Use of fish gill cells in culture to evaluate the cytotoxicity and photocytotoxicity of intact and photomodified creosote
Abstract
The influence of ultraviolet (UV) irradiation on creosote toxicity was investigated with the rainbow trout (Oncorhynchus mykiss) gill cell line, RTgill-W1, and two indicator dyes, alamar Blue™ and 5-carboxyfluorescein diacetate acetoxymethyl ester. These monitor redox potential and membrane integrity, respectively. After solubilization and chemical analysis, creosote was presented to cells in the dark to measure cytotoxicity or concurrently with UV irradiation to evaluate photocytotoxicity. Additionally, creosote was photomodified by 2 h of UV irradiation before presentation to cells in the dark or together with UV. Cytotoxicity was detected only at high nominal creosote concentrations, but photocytoxicity occurred at creosote concentrations 35-fold lower. All the aromatic hydrocarbons in creosote appeared to contribute to cytotoxicity, but photocytotoxicity was due only to the fluoranthene, pyrene, anthracene, and benzo[a]anthracene in the mixture. Photomodified creosote was much more cytotoxic than intact creosote and this difference was most pronounced in the alamar Blue assay. Likely, this was due to photomodification products that impaired the mitochondrial electron transport chain. Photomodified creosote was slightly less photocytotoxic than intact creosote. Overall these results indicate that UV irradiation potentially enhances the toxicity of creosote to fish in several different but significant ways.
INTRODUCTION
Despite the fact that the toxicity of selected polycyclic aromatic hydrocarbons (PAHs) in the presence of ultraviolet (UV) radiation had first been shown with cells in culture in 1935 [1], UV radiation exposures have only recently been considered in the environmental risk analysis of PAHs. The pioneering work of Bowling et al. [2] on the acute toxicity of UV-irradiated, anthracene-exposed sunfish was followed by the screening of PAHs in invertebrates and plants (reviewed in [3]). However, because of the costs involved, studies on the photoinduced toxicity of PAHs to fish are limited to only a few PAHs [2, 4-6] and none have been conducted on complex PAH mixtures.
Recently, a methodology was developed that allowed the testing and ranking of 16 priority PAHs rapidly and inexpensively for their direct and photoinduced toxicity to a cell line from the rainbow trout (Oncorhynchus mykiss), RTgill-W1 [7-9]. This cell line was derived from a target tissue of photoinduced toxicity, the fish gill epithelium [5, 10] and thus can serve as a model system for the photoinduced toxic effects in fish. In this culture system, the rapid killing of cells by PAHs in the absence of UV radiation was designated direct cytotoxicity [8], and was in contrast to the damaging or killing of cells due to photochemical reactions that followed the absorption of UV radiation by the PAH, which was termed photocytotoxicity [7, 9]. The establishment of toxic potencies showed naphthalene to be most environmentally relevant for its direct cytotoxicity [8], whereas fluoranthene and pyrene appeared to have the most potential to impact fish through photocytotoxicity [9]. These potencies should also be useful in predicting or explaining the cytotoxicity and photocytotoxicity of PAHs in mixture.
Although most previous studies on the UV-mediated toxic effects of PAHs have dealt with intact compounds, Greenberg and his group have shown that the UV irradiation of PAH solutions before exposure to plants and bacteria also leads to a significant increase in toxicity [11-13]. This toxicity is apparently caused by PAH photomodification products, which most commonly arise in large numbers from the direct chemical reaction of UV-irradiated PAHs with oxygen. Potentially, these photooxidation products can be toxic by themselves [12] or by a concurrent UV radiation exposure [13]. As for intact PAHs, photooxidized PAH products are difficult to study in vivo because of their complexity but also because of their potentially different modes of toxic action. Such different modes of toxic action can be studied in vitro with various fluorescent indicator dyes, and two of them are utilized in our RTgill-W1 cell bioassay. The indicator dye alamar Blue™ measures the redox potential, and 5-carboxyfluorescein diacetate acetoxymethyl ester (CFDA-AM) measures the membrane integrity of a cell [7]. Thus, compounds that preferentially act on the electron transport chain will be detected specifically with alamar Blue, whereas the more general damage to membrane integrity will be measured with CFDA-AM.
The goal in this paper was to apply the RTgill-W1 cell bioassay to a complex chemical mixture, creosote. Creosote was chosen because it is a widely used wood preservative and because it can contain up to 85% PAHs [14]. The study comprised three major steps. One was the solubilization of creosote in a modified cell culture medium without the use of a carrier solvent and the chemical analysis of the 16 priority PAHs and some additional hydrocarbons in the final creosote stock solution. This analysis allowed predictions of the cytotoxicity and photocytotoxicity of creosote, using previously established toxic equivalent factors, which summarized the relationships between the physicochemical properties of PAHs and their toxicities [8, 9]. The second step of this study was the application of the creosote solution to RTgill-W1 cells in the absence or presence of UV radiation and the measurement of the direct cytotoxicity and the photocytotoxicity of creosote using the fluorescent indicator dyes alamar Blue and CFDA-AM. This procedure allowed the biological response of cells to creosote to be evaluated and compared to the predictions derived from the chemical analysis. Finally, the direct cytotoxicity and the photocytotoxicity of creosote that had been modified by a 2-h UV irradiation in the absence of cells was studied.
MATERIALS AND METHODS
Cell line and culture media
The cell line from rainbow trout gills, RTgill-W1, was developed in this laboratory [15]. This cell line, when tested as outlined in Clemons et al. [16], was found to lack inducible 7-ethoxyresorufin O-deethylase (EROD) activity, which is a measure of cytochrome CYP1A catalytic activity (N.C. Bols, unpublished data). Cells were cultured in 75-cm2 culture flasks at 22°C in Leibovitz's L-15 medium supplemented with 10% fetal bovine serum. The source of the tissue culture supplies and a description of the subcultivation procedure have previously been presented in detail [17, 18].
A modification of the basal medium, L-15, was used for exposure of cells to creosote and UV radiation. This was necessary because treatment of conventional growth media with UV radiation generates toxicants that appear to arise from UV modification of medium components such as vitamins and aromatic amino acids. A description of the constituents of the modified L-15 medium, which was designated L-15/ex, and preparation procedures have been outlined in Schirmer et al. [7].
UV radiation exposure
Cells were irradiated at room temperature in an atmosphere of air and in the presence of tissue culture plate lids as described in Schirmer et al. [7]. The UV irradiation was done with two UV-B fluorescent lamps (Southern N.E. Ultraviolet, Branford, CT, USA) at a photon fluence rate of 1.4 μmol/m2/s UV-B. This photon fluence rate, which approximates an energy fluence rate of 53 μW/cm2, previously has been shown to be environmentally relevant [5, 7]. In addition to UV-B (290–320 nm), the UV-B lamps emitted some visible (400-700 nm) and some UV-A (320–400 nm) radiation. However, with a visible to UV-A to UV-B ratio of 5:1.5:1, compared to 200:10:1 in natural sunlight [11], the spectrum was weighted toward its UV-B component. Irradiation was measured with an InstaSpec™II photodiode array spectroradiometer calibrated with a 1-kW quartz halogen lamp (Oriel, Stratford, CT, USA). The values represent the photon fluence rates at the surface of the medium in the wells. A 500-μl/well aliquot of L-15/ex previously has been shown to have little discernible effect on these fluence rates [7]. The duration of irradiation was 2 h for all experiments.
