Role of chemical and ecological factors in trophic transfer of organic chemicals in aquatic food webs
Abstract
Trophic transfer of chlorinated organic contaminants was investigated in an aquatic community composed of zooplankton, benthic invertebrates, and fish. Biomagnification, measured as the increase in lipid-based chemical concentrations in predator over that in prey, was observed for high-Kow chemicals (logKow > 6.3). Low-Kow chemicals (log Kow < 5.5) did not biomagnify in the food web, and chemicals with log Kow between 5.5 and 6.3 showed some evidence of biomagnification. Trophic level differences in chemical accumulation in the food web could not be attributed to bioconcentration into increasing trophic levels with increasing lipid levels, as no relationship was observed between trophic position and lipid content of organisms. Plots of contaminant-ordinated principal component scores in component space predicted the detailed diets of the species examined. It is concluded that (1) trophic interactions play a crucial role in the distribution of high-Kow chemicals but not for low-Kow chemicals and that (2) contaminant distributions provide a means to determine structure in aquatic communities.
INTRODUCTION
Hydrophobic organic chemicals in the environment accumulate mainly in lipid tissues of aquatic organisms by the processes of equilibrium partitioning from water [1] and dietary uptake by absorption across the gastrointestinal tract [2, 3]. Considerable debate exists regarding the relative importance of chemical accumulation by partitioning from water and from dietary uptake. Certain investigators have argued that equilibrium partitioning from water is sufficient to describe organochlorine contaminant accumulation in lipid tissues of aquatic biota regardless of trophic position [4, 5]. The observed increase in concentration of certain chemicals with trophic position is explained in terms of an increase in the organism's lipid content with increasing trophic position. Following this rationale, lipid-adjusted biomagnification factors should approach unity for hydrophobic organic chemicals [4, 6] if equilibrium partitioning is the primary process determining chemical accumulation in aquatic organisms.
Authors have argued that equilibrium partitioning cannot explain the observed increase of concentrations of certain chemicals in food webs. A thermodynamic analysis of the distribution of polychlorinated biphenyl (PCB) congener concentrations in organisms of the Lake Ontario and Lake Michigan food webs showed that fugacities of PCBs in most organisms of these food webs are much greater than those in water [7]. This observation cannot be explained by lipid–water partitioning, which results in equal fugacities in water and organisms. The analysis also showed that chemical fugacities of the most hydrophobic chemicals increase with every step in the food chain. Laboratory experiments with fish demonstrated that food digestion and absorption in the gastrointestinal tract provide a mechanism by which the chemical fugacity can be raised when one organism is consumed by another [8]. An important implication of the occurrence of “biomagnification” is that feeding relationships play a crucial role in exposing organisms to chemical contaminants. From an ecological viewpoint, biomagnification theory implies that biomagnifying contaminants can play a useful role in determining feeding relationships and consequently community structure in food webs [9].
We present a field study of the distribution of some nonmetabolizable chemical compounds in a natural aquatic community composed of zooplankton, benthic invertebrates, and fish to quantify biomagnification for chemicals of differing Kow. The objective is to resolve the relative importance of chemical factors and ecological factors in the distribution of chemical contaminants in this ecosystem. This study provides two strategies for assessing the role of biomagnification (and ecological factors) versus “equilibrium partitioning.” The first strategy involves the analysis of chemical concentrations in organisms of an aquatic community on a lipid-wet-weight basis. If chemical partitioning is the principal mechanism of the distribution of nonmetabolizable chemicals in the food web, lipid-weight-based chemical concentrations in all organisms of the food web should be similar. If chemical biomagnification occurs, lipid-based chemical concentrations should increase with increasing trophic level. The second strategy involves a principal component analysis of chemical concentrations in organisms of the food web to test the assumption that feeding relationships play an important role in the distribution of hydrophobic organic chemicals in aquatic food webs. The merit of this approach is that the data analysis relies solely on contaminant concentration data and disregards data collected on feeding interactions. Data on feeding interactions can exhibit considerable uncertainty because of the “snapshot” nature of gut content analysis. The results of the PCA are compared to an independent gut content analysis of feeding relationships in the food web to determine whether chemical concentration profiles reflect trophic interactions.
