Correlation of measures of ambient toxicity and fish community diversity in a Chesapeake Bay Tributary, Maryland, USA: A biological, chemical, and geological assessment
Abstract
A battery of water column and sediment toxicity bioassays measuring lethal and sublethal endpoints were employed with fish, invertebrates, vascular plants, and bacteria. Monthly water column bioassays were conducted with water from three stations in the South River, Maryland, USA, and from a reference station in the Wicomico River (Maryland, USA). Sediment bioassays were conducted with five discrete samples taken from each station. Water column assays indicated low-level effects at the upstream stations in the South River. Animal species demonstrated higher responses than did plants. Chemical analyses revealed only trace levels of heavy metals. Sediment in the upper stations of the South River demonstrated significant toxicity to animal but not plant test species. Sediment chemistry indicated elevated levels of heavy metals and polycyclic aromatic hydrocarbons. The sediments are enriched with chromium, copper, iron, manganese, cadmium, and lead—above normalized background geochemical ratios. The toxicological risk ranking model identified a strong gradient from upstream to downstream. Toxicological risk scores for the downstream station in the South River were comparable to those for the reference station. The major source of contaminants in the South River is probably derived from a multitude of non–point sources associated with urbanization within the watershed. The upper stations are in a zone of deposition of riverine material, whereas the downstream station is dominated by open Chesapeake Bay dynamics.
INTRODUCTION
In order to further our understanding of how toxic contaminants are affecting habitat quality and living resources in Chesapeake Bay, the Maryland Department of Natural Resources (USA) established a long-term ambient toxicity testing program in 1991. The intent of this program is not to perform dilution bioassays to establish acceptable levels but rather to assess in situ habitat quality, using a battery of bioassays to evaluate hazards to an array of resident species. Toxicity studies were coordinated with an independent fish community assessment program in an attempt to determine if quantitative links between the two approaches are possible. Previous studies in the program have demonstrated correlations between measures of ambient toxicity and fish community diversity indices in Chesapeake Bay tributaries [1-3]. This paper reports on the third year of field testing of the program, integrating ambient toxicity and fish community metrics. It describes the results of sampling for ambient toxicity, fish community condition, and chemical analyses in the South River, Maryland, USA.
Based on initial studies, four tidal tributaries were selected for paired ambient toxicity/fish index of biotic integrity sampling in 1994 to assess the impact of watershed urbanization on receiving-stream habitat quality. Results from that study indicated toxic contaminant impacts in the several estuaries, including a distinct gradient of contaminant effect from upstream to downstream in the South River estuary. This system was selected for more intensive sampling in order to generate data for analysis of intrasite variability, to address the sensitivity of the ranking model employed in the program [4], and to begin to assess contaminant loadings to the estuary. The South River watershed does not have large concentrations of heavy industry or extremely dense urban areas with conspicuous point sources. However, the watershed is subject to increasing urbanization, and it is typical of northwestern shore tributaries of Chesapeake Bay.
This project is complementary to an effort by the Chesapeake Bay Program, which is utilizing a scheme devised to classify all areas of the Bay into one of four categories: contaminant impacted; contamination effects suspected; clean; and insufficient data. One of the questions the identification protocol has yet to address is how to define the geographical extent of a given region. Knowledge of the sensitivity, and therefore of the spatial acuity of the ambient toxicity approach, will help define the areal extent of an impacted area for the purpose of management or regulatory practices. This point is an important one for coastal zone management programs. Implementation of effective habitat protection, enhancement, or restoration policies is contingent upon a knowledge of cause and effect. This requires an understanding of the relative areal extent of the sources and sinks of point and non–point source pollution.
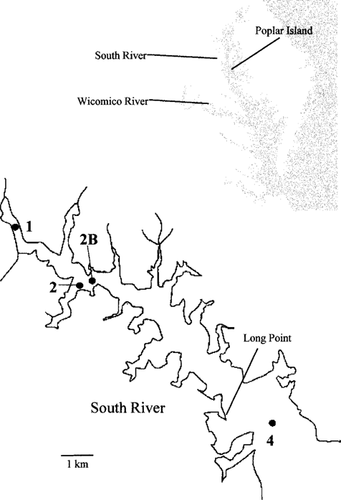
Map of the South River sampling stations.
MATERIALS AND METHODS
Stations were established at three (1, 2B, and 4) of the five locations in the South River used in the 1994 study [2] (Fig. 1). Samples were also taken from a single station in the Wicomico River; these samples served as a reference. Depth-integrated water samples were collected in July, August, and September of 1995. Sampling was coordinated with a fish community sampling program. Samples were taken twice from each site during the course of 7-d bioassay test periods to provide fresh renewal water. Samples were filtered through 37-μm mesh and adjusted to a salinity of 15 ppt with hypersaline brine. All water was stored in amber bottles at 4°C until use. Basic water-quality parameters were recorded at the time of collection (salinity, dissolved oxygen, temperature, and secchi depth). Heavy metals and chlorinated pesticides were analyzed on August samples only using standard U.S. Environmental Protection Agency (U.S. EPA) methods [5].
Sediment samples were taken from five sites at each station in August. Samples were obtained from the top 2 cm of grab samples using a petite ponar sampler. The sampling plan included a centrally located sample site (1), which corresponded to the location of the water sample site and the fish trawl sampling location. Using this location as a central point, a 100-m-square grid was located around the central point, and a discrete sediment sample was also taken at each of the four corners of the square (sites 2–5). Throughout the text, ‘station’ refers to the main sample location in the river and ‘site’ refers to the discrete locations from which sediment was taken. The central site was located in the channel of the tributary. Sampling at the corners of the square provided samples both upstream and downstream of the central location as well as samples taken away from the channel center. The absolute width of the channel varied from station to station. Sediment samples from each site were maintained as discrete samples for testing and analysis. The sampling plan provided true station replication for statistical purposes. Samples were held out of direct sunlight at 4°C and were used within 2 weeks.
