Effects of polychlorinated biphenyl 126 on green frog (Rana clamitans) and leopard frog (Rana pipiens) hatching success, development, and metamorphosis
Abstract
Although increasing evidence links planar chlorinated hydrocarbons, such as polychlorinated biphenyls (PCBs), to decreases in survival and reproduction of fish, mammals, and birds near Green Bay, Wisconsin, and the Great Lakes, USA, relatively little is known of their bioaccumulation or of their possible effects in amphibians. We exposed embryos and larvae of two ranid species commonly occurring in the Green Bay ecosystem, the green frog (Rana clamitans) and the leopard frog (Rana pipiens), to PCB 126, a model coplanar PCB compound. Nominal concentrations ranged from 0.005 to 50 μg/L, and exposure lasted through metamorphosis. Tissue concentrations of PCB 126 in tadpoles that did not metamorphose by the end of the experiment ranged from 1.2 to 9,600 ng/g wet mass. No significant mortality of embryos occurred before hatching; however, survival of larvae was significantly reduced at the highest concentration for both species. Few deformities were observed, but the incidence of edema was significantly higher in tadpoles exposed to 50 μg/L. Swimming speed and growth of tadpoles was also significantly reduced in this treatment. The percent of tadpoles that reached metamorphosis was significantly lower in green frogs at the highest concentration, and no leopard frogs survived past day 47 of the experiment in this treatment. At high concentrations, PCB 126 affected both ranid species; however, sublethal effects were not apparent for the parameters we measured at concentrations that occur in water in the Green Bay ecosystem.
INTRODUCTION
The Green Bay watershed in Wisconsin (USA) is polluted with polychlorinated biphenyls (PCBs), dioxins, heavy metals, and over 100 organic contaminants [1-3]. Polychlorinated biphenyl contamination of water and sediments in this ecosystem has been associated with the industrial processes of paper and pulp mills that line the Fox River, the main tributary to Green Bay. Although they are no longer being released into the watershed, PCBs persist in the Green Bay ecosystem because of slow biodegradation, sediment contamination, continued atmospheric deposition, and bioaccumulation up the food chain. Polychlorinated biphenyl accumulation in tissue has been linked to decreases in survival and reproduction of fish, mammals, and birds in Green Bay and in the Great Lakes [4-8]. However, in amphibians, relatively little is known of PCB bioaccumulation or its possible effects [9, 10].
Coplanar PCBs and mono-ortho derivatives are particularly toxic PCB congeners. These chemicals have been shown to bind a cytosolic aryl hydrocarbon (Ah) receptor [11], thus forming an activated complex that can induce cytochrome P4501A1 activity (detoxification enzyme activity) or that can bind to specific sequences of DNA, known as dioxin-response elements, that mediate the cell's toxic response. Toxic responses in animals may include body-weight loss, thymic atrophy, edema, teratogenesis, carcinogenesis, decreased immune function, hepatotoxicity and porphyria, and reproductive toxicity [12].
A recent study [13] (R.E. Jung and W.H. Karasov, unpublished data) determined percent hatching success of green frog (Rana clamitans) and leopard frog (Rana pipiens) embryos in enclosures located at sites situated along a PCB gradient in the Fox River and Green Bay. This field study found a significant negative correlation between percent hatching success and sediment PCB concentrations. We were interested in conducting a laboratory study in which confounding environmental factors present in the field would be minimized and in which a cause-effect relationship between PCBs and anuran hatching success, growth, and metamorphosis could be measured.
Polychlorinated biphenyl 126 (3,3′,4,4′,5-pentachlorobiphenyl), the congener we used in this study, was chosen because it has a high affinity for the Ah receptor and is therefore considered a good model for the class of coplanar PCBs known to exhibit the greatest toxicity [14]. Polychlorinated biphenyl 126 induces P4501A1 activity in both leopard frogs and green frogs [15] (Y.W. Huang, personal communication), the two species used in this study.
Our study of the acute and chronic exposure of anurans to PCB 126 tests two major hypotheses: (1) PCB 126 will show toxic effects in anurans that are similar to those reported for other animal groups that have been studied (PCBs have been correlated with depressed hatching success in bird and fish embryos [7, 8, 16], depressed survival and growth of fish fry and young mammals [17, 18], and increased edema in bird hatchlings and fish fry [19, 20]), and (2) PCB 126 will cause negative effects in anurans at ecologically relevant concentrations.
We studied the green frog and the northern leopard frog because they are common residents of the Green Bay ecosystem and because their populations may be affected by pollutants in the field. We exposed animals in a static-renewal experiment to increasing concentrations of PCB 126, as well as to negative and vehicle controls, from an early egg stage and up until metamorphosis. We chose to study eggs and larvae because amphibian early life stages are thought to be more sensitive to waterborne pollutants than are adults [9, 21]. We assessed PCB 126 effects on embryo hatchability and tadpole survivorship, deformities, edema, growth, swimming performance, and metamorphosis. Swimming performance may act as an indicator of underlying physiological stress and has been shown to decrease with exposure to certain toxicants [22]. It is also an ecologically relevant parameter, because tadpoles that swim slowly may be more susceptible to predation [22, 23]. As a quantitative index of actual (as opposed to nominal) exposure levels, we also measured bioconcentration of PCB 126 in tissues of tadpoles that did not metamorphose by the end of the experiment. Overall, this study is the first to look at the sensitivity of North American anuran amphibians to a model coplanar PCB compound, and it provides range-finding data for planning future PCB dose-response experiments.
