Correlation of measures of ambient toxicity and fish community diversity in Chesapeake Bay, USA, tributaries—urbanizing watersheds
Abstract
This study was performed to evaluate ambient toxicity conditions in Chesapeake Bay tidal tributaries whose watersheds are impacted by urban development and to further evaluate an existing toxicological risk ranking model. A battery of water-column and sediment bioassays were employed with animals and plants. The study was conducted in coordination with a fish community sampling program. Tests were conducted monthly from April through August 1994 at five sample sites in each of four tidal tributaries. Mortality, reproduction, and growth rates in the water-column assays did not consistently indicate chemical contamination in any system. Chemical analyses did not indicate elevated levels of contaminants in the water column. Sediment bioassays demonstrated greater responses than water-column assays. Sediment in the upstream reaches of the South River demonstrated significant toxicity. Toxicity was also observed at the uppermost Severn River station and the middle Patuxent River station. Chemical analyses of composite sediment samples indicated elevated metals levels in the South River. Some metals were above threshold values in the Patuxent and Wicomico rivers. The AVS/SEM ratios in pore water were below 1 in all cases. Organic analyses demonstrated low level polycyclicaromatic hydrocarbon contamination in all four systems. The toxicological risk ranking model ranked the South River as the most contaminant-impacted site. The Patuxent and Severn rivers were ranked below the South River; however, the ranking model identified specific locations in the Severn and Patuxent rivers that indicate sediment contamination. The Wicomico River had the lowest overall risk score. The Patuxent River requires more intense sampling due to its relatively larger size. The toxicological risk ranking results for sediment were significantly correlated with species diversity forfish communities sampled by bottom trawl. Results were consistent with data from previous years. Regression analysis of 2 years of data indicate that fish community impairment can be predicted with ambient toxicity results.
INTRODUCTION
This paper reports results of the second year of a program to integrate an ambient toxicity testing approach with aquatic community health metrics. It describes the results of tributary-specific toxicity testing, summary results from a parallel study on estuarine fish community Index of Biotic Integrity (IBI) investigations, and correlations between the two. The project was undertaken to further our understanding of how toxic contaminants are affecting habitat quality and resource populations in Chesapeake Bay.
It is unknown to what extent toxic contaminants directly or indirectly affect fish population levels in Chesapeake Bay. Some areas (e.g., Baltimore Harbor) are severely contaminated and others demonstrate localized ambient toxicity. It is unknown if localized toxic contamination affects populations in the Bay as a whole, or if low level but widespread contamination is a greater problem, or if a combination of the two affect living resource populations.
The current estuarine ambient toxicity approach was developed in pilot programs sponsored by the Maryland Department of Natural Resources (DNR) and the Environmental Protection Agency (EPA) Chesapeake Bay Program [1-3]. The objective was to assess biologically significant environmental contamination, as opposed to simple comparison of contaminant levels with chemical benchmarks. A toxicological risk ranking method was developed to integrate the array of toxicological data results into a site-specific risk score [4]. The rationale of the ranking system is to quantify the degree of concern for the possible presence of toxic contamination, or the risk, not merely to catalog presence or absence of toxic effects.
Previous fish community assessments indicate that fish assemblages in tributaries whose watersheds were dominated by urban development were less diverse than tributaries whose watersheds were dominated by forest and wetlands [5, 6]. The initial ambient toxicity/IBI studies conducted in four tidal tributaries of the Chesapeake Bay in 1993 [7] demonstrated toxic impact in industrial areas (Curtis Creek) and to a lesser extent in urbanized areas (Rock Creek), relative to relatively unimpacted areas (Fishing Bay and Wicomico River). The toxicity risk ranking model identified spatial trends between sampling stations. The risk ranking scores were significantly correlated with the diversity index of the bottom fish community. The sensitivity of the ranking procedure to toxicological gradients is the subject of ongoing investigations.
Based on these studies, four tidal tributaries were selected for paired ambient toxicity/fish IBI sampling in 1994 to assess the impact of urbanization of watersheds on receiving stream habitat quality. Information on ambient toxicity and fish community health in these areas may provide quantification of the impacts of nonpoint source pollution and sediment contamination on resident populations.
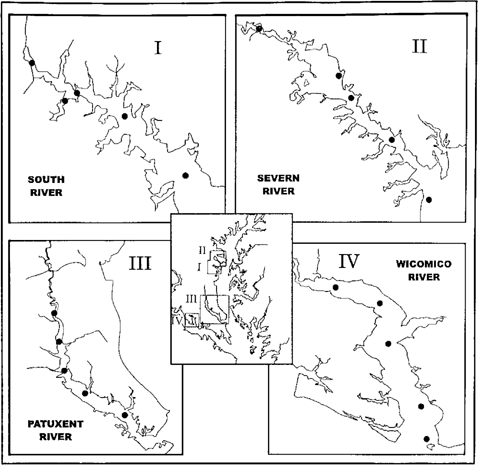
Maps of Chesapeake Bay tributaries showing the location of rivers and stations sampled for ambient toxicity in 1994.
A series of EPA studies to address ambient toxicity and biological community health in streams known as the Complex Effluent Toxicity Testing Program was carried out in the 1980s. These studies have been reviewed and further analyzed by Dickson et al. [8]. They concluded that in spite of spatial and temporal variations, statistically significant relationships could be discerned between measures of ambient toxicity and biological community health metrics in freshwater streams. In addition, Munkittrick and coworkers have explored specific fish population response indicators to sublethal contaminant effects [9]. They identified specific response patterns in fish population structures that correlate with specific modes of toxic action.
The ongoing investigations reported here are designed to identify and rank locations in the Chesapeake Bay system where contaminant problems exist and that result in demonstrable fish community impairment. These studies will provide guidance for where to investigate cause-and-effect relationships, explore the underlying ecological processes resulting in those effects, and the site-specific parameters that influence the system response. In addition, it is anticipated that regression relationships between community health metrics and toxicological risk can be used as a predictive tool for making inferences about ecological damage resulting from low-level contamination. This paper does not address the development of the IBI approach.
METHODS
Sample sites
Test sites were located in the Severn, South, Patuxent, and Wicomico rivers (Fig. 1). The Wicomico is a tributary of the Potomac River and served as a reference. The other three discharge to the mainstem of Chesapeake Bay on the western shore, south of Baltimore, MD, USA.
The Severn River watershed is 209 km2. With 36% of this area developed, it was the most urbanized watershed studied. Residential and forested areas dominate the shoreline. The river is subject to intense boating pressure. The United States Naval Academy and several marina facilities are located at or near its mouth.
