Classification of explosives transformation products in plant tissue†
Citation of trade names does not constitute an official endorsement or approval of the use of such commercial products.
Abstract
Explosives contamination in surface or groundwater used for the irrigation of food crops and phytoremediation of explosives-contaminated soil or water using plant-assisted biodegradation have brought about concerns as to the fate of explosives in plants. Liquid scintillation counting, high-performance liquid chromatography, and gel permeation chromatography were utilized to characterize explosives (hexahydro-1,3,5-trinitro-1,3,5-triazine and trinitrotoluene) and their metabolites in plant tissues obtained from three separate studies. Analyzing tissues of yellow nutsedge (Cyperus esculentus), corn (Zea mays), lettuce (Lacuta sativa), tomato (Lyopersicum esculentum), radish (Raphanus sativus), and parrot feather (Myriophyllum aquaticum) from three studies where exposure to explosives at nontoxic levels (1–2 mg/L in water) occurred showed that extensive transformation of the explosive contaminant occurred, variations were noted in uptake and transformation between terrestrial and aquatic plants, the products had significantly higher polarity and water solubility than the parent compounds, and the molecular sizes of the transformation products were significantly greater than those of the parent compounds (approximately 300 times greater).
INTRODUCTION
Explosives such as trinitrotoluene (TNT) and hexahydro1,3,5-trinitro-1,3,5-triazine (RDX) and their degradation products are substances that pose a threat to the environment because of their toxicity and mutagenicity and that are found at a number of sites used for munitions manufacture [1-3]. Phytoremediation has been suggested as a potential cleanup method for water and soil in wetland and upland areas [4, 5]. The products of the transformation of explosives have not been fully characterized, partially because of a lack of analytical techniques for detecting these compounds. Levels of the reactive parent compounds have been shown to be reduced by plant action [6], and the residues produced during bioaction on explosives have been investigated for two reasons. The first is the possibility that dangerous compounds may bioaccumulate in the lower rungs of the food ladder and become concentrated through predation, ultimately causing health problems in higher organisms, including humans. Groundwater plumes containing explosives and their degradation products exist in rural areas, and in some cases, this water is utilized for the irrigation of agricultural fields and gardens [6]. Recent studies have reported that levels of RDX in a number of plant tissues following irrigation with explosives-contaminated water have dry weight RDX concentrations that are above the action level for remediative cleanup of RDX in soils [6]. Peterson et al. [7] reported developmental effects due to TNT and 4-amino-2,6-dinitrotoluene (4-aDNT) in tall fescue. The second reason is the need to evaluate treatment technologies that utilize biological systems to clean up contaminated water and soil [8]. In order for these treatment technologies to acquire regulatory and community approval, the final fate of the contaminants in these systems must be determined. The degradative action that occurs in these systems is a combination of microbial and plant action on the contaminants. Recent work with axenic cell cultures has shown that plants are capable of explosives degradation in the absence of microbes [9]. An assay for the plant enzyme nitroreductase has been developed, and levels of this enzyme in plant species appear to correlate with the ability to degrade explosives [8]. Knowledge of the fate of explosives compounds following this type of biotreatment is vital in assessing the success or failure of these systems. A key to understanding the products of bioaction on explosives is the development of analytical techniques for classification of explosives transformation products in plant tissues.
The analytical tools used for the identification of explosives, by-products of their manufacture, and some degradation products of explosives have included high-performance liquid chromatography (HPLC) and gas chromatography (GC) for the separation and identification of contaminants [10, 11]. These techniques have been refined to analyze a number of explosives-related contaminants. However, because of the extent of transformation of the parent compounds by biological systems (resulting in compounds with significantly different molecular weight and polarity from the parent compounds for which the analytical techniques are designed), alternative techniques for the detection of the ultimate products of biodegradation of explosives are required.