MATERIALS AND METHODS
Study organisms
Two green frog egg clutches (approximately 1,000 eggs/clutch) were identified [24] and collected by netting in a pond near Deerfield, Dane County, Wisconsin, USA. Egg clutches were held in plastic containers and were transported to the Water Science and Engineering Laboratory at the University of Wisconsin-Madison. Two leopard frog egg clutches (approximately 300 eggs/clutch) were purchased from NASCO (Fort Atkinson, WI, USA). Egg clutches were fertilized and then transported in plastic containers to the laboratory in Madison. Embryos and tadpoles were staged during the experiment following the table proposed by Gosner [25].
Exposure of eggs
Two clutches of each species were exposed to the range of PCB concentrations. Green frog eggs were in the neurula stage (stages 15 to 16) in clutch 1 and in the mid-gastrula stage (stage 11) in clutch 2 when they were placed into treatment solutions for the hatchability study. Both leopard frog egg clutches were in the 4 to 16 cell stages (stages 4 to 6) at the beginning of exposure. Clutches of eggs (eggs released by one female and fertilized by one male) were kept separate throughout the experiment; however, there were no replicate treatments within clutches. Eggs were exposed to four or five levels of PCB 126 (Ultra Scientific, Kingstown, RI, USA, and AccuStandard, New Haven, CT, USA), 0.005 (green frog only), 0.05, 0.5, 5, and 50 μg/L, and to two control treatments. The positive control (0+) contained water plus 0.08% acetone (99.9+% pure, HPLC grade; Sigma Chemical, St. Louis, MO, USA), as a carrier for the PCB, and the negative control (0) contained only water. Two hundred and ten randomly drawn eggs from each clutch were subdivided into groups of 30 eggs, and each of these groups was exposed to 70 ml of one treatment solution in 100 × 20-mm glass petri dishes. Petri dishes were placed into a 23°C incubator on a 14:10 light:dark cycle. Treatment solutions were changed every 24 h (static renewal system) and were prepared with dechlorinated, charcoal-filtered water (pH 8.2; hardness 324 mg/L as CaCO3; dissolved oxygen 11.5 μg/L).
Green frog embryos were exposed to treatments for 5 d. Leopard frog embryos were exposed to treatments for 6 d. On the day embryos hatched, hatching success, deformities (categorized as bent or asymmetric tails or other grossly deformity), edema (distension of the body with fluid), and abnormal swimming performance were recorded.
Exposure of tadpoles
After all embryos had hatched, 20 surviving tadpoles (green frog) or 11 to 28 surviving tadpoles (leopard frog) from each petri dish were transferred to tanks containing 8 (green frog) or 6 L (leopard frog) of the same treatment solutions described in the egg experiment. Forty green frog tadpoles were exposed to each treatment, but numbers varied for leopard frogs, as follows: 40 tadpoles in 0+, 25 in 0, 42 in 0.05 μg/L, 37 in 0.5 μg/L, 36 in 5 μg/L, and 41 in 50 μg/L. A larger number of tadpoles was used in the leopard frog experiment (up to 28) because of the lower survival rate of animals purchased from NASCO (leopard frogs), compared with that of animals collected in the field (green frogs). Clutches were kept separate throughout the experiment; however, there were no replicate treatments within clutches. Tanks (two for each species/treatment) were placed in a thermoregulated water bath that was maintained at 23 to 24°C, with a 14:10 light:dark cycle. Water treatments in tanks were changed and tadpoles were fed every 3 d. Tadpole food consisted of boiled romaine lettuce blended into a puree and combined with a 3:1 Rabbit Chow:Tetra Min mixture (LM Animal Farms, Pleasant Plain, OH, USA; TetraMin Flake Food, TetraSales, Blacksburg, VA, USA). When the front legs of a tadpole emerged, the animal was measured and was transferred to a tilted plastic tub containing 1 L of treatment solution. The tilted tubs provided tadpoles with both dry and wet surfaces until they completed metamorphosis. Once placed in the tubs, tadpoles were not fed (metamorphosing tadpoles live off of fat stored in their tails), and treatment solutions were changed every 3 d.
Green frog tadpoles were exposed to treatments for 125 d. Leopard frog tadpoles were exposed to treatments for 104 d. The tanks were checked every day for mortality, and all dead tadpoles were removed and preserved in 10% formalin. Any deformities or abnormal swimming behaviors were recorded, either daily (green frog) or every 3 d (leopard frog). Snoutvent length (SVL), which was the total tadpole length minus the tail length (from day 23 after hatch), and total length (from day 50 after hatch) of 10 green frog tadpoles that had been chosen randomly from each tank were measured every 9 d. Total length of six leopard frog tadpoles (chosen randomly from each tank; from day 13 after hatch) was measured every 6 d and then every 9 d (from day 50 after hatch). We began length measurements of leopard frogs 10 d earlier than measurements of green frogs, because leopard frogs metamorphose much faster than do green frogs [26]. At day 50 after hatch, five green frog tadpoles (chosen randomly from each tank) were tested for swimming performance; we performed this test by chasing them, in a 25-cm plastic swimming channel, with a paintbrush, as described by Jung and Jagoe [22].
At metamorphosis (when tail length ≤ 2 mm), frogs were weighed, measured for SVL, and euthanatized. Frogs were then dissected to determine masses (±0.001 g) of liver, kidneys plus gonads, and fat bodies. Time to metamorphosis for each frog was recorded. Tadpoles that failed to metamorphose by the end of the experiment were weighed, measured for total length, staged, and euthanatized by immersion in a MS222 solution (3-aminobenzoic acid ethyl ester: 0.05% solution, Sigma Chemical). After euthanatization, tadpoles were frozen for contaminant analysis (leopard frogs: 14 tadpoles from 0+, 15 from 0.05 μg/L, 8 from 0.5 μg/L, 10 from 5 μg/L; and green frogs: 20 tadpoles from 0.5 μg/L, 23 from 5 μg/L, and 6 from 50 μg/L. One pool of tadpoles per treatment was used for both species).