The South River watershed is 176 km2. Roughly 25% of the watershed is developed, and about 17% is agricultural. Residential and forested areas compose much of the shoreline but with many public and private marinas and their resultant boating traffic. Land use ratios are nearly identical to those of the Severn River watershed.
The Patuxent River is the largest tributary studied to date. The watershed covers 1,946 km2, of which about 11% is developed. The dominant land uses are agricultural and forest. Two large reservoirs are located in the upland areas of the watershed. The Patuxent River was chosen for this study in part because of its great potential for further urbanization. Fast-growing areas within the watershed include satellite communities of the Baltimore-Washington corridor. Also, a comprehensive watershed assessment and restoration program is in progress in this watershed.
The Wicomico River watershed is 247 km2. Agriculture and forest are the dominant land uses, at about 30% and 40%, respectively. Less than 6% of this watershed is developed. The Wicomico River served as the saltwater reference tributary in the studies by Carmichael et al. [6] and Hartwell et al. [7] and has consistently demonstrated healthy fish community metrics. The Wicomico River was retained from the previous study to serve as an established field reference site and to lend continuity to the project from 1 year to the next. A variety of more detailed habitat parameters and watershed land use practices are routinely quantified as part of the IBI development program but are beyond the scope of this paper.
Five stations were established along the axis of each tributary from the upstream extent of saltwater excursion to the mouth of the tributary. Fish community sampling and ambient toxicity bioassay methods followed established procedures [2, 6, 7]. Fish sampling methods were designed to assess the fish community as it reaches its peak diversity in late summer. Ambient toxicity testing was initiated during this period to assess the potential impact of toxic contamination as the fish communities matured and to assess any short-term spikes in toxic effects that may be detectable by this sampling regime. Fish were sampled with beach seines and bottom trawls deployed near midchannel. All fish captured were identified, measured, counted, and released. Data from each station were summed over the summer sampling period, and an IBI metric and diversity indices were calculated for each station and tributary. Further details of the methods are found in Carmichael et al. [6].
Water column bioassays
The following static water-column bioassays were conducted monthly from April through August 1994: 7-d sheepshead minnow (Cyprinodon variegatus) survival and growth test; 7-day grass shrimp (Palaemonetes pugio) survival and growth test; a copepod (Eurytemora affinis) life-cycle survival and reproduction test; and a bacterial luminosity bioassay (MicrotoxR). Water-column tests that measured growth and reproduction were also conducted in July and August with the submerged aquatic plant species, sago pondweed (Potamogeton pectinatus). Fish and grass shrimp bioassays were conducted at DNR's Aquatic Toxicology Laboratory (Glen Burnie, MD, USA). The copepod bioassays were conducted at the University of Maryland, Chesapeake Biological Laboratory (CBL). The vascular plant bioassays were conducted at the Anne Arundel Community College, Environmental Center.
Depth-integrated water samples were collected monthly at the central station in each of the four rivers. Standard water quality parameters were measured at the time of collection (dissolved oxygen [DO], °C, salinity, pH, etc.). Samples were taken twice from each site during the course of each 7-d test to provide fresh renewal water for the bioassays. The sampling interval was 4 d. This timetable was dictated by the fish collection cruise schedule. Water samples were filtered through 37-μm mesh and adjusted to a salinity of 15 ppt, except for the copepod assays that were run at 10 ppt. All water was stored in amber bottles at 4°C until use. When the sample salinity exceeded 15 ppt, no adjustment was made. Heavy metals, acid and base/neutral semivolatile organic compounds, and chlorinated pesticides were analyzed on August samples only, using standard EPA methods [10].
Culture, maintenance, and bioassay procedures for grass shrimp and sheepshead minnow used methods contained in American Public Health Association (APHA) 8720 and EPA [11, 12], respectively. Detailed handling and bioassay methods have been described previously [2, 7]. Reference water for the copepod bioassays was taken from Wachepreague Bay on the Atlantic coast of Virginia each month. It was autoclaved, filtered to 1 μm, and diluted from its original salinity (33 ppt) to match the salinity of the test water. Dilutions were made with CBL well water. All media were adjusted to laboratory temperature (24°C) prior to the test. Detailed handling and bioassay methods have been previously described [2]. Copepod development was followed through one life cycle, (∼10–14 d). Numbers of surviving adults were counted and eggs and nauplii and subadults from the F1 generation were also counted at the end of the test.
The techniques for the P. pectinatus bioassays used in this study were originally developed by the U.S. Fish and Wildlife Service, Patuxent Wildlife Research Center and the Anne Arundel Community College Environmental Center [13, 14]. Laboratory-propagated stocks of sago pondweed, originally collected from Chesapeake Bay, were weighed and rooted in a nutrient agar medium. Eight replicates were submersed in 750 ml of ambient water from each river. Light levels were maintained at 70 mol/m2/s photosynthetically active radiation (PAR) on a 12L:12D cycle. Temperature was held constant at 22°C. Filtered air supplemented with CO2 to approximately 3% was continuously pumped into each test chamber. After 4 weeks, the plants were removed and weighed. The number of rhizome tips on each plant was counted as a measure of reproduction. The plants were then dried and weighed again as a measure of growth.
The MicrotoxR assay was performed on water samples from April through August. Each of the two water samples taken each month was tested (except April). Tests were run as dilution series bioassays for each sample. Samples were tested at concentrations of 0 (control), 60, 70, 80, 90, and 100%. Lowest observed effect concentrations (LOEC) were determined by Durmetts' procedure [15] on inhibitory samples only.
Sediment bioassays
Sediment toxicity tests were conducted in August using the following bioassays: 10-d sheepshead minnow (C. variegatus) embryo-larval survival and teratogenicity test; 10-d amphipod (Lepidactylus dytiscus) survival and growth test; 10-d amphipod (Leptocheirus plumulosus) survival and growth test; 10-d polychaete worm (Streblospio benedicti) survival and growth test; and a Spartina alterniflora seed germination test. The L. plumulosus bioassays were conducted at the DNR Toxic Aquatic Contaminants Laboratory. The S. alterniflora seed tests were conducted at Anne Arundel Community College. All the other sediment bioassays were conducted at Old Dominion University, Applied Marine Research Laboratory (AMRL).
Sediment samples were collected with a petit ponar grab sampler. Preliminary samples were taken in April for initial grain size analysis. In August 1994, discrete field samples were collected from each of the five fish sampling stations in each tributary for toxicity testing. The top 2 cm were retained for testing. The sampling plan does not provide for true field replication for statistical purposes but does allow a contrast of upstream versus downstream locations. The decision to sample discrete locations throughout each river was based on the intent to assess the condition of the entire system, relative to the fish community indices. This was unnecessary in the water samples because all the systems were tidal. However, in larger systems, such as the Patuxent River, this rationale may not be entirely valid. Sediment samples were segregated throughout the collection and toxicological tests. Samples were held out of direct sunlight at 4°C and used within 2 weeks.