Preparation of creosote in L-15/ex
Liquid-phase creosote was provided by Carbochem (Mississauga, ON, Canada) and was found to have a density of approximately 1.0 g/ml. For application to cultured fish cells, creosote was dissolved in L-15/ex by a technique recommended by Tadokoro et al. [19] for dissolving creosote in water. This technique facilitated the solubilization of creosote in a manner that closely reflected the solubilization process in the environment. A volume of 70 μl (70 mg) of liquid creosote was added to 1 L of sterile L-15/ex in a sterilized amber glass storage bottle with a Teflon®-lined cap (VWR Canlab, Mississauga, ON, Canada) and a sterilized, Teflon-coated magnetic stirrer (VWR Canlab). This nominal creosote concentration was chosen because at some creosote treatment and storage sites the maximum aqueous concentration of PAHs was in this range [20]. To fully protect the solution from light, the bottle was wrapped in aluminum. The solution was stirred at room temperature and the solubilization process was monitored after stirring for 1 h, 1 d, 3 d, and 7 d. At the end of each stirring period, the solution was allowed to settle for 1 h to allow undissolved particles to settle to the bottom of the bottle before 150 ml was carefully removed for application to cell cultures, and for extraction and chemical analysis.
Extraction and chemical analysis of creosote
A sample volume of 120 ml creosote in L-15/ex was extracted as described by Bestari et al. [21] with minor modifications. Briefly, each sample was extracted three times with methylene chloride (Fisher Scientific, Nepean, ON, Canada). The three extracts were combined in an Erlenmeyer flask, which was equipped with a glass wool-plugged funnel containing a layer of sodium sulfate to facilitate the removal of water. The Erlenmeyer flask was then swirled several times in the presence of sodium sulfate followed by passage of the entire extract into a round-bottomed flask, which was equipped with a glass wool-plugged funnel and contained a few glass beads. The methylene chloride was evaporated with a rotary evaporator at 28°C to concentrate the extract to approximately 5 ml, followed by a stream of nitrogen to achieve dryness. The final extracts were obtained by adding 5 ml of methylene chloride to the round-bottomed flasks. The extracts were stored in 5-ml amber glass vials at −20°C until chemical analysis. Naphthalene and fluoranthene could be recovered from spiked samples to 82 and 90%, respectively, which was similar to the recovery rates reported by Bestari et al. [21]. The deviations between two extractions of the same stock solution were less than 12% for all compounds.
For chemical analysis, 3 μl of extract was injected into a Hewlett Packard 5890 Gas Chromatograph (GC) equipped with an HP7673A autosampler and flame ionization detector (FID) (Hewlett Packard, Mississauga, ON, Canada). The temperature was held at 35°C for 2 min, followed by a 15°C/min increase to 165°C, followed by a 30°C/min increase to 300°C and a constant temperature of 300°C for 10 min. The carrier gas was helium with a flow rate of 30 ml/min. The injector temperature of the GC was 200°C, and the FID temperature was 300°C. The deviations between repeated measurements of the same creosote sample were less than 10% for all chemicals.
Chemical analysis focused on PAHs because they can account for 85% of the compounds in creosote [14, 20]. However, a few other compounds were analyzed as well. These included the heterocyclics dibenzofuran and carbazole, and the aromatic hydrocarbons benzene, toluene, and xylene. The detection limits of the GC method for each of the compounds are given in Table 1.
| Concentration in creosote/L-15/ex stock solution (μg/L)a | |||||
|---|---|---|---|---|---|
| Compound | Detection limit (DL) (μg/L) | 1 h | 1 d | 3 d | 7 d |
| Priority PAHs | |||||
| Naphthalene | 5.2 | 16.29 | 2.46b | 516.7 | 207.8 |
| Acenaphthylene | 5.2 | 63.62 | 75.70 | 101.0 | 74.96 |
| Acenaphthene | 5.2 | 726.1 | 901.9 | 1,226 | 874.1 |
| Fluroene | 7.3 | 606.5 | 781.0 | 767.6 | 577.7 |
| Phenanthrene | 5.2 | 1,239 | 1,570 | 1,224 | 905.4 |
| Anthracene | 5.2 | 131.5 | 162.5 | 136.2 | 109.6 |
| Fluoranthene | 5.2 | 511.9 | 602.9 | 409.8 | 282.7 |
| Pyrene | 2.1 | 399.8 | 469.2 | 313.7 | 208.0 |
| Benzo[a]anthracene | 6.3 | 119.8 | 144.4 | 90.75 | 59.71 |
| Chrysene | 8.3 | 105.5 | 113.4 | NDc | ND |
| Benzo[b+k]fluuoranthened | 14.6 | ND | ND | ND | ND |
| Benzo[a]pyrene | 14.6 | ND | ND | ND | ND |
| Indeno[l,2,3-cd]pyrene | 16 < DL < 208 | ND | ND | ND | ND |
| Dibenzo[a,h]anthracene | 16 < DL < 208 | ND | ND | ND | ND |
| Benzo[g,h,i]perylene | 16 < DL < 208 | ND | ND | ND | ND |
| Other aromatic hydrocarbons | |||||
| Benzene | 19.8 | 234.2 | 147.2 | 234.4 | 131.7 |
| 2-Methyl-naphthalene + indoled | 9.4 | 363.2 | 342.9 | 930.3 | 587.4 |
| 1-Methyl-naphthalene biphenyl | 6.3 | 66.96 | 54.17 | 283.3 | 161.2 |
| 5.2 | 168.3 | 186.1 | 319.6 | 218.3 | |
| Heterocyclics | |||||
| Dibenzofuran | 7.3 | 704.8 | 891.2 | 1,043 | 774.7 |
| Carbazole | 9.4 | 372.6 | 459.4 | 481.6 | 397.5 |
| Other compounds analyzed for but not detected | |||||
| Toluene | 13.5 | ND | ND | ND | ND |
| Ethylbenzene | 8.3 | ND | ND | ND | ND |
| p-m-Xylenes; o-xylene | 8.3 | ND | ND | ND | ND |
| Trimethylbenzenes: 1,3,5; 1,2,4; 1,2,3 | 9.4 | ND | ND | ND | ND |
- aTime refers to the solubilization time of creosote.
- bValue is below the detection limit of the gas chromatography method.
- cND = not detected.
- dCompound coeluted in the applied gas chromatography method.