Tadpoles were analyzed for PCB contaminant levels at the Wisconsin State Laboratory of Hygiene, University of Wisconsin-Madison. Levels of six PCB congeners (77, 123, 105, 156, 157, and 169) as well as of PCB 126 were analyzed. Organic analyses followed the procedures described in the Laboratory's Methods for Organic Analysis [27].
Statistical analysis
Percent hatching, survival, edema, deformities, and metamorphosis were transformed to logit values (using one value per tank), because proportions do not tend to be normally distributed. These logit values were used to create nine models that were conditional on three explanatory variables; species, clutch nested within species, and log [PCB concentration]. We used Mallow's Cp [28] as the basis for selecting the most appropriate model to explain variation in the data. The F and p values for log [PCB concentration] are given even when log [PCB concentration] is not included in the best model. The values for total length of tadpoles were compared between treatments using two-way analysis of variance, with treatment and clutch as the factors that distinguished between separate species. When analysis of variance results were significant, Tukey's honestly significant difference test for multiple comparisons was used. Swimming speed in green frog tadpoles was compared using analysis of covariance, with clutch as factor and log [PCB concentration] and total length as covariates. Body mass, SVL, logit-transformed percent edema, and time to metamorphosis in metamorphosed frogs were also compared by creating nine models conditional on species, clutch nested within species, and log [PCB concentration]. The procedures recommended by Mallows [28] were followed for selecting the most appropriate model. Models use positive (+1) but not negative (0) controls in the analyses. Organ masses for metamorphosed frogs were compared using analysis of covariance, with species as factor and log [PCB concentration] and SVL as covariates. Linear regression was used to relate concentration of PCB 126 in tissues of tadpoles that failed to metamorphose by the end of the experiment to nominal concentration of treatment water. Values for p of <0.05 for main effects and of <0.1 for interaction terms were considered to be statistically significant. Values for p for main effects that were <0.10 and >0.05 were considered to reflect trends.
RESULTS
Egg exposure
Treatment was not included in the best model to explain hatching success data (Fig. 1A). Hatchability was significantly different between species (F1,18 = 269; p < 0.001), with green frog eggs hatching at higher percentages than leopard frog eggs. Green frog eggs were collected in the field; leopard frog eggs were bought from NASCO. The percent fertilization of leopard frog eggs from NASCO is approximately 30% lower than that of wild-caught leopard frog eggs (K. Loeffler and M. Rosenshield, personal observations). The rates of hatching success of clutches within each species were not significantly different (F2,18 = 2.82; p = 0.086). If treatment is added to our model with species and clutch, treatment is not significant (F1,17 = 1.66; p = 0.22). Hence, PCB concentration had no significant effect on hatchability.
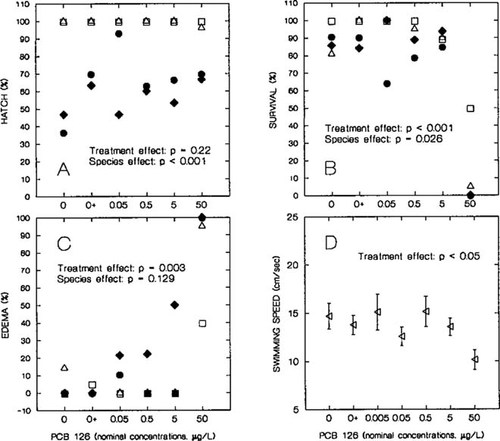
(A) Percent hatching success of embryos, (B) survivorship of tadpoles, and (C) edema in tadpoles for leopard frogs (•, clutch 1; ♦, clutch 2) and green frogs (▵, clutch 1; □, clutch 2) exposed to polychlorinated biphenyl (PCB) 126 and controls with (0+) and without (0) acetone vehicle. (D) Swimming speed of green frog tadpoles on day 50 after hatch during exposure to PCB 126 (n = 10 tadpoles per treatment). Points represent mean speed (±SE) of tadpoles of two green frog clutches.
Tadpole survival
Survival of tadpoles evaluated at the end of the experiment was significantly lower in both species at the highest PCB concentration (F1,19 = 20.6; p < 0.001)(Fig. 1B). Survival also differed between species (F1,19 = 5.81; p = 0.026). For the green frog, 6 out of 30 tadpoles survived after 125 d of exposure to the highest PCB 126 concentration of 50 μg/L (all in Gosner stages 30 to 39). Ten green frog tadpoles were lost during the experiment from the highest concentration treatment because of the flooding of one tank (thus, n = 30 for both clutches). For the leopard frog, no tadpoles survived after 47 d of exposure to 50 μg/L PCB 126.
Edema and deformities
Incidence of edema increased significantly in both species at high PCB concentrations (F1,20 = 11.373; p = 0.003) (Fig. 1C): 100% of leopard frog tadpoles and 77% of green frog tadpoles exposed to 50 μg/L PCB 126 exhibited edema at some point during the experiment.
The incidence of deformities (bent, kinked, or asymmetric tails or asymmetric bodies) in tadpoles of both species exposed to PCB 126 was relatively low; it never exceeded 10% in any treatment. Treatment was not included in the best model to explain incidence of deformities. There was no significant difference in deformities between leopard and green frogs (F1,18 = 0.48; p = 0.50); however, clutches within each species had significantly different incidences of deformities (F2,18 = 3.725; p = 0.044). When treatment was included in the model with species and clutch, it was not significant (F1,17 = 0.19; p = 0.67). For green frogs in clutch 1, one tadpole in the negative control treatment had a right back leg with only three toes, an undeveloped left back leg that was reduced to a gelatinous mass, and an asymmetric tail. In clutch 2, one tadpole in the 0.5 μg/L treatment had a bent tail and an asymmetric body, in which the right side was less developed than the left (front right leg was developed but never emerged). This tadpole died before completing metamorphosis. Two tadpoles from clutch 2 exposed to the 50 μg/L treatment showed large protuberances on the head between the eyes at the end of the experiment.