The three upstream Patuxent River samples were 1–2% sand while the lower two ranged from approximately 19 to 35% sand. The upper- and lowermost Severn River samples were muddy, with between 3 and 6% sand, while the middle three samples averaged approximately 93% sand. The downstream South River station was approximately 70% sand, and the other four were muddier and averaged approximately 9% sand. The Wicomico River stations were uniformly muddy, with an average sand content of only 1.6%.
Inorganic and organic contaminants were evaluated concurrently with toxicity tests on a composite sample from each river system. Acid and base/neutral semivolatile compounds and bulk metals were analyzed using standard EPA methods [16]. Pore-water samples were extracted by squeezing with a nitrogen press. Samples were filtered and analyzed for ammonia, nitrite, and sulfides. Sediments were also analyzed for acid-volatile sulfides (AVS) and total organic carbon (TOC). Samples were analyzed for AVS using the method of Di Toro et al. [17]. Simultaneously extractable metals (SEM) analysis was conducted on all samples to use with the AVS data in order to determine the potential toxicity of the sediment due to metals. The concentrations were then converted to micromoles per gram dry sediment and were added together to provide the total SEM value.
Detailed handling and bioassay methods for the animal species have been described previously [2, 7]. For the polychaete and amphipod species, handling and bioassay methods followed guidance in American Society for Testing and Materials (ASTM) [18] and DeWitt et al. [19]. Control sediments for each animal species consisted of native sediments from the area in which the test organisms were collected or naturally occur. Most tests had a reference sediment treatment as well.
The reference sediments were defined as sediment that brackets the particle size range of the sediment being tested. Thus, species normally from sandy sediment have a sand control and mud reference, while species from a muddy environment have a mud control and a sand reference. The purpose of these reference sediments was to assess what effect normal physicochemical parameters (primarily particle size) would have on the survival of the organism being exposed to those sediments, in the absence of toxicants. Because of the large range in particle size between test sites, two reference sediments were used with the L. dytiscus, C. variegatus, and S. benedicti bioassays.
A fine-grained reference sediment (99% silt/clay) was obtained from a small tidal creek within the Poropotank River, Virginia, USA. The sand control sediment (98% sand) was collected from Lynnhaven Inlet, Virginia Beach, Virginia, USA. A mud control sediment was also collected from Lynnhaven, which was approximately 37/50/13% sand/silt/clay.
Reference and control sediments were from the designated sites and are indicated throughout the text as follows: (1) Lynnhaven Sand, (2) Lynnhaven Mud, and (3) Poropotank Mud.
Lynnhaven mud was used as the control sediment for S. benedicti and C. variegatus eggs. Lynnhaven sand and Porpotank mud were used as reference sediments. Lynnhaven sand was used as the control for L. dytiscus, with Poropotank mud as a reference. The control sediment for the L. plumulosus bioassays was laboratory culture sediment obtained from Fishing Bay on the eastern shore of the Chesapeake Bay (sand/silt/clay = 18/57/25). L. plumulosus survives well in a wide range of sediment grain sizes and does not require a reference sediment treatment.
Following bioassays, the animals were preserved for weight measurements. Statistical evaluations of L. dytiscus mortality data were made relative to particle size effects based on the response to the reference sediments. Mortality was corrected for particle size effects using the regression equation previously established for L. dytiscus % survival = (observed survival)/(98.41 – 0.35066 × % silt/clay) [2].
The S. alterniflora bioassays were conducted by placing 100 seeds in porous bags that were then buried in sediment samples from each sample site. Ten replicates per test were employed. Control tests consisted of sand adjusted to salinities of 0 to 9 ppt. Seeds were incubated in the sediment for 3 d at 2°C. The bags were then removed from the sediment, washed, and incubated at room temperature. Percent germination was recorded at intervals of 2, 7, and 14 d.
For all water-column and sediment tests, percent survival was compared to controls using the t-test following arc sine transformation, or a Wilcoxon rank sum test if the data were not normal. Growth and reproductive parameters were also compared using t-tests and the Wilcoxon rank sum test. Differences between means were considered significant at the p = 0.05 level.
Risk ranking calculations
The risk ranking scheme has five components: (1) severity of effect, (2) degree of response, (3) test variability, (4) site consistency, and (5) number of measured endpoints. A detailed discussion and evaluation of the model can be found in Hartwell [4], and a brief summary is provided below. The rationale of the ranking system is to quantify the degree of concern for the possible presence of toxic contamination, or the risk, not merely to catalog presence or absence of toxic effects. The term risk is used here in the sense of jeopardy. If the toxicological risk score is high, due to high toxicity or high variability, this implies resource populations in the test area may be in more danger than areas with low scores. The model is not designed to replace a classical risk assessment but may be a useful component of, or a companion to, the hazard or exposure assessment components of a risk assessment. It specifically addresses ecological impact, without the necessity for demonstrating detailed, chemical-specific cause and effect relationships, which is one of the greatest difficulties in ecological risk assessment.
Severity, response, and variability are characteristics of the individual bioassays conducted for each sample/site, whereas consistency and the number of endpoints measured are tributary-specific attributes. Severity refers to the degree of effect that the bioassay endpoints measure (i.e., mortality vs growth). Severity multipliers were arbitrarily set at mortality = 3, reproduction = 2, and growth = 1. Response is the measure of the proportion of organisms responding in each bioassay (adjusted for control) regardless of statistical significance (e.g., 5% mortality, 45% growth inhibition, etc.). Statistical significance is based on arbitrary, albeit logical, confidence limits. Negative values were assigned a value of zero in the model. Variability was expressed as the coefficient of variation of response for each set of laboratory replicates. Consistency refers to the agreement between the various bioassay endpoints measured from one site. The number of endpoints measured at each site refers to the number of bioassays (species) and measured parameters (survival, growth, etc.) that are monitored.
Each site was ranked by the following scheme; endpoint severity was multiplied by the percent response of the test organisms for each bioassay endpoint and the coefficient of variation for that test endpoint. The products from all bioassays were summed for each test site. The site sum was adjusted by the site consistency factor and divided by the square root of the number of test endpoints for each site.