Using standard methods, inorganic contaminants were evaluated on a composite sample from each station for acid volatile sulfides and, simultaneously, for extractable metals, total organic carbon, bulk metals, and acid and base/neutral extractable semivolatile compounds [6, 7]. Concurrent with toxicity tests, pore-water samples were extracted by squeezing with a nitrogen press, and these samples were analyzed for ammonia, nitrite, and sulfides.
Water column bioassays
Monthly water column static bioassays included the following: 7-d larval sheepshead minnow (Cyprinodon variegatus) survival and growth test; 7-d juvenile grass shrimp (Palaemonetes pugio) survival and growth test; copepod (Eurytemora affinis) life-cycle survival and reproduction test; and Microtox® bacterial luminosity test (Microtox, Azur Chemicals, Carlsbad, CA, USA). Bioassays were also conducted in August with the submerged aquatic vegetation species sago pondweed (Potamogeton pectinatus); these bioassays measured 14-d growth and reproduction. Fish and grass shrimp bioassays were conducted at the Maryland Department of Natural Resources Aquatic Toxicology Laboratory. Copepod bioassays were conducted at the University of Maryland Chesapeake Biological Laboratory. Vascular plant bioassays were conducted at the Anne Arundel Community College Environmental Center. All culturing, handling, and testing methods have previously been described in detail [1, 2].
Culture, maintenance, and bioassay procedures for grass shrimp and sheepshead minnow used standard methods [8, 9]. Copepod methods were based on the methods of Hartwell et al. [10], which follow the development of 24-h-old nauplii through one life cycle (e.g., 10–14 d). Numbers of surviving adults, eggs, nauplii, and subadults from the F1 generation were counted at the end of the test. The culture and bioassay techniques used for the sago pondweed tests were based on methods developed by Fleming et al. [11] and Ailstock et al. [12]. The number of photosynthetic shoots and rhizome tips on each plant were counted as a measure of reproduction. Dry weight was taken as a measure of growth. Dilution series Microtox bioassays were performed on each of the two water samples taken each month [13].
Sediment bioassays
Sediment toxicity bioassays included the following: 10-d sheepshead minnow (C. variegatus) embryo–larval survival and teratogenicity test; 10-d amphipod (Lepidactylus dytiscus) survival and growth test; 10-d amphipod (Leptocheirus plumulosus) survival and growth test; 10-d polychaete (Streblospio benedicti) survival and growth test; and a lettuce (Lactuca sativa) seed germination test. Leptocheirus plumulosus bioassays were conducted at the Department of Natural Resources Laboratory. Seed tests were conducted at Anne Arundel Community College. All other sediment bioassays were conducted by the Old Dominion University Applied Marine Research Laboratory. All culturing, handling, and testing methods have previously been described in detail [1, 2]. Test methods for animal species were adapted from standard methods [14] and from the methods of DeWitt et al [15]. The seed bioassay followed the methods outlined in Hartwell et al. [2].
Particle size analysis of test sites ranged from <1 to >50% sand. Because of the large range in particle size between sites, two reference sediments were used with the L. dytiscus, C. variegatus, and S. benedicti bioassays. The purpose of these reference sediments was to assess what effect normal physicochemical parameters (primarily particle size) would have on the survival of the organism being exposed in the absence of toxicants. The reference sediments that were used bracketed the sediment particle sizes found at the selected test sites. Control sediments for the animal bioassays consisted of native sediments from the area in which the test organisms were collected or naturally occurred. Control sediments for the seed germination test was commercial play sand with water of the appropriate salinity. The unaltered salinity of the South 1, 2B, and 4 and Wicomico 3 test sediments were 10.1, 10.8, 11.7, and 10.4, respectively.
For all tests, percent survival was compared with controls using the t test following arcsine transformation or the Wilcoxon rank-sum test. Measures of growth and reproduction were compared with controls using t tests and the Wilcoxon rank-sum test. Differences between means were considered significant at the p = 0.05 level.
Toxicological ranking model
The ranking scheme was developed to evaluate the toxicological results on a site-by-site basis. The objective of the ranking system is to quantify environmental risk, not merely to rank presence or absence of toxic effects. Thus, high uncertainty or variability will result in increased risk scores similar to positive toxicological responses. A detailed discussion and evaluation of the model can be found in Hartwell [4], and a brief summary is provided below. The term risk is used here in the sense of jeopardy. If the toxicological risk score is high because of high toxicity or high variability, that implies that resource populations in the test area may be in more danger than those in areas with low scores. The model is not designed to replace a classical risk assessment, but it may be a useful component of, or a companion to, the ecological response analysis components of a risk assessment. It was designed to provide a quantitative toxicological indicator of impact for integration with living resource or habitat indicators. It specifically addresses ecological impact without the necessity for demonstrating detailed, chemical-specific cause-and-effect relationships. It may be viewed more as a measure of ecological effect or possibly as an assessment endpoint in the U.S. EPA Ecological Risk Assessment Guidelines. It does not specifically address exposure levels, as the underlying assumption of the ambient toxicity approach is that we do not always know what chemical or combination of chemicals is causing toxicity.
Three risk ranking scores were calculated: water column, sediment, and water and sediment combined. Separate risk scores were also calculated using animal and vegetative data. The term vegetative is used here to include both plant and bacterial (Microtox) data. Since water column bioassays were replicated in the laboratory, a risk score was calculated for each station and month, and risk scores were averaged over months. Sediment samples were collected and tested as discrete field samples from each sampling site. Risk scores were calculated for each station.
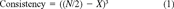 (1)
(1)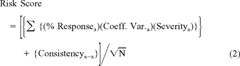 (2)
(2)Correlation of toxicological risk scores with index of biotic integrity metrics
Risk scores were contrasted with the Margalef diversity index [16] for bottom and resident fish communities used by the Maryland Habitat Assessment Program's Fish Index of Biotic Integrity Project (Table 1). Pearson correlation coefficients were calculated for every combination of toxicological risk score (water, sediment, and water and sediment combined) and fish diversity index for stations 1 and 4 in the South River and for the Wicomico River station. Station 2B in the South River was not a routine fish sampling station, so community data from station 2 was used. Bottom species included all species captured by trawl. Resident species included all species considered to be estuarine spawners captured in both bottom trawl and beach seines.