Only six leopard frog tadpoles exhibited deformities during the experiment. Three tadpoles from clutch 2 in the 0.05 μg/L treatment exhibited asymmetric bodies on day 40 after hatch. One tadpole from clutch 2 in the 5 μg/L treatment exhibited an asymmetric body on the last day of the experiment (day 104 after hatch). Also in clutch 2, one tadpole from the negative control treatment and one tadpole from the positive control treatment had asymmetric tails.
Swimming performance
There was a significant decrease in swimming speed (centimeters/second) in green frogs on day 50 after hatch at the highest concentration of PCB 126 (F1,167 = 9.69; p < 0.05)(Fig. 1D). The difference in swimming speed between clutches was significant (F1,167 = 8.57; p < 0.05), as was the interaction between clutch and total length (F6,167 = 7.56; p < 0.05). Tadpole total length was a significant covariate for swimming speed (F1,167 = 5.57; p < 0.05).
Total length
Green frog tadpoles exposed to 50 μg/L were smaller than tadpoles exposed to all other treatments on all dates measured (n = 12 dates), but the difference was significant only on six dates (days 50, 56, 62, 99, 105, and 117 after hatch, all p ≤ 0.05)(Fig. 2A). The effects of clutch and interaction of treatment and clutch were not significant on any of the dates on which tadpoles were measured (all p ≥ 0.064). Leopard frog tadpoles exposed to 50 μg/L were significantly smaller than tadpoles exposed to all other treatments on all dates during which both clutches were alive in this treatment (days 13, 19, and 25 after hatch, all p ≤ 0.002)(Fig. 2B).
There were no significant differences in total length of tadpoles in the five remaining treatments throughout the rest of the experiment (days 31, 37, 43, 50, 59, 68, 77, 86, and 95 after hatch; all p ≥ 0.272). The effects of clutch and interaction of treatment and clutch were not significant on any of the dates on which tadpoles were measured (all p ≤ 0.082), except on the first date, when interaction of treatment and clutch was significant (F5,60 = 3.54; p = 0.007).
Percent metamorphosis
The total numbers of leopard frog tadpoles that survived in the experiment were as follows: 35 tadpoles in 0+, 22 in 0, 32 in 0.05 μg/L, 31 in 0.5 μg/L, and 32 in 5 μg/L. There are no data for leopard frogs at 50 μg/L because no animals survived at this concentration. The total numbers of green frog tadpoles that survived were as follows: 39 tadpoles in 0+, 37 in 0, 39 in 0.05 μg/L, 38 in 0.5 μg/L, 35 in 5 μg/L, and 30 in 50 μg/L (treatment effect: p = 0.06; species effect: p < 0.001). The numbers of tadpoles that metamorphosed were as follows: leopard frogs: 20 metamorphoses in 0+, 13 in 0, 14 in 0.05 μg/L, 22 in 0.5 μg/L, and 18 in 5 μg/L; green frogs: 8 metamorphoses in 0+, 4 in 0, 14 in 0.05 μg/L, 17 in 0.5 μg/L, and 12 in 5 μg/L.
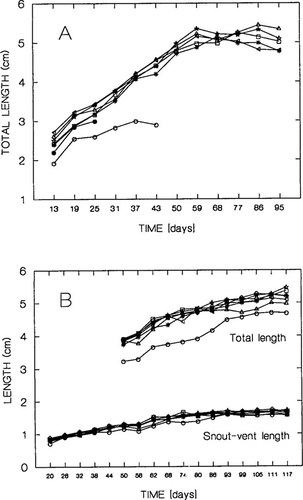
Growth of (A) leopard frog and (B) green frog tadpoles exposed to polychlorinated biphenyl (PCB) 126 and controls with (0+) and without (0) acetone vehicle (* = 0 μg/L; ★ = 0 μg/L (+); ◃ = 0.005 μg/L; □ = 0.05 μg/L; ⋄ = 0.5 μg/L; ▵ = 5 μg/L; ○ = 50 μg/L [•, clutch 1; ○, clutch 2 for leopard frogs]). In these plots, the clutches (each a mean value from six [leopard frog] or ten [green frog] tadpoles in a tank) are pooled within each exposure concentration (except for leopard frogs in the 50 μg/L concentration).
There was a trend for decreased percent metamorphosis in tadpoles at the highest concentration of PCB 126 (F1,20 = 3.97; p = 0.052) (Fig. 3A). When percent metamorphosis was analyzed without the 50 μg/L group, there was a significant increase in percent metamorphosis, with increased concentration of PCB 126 (F1,13 = 7.77; p = 0.015). A higher percentage of leopard frogs metamorphosed, compared with this same rate in green frogs (F1,13 = 36.7; p < 0.001), and clutches within species had significantly different numbers of tadpoles that metamorphosed (F2,13 = 18; p < 0.001). Four green frog tadpoles died during the period of tail resorption, just before completing metamorphosis (stages 41 to 45; 2 exposed to 0.5 μg/L and 2 exposed to 5 μg/L), and nine leopard frog tadpoles died during this period (1 exposed to 0+, 3 exposed to 0.05 μg/L, 1 exposed to 0.5 μg/L, and 4 exposed to 5 μg/L).