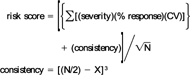

Three risk ranking scores were calculated: water, sediment, and combined water and sediment. A water risk score was calculated for each sampling month, and the response scores were averaged by river over months. Sediment samples were collected and tested as discrete samples without laboratory replication. Therefore, calculation of a risk score was done by pooling the data together by river to calculate the CV and consistency factors. The rationale for sampling sediment in this way was the assumption of low temporal variation in sediment relative to the water column and for the purpose of examining sediment contamination effects on a systemwide basis, which is consistent with the IBI community approach.
| Station/year | Bottom diversity index | Resident diversity index | IBI | Water risk | Sediment risk | Combined risk |
|---|---|---|---|---|---|---|
| 1994 | ||||||
| Patuxent River | 1.991 | 1.478 | 34.6 | 25.73 | 20.84 | 42.33 |
| Severn River | 1.836 | 1.180 | 26.3 | 23.80 | 13.11 | 6.14 |
| South River | 1.400 | 1.402 | 28.0 | 32.38 | 89.45 | 77.39 |
| Wicomico River | 2.037 | 1.409 | 31.4 | 25.55 | -2.59 | -33.25 |
| 1993 | ||||||
| Curtis Creek | 0.000a | 0.865 | 29.0 | 21.04 | 137.20 | 158.45 |
| Rock Creek | 1.428 | 1.761 | 35.0 | 17.90 | 76.48 | 47.02 |
| Fishing Bay | 1.760 | 1.086 | 28.5 | 10.46 | 58.24 | 24.73 |
| Wicomico River | 1.609 | 1.244 | 33.8 | 15.20 | 59.09 | 42.28 |
- a No fish caught, index = 0.
Sediment and water data were combined together by river system to calculate a toxicity risk factor for the whole system.
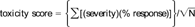
The risk scores were contrasted to diversity indices and the IBI values (Table 1). Pearson correlation coefficients were calculated for every combination of toxicological risk score (water, sediment, and combined) and fish community indices. Three community index categories were used: the IBI developed by Carmichael et al. [6], bottom species diversity, and resident species (estuarine spawners) diversity.
Briefly, the IBI derivation method is based on the methods of Karr [20] and includes nine metrics from measures of species richness, dominance, abundance, and trophic structure [21, 22]. Following transformations and salinity calibrations, the individual metric values were ranked between stations and divided into three groups: low, medium, and high. A ranking value of 1, 3, or 5 is then assigned to each metric. The ranked values of all nine metrics at each station were then summed to compute an IBI score. The mean values, averaged over a given sampling year, were calculated for each tributary. The IBI was designed to reflect the diversity and trophic structure of the entire fish community. This also means the IBI score should respond to a variety of factors in the habitat, including, but by no means limited to, toxic impacts. The IBI score effectively incorporates all resident and migratory species in both the trawl and beach seine data. Calculation of an IBI score with only the trawl data would not be effective because it would incorporate an incomplete set of species, relative to the number of metrics in the IBI derivation.

In addition, correlation coefficients were calculated for the toxicological risk scores and fish community diversity indices for the combined ambient toxicity/IBI data base for 1993 and 1994. (Table 1).
RESULTS
Statistically significant mortality did not occur in watercolumn bioassays with fish or grass shrimp (Table 2). Grass shrimp survival values were lower than normal in the August tests in the South and Severn River bioassays but were not statistically significant. Growth rates in fish were slightly, but significantly, reduced in April in the Severn River water sample (Table 3). The difference between ambient and control values was small, but variability was very low. All of the August fish bioassays resulted in poor growth. It is unclear if this is a real effect or a sample handling artifact. Control growth was very good, and all tissue samples were handled and weighed with exactly the same methods and all at the same time. Significant inhibition of grass shrimp growth was not observed (Table 3).
In the copepod assays, reproduction, as measured by the number of eggs, nauplii, and subadults present, was highly variable between stations and months (Table 4). Significantly reduced reproduction was observed in June and July samples from the South and Patuxent rivers and the Severn River in June. Reproduction in the Wicomico River in May was completely absent. However, survival and reproduction in May was reduced in all tests, including the controls. This was likely due to a failure of temperature control in the test chamber, which cooled to as low as 17°C at one point during the test. Survival to adult stage was significantly reduced in the July tests in the Patuxent River (Table 4). The South River had the lowest overall average survival, but no river was consistently lower than others in all months.
| Month/station | April | May | June | July | August |
|---|---|---|---|---|---|
| Fish | |||||
| Control | 2 | 0 | 5 | 6.7 | 10 |
| Patuxent River | 0 | 0 | 5 | 0 | 10 |
| Severn River | 0 | 0 | 5 | 3.3 | 3 |
| South River | 0 | 0 | 2.5 | 0 | 10 |
| Wicomico River | 0 | 2 | 2.5 | 10 | 0 |
| Shrimp | |||||
| Control | 0 | —a | — | 0 | 5 |
| Patuxent River | 5 | — | — | 0 | 2.5 |
| Severn River | 5 | — | — | 0 | 12.5 |
| South River | 0 | — | — | 0 | 17.5 |
| Wicomico River | 7.5 | — | — | 2.5 | 10 |
- a Organisms not available.
| Month/station | April | May | June | July | August |
|---|---|---|---|---|---|
| Fish | |||||
| Initial | 0.139 | 0.108 | 0.087 | 0.029 | 0.049 |
| Control | 0.538 | 0.379 | 0.111 | 0.317 | 0.483 |
| Patuxent River | 0.558 | 0.432 | 0.126 | 0.249 | 0.038c |
| Severn River | 0.529b | 0.429b | 0.153 | 0.226 | 0.026c |
| South River | 0.570 | 0.416 | 0.070 | 0.265 | 0.031b |
| Wicomico River | 0.583 | 0.417 | 0.124 | 0.237 | 0.023c |
| Shrimp | |||||
| Initial weight | d | —e | — | 0.183 | 0.070 |
| Control | 0.110 | — | — | 0.359 | 0.134 |
| Patuxent River | 0.110 | — | — | 0.356 | 0.184b |
| Severn River | 0.102 | — | — | 0.401 | 0.122 |
| South River | 0.090 | — | — | 0.366 | 0.110 |
| Wicomico River | 0.106 | — | — | 0.334 | 0.147 |
- a Values are mean terminal weight (mg).
- b Significant at p = 0.05.
- c Significant at p = 0.01.
- d Sample lost.
- e Organisms not available.