Sediment elemental enrichment analysis
Samples were analyzed for water content, bulk density, and grain size composition using the procedures of Kerhin et al. [17]. Samples were pretreated with hydrochloric acid and hydrogen peroxide to remove carbonate and organic matter, respectively; this was followed by wet-sieving through 62-μm mesh. The silt–clay fraction was analyzed using the pipette method [18].
| Station | Bottom diversity index | Resident diversity index | Water risk | Sediment risk | Combined water and sediment risk |
|---|---|---|---|---|---|
| South River 1 | 0.000 | 1.347 | 58.62 | 102.24 | 103.14 |
| South River 2B | NAa | NA | 34.17 | 71.26 | 63.97 |
| South River 4 | 0.330 | 0.818 | 16.49 | 25.79 | −30.73 |
| Wicomico 3 | 0.736 | 1.592 | −3.08 | 36.64 | −31.95 |
- aNA = not available (no fish sampled at this site).
The elements analyzed in this portion of the study were chromium, copper, nickel, zinc, iron, manganese, lead, and cadmium; we analyzed these elements using a microwave digestion technique followed by inductively coupled argon plasma analysis. The digestion procedure was modified from U.S. EPA Method 3051 for total recoverable metals to conform to U.S. EPA/Environmental Monitoring and Assessment Program and National Oceanic and Atmospheric Administration/National Status and Trends (NOAA/NST) programs. Following homogenization, subsamples were dried overnight at 105 to 110°C in Teflon® (DuPont, Boston, MA, USA) evaporating dishes. Dried samples were hand-ground with an agate mortar and pestle and powdered in a ball mill. Subsamples were transferred to Teflon digestion vessels with trace metal grade HNO3 and HCl plus ultrapure water. Preparation blanks were made with high-purity water plus the acids. Samples were irradiated using programmed steps optimized for pressure and percent power to 175°C in 5.5 min, then maintained at between 175 and 180°C for 9.5 min (approximately 6 atm peak pressure). After cooling, the samples were diluted to 100 ml in volumetric flasks and stored in polyethylene bottles prior to analysis in a Thermo Jarrel-Ash Atom-Scan 25 sequential inductively coupled argon plasma analyzer (Thermo Jarrel Ash, Franklin, MA, USA). The wavelengths and conditions selected for the elements of interest were determined using sediments from the vicinity of Poplar Island Habitat Restoration Project and standard reference materials from the National Institute of Standards and Technology (Silver Springs, MD, USA; #1646—Estuarine Sediment; #2704—Buffalo River Sediment) and the National Research Council of Canada (Montreal, PQ; PACS-1—Marine Sediment). Quality control was maintained through the following steps: we included blanks every 20 samples; one sample in every ten was replicated; and a standard reference material was analyzed after every 10 samples. Recoveries for the reference standards ranged from 93 to 101% (mean = 97.2%).
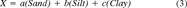 (3)
(3)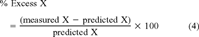 (4)
(4)The differences between the measured and predicted levels of metal X were normalized to predicted levels. This means that a value of 0% excess metal is at the regional norm, positive values are enriched, and negative values are depleted compared with the regional baseline. Direct comparisons of different metals in all sediment types can be made because of the method of normalization. The percent excess metal values alone do not give an accurate picture of the loading to the sediments because of natural sample and analytical variations, but because the data are normalized, Gaussian statistics can be applied to the interpretation of the data. Data falling within ±2σ are considered to be within normal background variability for the region. Samples with a value of ±3σ can fall within accepted background variability, but the value is marginal depending on the trends in the distribution. Values outside this range indicate a significant deviation. The standard deviations of the baseline data set and the data used to determine the coefficients in Equation 3 are the basis for determining the sigma level of the South River data.
RESULTS
Statistically significant mortality did not occur in water column bioassays with fish or grass shrimp (Table 2). Growth rates in fish were significantly reduced in July at station 1 of the South River and in all of the South River stations in September (Table 2). Grass shrimp growth in the Wicomico River sample was significantly higher than the control in August. Grass shrimp growth was significantly lower in stations 1 and 2B of the South River in September.
Copepod reproduction was variable between months. Control reproduction was unusually low in the July tests (Table 3). Reproduction in the South River increased from upstream to downstream in August and September. The results from the Wicomico River tests were unremarkable. Control mortality was also variable between months. Mortality rates in the Wicomico River and at station 1 in the South River were significantly lower than controls in July. Copepod mortality at station 1 of the South River was significantly higher than controls in August.
| % Mortality | Mean terminal weight | |||||
|---|---|---|---|---|---|---|
| Month/station | July | August | September | July | August | September |
| Fish | ||||||
| Control | 0 | 0 | 2.50 | 0.422 | 0.444 | 0.493 |
| South River 1 | 0 | 0 | 0 | 0.364a | 0.406 | 0.374b |
| South River 2B | NSe | 0 | 0 | NS | 0.365 | 0.390b |
| South River 4 | 0 | 0 | 0 | 0.374 | 0.389 | 0.388a |
| Wicomico 3 | 0 | 0 | 0 | 0.418 | 0.402 | 0.453 |
| Initial weight | 0.113 | 0.103 | 0.135 | |||
| Shrimp | ||||||
| Control | 0.319 | 0.178 | 0.291 | |||
| South River 1 | 0 | 3.33 | 0 | 0.301 | 0.159 | 0.248a |
| South River 2B | 0 | 0 | 0 | NS | 0.164 | 0.245a |
| South River 4 | NS | 6.67 | 2.50 | 0.303 | 0.180 | 0.273 |
| Wicomico 3 | 2.50 | 6.67 | 0 | 0.320 | 0.200a | 0.286 |
| Initial weight | 2.50 | 0 | 2.50 | 0.120 | 0.067 | 0.142 |
- aSignificant at p = 0.05.