| Common name | Species | N | Length (mm) | Weight (g) | Lipid % |
|---|---|---|---|---|---|
| Plankton | Copepods, cladocerans | 3 pools | 0.65 | ||
| Zebra mussels | Dreissena polymorpha | 4 pools | 0.80 | ||
| Amphipods | Gammarus fasciatus | 1 pool | 1.52 | ||
| Caddis fly larvae | Hydropsyche | 2 pools | 1.84 | ||
| Mayfly larvae | Hexagenia spp. | 1 pool | 0.55 | ||
| Crayfish | Orconectes propinquus | 12 | 1.15 | ||
| Emerald shiner | Notropis atherinoides | 6 pools of 4 | 52.2 (4.3) | 4.2 (0.1) | 4.14 |
| Spottail shiner | Notropis hudsonius | 9 pools of 4 | 68.8 (7.1) | 6.7 (1.1) | 3.27 |
| Brook silversides | Labidesthes sicculus | 3 pools of 6 | 51.0 (1.7) | 3.3 (0.3) | 4.63 |
| Gizzard shad | Dorosoma cepedianum | 7 | 65.5 (2.9) | 3.3 (0.4) | 1.58 |
| Alewife | Alosa pseudoharengus | 3 | 65.4 (5.1) | 3.0 (0.03) | 3.49 |
| Mottled sculpin | Cottus bairdi | 3 | 89.7 (0.9) | 5.4 (0.1) | 0.93 |
| Freshwater drum | Aplodinotus grunniens | 5 | 309.2 (24.2) | 338.2 (58.6) | 0.91 |
| Shorthead redhorse | Moxostoma macrolepidotum | 3 | 308.7 (31.4) | 370.6 (77.8) | 1.38 |
| Stonecat | Noturus flavus | 8 | 203.8 (20.6) | 95.6 (26.9) | 0.28 |
| Rock bass (adult) | Ambloplites rupestris | 6 | 147.8 (8.1) | 76.1 (16.4) | 0.17 |
| Rock bass (young of year) | 13 | 51.5 (1.5) | 2.9 (0.3) | 3.83 | |
| Yellow perch | Perca flavescens | 6 | 155.0 (9.7) | 55.4 (8.2) | 0.97 |
| White perch | Morone americana | 40 | 167.7 (2.4) | 84.9 (3.4) | 1.36 |
| White bass | Morone chrysops | 3 | 291.7 (5.8) | 376.1 (12.8) | 3.16 |
- aNumbers in parentheses represent ± 1 SE.
METHODS
Sample collection
Organisms were collected from the head of the Detroit River (42°29′N, 82°91′W) from May to September 1991. Fish were captured by hook, fish trap, gill net, and seine net. Benthic invertebrates were captured by ponar dredge or by hand, and plankton was sampled by plankton net (100 μm). Plankton samples were centrifuged in Teflon® Oak Ridge tubes to separate phytoplankton from zooplankton, and zooplankton were retained for gas chromatographic analysis (GC). Large fish (>30 g) were prepared by removing approx. 5 g of dorsal muscle from each fish for GC and stored at −20°C. Small fish, benthic invertebrates, and zooplankton were analyzed whole or were pooled for analysis when individual weights were less than 5 g. The gastrointestinal tracts of large fish were excised and stomach contents examined to determine prey consumption and to define trophic links in the aquatic community.