No mortality, growth, or reproductive effects were seen in the plant bioassays in July or August (Table 5). The MicrotoxR assays demonstrated statistically significant inhibition in April, May, and August. None of the samples were inhibitory in June or July. The bioassays often showed significant stimulatory results. This may be due to eutrophic conditions in the tributaries. The LOECs for those tests that showed inhibitory results are shown in Table 6. Only one instance of effects below 70% test water was observed. Due to the experimental design, the true LOEC for the Severn River #2 run is unknown. In the single water samples from each tributary, some metals were above detection limits, but all values were relatively low, and all were below marine ambient water quality criteria. All organic analyses results were below detection limits.
| Month/station | April | May | June | July | August |
|---|---|---|---|---|---|
| Mortality | |||||
| Control | 15.0/35.0b | 38.3 | 21.7 | 10.0 | 20.0 |
| Patuxent River | 40.0 | 35.5 | 23.3 | 43.3c | 10.0 |
| Severn River | 24.9 | 53.3 | 45.0 | 40.0 | 11.7 |
| South River | 40.0 | 50.0 | 35.0 | 50.0 | 13.3 |
| Wicomico River | 30.0 | 70.0c | 26.7 | 25.0 | 17.7 |
| Reproduction (no. eggs, nauplii, and subadults) | |||||
| Control | 577.5/350.0b | 162.5 | 1,188.75 | 1,234.75 | 875.5 |
| Patuxent River | 445.25 | 210.67 | 747.25c | 738.5c | 791.75 |
| Severn River | 665.25 | 76.5c | 239.25c | 1,293.0 | 778.0 |
| South River | 547.0 | 310.75 | 320.0c | 588.25c | 1,161.0 |
| Wicomico River | 406.0 | 0.0d | 804.75 | 855.5 | 982.0 |
- a Values are the mean of four replicates.
- b Two controls were used during April due to wide differences in salinity of test water. Salinity of all test water was adjusted to 10 ppt in other months.
- c Significant at p = 0.05.
- d Significant at p = 0.01.
| Station | Initial weight | Terminal weight | Dry weight | No. rhizome tips |
|---|---|---|---|---|
| July | ||||
| Control | 1.28 | 6.03 | 0.63 | 20.7 |
| Patuxent River | 1.32 | 6.72 | 0.69 | 20.6 |
| Severn River | 1.33 | 6.32 | 0.70 | 23.0 |
| South River | 1.24 | 6.53 | 0.71 | 19.2 |
| Wicomico River | 1.21 | 6.18 | 0.66 | 20.3 |
| August | ||||
| Control | 1.33 | 4.95 | 0.61 | 24.7 |
| Patuxent River | 1.25 | 4.41 | 0.51 | 26.2 |
| Severn River | 1.32 | 5.26 | 0.59 | 24.3 |
| South River | 1.30 | 4.54 | 0.50 | 25.0 |
| Wicomico River | 1.23 | 4.40 | 0.50 | 23.4 |
- a Weight values are the mean of 10 replicates in units of g/plant.
Survival results from the sediment bioassays with amphipods, worms, and sheepshead minnow eggs are included in Table 7. High levels of mortality were observed in the L. dytiscus tests. After adjustment for grain size however, only the South River demonstrated obviously elevated mortality levels relative to controls. Mortality in the polychaete tests was highly variable, and statistically significant differences were not observed. Higher levels of polychaete mortality were observed in the Patuxent River relative to other sites. Polychaete mortality in the South and Wicomico rivers was marginally elevated. Elevated mortality was not observed in the L. plumulosus bioassays (Table 7), with the exception of the South River. Mortality in the fish bioassays was also highly variable (Table 8). The highest mortality level was observed in the South River. Fish mortality was also elevated above the controls in the Wicomico and Patuxent rivers. Fish mortality in the South and Patuxent rivers was primarily due to hatching failure as opposed to larval mortality (posthatch) observed at the single downstream replicate from the Wicomico. Fish mortality in the South River was primarily at the upstream sites as opposed to the Patuxent River where peak mortality occurred at the middle site. Growth of L. plumulosus was poor in the South River bioassays (Table 7); however, control growth was also poor. Growth of controls in the L. dytiscus and S. benedicti tests was also lower than ambient results (Table 7). No significant effects on germination were observed in sediment bioassays with either the lettuce or S. alterniflora seeds (Table 7).
| Month/station | April | May | August |
|---|---|---|---|
| Patuxent River | 80 | 70 | |
| Severn River (run 1) | 70 | 70 | 70 |
| Severn River (run 2) | ≤60 | ||
| South River | 70 | 90 | |
| Wicomico River | 90 | 99 |
- a Values are expressed as % river water, diluted with control water.
| % Mortality | Growth | |
|---|---|---|
| L. dytiscusa | ||
| Initial | 0.518 | |
| Patuxent River | 5.39 | 0.806 |
| Severn River | 9.10 | 0.735 |
| South River | 23.78 | 1.201 |
| Wicomico River | 0.00 | 0.724 |
| Lynnhaven sand (c)b | 2.08 | 0.531 |
| Poropotank mud (r) | 5.38 | 0.799 |
| S. benedicti | ||
| Initial | 0.103 | |
| Patuxent River | 23.00 | 0.771 |
| Severn River | 10.00 | 0.578 |
| South River | 17.00 | 0.650 |
| Wicomico River | 14.00 | 0.607 |
| Lynnhaven mud (c) | 5.00 | 0.529 |
| Poropotank mud (r) | 10.00 | 0.581 |
| L. plumulosus | ||
| Initial | 0.029 | |
| Patuxent River | 6.0 | 0.033c |
| Severn River | 8.0 | 0.063d |
| South River | 22.0 | 0.033 |
| Wicomico River | 10.0 | 0.040d |
| Culture (c) | 12.0 | 0.014 |
| Germination | ||
| S. alterniflora | ||
| Patuxent River | 6.75 | |
| Severn River | 7.34 | |
| South River | 10.27 | |
| Wicomico River | 8.55 | |
| Control | 11.29 | |
- a L. dytiscus survival is percent survival adjusted for predicted particle size effects.
- b (r) = Reference; (c) = control.
- c Significantly different than controls (p < 0.05).
- d Significantly different than controls (p < 0.01).
| Station | Total % mortality | % Unhatchedb | % Dead eggs | % Dead fish |
|---|---|---|---|---|
| Patuxent River | 58.00 | 54.00 | 32.00 | 5.56 |
| Severn River | 40.00 | 38.00 | 10.00 | 3.57 |
| South River | 72.00 | 70.00 | 20.00 | 4.76 |
| Wicomico River | 66.00 | 48.00 | 22.00 | 42.50 |
| Lynnhaven mud (c)c | 42.00 | 34.00 | 28.00 | 10.67 |
| Poropotank mud (r) | 46.00 | 38.00 | 22.00 | 11.07 |
| Lynnhaven sand (r) | 2.00 | 0.00 | 0.00 | 2.00 |
- a % Total mortality = (dead fish + unhatched)/(no. eggs exposed) × 100. % Dead fish = (dead fish)/(no. hatched) × 100. % Dead eggs = (dead eggs)/(no. exposed) × 100. %Unhatched = (no. unhatched)/(no. eggs exposed) × 100.
- b % Unhatched includes dead eggs.
- c (r) = Reference, (c) = control.