Cytotoxicity and photocytotoxicity of creosote
Confluent monolayers of RTgill-W1 cells in 48-well tissue culture plates were used to study the cytotoxicity and photocytotoxicity of creosote. Confluent cultures were achieved by plating 50,000 cells per well and allowing them to grow for 3 d. At confluency, the culture medium was removed and each well was rinsed once with 500 μl of L-15/ex. After the rinse, all wells received increasing volumes of creosote in L-15/ex together with fresh L-15/ex to yield a total volume per well of 500 μl. To determine cytotoxicity, cells were exposed to creosote for 6 h in the dark before the alamar Blue and CFDAAM cytotoxicity assays were carried out as described below. For photocytotoxicity, cells were exposed to creosote for 6 h in the dark before being UV irradiated for 2 h and measured with the alamar Blue and CFDA-AM cytotoxicity assays.
Cytotoxicity and photocytotoxicity of photomodified creosote
In order to obtain photomodified creosote, aliquots of creosote in L-15/ex were placed in glass jars and UV irradiated for 2 h. Glass jars were used to reduce losses due to adsorption. The jars were covered with 48-well plate lids to achieve photon fluence rates that were similar to those obtained in UV-irradiated 48-well tissue culture plates. The protocol that followed the preparation of photomodified creosote was identical to that described above for the cytotoxicity and photocytotoxicity of creosote but used photomodified creosote solution instead of intact creosote.
Alamar Blue and CFDA-AM cytotoxicity assays
The alamar Blue (Immunocorp. Science, Montreal, PQ, Canada) and CFDA-AM (Molecular Probes, Eugene, OR, USA) indicator dyes were used in combination as described in Schirmer et al. [7]. Briefly, alamar Blue and CFDA-AM were prepared together in L-15/ex to give final concentrations of 5% v/v and 4 μM, respectively. After removal of the creosote solutions, 150-μl aliquots of the dyes were added to each well and, 2 h later, fluorescence was quantified with a CytoFluor 2350 (PerSeptive Biosystems, Burlington, ON, Canada) at respective excitation and emission wavelengths of 530 (±30) and 595 (±35) nm for alamar Blue, and 485 (±22) and 530 (±30) nm for CFDA-AM.
Data analysis
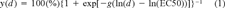 (1)
(1)| Compound | WS (μM)a | Kowb | WS × Kow (MAF)c | NEMAFd |
|---|---|---|---|---|
| Priority PAHs | ||||
| Naphthalene | 240 | 1,995 | 478,800 | 1.000 |
| Acenaphthylene | 26.0 | 12,589 | 327,314 | 0.684 |
| Acenaphthene | 23.0 | 19,953 | 458,919 | 0.958 |
| Fluorene | 12.0 | 15,136 | 181,632 | 0.379 |
| Phenanthrene | 7.00 | 28,840 | 201,880 | 0.422 |
| Anthracene | 0.409 | 28,184 | 11,527 | 0.024 |
| Fluoranthene | 1.28 | 165,959 | 212,427 | 0.444 |
| Pyrene | 0.667 | 75,858 | 50,597 | 0.106 |
| Benzo[a]anthracene | 0.048 | 407,380 | 19,554 | 0.041 |
| Chrysene | 0.013 | 812,830 | 10,567 | 0.022 |
| Other aromatic hydrocarbons | ||||
| Benzene | 22,000 | 135 | 2,970,000 | 6.203 |
| 2-Methyl-naphthalene + indole | 173 | 7,244 | 1,253,212 | 2.617 |
| 1-Methyl-naphthalene | 200 | 7,413 | 1,482,600 | 3.096 |
| Biphenyl | 48.6 | 12,589 | 611,825 | 1.278 |
| Heterocyclics | ||||
| Dibenzofuran | 28.2 | 13,183 | 371,761 | 0.776 |
| Carbazole | 6.160 | 5,248 | 32,328 | 0.067 |
- aWS = water solubility [34].
- bKow = octanol/water partition coefficient [34].
- cMAF = membrane accumulation factor.
- dNEMAF = naphthalene equivalent membrane accumulation factor (MAFcompound/MAFnaphthalene).
- eeCompounds coeluted in the applied gas chromatography method. The values in this table refer to 2-methyl-naphthalene only.
Predicting the cytotoxicity and photocytotoxicity of creosote. Predictions on the effects on cellular activity were made for the cytotoxicity and photocytotoxicity of creosote. These predictions were based on the chemical creosote analysis in this report and on the PAH toxicity data described in Schirmer et al. [8, 9]. In accordance with the toxicity data, naphthalene and fluoranthene were used as model compounds for, respectively, the cytotoxicity and photocytotoxicity of the hydrocarbons in creosote.
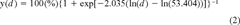 (2)
(2)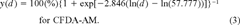 (3)
(3)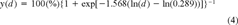 (4)
(4)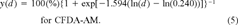 (5)
(5)| 1 h | 1 d | 3 d | 7 d | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | NEMAFb | Cc (μM) | NECd (μM) | C (μM) | NEC (μM) | C (μM) | NEC (μM) | C(μM) | NEC (μM) |
| Priority PAHs | |||||||||
| Naphthalene | 1.000 | 0.127 | 0.127 | 0.019 | 0.019 | 4.03 | 4.03 | 1.62 | 1.62 |
| Acenaphthylene | 0.684 | 0.418 | 0.286 | 0.497 | 0.340 | 0.664 | 0.454 | 0.492 | 0.336 |
| Acenaphthene | 0.958 | 4.71 | 4.51 | 5.85 | 5.60 | 7.95 | 7.62 | 5.67 | 5.43 |
| Fluorene | 0.379 | 3.65 | 1.38 | 4.70 | 1.78 | 4.62 | 1.75 | 3.47 | 1.31 |
| Phenanthrene | 0.422 | 6.95 | 2.93 | 8.81 | 3.72 | 6.87 | 2.90 | 5.08 | 2.14 |
| Anthracene | 0.024 | 0.738 | 0.018 | 0.912 | 0.022 | 0.764 | 0.018 | 0.615 | 0.015 |
| Fluoranthene | 0.444 | 2.53 | 1.12 | 2.98 | 1.32 | 2.03 | 0.901 | 1.40 | 0.625 |
| Pyrene | 0.106 | 1.98 | 0.210 | 2.32 | 0.246 | 1.55 | 0.164 | 1.03 | 0.109 |
| Benzo[a]anthracene | 0.041 | 0.525 | 0.021 | 0.632 | 0.026 | 0.397 | 0.016 | 0.261 | 0.011 |
| Chrysene | 0.022 | 0.462 | 0.010 | 0.497 | 0.011 | NDf | NAg | ND | NA |
| Other aromatic hydrocarbons | |||||||||
| Benzene | 6.203 | 3.00 | 18.6 | 1.89 | 11.7 | 3.00 | 18.6 | 1.69 | 10.5 |
| 2-Methyl-naphthalene + indole | 2.617 | 2.56 | 6.70 | 2.41 | 6.31 | 6.55 | 17.1 | 4.14 | 10.8 |
| 1-Methyl-naphthalene | 3.096 | 0.471 | 1.46 | 0.381 | 1.18 | 1.99 | 6.16 | 1.13 | 3.50 |
| Biphenyl | 1.278 | 1.09 | 1.39 | 1.21 | 1.55 | 2.07 | 2.64 | 1.41 | 1.80 |
| Heterocyclics | |||||||||
| Dibenzofuran | 0.776 | 4.19 | 3.25 | 5.30 | 4.11 | 6.20 | 4.81 | 4.60 | 3.57 |
| Carbazole | 0.067 | 2.23 | 0.149 | 2.75 | 0.184 | 2.88 | 0.193 | 2.38 | 0.159 |
| Total NEC | 42.2 | 38.1 | 67.3 | 41.9 | |||||
- aTime refers to the solubilization time of creosote.