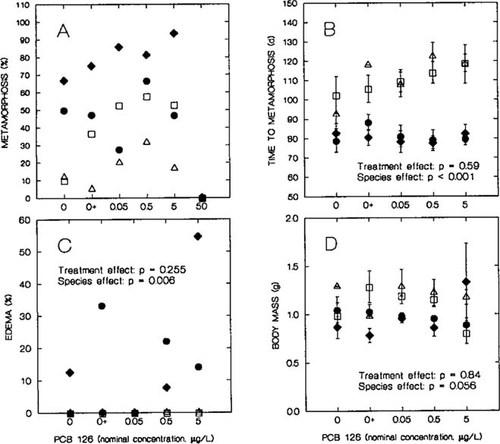
(A) Percent metamorphosis of leopard frog and green frog tadpoles exposed to polychlorinated biphenyl (PCB) 126 and controls with (0+) and without (0) acetone vehicle. (B) Mean time to metamorphosis (±SE) of leopard frog and green frog tadpoles exposed to PCB 126. (C) Percent edema and (D) mean body mass (±SE) of leopard frog and green frog metamorphs exposed to PCB 126. Leopard frog: •, clutch 1; ♦, clutch 2. Green frog: ▵, clutch 1; □, clutch 2.
We observed edema in leopard frog metamorphs but not in green frog metamorphs (F1,12 = 11.156; p = 0.006) (Fig. 3C). When species were analyzed together, the effect of treatment and the treatment-by-species interaction were not significant (respectively, F1,12 = 1.426; p = 0.255 and F1,12 = 1.426; p = 0.255). Within leopard frogs alone, the incidence of edema was significantly higher in the 5 μg/L treatment, as compared with this value in all other treatments combined (chi-square = 8.74; df = 1; p < 0.01). Two metamorphs in the 5 μg/L treatment exhibited such severe edema in the head, legs, and body that they were unable to leap.
Time to metamorphosis
The effect of treatment was not included in the best model to explain time to metamorphosis in either species. However, species differences were important, because leopard frogs metamorphosed significantly sooner than did green frogs (F1,123 = 146; p < 0.001) (Fig. 3B). When treatment was included in the model with species to explain variation in time to metamorphosis, it was not significant (F1,122 = 0.296; p = 0.59). Therefore, exposure to PCB 126 did not significantly affect the time at which tadpoles metamorphosed.
Body mass and SVL at metamorphosis
Treatment was not included in the best model to explain differences in body mass between metamorphs; however, there was a trend for green frogs to be larger than leopard frogs (F1,123 = 3.71; p = 0.056) (Fig. 3D), which is typical for these species. When treatment was added to the model with species to explain body mass data, it was not significant (F1,122 = 0.04; p = 0.84). Snout-vent length was not significantly different between treatments for both species (F1,118 = 1.92; p = 0.169), but green frogs (19.906 ± 0.198 mm) were significantly longer than leopard frogs (19.3 ± 0.158) (F1,118 = 10.2; p = 0.002). Clutches were significantly different within each species (F2,118 = 5.28; p = 0.006), and treatment and clutch interactions were significant as well (F2,118 = 3.58; p = 0.031).
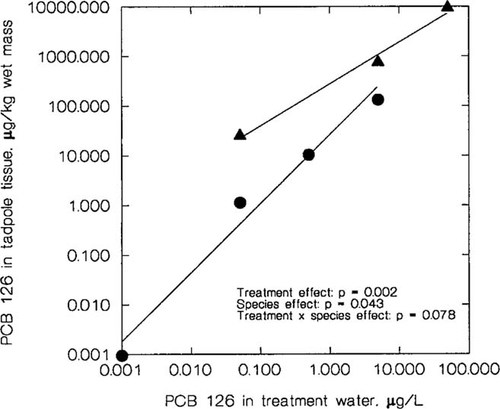
Relationship between nominal concentration of polychlorinated biphenyl (PCB) 126 in treatment water (μg/L) and concentration of PCB 126 in tadpole tissue (μg/kg wet mass). One pool per treatment containing the following numbers of individuals: leopard frogs (•), 14 tadpoles from 0+, 15 from 0.05 μg/L, 8 from 0.5 μg/L, and 10 from 5 μg/L;. Green frogs (▴), 20 tadpoles from 0.5 μg/L, 23 from 5 μg/L, and 6 from 50 μg/L.
Organ masses
Log [PCB concentration] was not a significant covariate for liver mass (F1,138 = 0.017; p = 0.898). However, there was a significant effect of species on liver mass (F1,138 = 5.87; p = 0.017) and a significant effect of SVL as a covariate (F1,138 = 112; p < 0.001). The adjusted least-squares mean liver mass was 0.025 ± 0.001 g in green frogs and 0.022 ± 0.001 g in leopard frogs. Log [PCB concentration] as a covariate for kidney-gonad mass was not significant (F1,135 = 0.777; p = 0.380). However, effect of species was significant (F1,135 = 35.9; p < 0.001), and SVL was a significant covariate (F1,135 = 53.5; p < 0.001). The adjusted least-squares mean kidney-gonad mass was 0.013 ± < 0.001 g in green frogs and 0.009 ± <0.001 g in leopard frogs. The effect of log [PCB concentration] as a covariate for fat body mass was not significant (F1,137 = 1.63; p < 0.204). However, there was a significant effect of species on fat body mass (F1,137 = 25.81, p < 0.001) and a significant effect of SVL as a covariate (F1,137 = 59.0; p < 0.001). The adjusted least-squares mean fat body mass was 0.006 ± 0.001 g in green frogs and 0.003 ± <0.001 g in leopard frogs.
Tadpoles that failed to metamorphose
A logistic regression showed no effect of treatment (F1,20 = 0.34; p = 0.57) or species (F1,20 = 0.08; p = 0.78) on the percent of tadpoles reaching the last stage before metamorphic climax (stage 40) by the last day of the experiment. Of 74 green frog tadpoles and 56 leopard frog tadpoles, 12.41 and 12.83% reached stage 40, respectively.