Bulk and pore-water chemistry results for sediments are shown in Table 9. Lead and zinc were above NOAA low effects range (ERL) levels in the Patuxent, South, and Wicomico rivers [24, 25]. The South River also had chromium levels near the ERL value. Contamination with routine organic contaminants was relatively low in all systems. Ammonia levels in pore water were relatively high in all samples, including the reference sediments (Table 9). The Severn River sample had less than 1% TOC. Data for SEM and SEM/AVS ratios for metals in sediment pore water are shown in Table 10. The Patuxent River sediments had the highest SEM levels, primarily due to zinc values, but no SEM/AVS ratios were above 1.0. The South River had the highest ratio of 0.384.
The water-column risk scores for each sampling period are shown in Figure 2. Results were highly variable between months and stations, with no apparent pattern. The May co-pepod data were excluded from the calculations. Mean water toxicological risk scores are shown in Figure 3. Risk scores in the four rivers were similar. The toxicity scores for discrete sediment samples are shown in Figure 4. There appears to be a strong upstream to downstream gradient in the South River. Data from the Severn River demonstrate a localized spike at the upstream station. The Patuxent River has a spike in the middle reach of the river. The Wicomico River scores are relatively uniform, with no extreme peaks. The pooled sediment risk scores are shown in Figure 5. The pooled scores integrate variability and consistency into the scores. These values indicate that the South River has a high risk for sediment toxicity impacts. The risk scores for combined water and sediment data are shown in Figure 6. The South River clearly has the highest risk value. The Patuxent River has the next lowest risk score, which is half of the South River value. The toxicological risk values for the Severn and Wicomico rivers are low or negative.
The correlation coefficients for the risk scores and the fish community metrics from 1994 are shown in Table 11. The bottom trawl diversity index was strongly correlated with the sediment toxicological risk score. The combined score, which was dominated by the sediment score, also tracks the bottom diversity score but was not statistically correlated due to increased variability. The resident species diversity index and the overall IBI did not have strong correlations with the toxicological risk scores. Linear regression relationships between the toxicological risk scores and bottom diversity indices can be seen in Figure 7. The pattern seen in 1994 is consistent with the correlations in the 1993 data. Combining the data sets yields similar results and incorporates a larger sample size (Table 11). Linear regression relationships between the 1993 and 1994 toxicological risk scores and diversity indices are shown in Figure 8.
| Chemical | Patuxent River | South River | Severn River | Wicomico River | NOAA ERL | NOAA ERMb |
|---|---|---|---|---|---|---|
| Antimony | <0.38 | <0.32 | <0.17 | 1.08 | 2 | 25 |
| Arsenic | 7.96 | 11.9 | 5.91 | 7.65 | 33 | 85 |
| Beryllium | 1.31 | 1.45 | 0.66 | 1.69 | — | — |
| Cadmium | 1.69 | <1.29 | <0.69 | <1.54 | 5 | 9 |
| Chromium | 40.0 | 78.7 | 33.6 | 46.2 | 80 | 145 |
| Copper | 17.2 | 30.8 | 12.8 | 19.7 | 70 | 390 |
| Lead | 50.8 | 60.6 | 23.4 | 72.3 | 35 | 110 |
| Nickel | 17.7 | 15.3 | 6.69 | 20.4 | 30 | 50 |
| Selenium | 2.54 | 2.19 | 0.52 | 4.31 | — | — |
| Zinc | 155 | 219 | 77.8 | 141 | 120 | 270 |
| Fluoranthene | <58 | 30 | 70 | <58 | 600 | 3,600 |
| Pyrene | <77 | <65 | 60 | <77 | 350 | 2,200 |
| Butylbenzylphthalate | <350 | 110 | 230 | 560 | — | — |
| Chrysene | <58 | <49 | 60 | <58 | 400 | 2,800 |
| Benzo(a)anthracene | <67 | <57 | 30 | <67 | 230 | 1,600 |
| TOC | 3.04 | 2.17 | 0.99 | 2.58 | — | — |
| Ammonia | 15.62 | 10.29 | 13.49 | 25.50 | ||
| Unionized ammonia | 0.2493 | 0.2580 | 0.2152 | 0.4069 | ||
| Nitrite | 0.0165 | 0.0058 | 0.0064 | 0.0078 | ||
| Sulfide | 0.009 | 0.011 | <0.006 | 0.007 |
- a Units are mg/kg and μg/kg for metals and organics, respectively.
- b ERM = median effects range.
DISCUSSION
Observed toxicity was highly site specific within and between tributaries. The South River bioassays displayed greater toxicological risk scores than any other tributary. There was a marked difference between the upstream and downstream portions of the river. Most of the observed sediment mortality effects were concentrated in the upper three stations (Fig. 4).
A more intense evaluation of the South River has been performed and results are in preparation. The Severn River sediment bioassays demonstrated toxic effects only at the uppermost station. In both cases, sediment results were not statistically significant due to high variability resulting from pooling data within tributaries. Because data must be pooled by river to make statistical tests of river versus control, the mean level of response from a given station may be masked by lack of response at other stations. The Patuxent River results demonstrated both water column and sediment toxicity, primarily at one station in the middle reach of the river. In spite of the high mortality rate for worms, the highest rate of growth by the worms was achieved at this site; however, these data are based on fewer animals than for other sites. The Patuxent River had the highest SEM metals levels. It also had the highest AVS level, resulting in a low SEM/AVS ratio. The levels of lead and zinc exceeded NOAA ERL levels. The Wicomico River bioassays resulted in very few toxic responses. One sediment sample yielded high mortality for fish larvae. In the South, Severn, and Patuxent rivers, the impact on fish was primarily on the embryonic (egg) stage. The eggs did not die, but up to 100% failed to hatch. In the downstream Wicomico River station the effect was on larval (posthatch) survival. The eggs hatched, but larval survival was poor. Bulk sediment analyses demonstrated that lead and zinc concentrations were above NOAA ERL levels in the composite Wicomico sample (Table 9).
| Site | Cadmium | Lead | Copper | Nickel | Zinc | Sum | Mean AVS | Ratio |
|---|---|---|---|---|---|---|---|---|
| Patuxent River | 0.036 | 0.202 | 0.298 | 0.155 | 2.176 | 2.868 | 31.09 | 0.092 |
| Severn River | 0.036 | 0.069 | 0.149 | 0.160 | 0.912 | 1.326 | 9.96 | 0.133 |
| South River | 0.012 | 0.064 | 0.167 | 0.078 | 0.814 | 1.140 | 2.98 | 0.384 |
| Wicomico River | 0.172 | 0.169 | 0.432 | 0.445 | 1.155 | 1.301 | 11.54 | 0.113 |
| Lynnhaven sand | 0.000 | 0.000 | 0.000 | 0.000 | 0.013 | 0.013 | 0.63 | 0.021 |
| Lynnhaven mud | 0.000 | 0.021 | 0.036 | 0.029 | 0.441 | 0.527 | 3.17 | 0.166 |
| Poropotank mud | 0.000 | 0.021 | 0.000 | 0.054 | 0.539 | 0.613 | 5.46 | 0.112 |
| Detection limits | 0.001 | 0.004 | 0.003 | 0.007 | 0.005 |
- a Mercury values for all sites were <0.0001 μmol/g. Detection limit = 0.0001 μmol/g.