- b Significant at p = 0.01.
- cNS = no sample.
Growth and reproductive results from the plant bioassays did not demonstrate effects at any station (Table 4). The Microtox assays did not demonstrate statistically significant inhibition at any station or during any month.
Chemical analyses of the August water samples did not detect any organic contaminants. Arsenic, lead, selenium, and zinc were above detection limits in the South River, but at concentrations well below acute and chronic water quality criteria for marine water. All metals were below detection limits in the Wicomico River water sample.
Significant levels of mortality were observed with the L. dytiscus bioassays in all stations, except in the control sand. After adjustment for grain size, however, only mortality at station 2B of the South River was significant relative to controls (Table 5). Adjusted mortality at station 1 was higher than at station 2B, but variability was higher, and the result was not statistically significant. A gradient of decreasing mortality from upstream to downstream was present in the South River. Significant levels of mortality were also observed for S. benedicti at all stations, including the mud reference. A gradient of decreasing mortality from upstream to downstream was present in the South River for this species also. The only significant mortality observed in the L. plumulosus bioassay was in the Wicomico River sample. Mortality rates for L. plumulosus were low in all tests. No significant effects on germination were observed in the seed bioassays. Control germination was strongly affected by salinity. Germination in all the test sediments was better than that observed in controls at a comparable salinity. The 0-ppt control was used for statistical testing purposes. Mortality in the fish egg bioassay was highest at station 2B, followed by station 1 (Table 6). Mortality was primarily attributable to hatching failure. Larval mortality was only observed at stations 1 and 2B in the South River. However, none of the results were statistically significant.
| Month/station | July | August | September |
|---|---|---|---|
| Mortality | |||
| Control | 40.0 | 10.0 | 41.7 |
| South River 1 | 5.0b | 40.0b | 21.7 |
| South River 2B | NS | 20.0 | 0.0 |
| South River 4 | 26.7 | 21.7 | 5.0 |
| Wicomico 3 | 16.7b | 15.0 | 15.0 |
| Reproduction (# eggs, nauplii, and subadults) | |||
| Control | 133.00 | 747.50 | 409.50 |
| South River 1 | 714.25b | 573.50 | 529.75 |
| South River 2B | NS | 853.50 | 710.50 |
| South River 4 | 227.25 | 1,039.75 | 812.25 |
| Wicomico 3 | 289.00 | 979.75 | 545.75 |
- a Values are the mean of four replicates; NS = no sample.
- b Significantly different than controls (p < 0.05).
Growth of L. plumulosus was higher than that of controls in all treatments and was significantly higher than that of controls at station 2B of the South River and the Wicomico River (Table 5). Growth of L. dytiscus was poor in most tests, especially for the controls. However, the apparent reduction in growth appears to be an artifact of variability. Significantly higher growth was seen in South River 1 and Wicomico River samples. Growth rates of S. benedicti were not statistically significant compared with controls, but a gradient of increasing growth rates was seen from upstream to downstream in the South River.
| Station | Initial weight | Terminal weight | Dry weight | Reproduction |
|---|---|---|---|---|
| Control | 1.23 | 3.41 | 0.44 | 47.80 |
| South River 1 | 1.26 | 4.37 | 0.50 | 44.90 |
| South River 2B | 1.25 | 2.76 | 0.37 | 40.00 |
| South River 4 | 1.32 | 3.83 | 0.44 | 46.70 |
| Wicomico 3 | 1.24 | 3.00 | 0.39 | 44.50 |
- a Weight values are the mean of 10 replicates (g/plant).
- b Defined as the number of rhizome tips plus the number of photosynthetic shoots.
| % Mortality | Growth | |
|---|---|---|
| L. dytiscusa | ||
| South River 1 | 44.30 | 0.351b |
| South River 2B | 36.21c | 0.303 |
| South River 4 | 20.18 | 0.306 |
| Wicomico 3 | 28.45 | 0.357b |
| Lynnhaven sand (C) | 14.54 | 0.280 |
| Poropotank mud (R) | 30.05 | 0.312 |
| Initial | 0.338 | |
| S. benedicti | ||
| South River 1 | 48.00c | 0.117 |
| South River 2B | 33.33c | 0.127 |
| South River 4 | 10.67b | 0.131 |
| Wicomico 3 | 24.00c | 0.152 |
| Poropotank mud (R) | 9.33b | 0.155 |
| Lynnhaven mud (C) | 0.00 | 0.143 |
| Lynnhaven sand (R) | 42.67c | 0.077c |
| Initial | 0.066 | |
| L. plumulosus | ||
| South River 1 | 7.0 | 0.128 |
| South River 2B | 9.0 | 0.152b |
| South River 4 | 5.0 | 0.228 |
| Wicomico 3 | 13.0b | 0.130b |
| Culture (C) | 3.0 | 0.110 |
| Initial | 0.065 | |
| % Germination | ||
| L. sativa | ||
| South River 1 | 91.2 | |
| South River 2B | 96.4 | |
| South River 4 | 85.0 | |
| Wicomico 3 | 94.0 | |
| Sand (0 ppt) | 98.7 | |
| Sand (10 ppt) | 43.6 | |
| Sand (12 ppt) | 8.0 | |
| Culture (15 ppt) | 15.2 | |
- a Survival adjusted for predicted particle-size effects.
- b Significantly different than controls (p < 0.05).
- c Significantly different than controls (p < 0.01).
| Station | % Mortalitya | % Unhatched | % Dead eggs | % Dead fish |
|---|---|---|---|---|
| South River 1 | 48.00 | 24.00 | 20.00 | 7.29 |
| South River 2B | 62.00 | 34.00 | 24.00 | 8.00 |
| South River 4 | 0.00 | 0.00 | 0.00 | 0.00 |
| Wicomico 3 | 12.00 | 8.00 | 4.00 | 0.00 |
| Poropotank mud (R) | 12.00 | 10.00 | 2.00 | 0.00 |
| Lynnhaven sand (R) | 0.00 | 0.00 | 0.00 | 0.00 |
| Lynnhaven mud (C) | 2.00 | 0.00 | 2.00 | 0.00 |
- a % Mortality = (Dead fish + unhatched eggs + dead eggs at test termination)/(# eggs exposed)·100. % Dead fish = (Dead fish)/(# hatched)·100. % Dead eggs = (Dead eggs)/(# exposed)·100. % Unhatched = (# unhatched)/(# eggs exposed)·100.