Sample preparation
Sample preparation was by the method of Lazar et al. [10]. Samples (5 g) were prepared by grinding by mortar and pestle in 20 g anhydrous sodium sulfate (J.T. Baker, Toronto, ON, Canada) and then added to a 0.025 × 0.60-m glass column containing 10 g anhydrous sodium sulfate and 70 ml of 1:1 dichloromethane/hexane (BDH, Toronto, ON, Canada). After 1 h, the column was eluted with 250 ml of 1:1 dichloromethane/ hexane solution. The extract was concentrated to 2 ml by rotary evaporator and then added to a 0.01 × 0.55-m glass column containing 40 g activated Florisil (60/100-mm mesh, Supelco, Mississauga, ON, Canada) and 3 g anhydrous sodium sulfate for cleanup. The column was eluted with 50 ml hexane and the extract concentrated to 10 ml for gas chromatography. Chemical recoveries were greater than 90%. Two milliliters of extract were removed for gravimetric lipid determination at the beginning of the cleanup step.
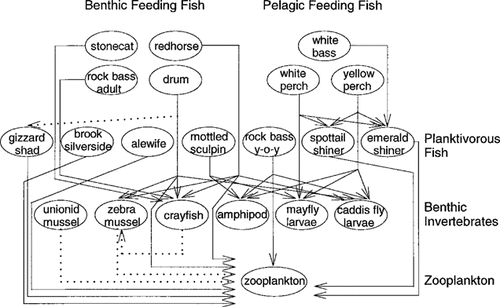
Detroit River food web based on feeding relationships.
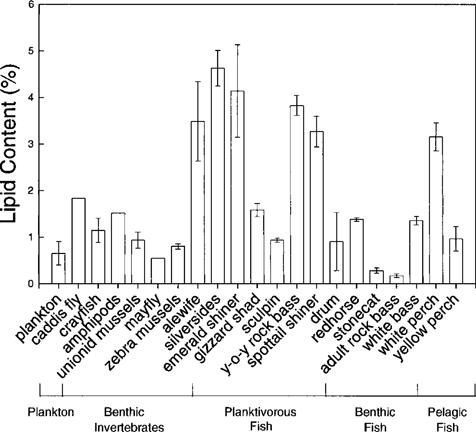
Lipid contents of Detroit River aquatic organisms. Error bars represent ±1 SE.
Gas chromatography
Gas chromatographic analysis was performed on a Hewlett Packard 5890/ECD equipped with an HP-3396 integrator and an HP-7673 autosampler. The analytical column was a DB-5 (J&W Scientific, Folsom, CA, USA), dimensions were 30 m × 0.25 mm, and film thickness was 0.25 mm. Injection was 1-ml splitless at 250°C. Carrier gas was ultra-high-purity He at a 30-cm/s flow rate, and makeup gas was Ar/CH4 (95%/ 5%) at a 40-ml/min flow rate. The oven was temperature programmed from 100 to 270°C at 3°C/min. Samples were analyzed for pentachlorobenzene (QCB; log Kow = 5.0; [11]), hexachlorobenzene (HCB; log Kow = 5.5; [12]), octachlorostyrene (OCS; log Kow = 6.29; [13]), PCB 52 (log Kow = 6.1; [11]), PCB 87 (log Kow = 6.5; [11]), PCB 101 (log Kow = 6.44; [13]), PCB 138 (log Kow = 6.83; [14]), PCB 153 (log Kow = 6.9; [15]), PCB 180 (log Kow = 7.36; [14]), and 2,2-bis(p-chlorophenyl)-1,1-dichloroethylene (p,p′-DDE; log Kow = 5.69; [13]). Detection limits were less than 0.05 μg/kg [10].