- bNEMAF = naphthalene equivalent membrane accumulation factors, obtained from Table 2.
- cC = concentration in μ/L obtained from Table 1 and converted into mol/L according to the molecular weight of each compound.
- dNECs were calculated by multiplying C by the corresponding NEMAF.
- eCompounds coeluted in the applied gas chromatography method.
- fND = not detected.
- gNA = not available.
Statistical analysis. The cytotoxicity and photocytotoxicity of creosote, which were predicted as described above, were compared to the observed values of cytotoxicity and photocytotoxicity to RTgill-W1 cells in both the alamar Blue and the CFDA-AM assay. Four observed values of cytotoxicity or photocytotoxicity were available for each creosote concentration in each of the four creosote samples and stemmed from four replicate wells. Each of these replicate wells contained alamar Blue and CFDA-AM in mixture and was read twice with the CytoFluor to measure either alamar Blue or CFDAAM. These observed values, which were expressed as % of control wells that contained no creosote, were plotted along the y axis.
For predicted values of cytotoxicity or photocytotoxicity, only one value was available for each creosote concentration in each of the four creosote samples because predictions were based on the concentrations of the chemicals in the creosote. These predicted values, expressed as % of control, were plotted along the x axis.
To describe the goodness of fit between the observed and the predicted values, three regression analysis parameters had to be taken into account. These were the coefficient of determination (r2), the slope, and the y intercept. For a perfect match between the observed and the predicted values, these parameters would have to be one for r2, one for the slope, and zero for the intercept.
RESULTS
Preparation and chemistry of creosote solutions
The amount of creosote that could be dissolved in L-15/ex was dependent on the time of stirring. After 1 d of stirring, all priority PAHs were found at higher concentrations in L-15/ ex than after 1 h of stirring, with the exception of naphthalene (Table 1). After 3 d, some PAHs had further increased in concentration whereas others slightly decreased. In contrast, all PAH concentrations decreased between days 3 and 7 of stirring. This was also reflected in the total amount of priority PAHs detected, which was lowest after 7 d with 3,300 μg/L and highest after 1 and 3 d with 4,823 μg/L and 4,785 μg/L, respectively (Table 1). However, with the exception of naphthalene, the relative contribution of each PAH to the total mass detected remained relatively constant for each time point with acenaphthene, fluorene, and phenanthrene being the main contributors (Table 1).
Peak concentrations were observed after 3 d of stirring for the other aromatic hydrocarbons and for the heterocyclics (Table 1). Among the heterocyclics, dibenzofuran was more abundant than carbazole whereas among the aromatic hydrocarbons, 2-methyl-naphthalene + indole were the main contributors.
If all detected compounds were added together, the highest total mass was found to be 8,077 μg/L after 3 d of stirring, followed by 6,904 μg/L, 5,830 μg/L, and 5,571 μg/L after 1 d, 1 h, and 7 d of stirring, respectively. Thus, with the method used to solubilize creosote and with taking 31 compounds into account, 7.9 to 11.5% of the 70 μg/L creosote originally added to L-15/ex could be accounted for.
Cytotoxicity of creosote solutions
After RTgill-W1 cell cultures had been exposed to creosote solutions for 6 h in the dark, the two fluorescent dyes, alamar Blue and CFDA-AM, indicated cytotoxic responses. With both indicator dyes, fluorometric readings were greatly reduced at the highest nominal creosote concentration, which was 70 mg/L (Fig. 1). For the creosote sample that was obtained after 1 d of stirring, cytotoxicity was observed also at the second highest creosote concentration, 50 mg/L. For this creosote sample, EC50 values were 53 mg/L for alamar Blue and 52 mg/L for CFDA-AM and were the lowest observed for the cytotoxicity of the creosote solutions (Fig. 1). In contrast, EC50 values were highest for the creosote sample that had been stirred for 1 h before dosing. The EC50 values were 71 mg/L and 77 mg/L, respectively, for the alamar Blue and the CFDA-AM cytotoxicity assay (Fig. 1). Differences that were observed between the alamar Blue and CFDA-AM cytotoxicity assays were minor in the creosote samples that had been stirred between 1 h and 3 d. However, for the 7-d creosote sample, the alamar Blue assay revealed a higher level of cytotoxicity for 70 mg/L than did the CFDA-AM assay (Fig. 1).
| 1 h | 1 d | 3 d | 7 d | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Photocytotoxic compound | FEFb | Cc (μM) | FECd (μM) | C (μM) | FEC (μM) | C (μM) | FEC (μM) | C (μM) | FEC (μM) |
| Anthracene | 1.898 | 0.738 | 1.40 | 0.912 | 1.73 | 0.764 | 1.45 | 0.615 | 1.17 |
| Fluoranthene | 1.000 | 2.53 | 2.53 | 2.98 | 2.98 | 2.03 | 2.03 | 1.40 | 1.40 |
| Pyrene | 1.691 | 1.98 | 3.35 | 2.32 | 3.92 | 1.55 | 2.62 | 1.03 | 1.74 |
| Benzo[a]anthracene | 3.321 | 0.525 | 1.74 | 0.632 | 2.10 | 0.397 | 1.32 | 0.261 | 0.867 |
| Total FEC | 9.02 | 10.73 | 7.42 | 5.18 | |||||
- aTime refers to the solubilization time of creosote.
- bFEF = fluoranthene equivalent factors obtained from Schirmer et al. [9]. Although these FEFs were obtained from cytotoxicity assays 24 h after UV irradiation, they varied little from the 2-h FEFs.
- cC = concentration in μ/L, obtained from Table 1 and converted into mol/L according to the molecular weight of each compound.
- dFECs were calculated by multiplying C by the corresponding FEF.
Impairment of RTgill-W1 cells upon exposure to increasing concentrations of creosote in L-15/ex. Confluent cultures were exposed in the dark for 6 h to creosote that had been stirred in L-15/ex for 1 h, 1 d, 3 d, and 7 d. Immediately after the exposure, effects on cells were assayed with a mixture of alamar Blue (•) and 5-carboxyfluorescein diacetate acetoxymethyl ester (CFDA-AM) (□) and expressed as a percentage of the readings in control cultures that received no creosote. Each data point represents the mean of four culture wells. The vertical lines indicate the standard deviation.
