot blot data for vitellogenic responses in progeny were tested for differences in optical densities by analysis of variance (p < 0.05), and Tukey's test (p < 0.05) was used for post hoc comparisons between treatments.
In the YES assay, the response representing 25% of the maximum response observed over a range of test concentrations (IC25) was calculated by probit analysis using SoftTox software.
RESULTS
Among medaka exposed to aqueous solutions of o,p′-DDT from 1 to 100 d posthatch, there were a large number of mortalities in treatments with the highest concentration of DDT (50 μg/L), with only six fish surviving to the end of the experiment. In the other treatments, there was good survival (>85%) of medaka and no significant differences in the mean lengths or weights of the fish in these treatments (Table 1). Among the male medaka examined histologically, there were testis–ova observed in the gonads of males from treatments with 5, 10, and 50 μg/L o,p′-DDT (Table 1). There were significant differences in the incidence of testis–ova among treatments, with an apparent concentration-related response. Note that a testis–ova was observed in the only male surviving to the end of the experiment in the 50-μg/L treatment. When fish with testis–ova were included as males in an analysis of sex ratios, there was a significantly smaller ratio of males to females in the 10-μg/L treatment, but ratios were not significantly different in any of the other treatments (Table 1).
Testis–ova were readily observed as both testicular tissue and oocytes in the gonad, although the degree of development of oocytes varied from early to late previtellogenic stages in individual fish. In many cases, a gradient of gonadal differentiation was observed, with the anterior portion of the gonad as ovarian tissue and the posterior portion as testicular tissue. The oocytes present in the testis–ova appeared to be at the same stage of development as oocytes in female medaka.
| Measured concn. (%) | |||
|---|---|---|---|
| Hours | |||
| Nominal DDT concn. (μg/L) | 0 | 24 | 48 |
| 1 | 115.9 ± 22.3 | 14.2 ± 0.8 | 2.2 ± 0.5 |
| 5 | 87.1 ± 9.6 | 19.3 ± 0.4 | 10.0 ± 0.4 |
| 10 | 140.3 ± 24.4 | 7.9 ± 0.3 | 2.5 ± 0.2 |
In female medaka sampled during the exposure phase of the maternal transfer experiment, the concentrations of o,p′-DDT increased rapidly over the 2-week exposure period to a mean of 109.6 μg/g wet weight (3,257 μg/g lipid), which was probably not at equilibrium, judging by the rapid increase in concentration over time (Table 3). Once exposures ceased, tissue residues of o,p′-DDT declined rapidly over the 21-week postexposure monitoring period (Table 3). Low tissue residues (<0.1 μg/g wet weight) of o,p′-DDT were observed in female medaka from the control treatment at 0, 2, and 23 weeks of the experiment.
Eggs collected at the beginning (3 weeks after start of exposures) and end (6 weeks after start of exposures) of the period of egg production were individually pooled and analyzed (Table 4). The mean wet-weight concentration of these two samples was 91.2 μg/g of o,p′-DDT (3,622 μg/g lipid; n = 2); three orders of magnitude higher than o,p′-DDT concentrations in pooled eggs from control females (Table 4). The lipid-normalized o,p′-DDT concentrations in eggs were almost identical to the lipid-normalized tissue residues in the female medaka at the end of the exposure phase. At 23 weeks after start of exposures, the concentrations of o,p′-DDT in the progeny of the exposed females had declined markedly to a mean of 0.06 μg/g wet weight (Table 4).
| DDT concn. (μg/g) | ||
|---|---|---|
| Treatment | Wet wt | Lipid normalized |
| Control | ||
| 0 weeks (n = 3) | 0.02 ± 0.01 | 0.21 ± 0.14 |
| 2 weeks (n = 3) | 0.05 ± 0.03 | 0.28 ± 0.19 |
| 23 weeks (n = 3) | 0.04 ± 0.04 | 0.19 ± 0.09 |
| Exposed | ||
| 1 week (n = 3) | 10.91 ± 4.72 | 339.73 ± 22.11 |
| 2 weeks (n = 2) | 109.61 | 3,257.80 |
| 5 weeks (n = 3) | 24.01 ± 13.2 | 673.71 ± 74.19 |
| 9 weeks (n = 1) | 28.75 | 644.62 |
| 23 weeks (n = 3) | 0.28 ± 0.35 | 3.01 ± 1.33 |
| DDT concn. (μg/g) | ||
|---|---|---|
| Treatment | Wet wt | Lipid normalized |
| Control | ||
| 3 weeks (eggs) | 0.14 | 3.91 |
| 6 weeks (eggs) | 0.02 | 0.67 |
| 23 weeks (juveniles) | NDa | ND |
| Exposed | ||
| 3 weeks (eggs) | 102.45 | 3,483.33 |
| 6 weeks (eggs) | 80.02 | 3,761.07 |
| 23 weeks (juveniles) | 0.02 ± 0.01 | 0.56 ± 0.12 |
- aND = not detected.