Statistical analysis
All chemical concentrations were expressed on a lipid-wet-weight basis (μg chemical per kg lipid) and were logarithmically transformed to control heteroscedasticity. Heteroscedasticity was tested using an Fmax test. Linear regression was used to elucidate relationships between fish length and lipid proportions. The 10 chemicals in this analysis were grouped by a chemical-ordinated principal components analysis [16]. Principal component analysis (PCA) is commonly used to project the multidimensional space occupied by the original data set onto a reduced space while preserving the majority of information contained in the environmental data [17]. It allows the investigator to group highly related variables together, thus reducing the total variable number in further analyses, resulting in a more powerful statistical analysis. Species were grouped into five categories: zooplankton, benthic invertebrates (caddis fly larvae, mayfly larvae, crayfish, amphipods, and zebra mussels), planktivorous/insectivorous fish (emerald shiner, spottail shiner, brook silversides, alewife, gizzard shad, and young-of-year rock bass), benthic feeding fish (sculpin, drum, redhorse, stonecat, and adult rock bass), and pelagic feeding fish (white bass, white perch, and yellow perch) for the purpose of analysis of variance. These categories correspond to differing trophic levels based on the feeding relationships observed in this aquatic community. A one-way multivariate analysis of variance (MANOVA) was performed on the component scores generated by the PCA. Differences between cell means were tested by a Fisher's least significant difference procedure [16].
RESULTS AND DISCUSSION
Trophic links
Table 1 describes the species collected, sample sizes, lengths, weights, and lipid content of fish collected. The similarity in length and weight of individuals of the same species was due to the capture method (gill net mesh size) and the predisposition of certain species to travel in similar age class schools (white bass). Examination of stomach contents indicated that both stonecat and adult rock bass consumed crayfish exclusively. Stonecat stomachs contained considerable amounts of sediment, whereas adult rock bass consumed little sediment. The stomachs of young-of-year rock bass contained amphipods, copepods, larval caddis flies, cladocerans, and chironomid larvae. Yellow perch and white perch are known to be generalist predators [18]. Yellow perch fed on amphipods, caddis fly larvae, mayfly larvae, and small fish, whereas the stomachs of white perch contained mayfly larvae and small fish only. White perch consumed a greater numerical proportion of small fish than yellow perch (15% vs 4.2%). White bass were strict emerald shiner predators, in accord with a previous investigation [19]. Freshwater drum stomachs contained sediment and a wide variety of benthic invertebrates ingested with sediment, including copepods, cladocerans, chironomids, gastropods, zebra mussels, caddis fly larvae, and crayfish. Small fish have been documented as important diet constituents in freshwater drum [18], in which young-of-year gizzard shad were the principal fish eaten [20]. Redhorse consumed similar benthic invertebrates, but the major prey item observed was the zebra mussel. Considerable sediment was found in redhorse stomachs. Sculpin fed on amphipods, oligochaetes, and caddis fly larvae. Small fish were mainly planktivorous; however, emerald shiner and spottail shiner also consumed larval and adult insects, and adult flying insects composed a large portion of brook silversides' diets. On the basis of the results of the analysis of stomach contents of individual fish, Figure 1 provides a schematic diagram of the feeding interactions and trophic positioning in the Detroit River food web. Zebra mussels are known to be filter feeders; therefore, planktonic organisms constitute the majority of their diets. The omnivorous diet of crayfish is known to include zebra mussels [21].
Lipid content
There was no apparent relationship between trophic level and lipid content of aquatic biota (Fig. 2). Lipid contents of insectivorous fish were greater than their benthic invertebrate prey. Lipid contents in benthic and pelagic feeding fish were less than in planktivorous fish and were approximately the same as lipid contents in benthic invertebrates. These observations indicate that if biomagnification is observed, it cannot be explained by increases in lipid content with trophic position in the food web.
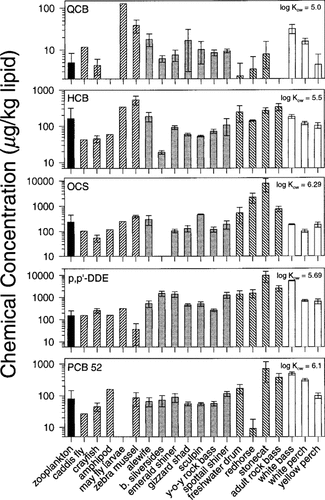
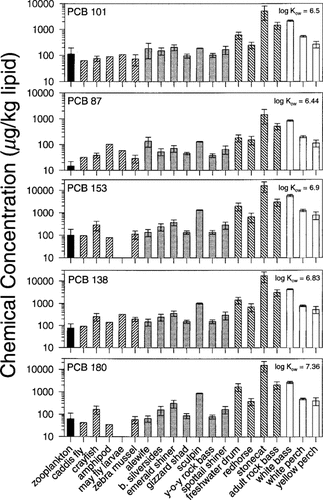
Distribution of chemical contaminants in Detroit River biota. Concentrations are expressed on a lipid-wet-weight basis. Error bars represent ±1 SE. Plankton are shown with black bars, benthic invertebrates with right-hatched bars, planktivorous/insectivorous fish with gray bars, benthic feeding fish with left-hatched bars, and piscivorous fish with white bars.