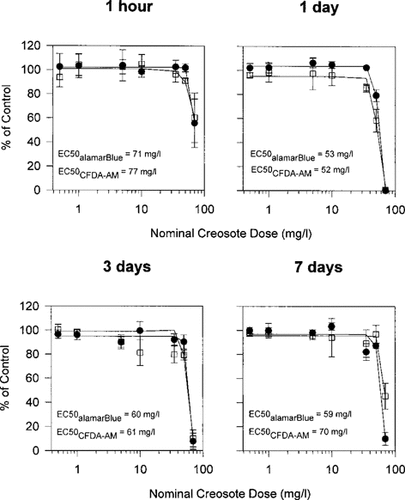
The observed and predicted abilities of creosote solutions to be directly cytotoxic were compared (Fig. 2 and Table 5). For predicted ability, the concentrations of all identified compounds in the creosote solutions were converted to NECs and summed to express each nominal creosote dose for each creosote solution as an NEC (Tables 2 and 3). The NEC of each nominal creosote dose for each creosote sample was subsequently applied to the appropriate logistic functions to yield dose–response curves of predicted direct cytotoxicity (Fig. 2). Generally, the predicted curves were too shallow at concentrations at which cytotoxicity occurred, leading to an underestimation of the direct cytotoxicity by the creosote solutions. Thus, it became apparent that not all cytotoxicity could be accounted for by NECs. The lack of fit between the predicted and observed values, particularly at high creosote concentrations, was also reflected by the regression analysis (Table 5). The values for r2 were below 0.82 for most cases and/or the slope or y intercept varied greatly from their ideal values of one or zero, respectively.
Impairment of RTgill-W1 cells upon creosote exposures as described in Figure 1 and as predicted from previously obtained dose-response curves of naphthalene cytotoxicity using naphthalene equivalent concentrations (NECs). Circled and squared symbols represent the observed values for the alamar Blue (•) and 5-carboxyfluorescein diacetate acetoxymethyl ester (CFDA-AM) (□) cytotoxicity assays in the 1- and 3-d-stirred creosote samples and are identical to those shown in Figure 1. Predicted values (+), joined by the solid lines, were obtained as described in the Materials and Methods. The 1- and 3-d-stirred creosote samples were chosen because they contained, respectively, the lowest and highest NECs (Table 3).
| Parameters describing linear regressiona | |||||
|---|---|---|---|---|---|
| Creosote solubilization time | Toxicity measured | Assay used | r2 | Slope (95% confidence interval) | y Intercept (95% confidence interval) |
| 1 h | Cytotoxicity | Alamar Blue | 0.47 | 0.94 ± 0.19 | 12 ± 17 |
| (0.54 to 1.3) | (−24 to 48) | ||||
| CFDA-AMb | 0.71 | 1.4 ± 0.17 | −34 ± 16 | ||
| (1.0 to 1.7) | (−67 to -1.2) | ||||
| Photocytotoxicity | Alamar Blue | 0.96 | 1.2 ± 0.050 | −4.9 ± 2.3 | |
| (1.1 to 1.3) | (−9.7 to -0.15) | ||||
| CFDA-AM | 0.96 | 1.3 ± 0.056 | −1.6 ± 2.5 | ||
| (1.2 to 1.4) | (−6.8 to 3.6) | ||||
| 1 d | Cytotoxicity | Alamar Blue | 0.84 | 2.4 ± 0.22 | −130 ± 20 |
| 1.9 to 2.8) | (−170 to -89) | ||||
| CFDA-AM | 0.95 | 3.3 ± 0.14 | −230 ± 13 | ||
| (3.0 to 3.6) | (−260 to -200) | ||||
| Photocytotoxicity | Alamar Blue | 0.95 | 1.2 ± 0.054 | −4.5 ± 2.3 | |
| (1.1 to 1.3) | (−9.3 to 0.20) | ||||
| CFDA-AM | 0.96 | 1.1 ± 0.043 | −0.35 ± 1.7 | ||
| (1.0 to 1.2) | (−4.0 to 3.2) | ||||
| 3 d | Cytotoxicity | Alamar Blue | 0.63 | 1.1 ± 0.18 | −8.1 ± 15 |
| (0.71 to 1.4) | (−39 to 23) | ||||
| CFDA-AM | 0.76 1.1 | ± 0.13 | −18 ± 11 | ||
| (0.88 to 1.4) | (−41 to 4.3) | ||||
| Photocytotoxicity | Alamar Blue | 0.93 | 1.0 ± 0.057 | −7.8 ± 2.8 | |
| (0.93 to 1.2) | (−13 to -2.1) | ||||
| CFDA-AM | 0.97 | 1.2 ± 0.043 | −2.3 ± 2.0 | ||
| (1.1 to 1.2) | (−6.4 to 1.7) | ||||
| 7d | Cytotoxicity | Alamar Blue | 0.81 | 2.0 ± 0.20 | −100 ± 18 |
| (1.6 to 2.5) | (−140 to -63) | ||||
| CFDA-AM | 0.66 | 1.6 ± 0.22 | −58 ± 21 | ||
| (1.1 to 2.0) | (−100 to -15) | ||||
| Photocytotoxicity | Alamar Blue | 0.96 | 1.0 ± 0.045 | −6.7 ± 2.3 | |
| (0.93 to 1.1) | (−11 to -2.0) | ||||
| CFDA-AM | 0.95 | 1.2 ± 0.054 | 5.2 ± 2.7 | ||
| 1.1 to 1.3 | (−0.31 to 11) | ||||
- aGoodness of fit between the observed and the predicted values increases with r2 approaching one, the slope approaching one, and the y intercept being close to zero.
- bCFDA-AM = 5-carboxyfluorescein diacetate acetoxymethyl ester.
Photocytotoxicity of creosote solutions
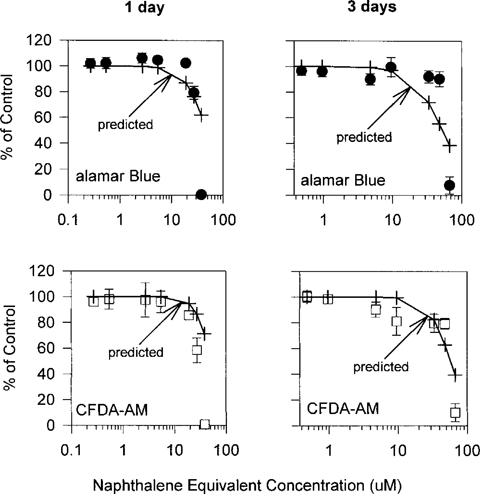
When RTgill-W1 cells were UV irradiated immediately after being exposed to creosote for 6 h in the dark, the two fluorescent dyes, alamar Blue and CFDA-AM, indicated more pronounced cytotoxic responses than dark controls. This photocytotoxicity was observed at concentrations above 1 mg/L. Except for minor differences, the two fluorescent dyes yielded similar dose–response curves for the creosote solutions that were obtained after 1 h to 3 d of stirring (Fig. 3). The EC50 values were lowest with the 1- and 3-d creosote samples but were slightly higher in the creosote solution that was obtained after 1 h of stirring (Fig. 3). For the 7-d creosote sample, the alamar Blue cytotoxicity assay yielded a dose-response curve and an EC50 value that were comparable to the dose-response curves and EC50 values obtained either with alamar Blue or CFDA-AM for the other three creosote samples. However, the CFDA-AM assay was less sensitive in the 7-d creosote sample, and resulted in an EC50 value that was three times above the EC50 value for alamar Blue (Fig. 3).