Bulk sediment chemistry results for sediments are shown in Table 7. Metals levels were generally higher in South River stations relative to the Wicomico sample. For both total and recoverable metals, the highest levels of (typically) toxic metals were always seen at South River stations 1 or 2B, except for recoverable nickel. All total values at these two stations were above NOAA effects range low levels [21]. Nickel at South River station 2B exceeded the effects range-median (ERM). Iron levels found in the South River were relatively high for this region of the Bay [19]. A variety of PAHs were detected in the South River samples. None were found in the Wicomico River sample. The two upstream samples from the South River had consistently higher levels than did station 4, and a larger number of compounds were detected. All values were below NOAA effects range low levels values.
Data for simultaneously extractable metals/acid volatile sulfide (SEM/AVS) ratios for metals in sediment pore water are shown in Table 8. All stations in the South River had higher SEM levels than did the Wicomico River samples and reference sediments. Zinc values were considerably higher than for other metals. The AVS levels were extremely high at the two upper stations of the South River. Consequently, the SEM/AVS ratios are well below 1. Unionized ammonia and TOC levels were higher in the two upstream samples from the South River (Table 9).
The mean water column, sediment, and combined risk scores are shown in Table 1. Results showed a gradient from upstream to downstream in the South River in both media. The Wicomico River scores were generally near or below those of the South River stations. Animal and vegetative bioassays yielded different results. The plant and Microtox bioassays did not exhibit significant responses for any measured endpoint. Calculation of toxicological risk scores using only vegetative data yielded uniformly low-level results, with no gradients.
The correlation coefficients for the risk scores and the fish community metrics are shown in Table 10. None of the correlations are significant. However, there are only three data pairs in any given comparison. The bottom diversity index values were quite low relative to other years and other areas (Table 1) [1, 2]. However, they clearly track the sediment and combined risk scores.
The results of the elemental enrichment analysis are presented in Table 11. The correlation coefficients between grain size and element were as follows: chromium, 98; nickel, 98; zinc, 96; iron, 99; cadmium, 32; lead, 82; manganese, 96; and copper, 99. Concentrations of all metals but cadmium are directly related to sediment grain size. The poor correlation for cadmium is a result of baseline concentrations that are at or near the detection limit, but because of the large number of samples (104), the confidence level is high, and the natural variability is taken into account by the standard deviation (sigma value), which is moderately high in the upper South River compared with other elements. Chromium, copper, manganese, cadmium, lead, and iron show significant ( >3σ) enrichment in the upper South River stations. Grain size analysis showed a transition from clay to silt–clay from station 1 through 4, with one site dominated by a sand spit at station 2B (site 3).
| Chemical | South River 1 | South River 2B | South River 4 | Wicomico | NOAA ERLa | NOAA ERMb | |
|---|---|---|---|---|---|---|---|
| Antimony | Recoverable | <0.10 | 0.17 | <0.10 | <0.10 | ||
| Arsenic | Recoverable | 3.6 | 3.9 | 3.6 | 2.4 | ||
| Beryllium | Recoverable | 0.42 | 0.46 | 0.6 | 0.32 | ||
| Cadmium | Recoverable | 0.67 | 0.91 | <0.31 | <0.31 | ||
| Total | 2.95 | 1.74 | 0.24 | 0.62 | 1.2 | 9.6 | |
| Chromium | Recoverable | 25.2 | 26.4 | 25.6 | 7.0 | ||
| Total | 271.8 | 232.2 | 171.2 | 95.9 | 81 | 370 | |
| Copper | Recoverable | 10.5 | 17.7 | 10.6 | 6.5 | ||
| Total | 62.2 | 107.7 | 34.1 | 20.6 | 34 | 270 | |
| Iron | Total | 10.8 | 8.6 | 5.9 | 4.2 | ||
| Lead | Recoverable | 17.3 | 26.4 | 20.9 | 11.5 | ||
| Total | 44.8 | 86.0 | 41.1 | 18.5 | 46.7 | 218 | |
| Manganese | Total | 940.8 | 1,176.6 | 2,426.2 | 1,839.4 | ||
| Nickel | Recoverable | 3.9 | 5.0 | 8.0 | 16.7 | ||
| Total | 46.5 | 53.5 | 47.4 | 35.7 | 20.9 | 51.6 | |
| Selenium | Recoverable | 0.12 | 0.15 | 0.13 | <0.10 | ||
| Zinc | Recoverable | 50.9 | 76.9 | 91.2 | 34.1 | ||
| Total | 289.0 | 347.4 | 278.6 | 130.0 | 150 | 410 | |
| Mean metals concn:ERM quotient | 0.506 | 0.581 | 0.400 | 0.249 | |||
| Phenanthrene | <150 | 130 | 110 | <86 | 240 | 1,500 | |
| Fluoranthene | 300 | 380 | 140 | <74 | 600 | 5,100 | |
| Pyrene 230 | 290 | 110 | <98 | 665 | 2,600 | ||
| Chrysene | 180 | 260 | <63 | <74 | 400 | 2,800 | |
| Benzo[a]anthracene | 170 | 140 | <74 | <86 | 230 | 1,600 | |
| Benzo[b]fluoranthene | 230 | 210 | <84 | <98 | − | − | |
| Benzo[k]fluoranthene | <270 | 190 | <140 | <160 | − | − | |
| Benzo[a]pyrene | 130 | 120 | <63 | <74 | 430 | 1,600 | |
| All chemicals Mean concn:ERM quotient | 0.312 | 0.335 | 0.282 | 0.249 | |||
- a National Oceanic Atmospheric Administration effects range low levels.