Survival of the progeny from exposed females monitored over the period from egg collection to swim-up (22 d) varied between 70% and 100%, but there were no significant differences in mortalities between experimental and control treatments. Similarly, there were no significant differences in mortalities among the progeny of DDT-exposed females when data were analyzed on the basis of the day of collection during the egg production phase of the experiment.
However, there were significant differences in the time to hatch of progeny from the control and exposed treatments. Among the progeny that were collected from the control and exposed treatments on a daily basis over the 24-d period of the egg production phase, the median time to hatch was calculated for each daily batch. The median time to hatch for progeny from the control treatment varied between 10.5 and 16.5 d and averaged 13.3 d. In fish from the exposed treatment, the median time to hatch varied between 11.9 and 19.3 d and averaged 16.0 d. There was a significant difference between the averages of the median time to hatch in progeny from the control and exposed treatments.
Among progeny from the maternal transfer experiment, subsamples were collected at 15 weeks after egg collection (Fig. 1) for histological examination. Fish were collected from the control treatment (n = 40 males; n = 33 females) and from the exposed treatment (n = 67 males; n = 53 females). No testis–ova were observed in any of the male medaka, and there were developing spermatocytes and spermatids and, in some cases, spermatozoa observed in males from both treatments. In females, ovarian development was assessed by classifying the maximum degree of development of oocytes observed in each fish according to previously established criteria for medaka [22]. A comparison of oocyte development in fish from control and exposed treatments revealed significant differences in stages of development of oocytes in the ovary. In the females from the control treatment (n = 33), only nine of the fish had oocytes in the advanced vitellogenic stage, and the remainder were in the previtellogenic stage. In the females from the exposed treatment (n = 53), there were 26 and 27 females in the vitellogenic and previtellogenic stages, respectively. Subsequent observations offish that were not sacrificed indicated that medaka progeny from both control and exposed treatments began to produce viable eggs at five months after hatch, and embryos from this second generation developed normally to swim-up.
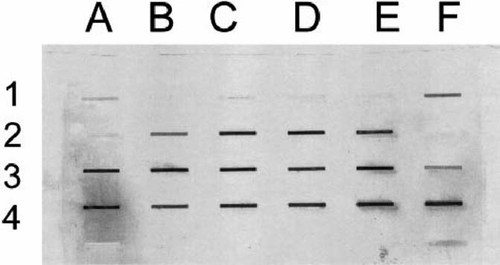
Slot blots, showing differences in slots that contain 5 (jig of protein prepared from homogenates of whole livers collected from medaka following exposure to 17β-estradiol at 12 mg/L for 4 d. The slots correspond to samples from individual animals: A1-E1 control males; F1-D2 control females; E2-C3 DDT-exposed males; D3-B4 DDT-exposed males; C4-D4 male positive control; E4-F4 female positive control. Because of small sample volumes, samples in A2 and B1 did not contain 5 g of protein and were excluded from quantitative analysis.
Among the progeny that were tested for a vitellogenic response to exposure to 17β-estradiol at 10 months after initial in ovo exposure (Fig. 1), western blot analysis of homogenized liver samples produced two vitellogenin bands. There were qualitative differences in band intensity, but the size and location of the bands did not vary among four groups: males and females from DDT treatments and males and females from control treatments. Slot blots of 5 μg of protein from each sample were prepared (Fig. 2) in order to quantify changes in total vitellogenin without isolating the two bands seen on western blots. The optical density data (Table 5) showed that there were significant differences among the four groups (n = 18) in the optical densities of the slots. Post hoc comparisons revealed a significant difference in the optical densities of slots for two of the four groups—males from the control treatment and males from the DDT treatment (n = 9)—indicating an enhanced vitellogenic response in the exposed males from the maternal transfer experiment that were subsequently exposed to 17β-estradiol.