An analytical technique developed for polymer classification and environmental sample preparation, gel-permeation chromatography (GPC), is utilized to determine the molecular size of explosives residues in plant extracts. The technique is capable of elucidating the molecular weight distribution in complex mixtures [12] and has been used in this study to show that RDX transformation products are larger than the parent compound. Chemists utilize GPC to determine molecular weight distributions during polymer characterization [13] and to perform the separation of synthetic macromolecules [14], to perform sample preparation before analysis of pesticides and polychlorobiphenyls in soils, sludges, animal fats, crops, feeds, and other environmental samples [15], and to determine the molar masses and sizes of plant products such as starches [16]. Incorporation of radioactive isotopes of H, N, and C atoms into contaminant compounds and use of these isotopically labeled molecules to probe the fate of these compounds has been performed [17-19]. Scintillation counting of the beta emissions resulting from the radiation characteristic of [14C] can be used to trace the fate of labeled atoms regardless of the molecular form [20].
Liquid scintillation counting, HPLC, and GPC are performed on extracts from terrestrial and aquatic plant tissues exposed to explosives contamination in order to further understand the process of biological transformation of explosives by terrestrial and aquatic plants. The result of this work provides an increased understanding of the extent of transformation of the explosive contaminant, the molecular properties of the residues, and the relative amount of these residues present in exposed plant tissues.
MATERIALS AND METHODS
Plant tissue generation
Aquatic plant tissues: Parrot feather (Myriophyllum aquaticum). Contaminated parrot feather tissue came from a continuous flow reactor (60 L) in which a 20-g/L density of parrot feather was exposed to an inlet TNT concentration of 2 μg/g for a period of 2 months. In order to give uniform concentration of the contaminant, the influent was diffused through 4 cm of rocks. A 20-g/L density of parrot feather was utilized in the reactor. After the reactor had operated for 1 month, a 10-g subsample of the plants was removed, washed with distilled water, blotted dry, placed in a plastic bag, and frozen. Another 10-g sample was taken in the same manner after the reactor had operated for 2 months. An identical reactor was operated with no TNT as a control.
Tissue from exposed garden crops. Plant tissue was obtained following a study designed to address the effects of RDX-contaminated irrigation water on plant (corn [Zea mays], lettuce [Lacuta sativa], radish [Raphanus sativus], tomato [Lyopersicum esculentum], and yellow nutsedge [Cyperus esculentus]) uptake of the contaminant. Corn, tomato, radish, and lettuce served as examples of common garden crops, and yellow nutsedge served as an index plant for explosives uptake. The experimental design included plants irrigated with reverse osmosis water as a control and plants irrigated with 1.0 μg/ml RDX.
The source of the seeds of the agronomic crops was BWI Companies (Jackson, MS, USA). Yellow nutsedge tubers were obtained from Wildlife Industries (Oshkosh, WI, USA). After planting, reverse osmosis water was added to the soil surface and to the outer bucket. Day length was maintained at an even photosynthetic active radiation distribution pattern of 1,200 μE/m2/s. Day length for the warm season crops (corn, tomato, and yellow nutsedge) was 16 h, and was 12 h for the cool season crops (lettuce and radish). Temperature for warm season crops was maintained at 32.2°C (maximum) daytime and 21.1°C (minimum) nighttime. Cool season crops were subject to a 23.8°C maximum day temperature and a 16.7°C minimum night temperature. Relative humidity was maintained near 50%. Soluble fertilizers, calcium nitrate (CaNO3) and Ferti Lome Geranium, Hanging Basket and Pansy Soluble Plant food, 20-20-20 (Voluntary Purchasing Groups, Bonham, TX, USA), were added to ensure optimum plant growth. Foliarapplied fungicides and insecticides were also used when necessary to control damaging insects and diseases. Analysis of control soils and plants grown in control soils showed no interferences due to the addition of these compounds. Plant mass was not significantly reduced in the contaminated plants when compared to the control plants.
Each crop was irrigated up to three times weekly, depending upon water requirements of the crop. Moisture content of the soil was monitored using soil tensiometers to between 30 and 60 megapascals (Mpa) (field capacity is normally 30 Mpa). Any additional water requirements in excess of three weekly applications were supplemented with reverse osmosis water.