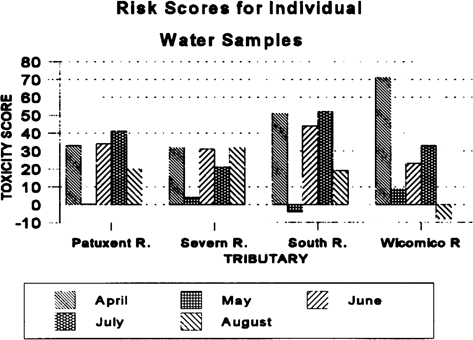
Risk scores for monthly water-column samples from four Chesapeake Bay tributaries evaluated for ambient toxicity in 1994.
No specific chemical or suite of chemicals has been identified in these samples, which can obviously explain the results. The standard suite of priority pollutant chemicals was analyzed. However, a thousand chemical contaminants have been identified in the Bay [26], most of which are not analyzed in standard surveys. Little or no information on potential synergism between these compounds exists. In addition, many more organic chemicals are simply regarded as unknowns in gas chromatography-mass spectrometry (GC/MS) analyses. Many chemicals cannot be analyzed by GC at all. Finally, the chemical analyses are performed on composites of all stations within each river due to cost constraints. Thus, high level concentrations of chemicals from a specific location or locations could essentially be diluted by sediment from cleaner portions of the estuary. This may be particularly important in the Severn River, where the sediment in the middle three stations was composed primarily of sand.
No mortality, growth, or reproduction effects were observed in any of the vascular plant bioassays. Except for pesticides, the relative sensitivity of plants and animals to environmental contaminants is largely an unknown factor. However, the sago pondweed has been tested with several individual chemicals and has been shown to be a reasonably sensitive species, especially to herbicides [13, 27, 28]. Swanson et al. [29] concluded that aquatic macrophytes are more sensitive to toxicants than commonly tested algal species. The potential for seasonal sensitivity of plants to low level contamination is also unknown. The impacts of herbicide runoff would be expected to be lower in late summer than in spring. This aspect of sampling design needs further investigation. A thorough review of the use of plant species in ecological biomonitoring studies was presented by Doust et al. [30].
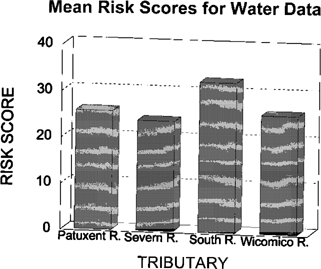
Mean risk scores for water column data from four Chesapeake Bay tributaries evaluated for ambient toxicity in 1994.
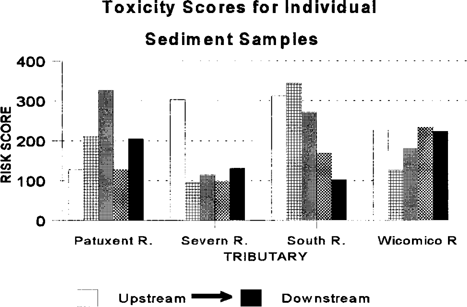
Toxicity scores for individual sediment samples from four Chesapeake Bay tributaries evaluated for ambient toxicity in 1994.
The risk ranking procedure results in comparable risk scores between sites. The use of multiple species, multiple sampling times/locations, and a correction for the number of significant data points on a tributary-by-tributary basis results in a robust scoring procedure. The combined toxicological risk scores do not respond strongly to small variations in data availability or isolated spikes, but small responses are not lost. It is instructive to look at the spikes on a sample-by-sample basis. This approach displays the existence of a downstream gradient in the Severn River and site-specific spikes in the Patuxent and Severn rivers. A more intense examination of the upper South and Severn and the middle Patuxent rivers may yield clearer pictures of the nature and extent of degraded habitats. Transient spikes, if present from spills or heavy runoff, may also be detectable in the water-column bioassays if sampling is timely. The ability of the data set to identify isolated hot spots is dependent on the size of the area relative to sampling intensity [31]. The Patuxent River sampling stations were distributed over two to three times the distance of stations in the other rivers. Thus the sensitivity of a river-specific combined score requires careful application of the data. Large areas of degraded habitat could be missed if the sampling stations are too far apart. The sheer size of the river will affect its assimilative capacity for environmental degradation. Also, the relative size and complexity of the Patuxent River watershed is greater than that of the other rivers.
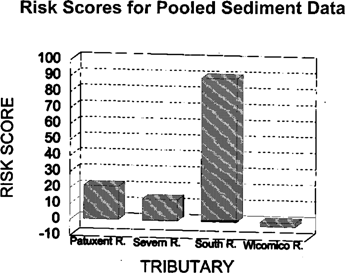
Risk scores for pooled sediment data from four Chesapeake Bay tributaries evaluated for ambient toxicity in 1994.
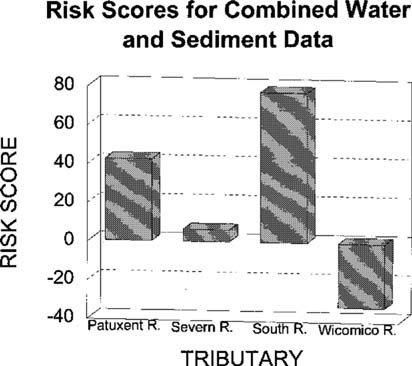
Risk scores for combined sediment and water data from four Chesapeake Bay tributaries evaluated for ambient toxicity in 1994.