Previously, several PAHs were found to be strictly photocytotoxic, and fluoranthene was established as a reference compound for this mode of toxic action [9]. Among the photocytotoxic PAHs, four were found in all creosote samples. These PAHs were anthracene, fluoranthene, pyrene, and benzo[a]anthracene (Tables 1 and 4). When the concentrations of these compounds in the stock solution of 70 mg/L creosote in L-15/ex were corrected by their appropriate FEF [9], FECs were obtained (Table 4). The sum of FECs in each creosote sample and each nominal creosote dose was subsequently applied to the appropriate logistic functions to yield dose–response curves of predicted photocytotoxicity. If these predicted dose–response curves were compared to the observed photocytotoxicity data, a good agreement between the two was seen (Fig. 4). Linear regression analysis revealed high r2 values along with values that were close to one and zero for the slopes and y intercepts, respectively (Table 5). Therefore, the four photocytotoxic PAHs, anthracene, fluoranthene, pyrene, and benzo[a]anthracene, were concluded to fully account for the observed photocytotoxicity of creosote to RTgill-W1 cells.
Impairment of RTgill-W1 cells upon exposure to increasing concentrations of creosote in L-15/ex in the presence of UV radiation. Confluent cultures were exposed to creosote in the dark for 6 h as described in Figure 1 followed by a UV radiation exposure for 2 h. Immediately after UV irradiation, effects on cells were assayed with a mixture of alamar Blue (•) and 5-carboxyfluorescein diacetate acetoxymethyl ester (CFDA-AM) (□) and expressed as a percentage of the readings in control cultures that received UV radiation but no creosote. Each data point represents the mean of four culture wells. The vertical lines indicate the standard deviation.
Cytotoxicity of photomodified creosote solutions
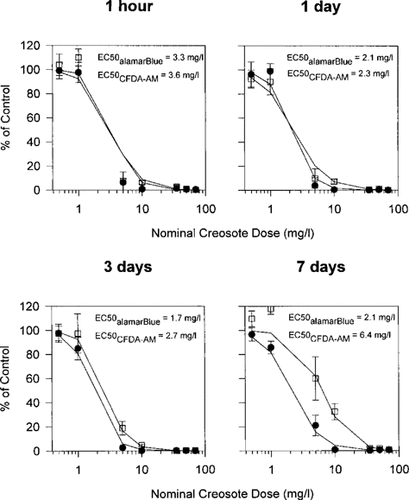
When RTgill-W1 cells were exposed for 6 h in the dark to creosote solutions that previously had been UV irradiated for 2 h, cytotoxicity continued to be detected for all four creosote solutions and with both assays of cellular activity (Fig. 5). However, in contrast to the cytotoxicity of unirradiated (intact) creosote, the two assays gave quantitatively different results. For the alamar Blue assay, cytotoxicity was seen at nominal creosote concentrations above 1 mg/L. However, for the CFDA-AM assay, nominal creosote concentrations of 5 mg/L and above were necessary to elicit an appreciable response (Fig. 5). Consequently, the EC50 values for the CFDA-AM assay were between 3.2 and 4.5 times higher than those for the alamar Blue assay.
Photocytotoxicity of photomodified creosote solutions
When RTgill-W1 cells were UV irradiated for 2 h immediately after being exposed to photomodified creosote for 6 h in the dark, photocytotoxicity continued to be detected for all four creosote solutions and with both assays of cellular activity (Fig. 6). In general, the alamar Blue and the CFDA-AM assays showed responses that were similar to those obtained for the cytotoxicity of photomodified creosote, although toxicity was much more pronounced for the two endpoints. For the alamar Blue assay, EC50 values were comparable to the EC50 values that were obtained for the photocytotoxicity of creosote and ranged from 2.1 mg/L for the creosote sample obtained after 1 d of stirring to 4.3 mg/L for the creosote sample obtained after 1 h of stirring (Fig. 6). For the CFDA-AM assay, EC50 values were 2.2 to 3.3 times above those obtained for the alamar Blue assay and ranged from 4.6 mg/L for 1 d of stirring to 11 mg/L for 7 d of stirring (Fig. 6).

Impairment of RTgill-W1 cells upon creosote exposures as described in Figure 3 and as predicted from previously obtained dose-response curves of fluoranthene photocytotoxicity using fluoranthene equivalent concentrations. Circled and squared symbols represent the observed values for the alamar Blue (•) and 5-carboxyfluorescein diacetate acetoxymethyl ester (CFDA-AM) (□) cytotoxicity assays in the 1- and 7-d-stirred creosote samples and are identical to those shown in Figure 3. Predicted values (1), joined by the solid lines, were obtained as described in the Materials and Methods. The 1- and 7-d-stirred creosote samples were chosen because they contained, respectively, the highest and lowest FECs (Table 4).
Comparison of cytotoxicity and photocytotoxicity of intact and photomodified creosote
The toxic potency of creosote to RTgill-W1 cells was strongly influenced by UV radiation. The most potent combination was the simultaneous presence of creosote and UV radiation, the photocytotoxicity of creosote. Compared to creosote alone, toxicity was increased 35 and 23 times in the alamar Blue and the CFDA-AM assays, respectively, in the creosote sample that was solubilized for 3 d (Table 6). If the same creosote sample was photomodified before exposure of the cells in the dark, toxicity was 7.6 (alamar Blue) and 2.0 (CFDA-AM) times that of intact creosote, but was lower than the photocytotoxicity of either creosote or photomodified creosote (Table 6). Similar ratios were found for the creosote samples that were obtained after 1 h, 1 d, and 7 d of solubilization. The average increase in toxicity compared to intact creosote for all creosote samples was 27 ± 5.9 and 19 ± 5.7 times for alamar Blue and CFDA-AM in the photocytotoxicity of creosote, 8.4 ± 1.1 and 2.3 ± 0.4 times for alamar Blue and CFDA-AM in the cytotoxicity of photomodified creosote, and 20 ± 4.0 and 8.5 ± 1.9 times for alamar Blue and CFDAAM in the photocytotoxicity of photomodified creosote.
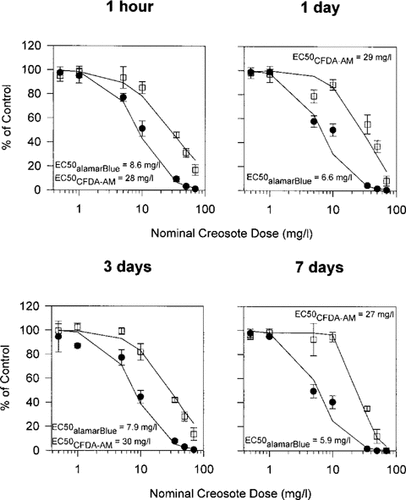
Impairment of RTgill-W1 cells upon exposure to increasing concentrations of photomodified creosote in L-15/ex. Confluent cultures were exposed in the dark for 6 h to creosote that had been stirred in L-15/ex for 1 h, 1 d, 3 d, and 7 d and subsequently modified by UV radiation exposure for 2 h. Immediately after the exposure of this photomodified creosote to cells, effects on cells were assayed with a mixture of alamar Blue (•) and 5-carboxyfluorescein diacetate acetoxymethyl ester (CFDA-AM) (□) and expressed as a percentage of the readings in control cultures that received no creosote. Each data point represents the mean of four culture wells. The vertical lines indicate the standard deviation.