Forty-five days after planting, lettuce, radish, and yellow nutsedge were harvested. Stainless steel scissors were used to cut the aboveground portion of lettuce and yellow nutsedge 5 cm above the soil surface. The tissue was washed in reverse osmosis water to remove dust or soil particles. Corn was harvested when the edible portions of the plants (kernels) were physically mature (76–80 d). Tomato fruit was harvested as it ripened, beginning as early as 50 d and ending on day 85. After collecting and washing was completed, tissues were placed into plastic bags and immediately frozen at 24°C. Plant mass measurements indicated no negative affects on plant growth due to the RDX-contaminated irrigation water.
Labeled lettuce, radish, and tomato. The study from which these tissues were obtained used irrigation water spiked with [U-14C] RDX to asses the fate of RDX in a garden setting [6]. Lettuce, radish, and tomato were chosen as common garden crops. Rectangular growth chambers were constructed of 0.635-cm Plexiglas. The tomato chambers were 71.12 cm high by 60.96 cm wide by 60.96 cm long. The radish chambers were 44.45 cm high by 40.64 cm wide by 40.64 cm long. Each chamber was equipped with an air inlet, air outlet, and a port through which plants could be watered. Air was taken into the chambers by pulling a vacuum on the system at 10–12 mmHg. Plants received a 14-h photoperiod from a light bank in the walk-in environmental room. Light at the upper surface of the plant was approximately 500 μE/m2/s for tomatoes and 300 μE/m2/s for radish. Temperature in the chambers averaged 23°C, and the humidity in the chambers remained near 100%. The experimental design included uncontaminated controls with plants, contaminated water with no plants, and contaminated water with plants. Total dry weights of plant tissues from RDX-treated and control chambers showed no significant differences in plant mass for tomato or lettuce plants and a significant reduction (approximately 25%) in plant mass for radishes. Transpiration by the plants was not measured.
Clean soil was dried for 24 h, mixed well, and transferred to plastic pails. Each pail had drain holes in the bottom and was contained in a saucer and one pail was placed into each chamber after planting. The RDX-contaminated irrigation water was prepared by adding radiolabeled [U-14C] RDX to distilled, deionized water (100 μg/L). Plants were watered as needed with this solution. Plants were started in peat moss from seeds and transferred to growth chambers at 2 to 3 weeks. Tomatoes (Burpee Tumbler Hybrid Catalog 15418AK Lot 9, Burpee, Warminster, PA, USA) were transplanted in triplicate and two were culled after survival of one was assured (one week). Radishes (Burpee) were transferred to each replicate. Four lettuce plants (White Icicle Lot 4301204, B.W.I., Texarkana, TX, USA) were transferred to each replicate. After approximately 11 weeks for tomato and 6 weeks for radish and lettuce the aboveground plant tissues, tomato fruits, and roots of radish plants were harvested and frozen.
Analytical systems
Analytical system for analysis of U.S. Environmental Protection Agency (U.S. EPA) Method 8330 analytes in plant tissues. The HPLC system used in these studies conformed to U.S. EPA Method 8330 for analysis of explosives in soils, including the use of two analytical columns for analyte confirmation. A column heater set at 30°C was used to ameliorate retention time shifts due to changes in room temperature. The solvents utilized were acetonitrile (CH2 Cl2 [HPLC grade]) and methanol (CH3OH [HPLC grade]). The water used was organicfree reagent water (18 mega-ohm Milli-Q, Milli-Q, Bedford, MA, USA). The analysis was performed according to U.S. EPA Method 8330 except for the modified sample preparation for plant tissues described below.
Frozen plant samples from the three studies described above were allowed to come to room temperature. A representative subsample of the plant tissue was placed onto a clean paper towel to remove excess moisture. Ten grams fresh weight of the plant tissue was cut into small pieces (less than 1 cm). The cut up sample was placed into a homogenizing chamber and Milli-Q water was added to cover the top of the sample, and the mixture was homogenized using a sawtooth generator probe, beginning at 500 rpm. Once 500 rpm was reached, the speed was increased to 2,500, 5,000, and 7,500 rpm. The sample was homogenized to a frothy paste.