- b National Oceanic Atmospheric Administration effects range median levels.
DISCUSSION
Results were consistent with data gathered in 1994 [2]. The upstream South River stations yielded greater toxicological responses than did station 4, and the risk ranking results show a strong gradient from upstream to downstream in both the water column and sediment. Sampling in 1994 could not assess the possibility of a gradient of effects in the water column because this sampling used a single, centrally located water sampling station in each tributary. The results of 1995 bioassays with water from discrete locations in the tributary allow a more thorough assessment of the impact of toxic contamination. The magnitudes of the observed effects in the water column were relatively small compared with those observed from the sediment bioassays. This is consistent with the previous studies [1, 2]. It is important to note that all sampling stations were located away from known point sources. There are only four permitted discharges in the entire watershed. Two are small, publicly owned treatment works; one at the river mouth and one several kilometers upstream in the nontidal watershed. The other two are small industrial point sources, also upstream in the nontidal watershed.
| Site | Cadmium | Lead | Copper | Nickel | Zinc | Sum | Mean AVS | Ratio |
|---|---|---|---|---|---|---|---|---|
| South River 1 | 0.129 | 0.193 | 0.531 | 0.141 | 3.235 | 4.229 | 239.02 | 0.018 |
| South River 2B | 0.072 | 0.362 | 0.777 | 0.197 | 3.580 | 4.987 | 114.25 | 0.044 |
| South River 4 | 0.051 | 0.182 | 0.298 | 0.326 | 2.782 | 3.638 | 7.38 | 0.493 |
| Wicomico 3 | 0.047 | 0.099 | 0.125 | 0.151 | 0.908 | 1.331 | 8.26 | 0.161 |
| Lynnhaven sand | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.93 | − |
| Lynnhaven mud | 0.000 | 0.039 | 0.066 | 0.050 | 0.643 | 0.798 | 4.33 | 0.184 |
| Poropotank mud | 0.026 | 0.027 | 0.000 | 0.064 | 0.721 | 0.838 | 5.51 | 0.152 |
| Detection limits | 0.0003 | 0.0006 | 0.0050 | 0.0004 | 0.0005 |
- a Mercury values for all sites were <0.00 μmol/g with detection limits of 0.00005 μmol/g.
| Site | Ammonia | Nitrite | Sulfide | Unionized ammonia | TOC |
|---|---|---|---|---|---|
| South River 1 | 9.76 | 0.0096 | 0.0189 | 0.6181 | 4.39 |
| South River 2B | 8.55 | 0.0066 | 0.0025 | 0.4966 | 4.29 |
| South River 4 | 3.64 | 0.0068 | 0.0031 | 0.0622 | 2.50 |
| Wicomico 3 | 3.86 | 0.0085 | 0.0000 | 0.0616 | 2.25 |
| Lynnhaven sand | 3.51 | 0.0158 | 0.0049 | 0.0205 | 0.12 |
| Lynnhaven mud | 4.85 | 0.0238 | 0.0213 | 0.0391 | 1.16 |
| Poropotank mud | 3.87 | 0.0057 | 0.0014 | 0.0248 | 5.04 |
Based on grain size gradients, station 2B appears to lie in or near the transition zone from tributary-dominated sediment dynamics to open Bay dynamics, and the individual and combined risk scores for that station are intermediate between scores from stations 1 and 4. To be truly representative of the system, ambient toxicity assessments cannot utilize only one or two stations in a given tributary but rather must cover the length of a tributary. Large areas of degraded habitat could be missed if the sampling stations are too far apart. Also, the sheer size of the river may affect its assimilative capacity for environmental degradation.
The contrast between the results from the animal and plant tests indicates differential environmental effects and may be useful in exploring the specific causes of observed results. None of the vegetative bioassays resulted in significant toxicological responses at any station in either sediment or water tests. Also, in the seed germination tests, all of the sites had substantially better germination than did the control at a similar salinity. This indicates that natural sediment allows the plant embryos to overcome osmotic stress. This may be related to a variety of effects—such as the counteracting of osmotic stress through competitive diffusion of small ionic molecules—or it may involve beneficial effects of nutrients and organic substrates available in pore water. Except for pesticides, the relative sensitivity of plants and animals to environmental contaminants is largely unknown, and species-sensitivity distributions are compound-specific. However, the sago pondweed has been tested with several individual chemicals and has been shown to be a reasonably sensitive species, especially in terms of herbicides [12, 22]. The potential for seasonal sensitivity of plants to low-level contamination is also unknown, but the impacts of herbicide runoff would be expected to be lower in late summer than in spring.
| Risks | Bottom diversity index | Resident diversity index |
|---|---|---|
| Water risk | −0.9129 (0.0872) | −0.1374 (0.8626) |
| Sediment risk | −0.7505 (0.2495) | 0.3480 (0.6520) |
| Combined water/and sediment risk | −0.8582 (0.1418) | 0.4152 (0.5848) |
It is also interesting that the germination rate in the culture sediment is low. The culture sediment is the sediment in which the L. plumulosus stock is maintained at the laboratory. It is obtained from Fishing Bay on the lower eastern shore of Maryland. We tested a variety of sediments for amphipod culture, and the amphipods did best in sediment from this location. In the 1993 ambient toxicity study [1], Fishing Bay was tested, and it was concluded that low resident fish diversity and index of biotic integrity scores were attributable to poor habitat, including the lack of submerged aquatic vegetation, among other possibilities [23], because there was no indication of toxic contaminant impact there. Unfortunately, the Fishing Bay sediment was tested at a salinity of 15 ppt, so possible inferences about phytotoxicity are only speculative at this time. The recent recognition that the toxic algae Pfiesteria sp. are present in eastern shore tributaries of Chesapeake Bay may indicate a direct impact on fish populations, or it may simply indicate eutrophication impacts. More data will be necessary to address these questions.