The cytotoxicity and photocytotoxicity of intact and photomodifed creosote caused different changes in cell morphology as judged by phase-contrast microscopy. For the cytotoxicity of intact and photomodified creosote, cells often were enlarged and their nuclei more distinct than in control cultures. However, membrane blebs were not observed and cells remained attached to the culture surface. In contrast, for the photocytotoxicity of intact and photomodified creosote at the highest concentrations, many cells rounded up and detached from the growth surface during the 2 h of UV irradiation. At lower concentrations many cells remained attached but had blebs.
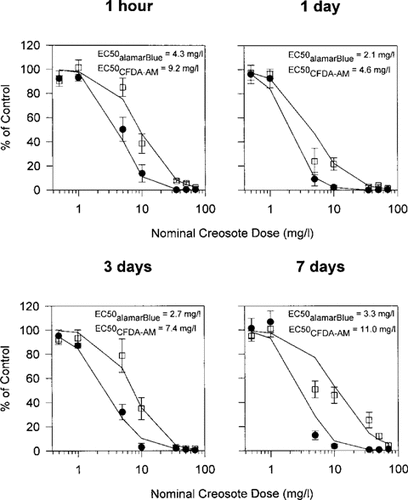
Impairment of RTgill-W1 cells upon exposure to increasing concentrations of photomodified creosote in L-15/ex in the presence of UV radiation. Confluent cultures were exposed to photomodified creosote as described in Figure 5 followed by a UV radiation exposure for 2 h. Immediately after UV irradiation, effects on cells were assayed with a mixture of alamar Blue (•) and 5-carboxyfluorescein diacetate acetoxymethyl ester (CFDA-AM) (□) and expressed as a percentage of the readings in control cultures that received no creosote. Each data point represents the mean of four culture wells. The vertical lines indicate the standard deviation.
DISCUSSION
Preparation and chemistry of creosote solutions
Creosote was solubilized in this study without the aid of a carrier solvent. Although the applied nominal creosote concentration was relatively high, chemical analysis showed compounds to be present in the final creosote solution at concentrations that well reflected those found in groundwater at different wood treatment sites [22, 23]. Compared to available data on the composition of the original creosote used in this study [24], creosote in L-15/ex was similar for major components such as phenanthrene, pyrene, and anthracene. However, naphthalene was found at lower concentrations than expected from the original creosote composition. This confirms studies by Tadokoro et al. [19], who found that the applied solubilization procedure led to creosote solutions that well reflected the original creosote with the exception of low-boiling chemicals. Alternatively, the relatively low naphthalene concentrations found in our study could be due to losses during the storage of the creosote before its application in the experiments described in this report.
| Alamar Blue | CFDA-AMb | |||
|---|---|---|---|---|
| Toxicity being measured | EC50 (mg/L)c | ∼ fold increased | EC50 (mg/L)c | ∼ fold increased |
| Cytotoxicity of creosote | 60.0 | 1.0 | 61.0 | 1.0 |
| Photocytotoxicity of creosote | 1.7 | 35.0 | 2.7 | 23.0 |
| Cytotoxicity of photomodifled creosote | 7.9 | 7.6 | 30.0 | 2.0 |
| Photocytotoxicity of photomodifled creosote | 2.7 | 22.0 | 7.4 | 8.2 |
- aTable contains values obtained in the creosote sample that had been solubilized for 3 d.
- bCFDA-AM = 5-carboxyfluorescein diacetate acetoxymethyl ester.
- cValues correspond to the EC50 values given for the 3-d creosote sample in Figures 1, 3, 5, and 6.
- dFactor refers to the increase in toxicity compared to the cytotoxicity of creosote (EC50 for the cytotoxicity of creosote/EC50 for each of the other properties).
Cytotoxicity of creosote solutions
The cytotoxicity elicited by creosote was similar in many ways to the direct cytotoxicity of individual low molecular weight PAHs [8]. Similar morphologic changes were observed in the cell cultures exposed in the dark to either creosote or individual low molecular weight PAHs. Both the alamar Blue and the CFDA-AM assay gave similar levels of impairment, which indicates that the two assays likely were measuring the same damage. This damage would be due to the specific inhibition of membrane-integrated enzymes or the general disturbance of membrane properties [8, 25]. In contrast, toxicity due to metabolic activation was unlikely to have occurred in the current experiments because of the absence of cytochrome CYP1A in the RTgill-W1 cell line, and because cytotoxicity occurred within such a short period.
High nominal creosote doses were necessary to elicit a cytotoxic response in RTgill-W1 cells. Comparisons to other studies that have used creosote are difficult because of the different ways in which creosote has been applied to test organisms and because the composition of creosote can vary significantly between manufacturers. However, mouse embryo cultures were affected by petroleum creosote at concentrations similar to those that were effective on RTgill-W1 cells [26]. Moreover, the supplementation of culture medium with a rodent hepatic S9 fraction did not modify the embryotoxicity of petroleum creosote.
Additivity has been an adequate model in the past for explaining the toxicity of mixtures of several creosote components, but in the current study such a model only partly described the cytotoxicity of the creosote solution. In the past, benzene, naphthalene, acenapthene, and 1-chloronaphthalene were shown to act additively in inhibiting mitochondrial respiration in vitro [27]. Naphthalene, acenaphthene, phenanthrene, and anthracene were slightly less than additive at equitoxic concentrations in eliciting acute toxicity to Daphnia magna, but additivity was still thought a sufficient model [28]. Presumably, in the current study, additional compounds were dissolved in the creosote solution that were not analyzed for chemically. These additional compounds could cause an underestimation of the cytotoxic potential of the creosote solutions in several ways.
The simplest cause of underestimation would be that one or more of the additional compounds was directly cytotoxic. In favor of this possibility is the fact that the chemistry of creosote is extremely complex, with up to 200 different compounds [14], whereas only 15 to 16 chemicals were identified in the current study and considered in the additive model. The phenols are one chemical class to consider. Phenols are frequently found in creosote [14], and have been shown to be cytotoxic to cultured fish cells [29] as well as to fish [30]. Another chemical class of potential importance is the polycyclic nitroaromatic hydrocarbons. In studies on the in vitro embryotoxicity of petroleum creosote, Iyer et al. [26] suggested that polycyclic nitroaromatic hydrocarbons were major contributors to the direct embryotoxicity. A different explanation for the underestimation is that the additional compounds might not be directly cytotoxic, but they could potentiate the cytotoxic actions of other compounds. Only further research with other complex solutions of aromatic hydrocarbons and more complete chemical analysis will allow these possibilities to be distinguished.