The homogenized samples were placed in a 150-ml freezedrier flask, covered with parafilm, and placed in the freezer until frozen (approximately 3–4 h). The freeze-drier flask containing the frozen sample was subsequently placed on the freeze drier (-40°C, 0.015 M Barr). The sample was removed from the freeze drier when no ice remained in the sample (approximately 48 h). A mass of freeze-dried sample corresponding to 1.0 g of fresh weight tissue based on dry weights for the specific tissue type was placed in an amber vial and 5.0 ml of acetonitrile was added. Using a vortex mixer, the sample was swirled for 1 min, and then placed in a watercooled (temperature did not exceed 30°C) ultrasonic bath for 18 h. Following sonication, the vials were centrifuged for 5 min and allowed to sit for approximately 1 h. Supernatant (2.5 ml) was removed and added to a cleanup column containing 0.25 g florisil and 0.25 g basic alumina wetted with acetonitrile. After the supernatant had completely passed through the cleanup column, an additional 2.5 ml of acetonitrile was passed through the column. The resulting 5 ml was vortexed for 1 min. This extract was matched to the chromatographic mobile phase by diluting with water and analyzed by HPLC [21].
Analytical system for GPC chromatography of plant extracts. The GPC separations were performed on either a GPC Autoprep Model 1002 A (Analytical Biochemical Laboratories, Assen, The Netherlands) equipped with UV–visible detection at 254 nm or a Waters 610 Fluid Unit pump capable of achieving 6,000 psi, a 200-μl loop injector, a Waters 996 Photodiode Array UV–Visible Absorbance detector, and Millennium 2.1 Chromatography Software (Waters Chromatography Division, Milford, MA, USA). The GPC columns (700 × 25 mm or 250 × 10 mm) were prepared using Bio Beads (S-X3) (200–400 mesh, 7 g, Bio-Rad Laboratories, Richmond, CA, USA). The mobile phase consisted of filtered methylene chloride. A GPC calibration solution was prepared in methylene chloride containing the following analytes: 25,000 μg/g corn oil, 1,000 μg/g bis(2-ethylhexyl)phthalate, 200 μg/g methoxychlor, 20 μg/g perylene, and 80 μg/g sulfur. Standards of the explosive compounds (2,2′-azoxytoluene, octahydro1,3,5,7-tetranitro-1,3,5,7-tetrazocine [HMX], TNT, and RDX) at 10 μg/g were prepared by placing 0.100 ml of 100 μg/g standards in acetonitrile in 0.9 ml methylene chloride.
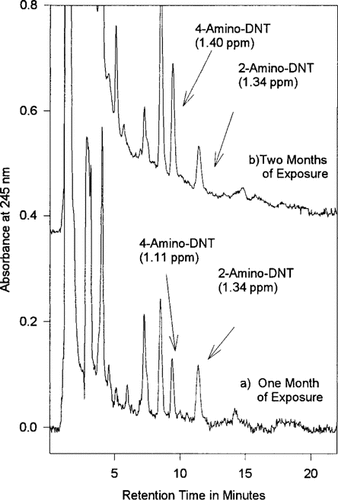
Parrot feather tissues at two sampling times from 2-μg/g trinitrotoluene flow-through reactor.