The Pearson correlation coefficients between the risk values and the fish community metrics were not statistically significant (Table 10). Unlike previous years, fish data are from station-specific data as opposed to tributary-wide data. Thus, the diversity indices are all relatively low (Table 1) compared with other years, in which the index was derived from the populations in the entire tributary. Community indicators of benthos are probably more sensitive than the fish community to local contamination, as the organisms are less mobile and live in intimate contact with the sediment. The Maryland Department of Natural Resources benthic monitoring program [24] has placed a variety of stations within 1 km of station 4 from 1995 to 1997, off Long Point (Fig. 1). The benthic community condition score (analogous to the fish community index of biotic integrity) was generally in the “good” range at these stations, which is consistent with the toxicological risk score. In 1998, a station approximately 1 km west of station 2B was found to have a degraded benthic community, whereas a station halfway between there and station 4, and at station 4 were in the good range [25].
This study demonstrates that the upper tidal portion of the South River is contaminated with toxic chemicals and/or overloaded with conventional pollutants, which has resulted in elevated levels of compounds such as ammonia or hydrogen sulfide. No single chemical was identified in these samples as being associated with the observed results, but the combined synergistic toxicity of the heavy metals is certainly implicated. Hyland et al. [26] have demonstrated that benthic community impacts become evident at mean ERM quotients as low as 0.05. Long et al. [27] have shown a relationship between ERM quotients above approximately 0.1 and acute toxicity as well as an increasing occurrence of toxicity with increasing numbers of ERL exceedances. In the upper South River samples, nickel exceeded the ERM, and both zinc and chromium approach their respective ERM levels.
| Station | Chromium | Copper | Nickel | Zinc | Iron | Manganese | Cadmium | Lead |
|---|---|---|---|---|---|---|---|---|
| SOU 11 | 4.9 | 1.8 | −1.2 | −0.8 | 8.0 | 0.9 | 9.3 | −0.4 |
| SOU 12 | 4.9 | 1.6 | −1.6 | −0.9 | 8.2 | 0.7 | 9.7 | −1.0 |
| SOU 13 | 5.0 | 0.9 | −1.6 | −0.9 | 8.4 | 0.9 | 10.5 | −0.5 |
| SOU 14 | 5.5 | 2.3 | −1.3 | −0.6 | 8.7 | 0.7 | 10.2 | −0.9 |
| SOU 15 | 4.7 | 1.3 | −1.8 | −0.9 | 7.7 | 1.7 | 10.4 | −0.1 |
| SOU 2B1 | 4.1 | 13.3 | 0.1 | 0.4 | 5.6 | 9.0 | 4.5 | 6.6 |
| SOU 2B2 | 3.6 | 5.8 | −0.4 | 0.3 | 6.0 | 1.9 | 5.5 | 2.7 |
| SOU 2B3 | 12.6 | 17.3 | −0.2 | 1.7 | 28.8 | 14.6 | 2.9 | 3.5 |
| SOU 2B4 | 4.5 | 10.4 | 0.2 | 0.5 | 1.9 | −2.0 | 6.9 | 4.9 |
| SOU 2B5 | 4.0 | 8.0 | 0.2 | 0.4 | 5.0 | 1.4 | 7.1 | 2.8 |
| SOU 41 | 3.4 | −0.5 | 0.4 | 0.3 | 3.6 | 15.9 | −2.2 | 2.0 |
| SOU 42 | 3.2 | −0.6 | 0.9 | 0.2 | 3.7 | 14.7 | −2.6 | 1.8 |
| SOU 43 | 2.9 | −0.8 | 0.1 | 0.1 | 2.8 | 9.0 | −1.7 | 0.2 |
| SOU 44 | 2.9 | −0.6 | 0.0 | 0.0 | 3.2 | 11.3 | −1.1 | 0.2 |
| SOU 45 | 3.2 | −0.7 | 0.6 | 0.1 | 3.7 | 13.5 | −1.9 | 1.6 |
| WIC 31 | −0.8 | −3.9 | −2.2 | −2.7 | −0.2 | 7.4 | −0.8 | −3.6 |
| WIC 32 | −0.8 | −3.9 | −2.5 | −2.6 | −0.2 | 9.0 | 0.5 | −2.9 |
| WIC 33 | −0.8 | −3.9 | −2.6 | −2.7 | −0.5 | 7.8 | −0.4 | −1.3 |
| WIC 34 | −0.7 | −3.9 | −1.9 | −2.7 | −0.5 | 7.3 | −0.1 | −2.7 |
| WIC 35 | −0.5 | −3.7 | −1.7 | −2.7 | −0.2 | 6.2 | −0.2 | −3.2 |
The mean metals concentration:ERM quotient [27, 28], plotted against the sediment risk scores, shows that the risk scores tend to increase with the quotient, even over the relatively small range of quotient values (less than one order of magnitude) (Fig. 2). Plotting species-specific mortality results against the calculated quotients (Fig. 2) indicates that the relative sensitivity of L. plumulosus and the seed germination assay to toxic chemicals may be lower than for the other species. Also, note that the highest quotient is at station 2B (Table 7), whereas South River station 1 had consistently higher mortality of the sensitive species. This may be another indication that biological testing is superior to chemical scans alone for assessing impacts, because the latter provide an incomplete picture of all the stressors present at a given location, whereas results from a battery of bioassays reflect the integration of all stressors present.
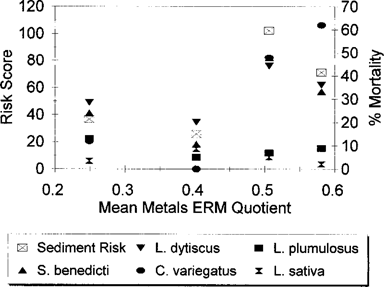
The relationship between the sediment risk ranking scores and species-specific mortality rates with the mean effects range-median (ERM) quotients.