The concentration of PCB 126 in tissues of tadpoles living to the end of the experiment increased in relation to nominal concentration of treatment water (Fig. 4). The log [concentration of PCB 126 in treatment water] was a significant factor in determining log [concentration of PCB 126 in tadpole tissues] (F1,3 = 116; p = 0.002). The species term in the model was also significant (F1,3 = 11.5; p = 0.043), indicating that there was a difference between the two species in terms of PCB bioconcentration. The value that equates to bioconcentration factor = PCB 126 concentration in wet tadpole tissue/PCB 126 concentration in treatment water ranged from 22 to 28 in leopard frogs and from 150 to 500 in green frogs. A significant interaction term of log [concentration of PCB 126 in treatment water] and species (F1,3 = 6.92; p = 0.078) indicated that the slopes of the regression lines for leopard frogs and green frogs were different. Control tadpoles did not have detectable concentrations of any PCB congener. No PCB congener other than 126 was detectable in treated animals, with the exception of the 50 μg/L green frog group, which had 5.3 ng/g PCB 77 and 1.1 ng/g PCB 169. These two congeners may have been the result of impurities in the initial PCB stock solution.
DISCUSSION
Bioconcentration in tissue
The PCB body burdens we determined from this study (0.0012 to 9.3 μg/g wet mass) were comparable to or higher than body burdens recorded in other studies of anurans in the Green Bay ecosystem. Huang [15] collected leopard frog adults from sites along the Fox River and Green Bay in the summers of 1994 and 1995. Adult frogs had total PCB levels between 0.0028 and 0.15 μg/g wet mass (without livers). During the summers of 1994 and 1995, Jung [13] and Jung and Karasov (unpublished data) raised green and leopard frogs from the egg stage through metamorphosis in enclosures placed along a pollution gradient in the Fox River and Green Bay. Green frog tadpoles collected 106 d posthatch had total PCB levels of 0.023 to 0.283 μg/g wet mass (without livers), and leopard frog tadpoles and metamorphs (without livers) collected approximately 60 d posthatch had total PCB levels of 0.020 to 2.74 μg/g wet mass. Tetrachlorinated biphenyls were the predominant homologues in tadpole tissue. Green frog tadpole and leopard frog tadpole and adult PCB body burdens were significantly correlated with sediment PCB levels in the river and the bay. Sediment PCB levels were also significantly correlated with decreased hatching success of embryos for both species. We did not observe decreased hatching success in the present study, even though the tadpole PCB body burdens from the 50 μg/L treatment were much higher than those from the field. However, it is important to consider that there could be differences between the effect of exposure levels in a controlled, stable laboratory environment and exposure levels in a fluctuating field environment, in which disease, predation, inclement weather, and other stressors may act in concert with toxicants.
The PCB levels in tadpoles exposed to the high-concentration treatments (0.14 and 0.75 μg/g wet mass for 5 μg/L and 9.3 μg/g wet mass for 50 μg/L) were similar to levels reported for invertebrates, fish, and birds in the Green Bay watershed. Annelids and chironomids collected in the Fox River/ Green Bay area had total PCB levels of 0.377 and 0.351 μg/g wet mass, respectively [29]. Sullivan et al. [30] reported total PCB concentrations in carp (fillets) captured in Little Lake Butte des Morts to be approximately 5 μg/g wet mass. In 1988, Forster's tern and common tern chicks, tree swallow nestlings, and red-winged blackbird adults collected from colonies near the mouth of the Fox River [31] had total PCB concentrations ranging from 0.053 to 14.1 μg/g wet mass. It is important to note that more than 100 PCB congeners are included in these total PCB body burdens. In the case of frogs in the Green Bay ecosystem, PCB 126 and other coplanar congeners occurred at very low or undetectable levels. Furthermore, congeners vary greatly in their ability to cause deleterious effects in organisms. For example, PCB 126 is over 10,000 times more potent than is 2,3′,4,4′,5,5′-hexachlorobiphenyl in a cytochrome P450 monooxygenase activity assay [32]. Therefore, the body burdens of PCB 126 recorded in this study would be expected to cause more toxicity than would comparable total PCB body burdens reported for animals in the ecosystem.
Embryos and larvae
Newly hatched green frog and leopard frog tadpoles exhibited increased mortality when compared with embryos exposed to PCB 126. The hatching success of ranid embryos exposed to PCB 126 throughout the egg stage at concentrations of up to 50 μg/L PCB 126 was not significantly lower than that of controls. However, tadpoles in this group, exposed during the egg and larval stages, exhibited high mortality. All leopard frog tadpoles exposed during the egg and larval stages to 50 μg/L PCB 126 (n = 41, combined for both clutches) were dead by day 47 of the experiment, and only six green frog tadpoles (n = 30, combined for both clutches) survived to day 125. (The higher survival of green frogs was possibly due to the difference in developmental stage at which exposure was begun for the two species.) Jung [13] also observed increased mortality of tadpoles compared to embryos when leopard frogs were exposed (during the egg stage) to graded doses of waterborne 2,3′,7,8-tetrachlorodibenzo-p-dioxin (TCDD), a highly toxic TCDD isomer that is stereochemically similar to PCB 126, for 24 h. 2,3′,7,8-tetrachlorodibenzo-p-dioxin binds the same receptor (Ah) as PCB 126 and is considered to be 10 to 10,000 times as potent, depending on the species tested. Jung did not see decreased hatching success at any concentration of TCDD tested. However, leopard frogs exposed for 24 h during the egg stage to 3 μg/L TCDD (approximately equivalent to 30 μg/L PCB 126, by conservative toxic equivalency factor estimates) had significantly increased mortality as tadpoles when compared with controls. Therefore, it is possible that toxicity occurring during the embryo stage is not manifest until the eggs have hatched into larvae.