RESULTS AND DISCUSSION
HPLC analysis of aquatic and garden plant tissues
Using U.S. EPA Method 8330 modified for analysis of plant tissues [6], it is possible to observe the buildup of the nitroaromatic and nitramine parent compounds in plant tissues. Matrix spiking shows recoveries between 80 and 100% for all Method 8330 analytes with method detection limits similar to those found in soil analysis [6, 21]. Figure 1 shows two chromatograms of extracts from plant material treated in a flow-through reactor in which a steady level of TNT-contaminated water was placed in contact with the aquatic plant parrot feather. The concentration of TNT in the flow-through reactor was maintained at 2 μg/g for a period of 2 months. Unlike recent studies of TNT phytotransformation [9, 22], the TNT used was not radiolabeled and as a result, the degradation products of TNT observable were limited to those listed in Method 8330. No TNT was detected in these tissues; however, two explosivebased degradation products were present in the tissue, 4-aDNT and 2-amino-2,6-dinitrotoluene (2-aDNT). The average concentrations of these compounds in three subsamples of tissue on a dry weight basis were 1.11 and 1.34 μg/g (standard deviation 0.12 and 0.14, respectively) after 30 d of exposure and 1.40 and 1.34 μg/g (standard deviation 0.18 and 0.09, respectively) after 60 d of exposure (analysis of unexposed control plant extracts indicated no interferences for these compounds). From these two data points, the levels of the amino-DNTs apparently remained essentially constant even though the plants in Figure 1a were exposed to twice the total amount of TNT as those in Figure 1b. This suggests that the level of these contaminants may have reached a steady state, a condition in which the amount of contaminant taken up by the plant is equal to that being removed from the plant. The fact that the concentration of these compounds does seem to increase over this time period suggests an active degradation of these compounds to less readily analyzed compounds.
| Contaminanta | Leaf (g/g) | SD | Stem (g/g) | SD | Root (g/g) | SD |
|---|---|---|---|---|---|---|
| TNT | 3.68 | 0.10 | <0.24 | 0.04 | 0.45 | 0.05 |
| 4-aDNT | 10.24 | 0.34 | 0.45 | 0.05 | 21.80 | 0.53 |
| 2-aDNT | 15.60 | 0.42 | 20.64 | 0.79 | 58.90 | 2.38 |
Explosive contamination in dissected parrot feather plant tissues. The aquatic plant parrot feather contains three easily separated tissue types: roots, leaves, and stems. Hughes, Shanks, and colleagues [9, 22] performed extensive studies of the phytotransformation of TNT and the distribution of metabolic products in this plant using HPLC and radiolabeled explosives. The parrot feather tissue used in this study was exposed to nonradiolabeled TNT in the same style of flowthrough reactor described above with aqueous TNT concentrations of approximately 5 μg/g and was dissected before homogenization. The concentrations of TNT, 4-aDNT, and 2-aDNT in the three tissue types are listed in Table 1 on a dry weight basis. Because parrot feather is a submerged aquatic plant, the contact between the plant and the explosives-laden water occurred at all three plant organs. The differences in the concentrations and contaminants found in the different parts of the plant are useful in suggesting where explosives adsorption and degradation occurs. The highest levels of TNT were found in the leaf tissue. The low TNT levels in the stem and roots suggested that either adsorption of TNT from the water was slow in these tissues or the rate of TNT removal from these tissues was high. The presence of the TNT degradation product 2-aDNT, and to a lesser extent 4-aDNT, suggest transformation of the parent TNT molecule either by parrot feather or microbial activity. In most studies where reductive degradation of TNT occurs by microbial action, the amination occurs at the 4- or para-position [23]. In this plant tissue the amination at the 2- or ortho-position of the toluene suggests a plant-based reduction.
Explosive contamination in dissected corn plant tissues. Corn plants have a number of discrete tissue types that can be analyzed for explosives contamination. Of the four tissue types analyzed (leaves, stalks, tassels, and husks), only the leaves (22 μg/g dry weight) and tassels (16 μg/g dry weight) contained appreciable concentrations of RDX. Because the only contact between the RDX-containing irrigation water and the plants occurred in the roots of these plants, it is possible to discuss the mobility of the contaminant within the plant. The levels of RDX in the bulk plant water traveling from the roots to the upper plant regions through the stalk were low, below detection limits (0.56 μg/g dry weight [6]) of the analytical method used. Transpiration rates were not measured for exposed or control plants during this study. The RDX contaminants are then removed from the bulk water of the plant and isolated in the specific tissue types leaves and tassels. The absence of RDX in the stalk and husk implies a plant process where the contaminant is isolated in specific tissue types.