Chemical concentrations in the food web
The distribution of organochlorine contaminants in Detroit River biota for all chemicals is illustrated in Figure 3. All chemical concentrations are reported on a lipid-weight basis, such that any observed increase in concentration with increasing trophic level cannot be explained in terms of equilibrium partitioning of the chemical from water into organisms of greater lipid content. When comparing HCB and QCB concentrations in predator to that in prey, no biomagnification was observed. The OCS concentrations in benthic feeding fish were elevated above prey, but this was not observed for pelagic feeding fish or for planktivorous/insectivorous feeding fish. Accumulation of DDE and PCBs in the food web showed a relationship to the trophic level of the organism, and biomagnification was evident. For most chemicals, stonecat, adult rock bass, and white bass contained the highest contaminant levels. These data indicate that the process of biomagnification is Kow dependent and that chemicals with log Kow of 5.5 or less show no biomagnification.
The large error associated with chemical concentrations in zooplankton (Fig. 3) might be an artifact of the collection process. Plankton tows conducted near shore out of the main current flow might capture mainly local zooplankton. Tows conducted nearer the center of the current, where chemical conditions might differ, might capture a larger proportion of upstream plankton. Although no known point sources for the chemicals measured in this study in the immediate collection area are known, point sources are known to be upstream. Chemical concentrations observed in zooplankton might better represent upstream chemical conditions. Benthic feeding fish appear consistently more contaminated than pelagic feeding fish despite the fact that pelagic fish are piscivorous. This might be due to benthic feeding fish ingesting large quantities of sediment with food.
| Univariate F tests Variable | SS | df | MS | F | p |
|---|---|---|---|---|---|
| Component 1 error | 70.84 | 4 | 17.71 | 35.60 | <0.001 |
| 66.16 | 133 | 0.50 | |||
| Component 2 error | 39.16 | 4 | 9.79 | 13.31 | <0.001 |
| 97.84 | 133 | 0.74 | |||
| Multivariate test Hotelling-Lawleya | 8,262 | 24.623 | <0.001 |
- aTrace = 1.504.
Principal components analysis
Principal components analysis grouped chemical concentration data into two nontrivial components [22]; component 1 explained 58.6% of the variance in the data and component 2 16.8%. Component 1 consisted of DDE and PCBs 52, 87, 101, 138, 153, and 180. Component 2 consisted of QCB, HCB, and OCS. This assignment might reflect the different underlying mechanisms of chemical accumulation, for example, partitioning for component 2 chemicals and biomagnification for chemicals constituting component 1. This assignment might also reflect the nature of the chemical contaminant source: diffuse and widespread sources for DDE and PCBs and point sources upstream in the St. Clair River for chlorobenzenes and OCS.
The MANOVA (Table 2) on component scores between the five ecological categories (zooplankton, benthic invertebrate, small planktivorous fish, benthic feeding fish, pelagic feeding fish) revealed an overall significant difference in chemicalordinated component scores between the categories (Hotelling-Lawley trace = 1.50, F(8,262) = 24.62, p < 0.001). Univariate F tests revealed significant differences between the categories for both components (p < 0.001; Table 2).