Our results are consistent with those found for other animal groups and other contaminants. Rainbow trout eggs that were injected with graded doses of PCB 126 at 24 to 50 h postfertilization [20] or that were exposed to waterborne TCDD for 48 h during the egg stage [33] also had high hatching success but low survivorship of newly hatched fry. In an experiment with fathead minnows exposed to Aroclor® 1242 (Monsanto, St. Louis, MO, USA) a commercial PCB mixture, eggs maintained at high concentrations (15 to 51 μg/L) hatched with good success, but none of the fry survived [17]. Dial and Bauer [34] found leopard frog eggs to be very resistant to paraquat, a herbicide, until approximately 3 d posthatch, when a significant increase in mortality was observed in all but one treatment group. Berrill et al. [35] also found that in experimental exposures of leopard and green frogs to low concentrations of pyrethroid insecticides, “newly hatched tadpoles are considerably more sensitive than embryo stages.”
Our results seem to differ from those of Jung [13] and Jung and Karasov (unpublished data), who noted decreased hatching success of anuran eggs that had been maintained in the field (Fox River and Green Bay) in water with concentrations of total PCBs as low as 0.12 μg/L. We suggest two hypotheses to explain these contrasting results. First, it remains a possibility that the effects of PCB 126 on frogs may be different from the effects of the PCB congeners that are found in higher concentrations in frogs in the field [13, 15] but that are supposedly less toxic to wildlife. Second, the possibility that environmental factors beside PCBs differentially influenced the field sites and therefore caused variation in hatching success is very likely. Wave action, extreme water-level fluctuations, or unmeasured parameters caused by degradation of the Fox River sites that coincidentally have high PCB sediment levels are confounding factors that might influence hatching success of eggs in these environments. Rosenshield and Karasov (unpublished data) performed a study to determine if the pattern of hatching success of anuran eggs exposed in the laboratory to water collected along the same pollution gradient in the Fox River would be different than the pattern of hatching success of eggs exposed in the field. Their study minimized the confounding environmental factors present in the Jung [13] field study. Rosenshield and Karasov found no significant differences in hatching success among sites or between sites and tap water controls in the laboratory experiment. Therefore, differences in hatching success between sites in the field study were likely due to factors other than toxicants in the water, including PCBs.
Growth of both green and leopard frog tadpoles was slowed at the highest concentration of PCB 126. By day 13 after hatch (the first day animals were measured), both clutches of leopard frog tadpoles exposed to the highest concentration were already significantly smaller in total length than were the tadpoles in the other treatments. In green frogs, body length of tadpoles exposed to the highest PCB concentration was also significantly less than that of tadpoles in all other treatments by day 20 after hatch. This suggests that the effects on tadpole growth of the contaminant occur quite early in development. Perhaps contaminated larvae are already at a disadvantage at the time of hatching. Jung [13] found a negative correlation between tadpole total length and TCDD dose for green frogs (31 d after exposure) for 24 h during the egg stage. This retarded growth of newly hatched tadpoles exposed to TCDD or to coplanar PCBs that likewise bind the Ah receptor could have detrimental effects on a frog population as a whole. Smaller tadpoles may take a longer period of time to reach metamorphosis than larger ones. Therefore, the time period during which animals remain in an aquatic environment is prolonged, thus leaving them vulnerable to predators and pond desiccation.
Green frog tadpoles exposed to the highest concentration of PCB 126 swam significantly slower on day 50 posthatch than did animals from other treatment groups. This result differs from that of Jung [13], who found that the swimming speed of American toad and green frog tadpoles, measured on days 6 and 41 posthatch, respectively, was not affected after an acute 24-h exposure to TCDD during the egg stage. Jung [13] also did not find an effect on swimming speed (on days 11, 32, and 131 posthatch) when green frog tadpoles were chronically exposed to water collected from the Fox River (total PCB concentration = 81 ± 20.9 ng/L). In this study, tadpoles that swam significantly slower in the high-concentration treatment (50 μg/L) also had a significantly higher incidence of edema, which may have caused a physical constraint on swimming performance. Therefore, swimming performance in tadpoles may indirectly be negatively affected by PCB exposure.
Metamorphs
The observed trend of decreased percent metamorphosis with increasing PCB 126 concentrations is probably due to a lack of metamorphosis in green frogs exposed to the highest PCB concentration, because no leopard frogs tadpoles survived in this treatment. Only six green frog tadpoles in the 50 μg/L treatment survived to the last day of the experiment; therefore, we should be cautious in relating this effect to PCB toxicity. Once percent metamorphosis was analyzed without the highest concentration group, it increased with increasing concentration of PCB.
The differences between species in time to metamorphosis are explained by different developmental schedules in green frogs and leopard frogs. In the laboratory, the duration of the tadpole stage for Rana pipiens (2 to 2.5 months) is shorter than that of Rana clamitans (3 to 4 months) [26]. Also, depending on the habitat and weather conditions, green frog tadpoles hatched from egg masses laid late in summer (during and after July) may not metamorphose that year but will overwinter as tadpoles to metamorphose the following summer [36]. This explains why our green frog study was extended to the end of October, whereas the leopard frog study was completed in July.
The edema observed in leopard frog tadpoles from the higher concentration treatment (5 μg/L) during the period of tail resorption of metamorphosis (stages 41 to 45) was an interesting result. These animals did not show signs of edema before metamorphic climax began (stage 40); however, edema (and in two cases, severe edema) became apparent during the 4 to 6 d required for the tail to be resorbed to a length of 2 mm. We hypothesize that PCBs stored in the fat of the tadpole tail were released and mobilized into the systemic circulation during these last stages of metamorphosis, thereby causing detrimental effects in some animals. Also, it has been shown that thyroid hormones amplify the toxicity of some chlorinated compounds, such as TCDD [37]. Thyroid hormone levels (T4 in particular) in tadpole blood and pericardial fluid are maximal at the time of metamorphic climax [38, 39], thereby increasing the susceptibility of metamorphosing tadpoles to the toxic effects of the chlorinated contaminant.