Detection of analytes not included in Method 8330. A number of products of remediation of RDX and HMX (nitramine explosives) have been suggested. They include hexahydro1-nitroso-3,5-dinitro-1,3,5-triazine (MNX); hexahydro1,3-dinitroso-5-nitro-1,3,5-triazine; hexahydro-1,3,5-trinitroso1,3,5-triazine; hydrazine; 1,1-dimethylhydrazine; 1,2-dimethylhydrazine; formaldehyde; and methanol [24]. More recently, Binks et al. [25] reported observing two unidentified metabolites following treatment of nitramines with a mixed microbial culture. Generally, all of the proposed degradation products are compounds with a reduced molecular weight when compared to the parent compound.
Degradation schemes for RDX have been proposed for specific treatment process, and many of the postulated metabolites and breakdown products have been detected [24, 25]. The nature of the metabolites and breakdown products depends on the treatment process or the weathering that the sample has undergone. A chromatographic peak of unknown origin was detected in tissue samples exposed to high levels of RDX contamination. Reference standards of MNX spiked into plant extracts were used to identify the unknown peak as the RDX degradation product MNX. Figure 2 contains chromatograms of yellow nutsedge extract that contain MNX. This degradation product of RDX is rarely seen in plant tissues. When it has been detected, as in Figure 2, it has always been found to be at low levels when compared to RDX. Samples have not been analyzed in which MNX was present and RDX was not detected. This suggests that concentrations of MNX may not build up in tissues. If MNXs do not build up large concentrations, then they may be minor products of the transformation of RDX or short-lived compounds that transform further into unknown compounds.
Gel permeation chromatography of tissues exposed to radiolabeled RDX
Gel permeation chromatography was utilized to determine the molecular size distribution of products resulting from the presence of contamination. A standard solution containing compounds with known molecular volumes was introduced onto a GPC column and the elution order was recorded. Fraction collection times were then determined based on the retention times of the known standards. Figure 3a shows a chromatogram achieved by injecting a standard solution. As can be seen, the larger molecules elute early in the chromatogram. The standard solution contains corn oil, bis(2-ethylhexyl) phthalate, methoxychlor, perylene, and sulfur. Corn oil is a complex mixture of large molecules that coelute first in a bimodal peak (many peaks with slightly different retention times). Bis(2-ethylhexyl)phthalate has a molecular volume that is smaller than the volume of the pores in the stationary phase and is retained longer than the corn oil. The slightly smaller methoxychlor molecules are next to elute followed by perylene and finally sulfur.
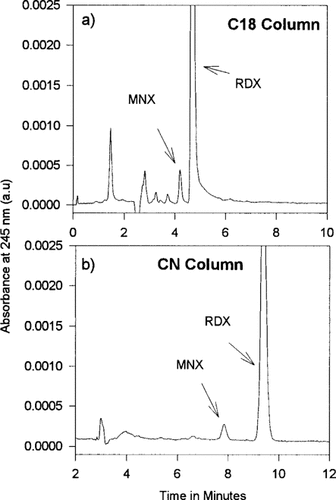
Chromatograms from high-performance liquid chromatography C18 and CN reversed phase of extracts of yellow nutsedge that had been exposed to irrigation water contaminated with 1.0 μg/g hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX). Chromatograms show RDX and hexahydro-1-nitroso-3,5-dinitro-1,3,5-triazine (MNX) contamination.
Figure 3b shows a chromatogram achieved by injecting four explosives standard solutions at 100 μg/g onto the small-scale GPC column. As was the case in the GPC standard, the larger molecules eluted early in the chromatogram. The largest standard is the TNT-based, dimerized degradation product 2,2′-azoxy toluene. This molecule contains two phenyl rings and occupies a substantial molecular volume. It elutes in a symmetric peak with a retention time only slightly less than that of sulfur in the GPC standard. The next explosives standard to elute is TNT. This molecule contains a single phenyl ring with three nitro and one methyl substituents. The compound HMX, a molecule containing a nonaromatic eight-membered ring with nitro substituents, is retained only slightly longer than TNT. The explosive RDX, a molecule containing a nonaromatic six-membered ring with three nitro substituents, is retained the longest.