Photocytotoxicity of creosote solutions
The photocytotoxicity of creosote appeared to arise from photosensitized reactions that led to the formation of reactive oxygen species. This was because the responses of cells to UV irradiation and creosote solutions were similar to the responses to UV irradiation and individual PAH congeners, which appeared to be photocytotoxic through the production of reactive oxygen species [7, 9]. The similar responses were the morphologic changes of cells and the decline in cellular activities that occurred to the same extent in either the alamar Blue or CFDA-AM assays. The photosensitized reactions potentially could be due to intact PAHs or products that arise from the photomodification of the original sensitizing compound. Alternatively, photomodification products could be directly cytotoxic. However, if cytotoxic photomodification products should indeed arise, they appear to be less important in the presence of UV radiation. This is because if the creosote solution was photomodified before being added to the cells in the dark, toxicity was less than for the photocytotoxicity of creosote and the two fluorescent indicator dyes showed significantly different results. One exception in the photocytotoxicity of creosote is seen in the sample that was solubilized for 7 d. The different responses in the alamar Blue and CFDAAM assays hint at the formation of oxidized products whose impairment of the mitochondrial electron transport system occurs before general membrane damage. Such oxidized products could have formed spontaneously during the prolonged stirring process.
The photocytotoxicity of creosote solutions could be fully accounted for by four PAHs, using previously developed FEFs [9]. These four PAHs were anthracene, fluoranthene, pyrene, and benzo[a]anthracene. In a study on the ahototoxicity of several coal tar constituents to guinea pig skin, Kochevar et al. [31] found anthracene, fluoranthene, and pyrene to be most potent, whereas phenanthrene, chrysene, and carbazole were not phototoxic. Other researchers have found relatively high phototoxic potencies for anthracene, fluoranthene, pyrene, or benzo[a]anthracene in a variety of biological systems [3]. However, it has not been shown previously that these four compounds can account for the photocytotoxicity of a complex mixture such as creosote. This is particularly intriguing as two of the above congeners, namely fluoranthene and pyrene, are prevalent not only in creosote-contaminated aquatic environments [20, 23] but also in air ambient to aluminum plants, wood heating sources, and rural or urban areas, as well as in contaminated fresh water and sediments [32].
The application of FEFs in our study was done under the assumption that the photocytotoxic compounds act in an additive fashion. Research on the phototoxicity of defined mixtures has just begun. Hatch and Burton [33] recently showed that fluoranthene and anthracene were additive in their phototoxicity to benthic organisms. This is in agreement with the proposed similar mode of phototoxic action for PAHs, the generation of reactive oxygen species upon absorption of UV radiation. Hence, higher concentrations of a phototoxic compound or mixtures of phototoxic compounds will give rise to higher concentrations of reactive oxygen species and consequently enhanced toxicity.
Cytotoxicity of photomodified creosote solutions
Photomodifying creosote through UV irradiation must have generated cytotoxic products because irradiation caused the creosote solutions to be more cytotoxic. Previously, fluoranthene was shown to be extensively photomodified under similar experimental conditions [7], and because fluoranthene has a half-life in solar radiation that is intermediate among the detected PAHs [34], significant modifications of other PAHs in creosote can be expected as well. Most commonly, the photomodification of a single PAH leads to a complex mixture that contains, among other classes of compounds, quinones and diols [12, 13]. For example, 9,10-phenanthrenequinone (PHEQ) was found to be the major component and primary toxicant in phenanthrene-containing solutions that were exposed to actinic radiation [12]. This PHEQ was unique in that it not only was resistant to further photooxidation but also in that it did not support photosensitized reactions. With phenanthrene being the most abundant PAH in all creosote samples, PHEQ thus likely contributes to the cytotoxicity of photomodified creosote in the current study.
The alamar Blue assay was more sensitive than the CFDAAM assay in detecting an inhibition of cellular activity by the photomodified creosote solutions. The EC50s were three- to fourfold lower with alamar Blue. This indicates that photomodification products had specific actions in mitochondria. Such an impairment could be due to compounds that structurally mimic components of the electron transport chain. For example, quinones can receive electrons from NADH that are normally donated to ubiquinone. Alamar Blue and natural receptors would then compete for electrons with the quinones of the photomodified creosote solution, which potentially leads to a diminished reduction of alamar Blue and thus diminished fluorescent readings. The reduced quinone can pass on its electron to oxygen, forming superoxide radical and the parent quinone. The quinone can then repeat the cycle while the superoxide radical is converted into hydrogen peroxide by superoxide dismutase or into hydroxyl radical in the presence of iron by the Fenton reaction [35]. Thus, quinones can lead to yields of oxygen radicals that exceed the capabilities of cellular antioxidant defense mechanisms and result in a more general damage to cell membranes and proteins. In cultured fish cells, this general damage due to oxidative stress has been measured with CFDA-AM in this report and with Neutral Red in previous reports [36, 37].
Photocytotoxicity of photomodified creosote solutions
As with the photocytotoxicity of the original creosote solution, the photocytotoxicity of photomodified creosote appears to arise from photosensitized reactions. Support for this comes from the fact that in combination with UV irradiation both the intact and photomodified creosote solutions caused similar changes in cellular morphology and a level of impairment that was the same with either the alamar Blue or CFDAAM assays. These photosensitized reactions would have been initiated by parent compounds that remained after the photomodification process or by photomodification products that themselves act as photosensitizers. One such photomodification product that has recently been identified is 2-hydroxyanthraquinone [13].
Comparison of cytotoxicity and photocytotoxicity of intact and photomodified creosote solutions
The results of this study indicate that assessing the environmental impact of creosote is complex. Ultraviolet irradiation consistently increased the toxicity of creosote to fish cells in culture and, at least in the case of the photocytotoxicity of creosote, this was attributable to PAHs. A positive association in fish between high PAH exposures near creosote-contaminated sites and the induction of cytochrome P4501A1 [38] or the occurrence of liver neoplasms [39] has been reported and constitute other ways in which creosote can be toxic to aquatic organisms. For the UV radiation exposures, the highest toxicity was seen when creosote and the fish gill cells were present simultaneously. This photocytotoxicity of creosote led to a 35-fold increase in toxic potency. Exposure conditions in which photocytotoxicity would be expected to occur in the environment are near the wastewater efflux of wood treatment facilities. Wood treatment and storage facilities are major contributors of creosote entry into the environment [20]. Furthermore, the toxicity of creosote was enhanced when creosote was photomodified before exposure to the cell culture system. This cytotoxicity of photomodified creosote led to an eightfold increase in toxic potency and implies that UV irradiation does not necessarily result in the toxicity deactivation of complex mixtures. Borthwick and Patrick [40] tested the deactivation of creosote in salt water due to exposure to direct sunlight and measured a half-life of less than 1 week. This study indicates that prolonged UV radiation exposures will likely lead to a significant decrease in toxic potency under static conditions. However, if the supply of creosote-contaminated water is continuous, as would be expected at wood treatment sites, deactivation due to UV irradiation is not likely to occur, but in contrast, can lead to a further activation of toxicity.
Acknowledgements
We would like to thank Kimberly Hamilton for helping with the chemical analysis of the creosote solutions. This research was supported by a Strategic Grant from the Natural Sciences and Engineering Research Council of Canada and by the Canadian Network of Toxicology Centers.