In this study, the effect of PCB treatment was not included in the models to explain differences in body masses or SVLs of metamorphs. We suspect that body-mass measurements were skewed by the presence of edema in metamorphs from the 5 μg/L treatment, possibly masking the effects that PCBs might have had on growth. Therefore, we recommend the use of dry body-mass measurements in future studies of this kind. Differences in body weights between species explain the interspecific differences in organ masses. Jung [13] found that in green frogs exposed to Fox River water, only kidney masses of metamorphs differed between treatments. In this study, the effect of treatment as a covariate was not significant for liver, kidney/gonad, or fat weights. However, because no animals metamorphosed in the highest concentration of 50 μg/L, we were unable to assess the effects of PCB 126 on all metamorphic parameters.
Ecological significance
Leopard frogs and green frogs were negatively affected by waterborne PCB 126 at high concentrations (5 to 50 μg/L). However, sublethal effects were not apparent for the parameters we measured at concentrations that occur in water in the Green Bay ecosystem or at tissue residue levels that occur in wild frogs. Based on sediment PCB concentrations, and using a sediment-water partition coefficient of 1.5 × 105 [40], Jung [13] approximated total PCB concentrations at Deposit A and Deposit X, two highly contaminated sites in the Fox River, to be 0.147 and 0.021 μg/L, respectively. Therefore, the most contaminated Fox River site, Deposit A, had total PCB levels that were more than one order of magnitude lower than the concentration of PCB 126 that caused the lowest level of observable effects in this study (5 μg/L).
The prevalence of deformities in this study was quite low. The number of deformities we observed was no greater than that number observed in enclosures in the field [13] (R.E. Jung and W.H. Karasov, unpublished data) and this number did not differ between PCB treatments and controls. Contaminants are one of the many putative causes of frog malformations that have been suggested by scientists working in the field [41]; however, our lab study does not support the hypothesis that coplanar PCBs or other contaminants acting via the Ah receptor may be the cause.
For both leopard frogs and green frogs, clutches of eggs (eggs released by one female and fertilized by one male) were separated throughout the experiment. For some of the parameters measured, such as swimming performance and percent metamorphosis of larvae and body mass and SVL of metamorphs, we found significant differences between clutches within species. This indicates that there may be substantial interindividual or genetic variation within populations in terms of responses to PCB toxicity. This variation could have important implications for adaptation of anurans to degraded environments. In areas in which habitat degradation and environmental pollution are an important problem, amphibians that are more able to cope with the onslaught of chemicals released around them will more likely survive to reproduce.
Biological marker of toxicity
The edematous response of larvae exposed to high concentrations of PCB 126 in this study was consistent with signs of toxicity seen in other vertebrate classes. Walker and Peterson [20] observed sac-fry mortality preceded by hemorrhages and severe fluid accumulation beneath the yolk-sac epithelial membrane in rainbow trout injected with PCB 126 24 to 50 h postfertilization. Edema formation was also a common toxic response in the embryos of chickens whose mothers were fed PCBs [42] or in cockerel chicks that were themselves fed PCBs [19]. Waterborne exposure to Aroclors 1016, 1242, and 1254 during the period extending from fertilization to 4 d posthatch was shown to cause acute abdominal edema in leopard frogs, American toads, and Fowler's toads [9]. This edematous response may be caused by induction of cytochrome P4501A1 in the vascular endothelium, thus resulting in changes in hemodynamic or vascular permeability [43], but this hypothesis has yet to be tested.
In this study, all leopard frog tadpoles exposed to the highest concentration of PCB 126 developed edema in the body (not the tail) within 16 d following hatch. Mortality followed severe edema (tadpole body bloated to a spherical shape) in every case. Not all green frog tadpoles in this highest concentration developed edema, but all those that were severely affected died. Those animals exhibiting milder signs of edema (some fluid accumulation in the body) in the 5 and 0.5 μg/L treatments survived throughout the experiment. We suspect that this pathologic response may be useful as a biological marker of PCB contamination in amphibians and thus deserves further examination. If scientists can identify edema in tadpoles in the field, the response could be used to indicate anuran exposure to chemicals that work by the same Ah-receptor-mediated mechanism as PCB 126.
In summary, our research is the first to relate the concentration of a coplanar PCB contaminant in ranid larvae to toxicity manifested during development from egg to frog. The signs of toxicity we observed at high concentrations of PCB 126 (5 to 50 μg/L) consisted of a decrease in tadpole survivorship, an increase in edema, decrease in growth, and decrease in swimming performance of larvae, as well as an increase in edema of leopard frog tadpoles during metamorphic climax. Edema in tadpoles is a pathologic response that may be useful as a biological marker of PCB exposure in the field. Although both ranid species were affected by the PCB contaminant at high concentrations, no sublethal effects were apparent for the parameters we measured at ecologically relevant concentrations for the Green Bay ecosystem.
Acknowledgements
This work and M. Rosenshield's research assistantship were funded by the University of Wisconsin Sea Grant Institute under grants from the National Sea Grant College Program, National Oceanic and Atmospheric Administration, U.S. Department of Commerce, and the State of Wisconsin (federal grant NA46RG0481, projects R/MW-54 and R/MW-73). M. Jofré was supported by a fellowship and a grant to project 9502 from Secretaría de Ciencia y Técnica, Facultad de Química Bioquímica y Farmacia, Universidad Nacional de San Luis, San Luis, Argentina. We thank A. Roberts, S. Cammisa, Y.W. Huang, A. Mangione, J. Carey, and B. Darken.