The retention of molecules during GPC depends nearly exclusively on the molecular volume. Within like classes of molecules, this tends to vary directly with molecular weight. The molecular volumes of specific molecules are not well-documented descriptors of chemical compounds. The fact that these volumes change considerably depending on the physical state of molecules (i.e., crystalline, amorphic, solubilized) in polar solvents or nonpolar solvents makes this an undesirable means of describing compounds. However, molecular weight remains constant regardless of the molecule's physical state, and is a long standing means of describing compounds. For these reasons, GPC fractions are classified by molecular weight. However, this is done with the understanding that the separation is based on volume, not weight, and that the conversion from volume to weight is an approximation.
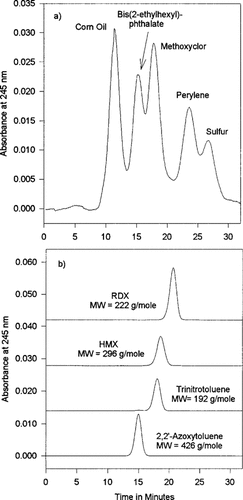
Chromatograms of standards separated by gel-permeation chromatography (GPC). (a) The GPC calibration standards. (b) Explosives standards by GPC.
Utilizing the information provided by injecting the GPC and explosive standards on the GPC column, a set of nine retention time windows was established in order to obtain fractions that are labeled as molecular weight ranges of >5,000, 5,000 to 1000, 1,000 to 750, 750 to 500, 500 to 350, 350 to 250, 250 to 175, 175 to 100, and <100 g/mole.
Extremely large or polar compounds would not be expected to be soluble in the methylene chloride mobile phase necessary for the GPC separation. As a result, the amount of radiolabel in the larger fractions may underrepresent the percentage of radiolabel in molecules of that size in the original tissue. Smaller, explosives-based molecules and the explosives parent compounds themselves have been shown to have high recoveries throughout the sample preparation process utilized [6]. No aqueous mobile phase GPC was performed on these samples.
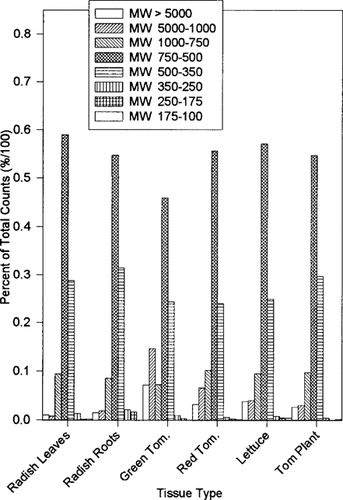
Results of scintillation counting of gel-permeation chromatography (GPC) fractions showing the partitioning of radioactivity (counts) in plant extracts by molecular size.
Figure 4 graphically displays the distribution of the radiolabel in each fraction. The relative count per fraction is similar for each tissue type utilized, with the majority of the radioactivity detected in fractions three, four, and five. The similarity in the labeled distribution of phytotransformation products in a variety of plant tissues suggests similar RDX metabolism in all of the biological systems studied.
The radioactivity introduced to these tissues was originally in the form of [U-14C] RDX. Standards of the parent compound, RDX, elute in GPC fractions six and seven. A very low percentage of the radioactivity detected is still in the form of the original molecule. The radiolabel that was originally in the molecular structure of the explosive has been transformed, and is now found in other size molecules. These molecules are significantly larger than the parent molecule.