Figure 3 illustrates the estrogenic response of o,p′-DDT relative to 4-NP and 4-tert-OP in the YES assay. All three compounds, plus the reference compound 17β-estradiol gave a sigmoidal dose–response curve in this assay, but the maximum response for o,p′-DDT and 4-NP did not reach the level observed for the reference estrogen. The IC25 values calculated for the estrogenic response were 0.04, 87.0, 317.8, and 5,309 ng/ml for 17β-estradiol, 4-tert-OP, 4-NP, and o,p′-DDT, respectively. These data indicate that o,p′-DDT is a less potent estrogen than the alkylphenol compounds in this in vitro assay.
| Treatment | Sex | N | Optical density |
|---|---|---|---|
| Control | Male | 4 | 14.1 ± 4.9a |
| Female | 4 | 43.0 ± 23.9 | |
| DDT exposed | Male | 5 | 69.3 ± 33.9a |
| Female | 5 | 76.7 ± 33.8 |
- a Posthoc comparisons for vitellogenic response.
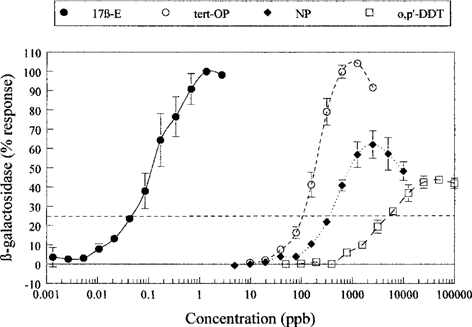
Results of the concentration–response curves in the yeast estrogen screening (YES) assay for 17-β-estradiol (reference estrogen), 4-tert-octylphenol (OP), 4-nonylphenol (NP), and o,p′-DDT. The horizontal dotted line crosses the response curves at the IC25.
DISCUSSION
Continuous exposure of medaka to aqueous solutions of o,p′-DDT from 1 d to 100 d posthatch resulted in the development of testis–ova in male medaka. This intersex condition has been observed previously in male medaka exposed to 4-NP and 4-tert-OP [16, 17] as well as to various synthetic and natural estrogen hormones [14]. It is not known whether this intersex condition effects the reproductive potential of male medaka [15]. It appears that chemicals that are estrogen agonists are capable of inducing development of intersex gonads in male medaka and other species offish that are gonochoristic, that is, fish that are naturally nonhermaphroditic [23, 24]. The lowest adverse effect levels for induction of testis–ova by 4-NP and 4-tert-OP were calculated from previously published data [16, 17] as 50 μg/L for both alkylphenol compounds on the basis of nominal exposure concentrations and 27.4 and 26.5 μg/L, respectively, on the basis of calculated average exposure concentrations. The lowest adverse effect levels for induction of testis–ova by o,p′-DDT were 5 μg/L on the basis of nominal concentration and 1.2 μg/L on the basis of the average measured concentration. These data indicate that o,p′-DDT has a greater potency than the alkylphenols for induction of testis–ova in medaka despite the relatively low estrogenic potency of o,p′-DDT in the in vitro YES assay.
Numerous variables might explain the differences in in vitro and in vivo potencies, some of which are differences in the kinetics of binding of these estrogenic compounds to estrogen receptors and estrogen-responsive elements in the recombinant human components of the YES assay relative to the medaka model, greater bioaccumulation of the more hydrophobic o,p′-DDT in medaka relative to bioaccumulation of the alkylphenols, low in vivo binding affinity of o,p′-DDT with estrogen-binding protein [25] relative to the binding of the alkylphenols, or in vivo metabolic activation of o,p′-DDT to a more potent estrogen [26]. In any event, these data demonstrate that in vivo and in vitro assays can give consistent results in screening for estrogenic compounds, but the relative potencies may not be comparable, as has been noted previously [27].
The number of females was statistically greater than the number of males in the treatment with 10 μg/L (nominal) of o,p′-DDT. This observation may have been due to complete feminization of medaka with the male genotype. Sex ratios skewed toward females have been observed in medaka exposed during early life stages to natural and synthetic estrogen hormones [14, 28]. However, these data should not be considered definitive evidence of the ability of o,p′-DDT to completely feminize fish at high exposure concentrations until experiments are conducted with genetic markers for the male genotype in medaka. For example, these results could be caused by differences in the susceptibility of male and female medaka to the toxic effects of o,p′-DDT due to sex-related differences in metabolic capability.
The analytical data for aqueous exposure solutions indicate that concentrations declined rapidly with time between renewals of test solutions. Since o,p′-DDT is relatively persistent in the aquatic environment, the declining concentrations in test solutions could have been due to binding to the walls of the exposure containers and to particulate material (e.g., food), uptake into medaka tissues, or volatilization out of solution into the air. Since the nominal concentrations of o,p′-DDT in the treatments at 5 and 10 μg/L exceeded the aqueous solubility of DDT [29], we speculate that adsorption onto surfaces and particulate material may have been a significant route for removal of this compound from solution. These data illustrate the importance of measuring the actual concentrations of test chemicals during aqueous exposure protocols.