Figure 4 illustrates the differences in chemically ordinated component scores between the five categories of aquatic organisms. With reference to component 1 (DDE + PCBs), all pairwise comparison probabilities by a Fisher's least significant difference test were significant (p < 0.001) except for differences between zooplankton and small planktivorous fish (which were significant at p = 0.02) and differences between zooplankton and benthic invertebrates (ns) and pelagic feeding fish and benthic feeding fish (ns). Benthic feeding and pelagic feeding fish occupied similar positions in the trophic hierarchy (Fig. 1), so it is feasible that they had similar tissue concentrations of the mostly high Kow component 1 chemicals. Considering the prey species outlined previously, all predators scored higher than prey species with the exception of benthic invertebrates and their zooplankton prey. Similarities in chemical concentrations between zooplankton and benthic invertebrates might be due to the method of preparation of plankton samples, where only the largest zooplankters were retained. Large zooplankton and filter-feeding invertebrates might consume similar smaller plankton species, resulting in similar chemical accumulation of DDE and PCBs. Component scores increased with trophic position, consistent with a biomagnification hypothesis, was observed for all combinations of predator and prey species with DDE and PCBs except with benthic invertebrates and zooplankton prey.
The significant differences seen between the five categories for component 2 (chlorobenzenes + OCS) (Table 2) were due solely to the high component score in the benthic feeding fish (Fig. 4). A Fisher's least significant difference test showed that benthic feeding fish had significantly higher component scores (p < 0.001) than all other categories, including pelagic feeding fish. All other pairwise comparisons were not significant. Small planktivorous fish had essentially the same scores as their prey, (benthic invertebrates and zooplankton), whereas benthic invertebrates were not significantly different in component scores than zooplankton. Pelagic feeding fish had the same component scores as zooplankton, invertebrates, and small planktivorous fish. Elevated component 2 scores in benthic feeding fish might be due to the ingestion of large amounts of chlorobenzene- and OCS-contaminated sediment, for which point sources exist upstream in the St. Clair River. Component 1 chemicals have no similar point sources.
Community structure
Figure 5 diagrams the component scores obtained for principal components 1 and 2 for each species in the Detroit River ecosystem. The chemically ordinated component scores reflect the community structure and trophic relationships in the Detroit River community. A diet analysis showed that white bass were strict piscivores in this community, and white bass can be found on the far right of the PC plot. Stonecat and adult rock bass were crayfish predators, and both are on the upper right of the PC plot. Redhorse fed mainly on zebra mussels and are on the upper left of the PC plot. Freshwater drum, yellow perch, and sculpin constitute another group. Diet analysis determined that freshwater drum and sculpin feed on similar organisms and are considered benthic feeding fish, whereas yellow perch were mainly pelagic feeding fish with a benthic component to their diet. The small-fish group (gizzard shad, emerald shiner, spottail shiner, alewife, and young-of-year rock bass) exhibit considerable overlap with the benthic feeding fish and benthic invertebrates on the PC plot and in diet. Although most members of the small-fish component were observed to feed on zooplankton, zooplankton are a heterogenous group, and planktivorous fish might have selectively fed on specific plankters or size classes of plankton. This might explain the “loose” association of these species on the PC plot. Brook silversides are found in the lower center of the plot, distant from other small fish. The relatively low component 2 scores for brook silversides compared to other small fish might be due to the large proportion of terrestrial insects in their diet; such insects have little or no direct contact with wateror sediment-borne chemicals. Differences in chemical concentrations in brook silversides relative to other small fish due to diet have been observed elsewhere [23]. White perch are found between the small planktivorous fish and benthic feeding fish groups (drum, sculpin, and yellow perch) and white bass, indicating a relationship to their diet composition, which was composed mainly of small fish but also included mayfly larvae. The amphipod Gammarus was grouped near gizzard shad, suggesting that amphipods and gizzard shad might have had similar detritivorus diets. An alternative explanation of the increasing horizontal compression at lower trophic levels in Figure 5 is that biomagnification of component 1 chemicals and therefore horizontal separation on the PC plot were minimal at lower trophic levels. The benthic invertebrates constituted the most morphologically and ecologically diverse group of organisms, so it is expected that diets would also be diverse within this group. The PC plot indicates that benthic invertebrates are not closely related by chemical accumulation pattern.