Gel permeation chromatography of tissues exposed to nonradiolabeled RDX. Figures 5a and 5b display the chromatograms of the HPLC analysis of extracts from yellow nutsedge fractions on C18 and CN columns, respectively. As expected, only fractions 6 and 7 contain significant amounts of RDX. When the amount of the parent molecule in each GPC fraction is compared to the radioactivity of each fraction we see that only a small amount of the radioactivity is due to RDX as the parent molecule. This is presented graphically in Figure 6 for green tomato fruit extracts. The left vertical axis represents the relative amount of radiolabel in a given fraction from the GPC results. Only a small portion of the radiolabel in these extracts results from [U-14C] atoms that are still in the RDX molecular state. For all tissue types, the vast majority of RDX atoms have been transformed to higher molecular weight molecules. The fact that appreciable levels of parent RDX molecules can be detected by HPLC in tissue extracts points towards large amounts of these transformation products in plants exposed to RDX contamination. Approximately 10 μg/g fresh weight RDX was detected in this green tomato fruit sample. The radioactivity associated with this 10 μg/g is nearly 1/100th of that observed in fractions labeled 1000-750, 750500, and 500-350. This suggests that, in a 100-g sample of green tomato fruit, milligram quantities of these transformation products are present, and even if a drastic reduction in toxicity was effected by their transformation, these products may still pose a threat if ingested.
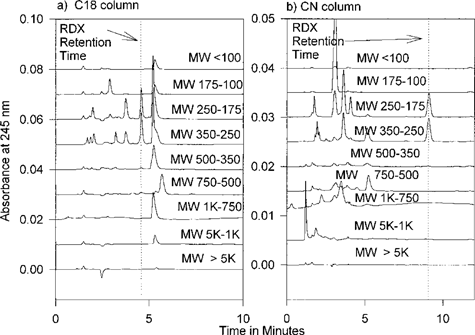
Chromatograms of gel-permeation chromatography (GPC) fractions of green tomato fruit (a) on C18 column, and (b) on CN column.
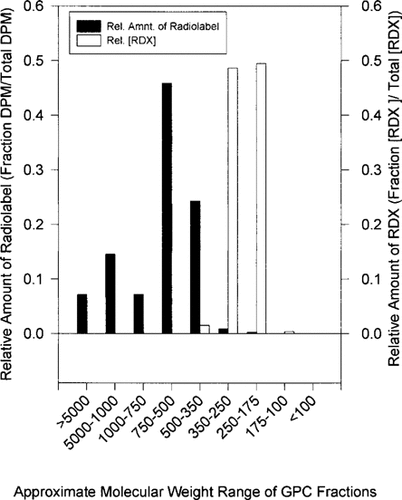
Comparison of radiolabel partitioning in gel-permeation chromatography (GPC) fractions to hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) partitioning in GPC fractions displaying the transformation of the RDX parent molecule to larger size molecules.
The fact that high concentrations of explosives transformation products can be extracted from exposed plants suggests a need for knowledge of these substances in order to provide adequate information regarding risk assessment. The introduction of yet unidentified functionalities to explosives molecules as a result of phytotransformation could possibly result in increased toxicity, bioavailability, or mobility in the environment.
CONCLUSIONS
The presence of high molecular weight explosives transformation products in plant tissues has been confirmed. High concentrations of these metabolites compared to those of the parent compound were found. This makes the possibility of transformation of explosives contaminants to biologically active compounds with increased toxicity, mobility, or bioavailability a concern. Coupling scintillation counting with HPLC and GPC separations provided a means of acquiring specific chemical information pertaining to transformation of explosives to compounds with increased molecular size and increased water solubility. Given that substantial amounts of extractable explosives transformation products have been shown to accumulate in exposed plants, the process by which transformation takes place should be studied further in order to provide accurate risk assessment.
Acknowledgements
We would like to thank the U.S. Department of Defense Installation Restoration Research Program and the U.S. Army Environmental Quality Technology Research Program for funding this research (Work Unit AF25-ET-001). We gratefully acknowledge Victor Medina (U.S. EPA Athens), Judith C. Pennington (Waterways Experiment Station), and Richard A. Price (Waterways Experiment Station), for providing exposed plant tissues. Thanks to John Curtis, Susan Sprecher, and Linda Nelson (Waterways Experiment Station) for helpful discussions. Thoughtful comments by the three anonymous reviewers were greatly appreciated.