The correlation between the toxicological scores and the fish community metrics indicates that the sediment toxicological risk score is strongly related to the bottom community diversity index (Table 11). The resident species metrics and the IBI were not strongly correlated with the risk scores. This is consistent with results from the 1993 sampling year [7]. The definition of resident species as estuarine spawners is important in this regard. This metric is dominated by species taken in the beach seines. The resident species are thus not living in close contact with the sediment at the bottom of the channels where the sediment samples were taken. The toxicological data clearly demonstrate that sediment toxicity is a dominant problem in the South River but that the water column scores were marginal there, as well as the other systems. However, the water samples were drawn from station 3, which is ∼4.8 km from the upper three stations where sediment toxicity was most obvious.
| Risks | IBI score | Bottom diversity index | Resident diversity index |
|---|---|---|---|
| 1994 | |||
| Water risk | -0.1615 | -0.8645 | 0.3988 |
| (0.8385) | (0.1355) | (0.6012) | |
| Sediment risk | -0.3205 | -0.9559a | 0.1806 |
| (0.6795) | (0.0441) | (0.8194) | |
| Combined risk | -0.0690 | -0.7730 | 0.2621 |
| (0.9310) | (0.2270) | (0.7379) | |
| 1993 and 1994 | |||
| Water risk | -0.1869 | 0.0423 | 0.2504 |
| (0.6578) | (0.9208) | (0.5498) | |
| Sediment risk | -0.1174 | -0.9028b | -0.3513 |
| (0.7819) | (0.0021) | (0.3936) | |
| Combined risk | -0.0999 | 0.9197b | -0.4214 |
| (0.8138) | (0.0012) | (0.2984) | |
- a Significant at p = 0.05.
- b Significant at p = 0.01.
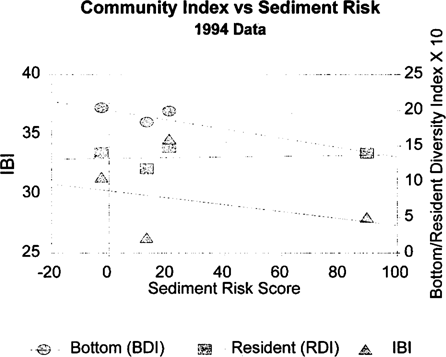
Plots of regression relationships between toxicological risk scores for sediment versus bottom and resident diversity index, and IBI for 1994 comparisons (bottom diversity index = BDI; resident diversity index = RDI; index of biotic integrity = IBI).
The relatively high fish community scores and low toxicological risk score in the Patuxent River are consistent. However, as indicated above, this system is much larger than any other tributary tested to date, and localized problems may exist, as indicated by the data from the middle station. This station was approximately 1.5 km downstream of a large steam electric power plant. Long-term benthic community monitoring data in the Patuxent River [32] demonstrate local regions where benthic community metrics indicate alternating healthy and degraded conditions down the length of the entire tributary.
The Severn River had a relatively high bottom diversity index but a low resident diversity index and IBI. The low IBI and resident indices in the Severn River system may be due to reasons other than ambient toxicity. This may not be the case in the upper reaches however.
Inclusion of this type of system in the correlation calculations affects the correlation results. As illustrated in Figure 7, the low IBI and resident diversity index values from the Severn River (Table 1) introduce scatter in the relationship between those parameters and the risk score at the low risk end (Severn River sediment toxicological risk score = 13.1). In contrast, the bottom diversity index for the Severn River is relatively high, consistent with a low sediment toxicological risk score.
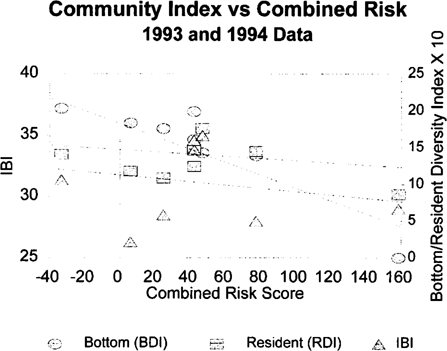
Plots of regression relationships between toxicological risk scores for sediment samples from seven tributaries of Chesapeake Bay sampled in 1993 and 1994 versus fish community metrics (bottom diversity index = BDI; resident diversity index = RDI; index of biotic integrity = IBI).
Correlation coefficients were calculated for the risk values and the IBI and diversity metrics for data pooled from 1993 and 1994 (Table 11). No factor to normalize the data for control results between years was attempted as in Dickson et al. [8]. The bottom diversity index is strongly correlated with the sediment and combined risk scores. The linear regression relationships between the community indices and sediment risk scores are shown in Figure 8. The sediment risk scores and the bottom diversity index show a clear relationship. The resident diversity index and IBI scores do not show as strong a correlation with the risk scores as does the bottom diversity index, but the trend for lower resident index values with higher sediment risk scores is evident.
Consistent with results from 1993, results from the Wicomico River displayed good fish community indices and low toxicological risk scores. The Wicomico River is an example of an area with high IBI/diversity indices and correspondingly low toxicological risk scores [4]. The South River and Curtis Creek correspond to areas with high toxicological risk scores and low IBI scores and/or diversity indices. Fishing Bay (sampled in 1993) and possibly the Severn River correspond to areas with low toxicological risk scores but with low IBI scores or diversity indices that do not necessarily reflect toxicological causes. These latter two stations demonstrated the lowest IBI and resident diversity index values in their respective sampling years, in contrast to the bottom diversity indices. None of the toxicological indices in either region indicate habitat degradation due to toxic contamination, with the exception of the upstream station in the Severn River. Low IBI scores in these areas may be due to habitat deficiencies, such as the absence of submerged aquatic vegetation in shallow areas [6]. Inclusion of these data tend to increase the statistical variability of the data set due to both low IBI and toxicological risk scores, which is contrary to the trend observed in other tributaries. There is no a priori justification to exclude these data from the analysis, but the fact that their bottom diversity indices are relatively high and the resident diversity indices are relatively low indicates a fundamentally different ecological situation than the South River and Curtis Creek, where the opposite is true.
The value of the toxicological risk ranking approach is that it was equally able to indicate where toxic contamination is and is not a likely impact, in the face of indications of impaired community health. Further studies will better define what is the sensitivity and background variability of the approach. As the toxicological data base expands, correlations with a variety of community monitoring data bases may also be possible (i.e., Maryland juvenile fish seine survey, long-term benthic monitoring program). Additional analyses need to be done to examine how well the toxicological risk ranking results from different years can be integrated. In addition, an assessment is needed of the importance of sampling intensity, relative to the size of the river system, on risk score sensitivity. Studies to address this latter question are in progress. Finally, the results of this study strongly indicate that the upper reaches of the South and Severn rivers are impacted by activities in the watershed as well as by facilities at specific locations along the shore. What those activities are, and what the chemical nature of the resultant toxicants released into rivers in developing watersheds need to be explored.
Acknowledgements
Invaluable field sampling assistance was provided by Margaret McGinty, Sandy Ives, Doug Randle, and Bill Rodney, under the direction of Stephen Jordan. Additional field assistance was provided by Randy Kerhin of the DNR, Maryland Geological Survey. This project was partially funded by NOAA through the Maryland Department of Natural Resources, Coastal and Watershed Resources Division grant NA 370 Z 0359.