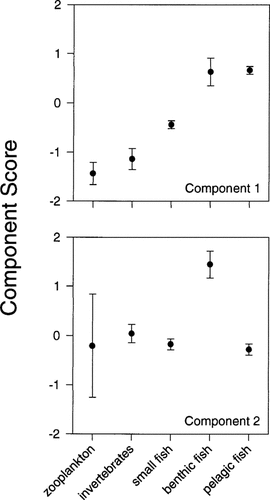
Component scores for five categories of Detroit River biota. Error bars represent ±1 SE.
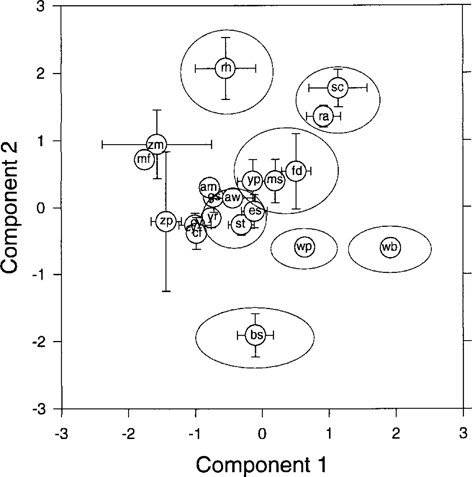
Component scores for components 1 and 2 from Detroit River food web. Error bars represent ±1 SE. Legend is as follows: am, amphipods; aw, alewife; bs, brook silversides; cf, caddis fly larvae; cy, crayfish; es, emerald shiner; fd, freshwater drum; gs, gizzard shad; mf, mayfly larvae; ms, mottled sculpin; ra, adult rock bass; rh, redhorse; sc, stonecat; st, spottail shiner; wp, white perch; wb, white bass; yp, yellow perch; yr, young-of-year rock bass; zm, zebra mussels; zp, zooplankton.
The PC score plot showed a greater similarity to the observed diets than to assigned trophic positions. Because the PCA was based on a chemical ordination of the data set, a connection can be stated between observed diets and chemicalaccumulation pattern in aquatic biota. The chemically ordinated PCA also revealed a greater resolution in community structure and trophic relationships than traditional food chain analyses (e.g., individual grouping of specialist predators). This methodology might be a useful tool to investigate the structure of ecological communities. This analysis indicates the importance of ecological factors to the distribution of hydrophobic chemical contaminants in aquatic ecosystems.
SUMMARY
The observation that lipid-based contaminant concentrations of very hydrophobic substances (log Kow > 6.3) increase with trophic position indicates that biomagnification of these substances in food webs occurs and that biomagnification is not the result of equilibrium partitioning of the chemical between water and lipids. For less hydrophobic substances of log Kow < 5.5 (QCB and HCB in this study), lipid-based concentrations do not show statistically significant trends with trophic position of the organisms. Biomagnification is not observed for these compounds, and the lipid–water equilibrium partitioning process can explain the observed concentration distribution in the food web. Substances with log Kow between 5.5 and 6.3 exhibit some degree of biomagnification. A principal component analysis of chemical concentrations in the Detroit River food web indicates that chemical accumulation patterns demonstrate an association with the feeding behavior of individual organisms. These findings illustrate that (1) from a toxicological viewpoint, feeding relationships play an important role in controlling the exposure of chemicals in food webs and that (2) from an ecological point of view, contaminant concentrations can be useful tools in elucidating trophic interactions in aquatic food webs.
Acknowledgements
We gratefully acknowledge the analytical chemistry supplied by the G.L.I.E.R. Laboratory and the financial assistance of the Ontario Ministry of the Environment and Energy, the Natural Sciences and Engineering Research Council of Canada, and the J.R. Mott Foundation.