Effects range-low and ERMs are primarily based on data from total metals extraction techniques, not recoverable methods. Therefore, comparison of these guidelines with recoverable metals levels are invalid (although such comparisons are often conducted). The partition between recoverable and total levels in clean versus contaminated locations has not been fully explored. Partition in clean areas would be expected to be element-specific as well as a product of the local geochemistry and hydraulic environment. How recoverable metals levels relate to ambient toxicity has not been quantified in the same fashion as for total metals. In the South River, the total:recoverable metals ratios varied from approximately 2 to 10, depending on element.
Pore-water SEM metals were generally higher at the two upstream stations. The AVS/SEM ratios were below 1.0 at all stations, but the mean AVS value for the upper stations was vastly higher than at all other locations. Using empirical analyses, Summers et al. [29] and O'Connor et al. [30] concluded that AVS/SEM ratios were not always effective predictors of ambient toxicity because of the sensitivity to high AVS concentrations.
Whereas the standard suite of organic priority pollutant chemicals was analyzed, a thousand chemical contaminants have been identified in Chesapeake Bay [31], and most of these are not analyzed in standard surveys. In addition, many organic chemicals are simply regarded as unknowns in gas chromatography/mass spectrometry analyses. Many potentially toxic chemicals cannot be analyzed by gas chromatography at all [32]. One of the basic assumptions of the ambient toxicity approach is that the bioassays will identify areas in which contamination is of biological significance, whereas typical scans of standard chemical contaminants cannot predict biological significance because of omitted chemicals and lack of knowledge of synergistic effects. This appears to be a case in point. A variety of PAHs were detected in samples from all South River samples. None of the samples had PAHs that were at or above NOAA effects range low levels concentrations, but the South River is clearly contaminated with by-products of petroleum combustion, as are most of the upper-western shore tributaries of the Chesapeake Bay [33]. Polycyclic aromatic hydrocarbon concentrations in eastern-shore tributaries are typically three to four times lower than those in the South River, with even lower values seen in the southern-Maryland portions of the Bay.
The correlations between grain size and all metals except cadmium were excellent, indicating that the concentrations of these metals are directly related to sediment grain size. The extremely high sigma level for metals at the South River stations 2B to 3 site is attributable to grain size normalization. The sample was 70.5% sand.
The major source of contaminants in the South River is probably derived from a multitude of non–point sources associated with urbanization within the watershed. The largest of the three permitted discharges in the nontidal watershed is a publicly owned treatment works plant that only discharges up to 0.024 million gallons/day. A more in-depth study of chemical flux relationships would be necessary to determine if low levels of contaminants are entering the system from upstream in the water column and concentrating in the sediments or if the sediments were previously contaminated and are acting as a low-level source to the water column. Iron shows the most consistent enrichment in the sediment in the South River stations. However, it is unlikely that this is attributable to anthropogenic loading; more likely, it is a natural source of iron. The samples with enrichment of chromium, copper, and cadmium were, for the most part, highly enriched. Copper is clearly partitioning to sediments in the vicinity of station 2B, whereas cadmium is being accumulated in the upstream zone, and manganese is passing through to the lower portions of the tributary. A more detailed picture of the sediment budget in the area would necessitate a sediment core study, which is currently under way. Data from the Maryland Geological Survey indicate that station 1 is in the turbidity maximum zone and is an area of high deposition of silt-sized material. Station 2B is an area of deposition of clay-sized material but is near the transition zone between tributary-dominated flow and open Bay forces. Station 4 is dominated by flows and sediment dynamics of the open water of the Chesapeake Bay (J. Halka, personal communication).
The South River watershed drains approximately 43,300 acres. According to census data, the population of the watershed was approximately 41,500 in 1990 [34]. Earlier watershed-specific data is not available, but census data indicate that the population of the region as a whole increased by 15% from 1980 to 1990. Land-use ratios indicate increased urbanization of the watershed. From 1984 to 1989, Maryland State Department of Planning data showed an increase of 19% in the “Urban Built-Up” land-use category in the watershed, with the greatest increases occurring in low- and medium-density residential areas. A decrease of 7.6% in forested land coverage occurred during the same period. More than 1,300 acres of road surface contribute to an estimated 4,316 acres of impervious surfaces and their associated run-off in the watershed. Development in the 1990s has continued unabated. Carmichael et al. [23] demonstrated a positive correlation between urbanized watersheds and depressed fish communities. Wang et al. [35] demonstrated a correlation between the extent of both agricultural and urban land use with declines in fish community index of biotic integrity in freshwater streams. Karr [36] provides several examples of correlations between watershed land-use practices and fish and benthic community degradation. Hall et al. [37] showed that the percent of low-density residential areas correlated positively with high concentrations of trace metals in major tributaries of the South River and with elevated concentrations of chromium, cadmium, and nickel in the water column.
Soil types in the South River watershed are primarily of the Monmouth and Collington varieties and are associated with glauconite. Glauconite can be strongly acidic at or near the surface and may contain high concentrations of metals such as cadmium, iron, zinc, and nickel. Oxidation of glauconite is microbially driven and occurs when the deposit is exposed to air (e.g., as a result of excavation). Exposed glauconite can release acidic water for extended periods of time, a process which essentially mimics acid mine drainage [38]. Problems with strongly acidic areas of glauconite have been identified in areas directly adjacent to the South River watershed (resulting from road construction excavations). Whether these problems are extensive in the watershed and have an effect downstream in the more pH-buffered environment of a tidal tributary is unknown at this time. More studies are currently in progress.
Acknowledgements
This paper is dedicated to the memory of Randy Kerhin, whose untimely passing was a significant loss to the scientific community in the Chesapeake Bay region. Field sampling assistance was provided by Margaret McGinty, Sandy Ives, Doug Randle, and Bill Rodney of the Chesapeake Bay Research and Monitoring Division Habitat Impacts Program, under the direction of Dave Goshorn. Celia Dawson, Eric Durell, Peter Adolphson, Gina Coelho, Mike Norman, and James Hill performed the bulk of the laboratory assays. This project was funded in part by the NOAA through the Maryland Department of Natural Resources, Coastal and Watershed Resources Division Grant NA 470 Z 0132.