Concentrations of o,p′-DDT in the tissues of female fish exposed to 2.5 μg/L of this compound in the maternal transfer test protocol quickly rose to about 100 μg/g wet weight (ppm) over a 2-week period, far higher tissue residues than would be expected in fish populations under conditions of gross DDT contamination [30]. The test compound was efficiently transferred into the lipids of eggs at concentrations approximately equal to the lipid-normalized tissue residues in the female medaka, which is consistent with previous studies on the transfer of lipophilic contaminants into fish eggs [31, 32]. The high concentrations of o,p′-DDT increased the time to hatch in developing medaka but did not appear to have any other toxicological effects in early life stages. Studies on the affects of p,p′-DDT on early development of Fundulus heterclitus showed that this compound can reduce hatching success, but in that study the time to hatch was not measured [33]. The concentrations of o,p′-DDT in eggs were within the range of tissue residues measured in adult mollies (Poecilia latipinna) exposed to o,p′-DDT that showed reduced growth and energy storage [19].
Despite indications that there were sublethal effects in developing medaka embryos, exposure to o,p′-DDT beginning in ovo did not induce intersex or other alterations to testicular development observable at 21 weeks after collection of eggs. The tissue residues of o,p′-DDT had declined by 21 weeks to near background levels, probably because of growth dilution. The progeny of the females exposed to DDT were capable of spawning and producing their own viable young at five months after hatch. However, there was evidence that the development of oocytes was more advanced in female medaka from the DDT treatment, as indicated by the greater proportion of females that had ovaries with oocytes in the vitellogenic stage of development. These data are important for evaluating the possible routes and duration of contaminant exposure in areas where natural populations of fish have high prevalences of intersex gonads [4]. The data in this study indicate that maternal transfer of contaminants resulting in relatively transient exposures to environmental estrogens during early life stages are not likely to induce intersex alterations, but these compounds may stimulate ovarian development in females. It may be that continuous exposures to elevated concentrations of estrogens, beginning in ovo and continuing throughout early development, are necessary in order to irreversibly affect gonadal differentiation or adversely affect reproduction in fish. These data are consistent with recent studies in which injection of marine turtle eggs with high concentrations of p,p′-dichlorodiphenyldichloroethylene did not cause any alterations to the differentiation of gonadal tissues in hatchling turtles [34]. However, these results with medaka appear to contradict earlier studies in which single injections of the eggs of California gulls with o,p′-DDT induced cortical localization of primordial germ cells, altered the morphology of the testis, and resulted in development of oviducts in male hatchings [11]. It is possible that the testes of these birds would have resumed normal differentiation if allowed to continue development. The appearance of oviducts in male birds is generally due to inhibition of the anti-Mullerian activity of testosterone during early development rather than to alterations to the differentiation of gonadal tissues [35].
It has been hypothesized that transient exposure to contaminants at early developmental stages may induce organizational effects that are manifested in disruptions to the neurological or reproductive capability of organisms once they reach sexual maturity [13]. Our observations of an enhanced vitellogenic response in the liver of male medaka tested 10 months after transgenerational exposure to o,p′-DDT is consistent with this hypothesis. It is difficult to speculate on molecular mechanisms for an enhanced vitellogenic response, although prior exposure to DDT could have enhanced the number of estrogen receptors or enhanced affinity for the estrogen receptor [36] or modulated the transcriptional signal at the level of estrogen-responsive-element binding. Studies conducted with mammalian models have indicated that perinatal exposure to chemicals that bind to the steroid/thyroid receptor superfamily can cause false imprinting of these receptors, possibly altering later responses to natural steroids [37]. These are preliminary data, and further work is needed to determine the relationship between vitellogenin levels in the liver and plasma of medaka and to investigate the environmental factors that can alter vitellogenic responses in fish.
Acknowledgements
This work was supported by a research contract from Fisheries and Oceans Canada and by a research grant from the Natural Sciences and Engineering Research Council of Canada to C.D. Metcalfe. Laboratory personnel who helped on the project include Karen Foster, Karen Bokvist, and Brenda Koenig. Vitellogenin assays were supported by a grant from the U.S. Environmental Protection Agency to W.H. Benson.




